Abstract
Cervical cancer is the fourth leading cause of cancer mortality in women worldwide. High-risk human papillomavirus infection is a major cause of cervical cancer. A previous study revealed the role of different oncogenes and tumor suppressors in cervical cancer initiation and progression. However, the complicated genetic network regulating cervical cancer remains largely unknown. The present study reported transcriptome sequencing analysis of three cervical squamous cell cancer tissues and paired normal cervical tissues. Transcriptomic analysis revealed that 2,519 genes were differently expressed between cervical cancer tissues and their corresponding normal tissues. Among these, 236 differentially expressed genes (DEGs) were statistically significant, including many DEGs that were novel in cervical cancer, including gastrulation brain homeobox 2,5-hydroxytryptamine receptor 1D and endothelin 3. These 236 significant DEGs were highly enriched in 28 functional gene ontology categories. The Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis suggested involvement of these DEGs in multiple pathways. The present study provides a transcriptome landscape of cervical cancer in Chinese patients and an improved understanding of the genetic regulatory network in cervical cancer tumorigenesis.
Keywords: transcriptome analysis, cervical squamous cancer, RNA sequencing, differentially expressed genes
Introduction
There were 485,000 women newly diagnosed with cervical cancer worldwide in 2013, leading to 236,000 mortalities (1). The incidence and mortality of cervical cancer exhibits an uneven distribution across the world; almost 85% of cases are identified in less developed countries. Cervical cancer ranks second among the most diagnosed cancers and is the third leading cause of mortality from cancer in females in less developed countries (2).
It is well known that infection with human papilloma virus is a major cause of cervical cancer (3). Although well-established screening programs and intervention systems have significantly reduced the incidence of cervical cancer in developed countries, patients are still diagnosed with advanced cancer. Limited treatment options for patients with advanced stages of cervical cancer results in a high recurrence rate and a poor prognosis (2). Currently, the combination of cisplatin chemotherapy and radiotherapy is the most common treatment strategy for advanced cervical cancer patients; however, the 5-year survival rate remains poor (<50%). Since the development of pharmacogenomics and personalized medicine, target therapy has dramatically increased the survival rate of cancer patients (4). Treatment of advanced cervical cancers would drastically benefit from the identification of novel therapeutic targets.
With the development of next generation sequencing technologies, RNA sequencing (RNA-Seq) has become a powerful tool for analyzing cancer transcriptomes, detecting such items as alternative splicing, isoform usage, gene fusions and novel transcripts with greater accuracy and higher efficiency (5,6). Initially, RNA-Seq was applied in the expression profile of yeast (7) and human embryonic kidney and B cell lines (8). RNA-Seq has wide application in transcriptome profile analysis in multiple cancers including breast (9) and colon cancers (10). However, the genomic landscape of cervical squamous cell cancer has yet to be elucidated.
To provide an improved understanding of the transcriptome of cervical squamous cell cancer, RNA-Seq of three cervical squamous cell cancer and matched normal tissues was performed. Differentially expressed genes (DEGs) were identified by statistical analysis, and a subset of novel DEGs were validated by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). In addition, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was performed.
Materials and methods
Patient samples
For human tissue samples, 27 cervical squamous tumor samples (stage Ib-stage IIb) and matched adjacent normal tissues were obtained from female patients undergoing curative surgery at Fujian Cancer Hospital from April, 2013 to February, 2014 (Fuzhou, China). The median age of the patients was 49 years, (range, 31–59 years). The number of patients in each staging was as follows: Stage Ib (10), stage IIa (12), stage IIb (5). None of the patients received preoperative adjuvant radiotherapy or chemotherapy. All the samples and matched clinical information were collected following written informed consent from the patients.
RNA isolation and RT-qPCR
Total RNA from human cervical squamous cancer tissues and corresponding normal tissues was prepared using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol. The RNA quality was determined using an Agilent 2100 Bioanalyzer (Agilent Technologies Inc., Santa Clara, CA, USA). Only RNA extracts with the following criteria were used for RNA-Seq analysis: RNA integrity number, ≥7; 28S/18S ratio, >1.8; OD range, 1.9–2.1. For reverse transcription, cDNA was synthesized using the ReverTra Ace qPCR RT kit (Toyobo Co., Ltd., Osaka, Japan) with 1 µg of total RNA. qPCR was performed using the SYBR Select Master Mix for CFX (Invitrogen; Thermo Fisher Scientific, Inc.). The reaction consisted of 1 cycle at 95°C for 15 min, followed by 40 cycles of 95°C for 15 sec, 55°C for 30 sec and 70°C for 30 sec. Primer sequences are presented in Table I. Relative quantification was achieved by normalization to the amount of GAPDH using the 2−ΔΔCq method (11). All measurements were repeated 3 times.
Table I.
Primers used for reverse transcription quantitative polymerase chain reaction.
| Genes | Forward primer | Reverse primer |
|---|---|---|
| PRAC | 5′-ATTCTGGTCCCCACCTTTGC-3′ | 5′-GGAGGTAGTAAGATGGGCCG-3′ |
| EDN3 | 5′-AGACGGTGCCCTATGGACT-3′ | 5′GGTCCTTGACTTCAACCTCCTT-3′ |
| SOSTDC1 | 5′-AAGTGCAAGAGGTACACCCG-3′ | 5′-GGCTCTTTTCCGCTCTCTGT-3′ |
| KLK12 | 5′-GTAACCAGCAGCGTTCAACC-3′ | 5′-AGAGTGGAGTTGCAAATATAGGT-3′ |
| KLK13 | 5′-AGCAGGTGAGGGAAGTTGTC-3′ | 5′-GAAAGGGGCAGGGTTTGGAT-3′ |
| KLK5 | 5′-TTTTCAGAGTCCGTCTCGGC-3′ | 5′-GGATGGATTTGACCCCCTGG-3′ |
| KLK6 | 5′-ATAAGTTGGTGCATGGCGGA-3′ | 5′-GGAACTCTCCCTTTGCCGAA-3′ |
| OLIG2 | 5′-TCAAGATCAACAGCCGCGAG-3′ | 5′-GTAGATCTCGCTCACCAGTCG-3′ |
| GBX2 | 5′-AGGGCAAGGGAAAGACGAGT-3′ | 5′-GTAGTCCACATCGCTCTCCA-3′ |
| MUC16 | 5′-TGAGGAGAACATGTGGCCTG-3′ | 5′-GCTGCATGACGTTGTCTGTG-3′ |
| CCL1 | 5′-CAGCTCCATCTGCTCCAATGA-3′ | 5′-TTCTGTGCCTCTGAACCCATC-3′ |
| HTR1D | 5′-CCCTCGGTGTTGCTCATCAT-3′ | 5′-CAGAGCCTGTGATGAGGTGG-3′ |
| GAPDH | 5′-TGGTATCGTGGAAGGACT-3′ | 5′-AGGGATGATGTTCTGGAGA-3′ |
PRAC; PRAC1 small nuclear protein; EDN3, endothelin 3; SOSTDC1, sclerostin domain containing 1; KLK, kallikrein related peptidase; OLIG2, oligodendrocyte transcription factor 2; GBX2, gastrulation brain homeobox 2; MUC16, mucin 16; CCL1, C-C motif chemokine ligand 1; HTR1D, 5-hydroxytryptamine receptor 1D.
Transcriptome deep sequencing
Total RNA extracted from human samples was treated with DNase I. Upon treatment, mRNA was isolated using Oligo d(T)25 Magnetic Beads (New England Biolabs, Inc., Ipswich, MA, USA). RNA preparation was performed as previously described (12). The mRNA was digested into short fragments and cDNA was synthesized using the mRNA fragments as templates. Short fragments were purified and resolved with elution buffer for end reparation and single nucleotide A (adenine) addition. Then the short fragments were connected with adapters and, following agarose gel electrophoresis, the suitable fragments were selected for the PCR amplification as templates. During the quality control steps, an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., and an ABI StepOnePlus Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) were used in quantification and qualification of the sample library. The library was sequenced using an Illumina HiSeq™ 2000 (Illumina, Inc., San Diego, CA, USA).
Screening of DEGs
The method for screening of DEGs was developed by Beijing Genomics Institute (BGI, Shenzhen, China) based on a pervious study (13).
The present study defined the number of unambiguous clean tags (which means reads in RNA-Seq) from gene A as × and, given that every gene's expression occupies only a small part of the library, × yields to the Poisson distribution.
The total clean tag number of the sample 1 is N1 and the total clean tag number of sample 2 is N2; gene A holds × tags in sample 1 and y tags in sample 2. The probability of gene A expressed equally between the two samples can be calculated with:
A P-value corresponds to the differential gene expression test. Since DEG analysis generates large multiplicity problems in which thousands of hypotheses (as in whether gene × is differentially expressed between the two groups) are tested simultaneously, a correction for false positive (type I errors) and false negative (type II) errors was performed using the false discovery rate (FDR) method (14). This method assumed that R differentially expressed genes have been selected in which S genes really demonstrate differential expression and the other V genes are false positives. If it is decided that the error ratio ‘Q=V/R’ must stay below a cutoff (e.g. 5%), then the FDR should be preset to a number no larger than 0.05. FDR ≤0.001 (14) was used and the absolute value of Log2Ratio≥1 as the threshold to judge the significance of a gene expression difference.
GO analysis of DEGs
All DEGs were mapped to GO terms in the database (http://www.geneontology.org/), calculating gene numbers for every term. Then a hypergeometric was used for the test to find significantly enriched GO terms in the input list of DEGs, based on ‘GO:TermFinder’ (http://search.cpan.org/dist/GO-TermFinder). Then a strict method was developed to perform this analysis:
Where N is the number of all genes with GO annotation; n is the number of DEGs in N; M is the number of all genes that are annotated to certain GO terms; and, m is the number of DEGs in M. The calculated P-value was adjusted through a Bonferroni Correction (15), and a corrected P-value ≤0.05 was used as a threshold. GO terms fulfilling this condition were defined as significantly enriched GO terms in DEGs.
Pathway enrichment analysis of DEGs
The formula was the same as that in GO analysis. Here N is the number of all genes with KEGG annotation, n is the number of DEGs in N, M is the number of all genes annotated to specific pathways and m is the number of DEGs in M.
Statistical analysis
Results from qPCR were analysed using SPSS version 19.0 (SPSS Corp., Armonk, NY, USA). Student's t-test was used to compare the differences in expression between cancer and normal tissues. Cluster analysis was performed using Cluster 3.0 (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm); the R version 3.4.0 (R Foundation) with heatmap package (https://stat.ethz.ch/R-manual/R-devel/library/stats/html/heatmap.html) was applied to the Pearson and Spearman clustering analysis. P<0.05 was considered to indicate a statistically significant result.
Results
Basic analysis of sequencing data
A basic analysis of sequencing data was performed, whereby the data was compared with the human genome using TopHat version 2.0.9 (https://ccb.jhu.edu/software/tophat/index.shtml); 48.47, 48.79 and 48.77 million clean reads were obtained from cervical squamous cell cancer samples 1 (stage I b), 2 (stage II a) and 3 (stage II b) with genome map rates (proportion of reads mapped to a reference genome) of 85.04, 84.74 and 83.79%, respectively. From the matched normal cervical samples 1–3, 47.31, 48.30 and 47.54 million clean reads were obtained with genome map rates of 86.90, 86.33 and 86.03% respectively.
Analysis of DEGs
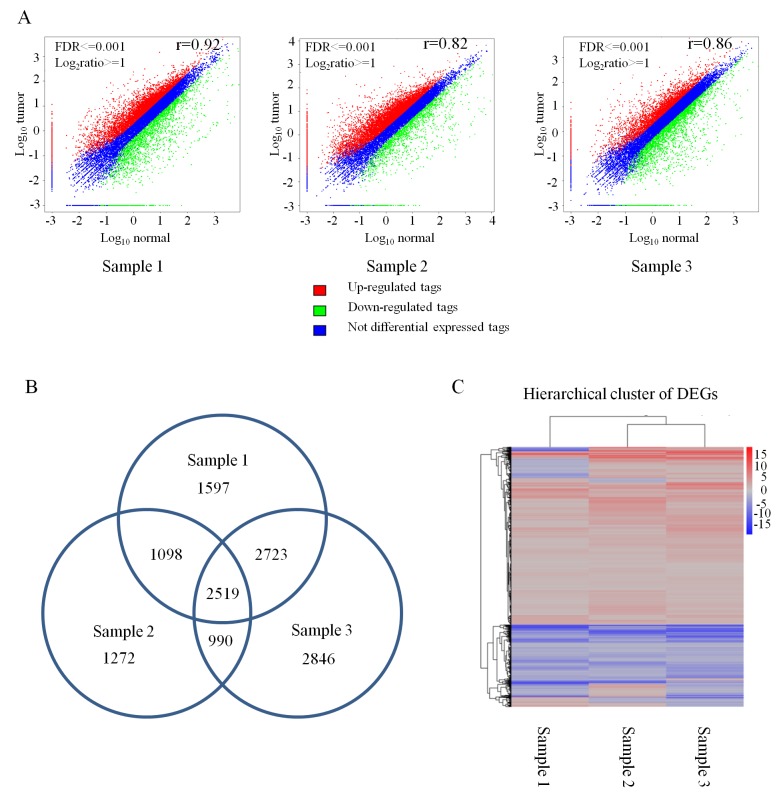
Next, the DEGs between cancer and normal tissue samples were screened. The gene expression level was normalized and measured by reads per kb of exon per million fragments mapped as described above. In total, 7,936 DEGs were detected in cancer/normal sample 1, 9,077 DEGs in cancer/normal sample 2, and 5,878 DEGs in cancer/normal sample 3 (Fig. 1A). Then, the overlapping DEGs in three pairs of samples were calculated and resulted in 2,519 overlapping DEGs in which 1,450 genes were consistently upregulated in the three cancer samples and 554 genes were consistently downregulated in the three cancer samples (Fig. 1B). However, the rest of the 515 DEGs were not consistently expressed in all three pairs of samples (Fig. 1B). Cluster analysis revealed that 2,519 DEGs were identical in the three pairs of samples (Fig. 1C). To screen for the statistically significant DEGs, DEGs with fold changes ≥3 were filtered out. With this threshold set, a total of 236 significant DEGs were detected in the three sample pairs, among which 84 DEGs were consistently upregulated and 152 DEGs were consistently downregulated (Table II).
Figure 1.
Analysis of differentially expressed genes in cervical squamous cancer tissues and matched normal tissues. (A) Scatter plots of global expression between cancer and normal samples 1–3 were produced by Pearson correlation coefficient analysis. (B) Venn diagram indicating the overlapping DEGs among the three pairs of samples. (C) Hierarchical clustering of DEGs among cervical cancer tissues and matched normal tissues from the three sample pairs. FDR, false discovery rate; DEGs, differentially expressed genes.
Table II.
List of significant differentially expressed genes.
| Gene symbol | Log2 (cancer vs. normal 1) | Log2 (cancer vs. normal 2) | Log2 (cancer vs. normal 3) | Average |
|---|---|---|---|---|
| GRP | 10.54868 | 9.411035 | 8.89707 | 9.61893 |
| NCRNA00313 | 9.817818 | 9.227841 | 9.280222 | 9.44196 |
| GBX2 | 8.452149 | 7.907159 | 11.89702 | 9.418777 |
| DUSP5P | 9.723775 | 8.01832 | 9.378824 | 9.040307 |
| OSTCL | 8.224955 | 9.28938 | 9.012454 | 8.842263 |
| SLC1A6 | 11.25474 | 6.720402 | 8.382081 | 8.785743 |
| MUC16 (CA125) | 9.088256 | 7.442924 | 9.262444 | 8.597873 |
| C8orf39 | 11.38619 | 10.93562 | 3.461771 | 8.594527 |
| CCL1 | 4.438704 | 9.430026 | 11.37549 | 8.41474 |
| HTR1D | 9.43695 | 7.39446 | 7.546875 | 8.126093 |
| SLFN12L | 5.035945 | 9.239662 | 9.971725 | 8.082443 |
| EN2 | 8.56846 | 5.597178 | 9.531558 | 7.899067 |
| OLIG2 | 6.906961 | 8.928602 | 7.520431 | 7.78533 |
| CAMP | 9.640688 | 9.946121 | 3.303074 | 7.62996 |
| SALL4 | 8.219391 | 10.70523 | 3.458123 | 7.460917 |
| TMED8 | 3.514758 | 12.33337 | 6.105627 | 7.31792 |
| FOXD3 | 3.729651 | 8.969243 | 9.12116 | 7.27335 |
| DNAJC5B | 9.583564 | 8.162562 | 3.478845 | 7.07499 |
| MMP3 | 7.707342 | 9.060809 | 4.216957 | 6.995037 |
| ONECUT2 | 8.016706 | 5.936664 | 6.709699 | 6.88769 |
| SLC24A2 | 7.428435 | 4.883445 | 8.076839 | 6.79624 |
| XIRP1 | 4.323227 | 6.049513 | 9.572409 | 6.648383 |
| TUBA3D | 4.153302 | 7.926962 | 7.772893 | 6.61772 |
| TM7SF4 | 7.775048 | 7.820021 | 4.203538 | 6.599537 |
| FOXD1 | 6.305949 | 8.09621 | 4.807546 | 6.403233 |
| DNAH5 | 7.543471 | 8.300234 | 3.291846 | 6.378517 |
| ZBED6 | 3.223498 | 8.059951 | 7.649948 | 6.311133 |
| INHBA | 5.49018 | 5.640679 | 7.472004 | 6.200953 |
| COL10A1 | 4.945116 | 9.64287 | 3.992286 | 6.193423 |
| CCL18 | 7.794751 | 7.065377 | 3.527205 | 6.12911 |
| HIST1H3G | 3.986192 | 10.74443 | 3.33624 | 6.022287 |
| ESM1 | 5.17567 | 7.531932 | 5.246043 | 5.984547 |
| PLA2G2F | 7.136285 | 7.286441 | 3.465107 | 5.96261 |
| GLDC | 5.663359 | 7.425411 | 4.524814 | 5.871193 |
| CSF2 (GMCSF) | 3.153302 | 10.14843 | 4.162211 | 5.821313 |
| GAS2L3 | 4.311731 | 8.448034 | 4.498861 | 5.752877 |
| FAM172BP | 3.960657 | 8.749995 | 4.269126 | 5.659927 |
| SCN8A | 8.282632 | 4.251928 | 4.299341 | 5.6113 |
| IL24 | 6.704049 | 5.178865 | 4.448351 | 5.443753 |
| AIM2 | 3.001299 | 9.176301 | 3.688599 | 5.288733 |
| KLHDC7B | 3.522536 | 6.009268 | 6.234041 | 5.25528 |
| DSCR6 | 8.119086 | 4.197481 | 3.278907 | 5.19849 |
| CXCL5 | 5.389794 | 5.913205 | 4.16949 | 5.157497 |
| TNNI3 | 4.841802 | 5.316369 | 5.269126 | 5.142433 |
| C1QL1 | 5.261826 | 3.796676 | 6.166828 | 5.07511 |
| DNAH11 | 5.814367 | 4.598159 | 4.538047 | 4.983523 |
| CELSR3 | 4.009208 | 6.425826 | 4.502799 | 4.979277 |
| PTPRR | 3.797158 | 7.448663 | 3.660185 | 4.96867 |
| MGAM | 5.090475 | 4.840449 | 4.606466 | 4.845797 |
| LOC100287559 | 6.917588 | 4.441899 | 3.124736 | 4.828073 |
| ITGB6 | 3.707891 | 7.290941 | 3.246702 | 4.74851 |
| C1orf152 | 3.551851 | 7.043164 | 3.615894 | 4.73697 |
| VWA3B | 3.908189 | 6.293915 | 3.985333 | 4.729147 |
| EPHB2 | 3.98941 | 6.179193 | 3.841365 | 4.66999 |
| LOC148696 | 3.153302 | 7.298488 | 3.33624 | 4.59601 |
| JPH2 | 3.888979 | 3.351702 | 6.529295 | 4.589993 |
| CDKN2A | 4.664062 | 5.672743 | 3.415469 | 4.58409 |
| TNNT1 | 5.645155 | 4.608629 | 3.449388 | 4.567723 |
| SIM2 | 5.530822 | 4.196787 | 3.783699 | 4.50377 |
| XIST | 3.744393 | 5.117718 | 4.556763 | 4.472957 |
| LOC100775107 | 3.563586 | 5.085756 | 4.595627 | 4.41499 |
| PLOD2 | 4.898069 | 4.881134 | 3.319208 | 4.366137 |
| CNGB1 | 4.806744 | 4.230395 | 3.852998 | 4.296713 |
| RSAD2 | 3.337828 | 4.916125 | 4.472057 | 4.242003 |
| FLJ43390 | 5.751652 | 3.737355 | 3.046734 | 4.17858 |
| EGFR | 5.416012 | 3.283692 | 3.41214 | 4.03728 |
| LOC100190986 | 3.642481 | 4.901331 | 3.562375 | 4.035397 |
| FAM63B | 3.302318 | 4.095168 | 4.669664 | 4.022383 |
| UBXN7 | 3.777425 | 4.331877 | 3.822488 | 3.977263 |
| LOC283299 | 3.47523 | 3.682908 | 4.577248 | 3.911797 |
| ARHGAP11B | 4.835126 | 3.582381 | 3.198737 | 3.87208 |
| BCAT1 | 4.92309 | 3.413721 | 3.162697 | 3.83317 |
| IGF2BP2 | 3.48637 | 4.974384 | 3.016948 | 3.8259 |
| IFIT3 | 3.33571 | 3.670937 | 4.105221 | 3.703957 |
| IFIT2 | 4.388168 | 3.335629 | 3.319752 | 3.681183 |
| GALNT4 | 4.730731 | 3.012215 | 3.124736 | 3.62256 |
| ATP13A3 | 3.28667 | 4.347695 | 3.224075 | 3.61948 |
| TRIO | 3.506362 | 3.683108 | 3.63553 | 3.608333 |
| KIAA0895 | 3.323227 | 3.2336 | 4.08545 | 3.547427 |
| PTPLB | 3.033159 | 4.04489 | 3.517358 | 3.531803 |
| WDR31 | 3.503799 | 3.477879 | 3.509077 | 3.496917 |
| ASPHD1 | 3.438704 | 3.304396 | 3.282801 | 3.341967 |
| FLJ34208 | 3.612733 | 3.230395 | 3.124736 | 3.322622 |
| IL1B | 3.113062 | 3.39834 | 3.179301 | 3.230234 |
| PRAC | −13.4151 | −13.6146 | −12.5618 | −13.1972 |
| EDN3 | −7.12592 | −15.8836 | −11.4601 | −11.4899 |
| FABP12 | −11.8695 | −10.8052 | −10.1364 | −10.937 |
| LOC644759 | −12.1577 | −10.3962 | −10.1672 | −10.907 |
| KLK12 | −9.45888 | −9.66283 | −12.3341 | −10.4853 |
| SOSTDC1 | −5.56369 | −13.3193 | −11.2345 | −10.0392 |
| PROK1 | −6.99645 | −8.77908 | −13.103 | −9.62617 |
| NCRNA00160 | −10.4406 | −9.28356 | −8.65661 | −9.46027 |
| HS3ST6 | −4.58141 | −14.2018 | −9.36341 | −9.3822 |
| LOC642366 | −9.06419 | −9.70611 | −8.97064 | −9.24697 |
| KLK5 | −6.72497 | −6.72175 | −14.0832 | −9.17663 |
| LGI3 | −9.66722 | −12.6499 | −4.90455 | −9.07387 |
| C18orf26 | −11.4915 | −8.05915 | −7.5957 | −9.0488 |
| KLK6 | −10.1991 | −6.49943 | −10.1097 | −8.93607 |
| FTLP10 | −9.17158 | −9.63008 | −7.80673 | −8.86947 |
| KRT13 | −7.04312 | −9.72684 | −9.80361 | −8.85787 |
| SDR9C7 | −8.99263 | −5.71915 | −11.7824 | −8.8314 |
| KLK13 | −7.35941 | −9.8484 | −8.89013 | −8.6993 |
| CYP4F22 | −7.35608 | −9.07243 | −9.50559 | −8.6447 |
| CWH43 | −8.37808 | −4.96987 | −12.2752 | −8.54107 |
| UPK1A | −8.75809 | −11.6327 | −5.03608 | −8.47563 |
| SPRR2C | −9.5305 | −6.16292 | −9.61626 | −8.43657 |
| ISL1 | −6.24758 | −11.9118 | −6.85564 | −8.33833 |
| CRNN | −9.03239 | −10.5285 | −5.43238 | −8.3311 |
| TMPRSS11BNL | −12.7646 | −7.64689 | −4.48074 | −8.29743 |
| SPRR3 | −9.08119 | −9.16385 | −6.62833 | −8.29113 |
| MUC21 | −7.66328 | −9.01786 | −7.95629 | −8.21247 |
| SFTA2 | −6.72934 | −6.03851 | −11.348 | −8.0386 |
| KRTDAP | −10.8126 | −9.41425 | −3.83469 | −8.0205 |
| HOXB13-AS1 | −9.26181 | −10.8682 | −3.93055 | −8.0202 |
| SPINK5 | −7.0192 | −8.64938 | −8.34653 | −8.00503 |
| ARSF | −12.2472 | −3.60249 | −8.14556 | −7.99843 |
| KRT4 | −6.28401 | −9.91166 | −7.48655 | −7.89407 |
| MAL | −9.13047 | −9.3469 | −5.19779 | −7.89173 |
| SPINK7 | −9.40574 | −9.496 | −4.58648 | −7.8294 |
| KLK11 | −5.43434 | −9.29366 | −8.63959 | −7.7892 |
| LOC144817 | −3.11972 | −10.3462 | −9.80503 | −7.757 |
| SPRR1B | −8.95135 | −5.98911 | −7.93974 | −7.62673 |
| SBSN | −8.96791 | −9.33194 | −4.57612 | −7.62533 |
| CLCA4 | −5.66679 | −5.92101 | −11.1121 | −7.56663 |
| TMPRSS11A | −5.85846 | −5.25567 | −11.315 | −7.4764 |
| TMPRSS11B | −8.1584 | −9.30062 | −4.81077 | −7.42327 |
| PRSS3 | −8.39642 | −8.84527 | −4.99691 | −7.41287 |
| PBMUCL1 | −8.68117 | −6.30909 | −7.22153 | −7.40393 |
| FADS6 | −7.76112 | −5.07643 | −9.35563 | −7.39773 |
| KLK9 | −8.21271 | −3.01753 | −10.9561 | −7.39543 |
| LRRTM4 | −6.356 | −7.06931 | −8.7054 | −7.3769 |
| ALOX12B | −9.21357 | −8.75139 | −4.12319 | −7.36273 |
| RHCG | −8.60154 | −4.29579 | −8.9801 | −7.29247 |
| RDH12 | −8.76324 | −7.91437 | −5.01249 | −7.23003 |
| TMPRSS11D | −5.68278 | −5.64144 | −10.3586 | −7.2276 |
| KLK10 | −4.85195 | −7.938 | −8.84348 | −7.21113 |
| DUOXA2 | −6.6414 | −7.13884 | −7.7198 | −7.16667 |
| KRT16P1 | −8.39166 | −6.41271 | −6.65457 | −7.15297 |
| WFDC5 | −5.94473 | −3.59185 | −11.6523 | −7.06297 |
| TMPRSS11F | −5.56643 | −4.27303 | −11.237 | −7.0255 |
| KRT78 | −8.71162 | −9.09812 | −3.24017 | −7.01663 |
| CYP2C18 | −5.67597 | −9.10234 | −6.21065 | −6.99633 |
| ENDOU | −8.373 | −6.00788 | −6.5908 | −6.99057 |
| TMPRSS11E | −7.36594 | −4.83162 | −8.75233 | −6.9833 |
| KLK8 | −5.28042 | −7.14018 | −8.49714 | −6.97257 |
| SLURP1 | −7.64971 | −9.05043 | −4.11415 | −6.9381 |
| HOXB13 | −11.8912 | −3.76729 | −5.14309 | −6.93387 |
| NR0B1 | −3.16863 | −8.78707 | −8.8382 | −6.9313 |
| DSG1 | −7.27882 | −9.56852 | −3.70815 | −6.85183 |
| OLFM4 | −4.41224 | −4.86631 | −11.2427 | −6.84043 |
| ASPG | −9.35845 | −5.80053 | −5.34926 | −6.83607 |
| SPRR2F | −11.0558 | −5.91653 | −3.44512 | −6.8058 |
| HCG22 | −6.72322 | −9.14481 | −4.41865 | −6.76223 |
| KRT16P3 | −6.29516 | −6.68591 | −7.20534 | −6.7288 |
| CPA6 | −4.97598 | −3.57612 | −11.5417 | −6.69793 |
| KRT6C | −7.20141 | −6.9751 | −5.85838 | −6.6783 |
| SCEL | −7.31534 | −4.10998 | −8.50819 | −6.6445 |
| CRHR1 | −4.20425 | −8.6737 | −6.95224 | −6.61007 |
| KLK7 | −4.16482 | −7.54068 | −8.07971 | −6.59507 |
| FABP4 | −3.60159 | −10.8444 | −5.24657 | −6.5642 |
| C18orf34 | −3.43166 | −8.03628 | −7.85036 | −6.43943 |
| IVL | −9.68789 | −3.53827 | −6.07053 | −6.43223 |
| SPRR2A | −8.33607 | −6.76353 | −4.16411 | −6.42123 |
| GJB6 | −5.78401 | −4.68417 | −8.7729 | −6.4137 |
| SPRR1A | −8.69142 | −5.35853 | −5.18329 | −6.41107 |
| CEACAM7 | −3.8467 | −6.03077 | −9.32939 | −6.4023 |
| PPP1R3C | −6.54484 | −9.23918 | −3.33717 | −6.37373 |
| C2orf54 | −8.04377 | −5.14492 | −5.76023 | −6.3163 |
| CCL14 | −3.97598 | −11.2998 | −3.62144 | −6.29907 |
| PRSS27 | −8.34943 | −4.60582 | −5.83476 | −6.26333 |
| FUT6 | −4.6948 | −6.47518 | −7.48074 | −6.2169 |
| LRP1B | −4.69219 | −8.09952 | −5.84579 | −6.2125 |
| KCNK10 | −4.25851 | −6.50135 | −7.80251 | −6.18747 |
| WISP2 | −5.54635 | −6.65683 | −6.06 | −6.08773 |
| SPRR2D | −8.64649 | −6.06525 | −3.46049 | −6.0574 |
| CYP2B7P1 | −4.29557 | −6.36724 | −7.48514 | −6.0493 |
| CLDN8 | −3.1579 | −5.95613 | −8.77604 | −5.96337 |
| GDF6 | −4.05615 | −7.32225 | −6.49891 | −5.9591 |
| TMEM45B | −5.61223 | −5.92896 | −6.3174 | −5.95287 |
| NEFL | −4.20425 | −3.92442 | −9.62952 | −5.9194 |
| DAPL1 | −5.71856 | −4.88481 | −7.14556 | −5.9163 |
| FAM3D | −5.63608 | −4.61094 | −7.45818 | −5.90173 |
| C10orf99 | −5.66475 | −3.59184 | −8.361 | −5.87253 |
| ATP13A4 | −4.13757 | −6.36183 | −7.11188 | −5.87043 |
| PSCA | −8.80031 | −5.31464 | −3.37421 | −5.82973 |
| GSTM5 | −4.86629 | −7.56056 | −4.82363 | −5.75017 |
| CRYM | −4.82557 | −7.89036 | −4.42697 | −5.7143 |
| ALOX12 | −6.72993 | −7.05622 | −3.27911 | −5.68843 |
| LYPD2 | −5.20351 | −6.50013 | −5.28866 | −5.6641 |
| DEGS2 | −3.47443 | −6.45172 | −6.87474 | −5.6003 |
| TP53AIP1 | −4.83451 | −6.02192 | −5.90829 | −5.58823 |
| APOD | −4.46756 | −8.75006 | −3.50099 | −5.57287 |
| PLA2G4F | −5.6344 | −3.88007 | −7.0168 | −5.51043 |
| TMEM132C | −3.09463 | −10.0647 | −3.37112 | −5.51017 |
| GJB2 | −6.73282 | −3.11385 | −6.60454 | −5.48373 |
| PTGDS | −5.8487 | −6.56155 | −4.02011 | −5.4768 |
| NCCRP1 | −6.9491 | −3.38815 | −6.05232 | −5.4632 |
| KRT16P2 | −4.82398 | −5.03598 | −6.48074 | −5.4469 |
| DUOX2 | −3.89572 | −4.21314 | −8.14256 | −5.41713 |
| LOC283392 | −3.65906 | −4.6075 | −7.9533 | −5.40663 |
| C21orf15 | −4.96246 | −7.22455 | −4.03008 | −5.4057 |
| HOPX | −4.52883 | −6.72314 | −4.68297 | −5.31163 |
| C7 | −4.26858 | −5.33946 | −6.24421 | −5.28407 |
| FXYD1 | −4.38736 | −8.16219 | −3.10047 | −5.21667 |
| VSIG10L | −5.64524 | −6.67538 | −3.28431 | −5.20163 |
| CXCL14 | −4.06317 | −4.69872 | −6.79264 | −5.18483 |
| C1orf177 | −7.12468 | −4.89746 | −3.40118 | −5.1411 |
| PGLYRP3 | −4.2413 | −5.30136 | −5.87213 | −5.13827 |
| NDRG4 | −3.11573 | −6.16805 | −6.10851 | −5.13077 |
| SH3GL2 | −3.43166 | −7.88472 | −4.03008 | −5.1155 |
| D4S234E | −6.32648 | −5.24772 | −3.58727 | −5.05383 |
| HSPB6 | −4.66679 | −6.44156 | −3.92896 | −5.01243 |
| KRT32 | −4.88012 | −4.74755 | −5.40859 | −5.0121 |
| C15orf59 | −3.52682 | −5.00472 | −6.41321 | −4.98157 |
| ECM1 | −5.89039 | −4.95876 | −3.74402 | −4.8644 |
| PKNOX2 | −3.80089 | −7.16728 | −3.44512 | −4.80443 |
| SLC22A3 | −5.01115 | −5.30293 | −4.01922 | −4.77777 |
| SCN2B | −3.37919 | −6.85042 | −3.98117 | −4.73693 |
| BNIPL | −3.30443 | −4.13255 | −6.42697 | −4.62133 |
| S100A12 | −5.68204 | −3.48731 | −4.64434 | −4.60457 |
| TPRG1 | −5.48765 | −5.09685 | −3.17304 | −4.58583 |
| F10 | −4.46875 | −5.39257 | −3.72098 | −4.52743 |
| KRT14 | −5.32 | −3.2456 | −5.00968 | −4.5251 |
| PGLYRP4 | −5.26736 | −3.75249 | −4.48259 | −4.5008 |
| CYP2F1 | −5.58817 | −3.60249 | −4.21065 | −4.4671 |
| SCARA5 | −3.8261 | −6.19246 | −3.34042 | −4.453 |
| DNASE1L3 | −4.77494 | −3.71102 | −4.65301 | −4.37967 |
| CD300LG | −5.09807 | −3.14682 | −4.72805 | −4.3243 |
| FABP5 | −3.59716 | −3.02627 | −6.02887 | −4.21743 |
| CORIN | −3.67113 | −5.92442 | −3.04919 | −4.2149 |
| TGM5 | −4.13436 | −3.93665 | −4.13218 | −4.06773 |
| TMPRSS2 | −3.3997 | −3.8615 | −4.73918 | −4.00013 |
| SCN4B | −3.0682 | −4.22699 | −4.44512 | −3.91343 |
| ANXA9 | −3.47853 | −3.71392 | −4.52455 | −3.90567 |
| NEFM | −4.29673 | −3.47696 | −3.66751 | −3.81373 |
| CD1E | −3.4358 | −3.40555 | −3.64926 | −3.49687 |
Validation of significant DEGs
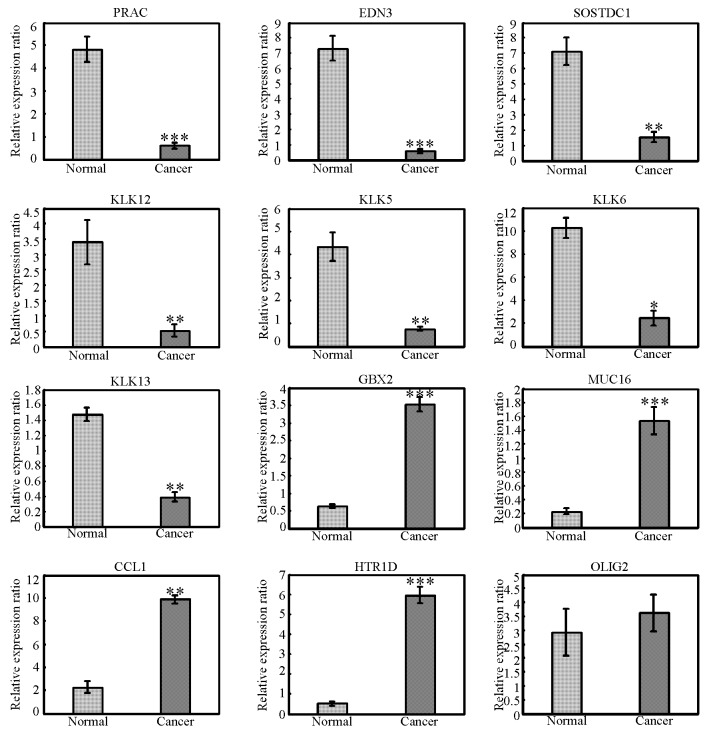
To validate the transcriptome analysis data, RT-qPCR was performed on cancer and normal tissue samples to examine the mRNA expression levels for several DEGs identified in the present study. First, established oncogenes and tumor suppressor genes in other types of cancers were examined; gastrulation brain homeobox 2 (GBX2), mucin 16 (MUC16), C-C motif chemokine ligand 1 (CCL1), 5-hydroxytryptamine receptor 1D (HTR1D) and oligodendrocyte transcription factor 2 (OLIG2) are putative oncogene candidates, while PRAC1 small nuclear protein (PRAC), endothelin 3 (EDN3), sclerostin domain containing 1 (SOSTDC1), kallikrein related peptidase (KLK) 12, KLK5, KLK6 and KLK13 are putative tumor suppressors. The mRNA expression levels for these genes were validated in the 27 pairs of cervical squamous cancer samples and matched normal tissue samples. As demonstrated in Fig. 2, expression of GBX2, MUC16, CCL1 and HTR1D was significantly upregulated in cancer samples compared with normal tissues, while expression of PRAC, EDN3, SOSTDC1, KLK12, KLK5, KLK6 and KLK13 was significantly downregulated in cancer samples compared with normal tissues. However, no significance change in expression of OLIG2 was observed in the 27 pairs of samples analysed. OLIG2 is a basic helix-loop-helix transcription factor expressed in the developing central nervous system (CNS) and the postnatal brain (16). OLIG2 is highly expressed in glioblastoma cells, and in glioblastoma initiating cells in particular (16), while expression of OLIG2 is barely detected in other types of cancer. Tissue-specific expression of OLIG2 in the CNS may explain why no significant change in OLIG2 expression was observed in cervical cancer samples.
Figure 2.
Validation of differentially expressed genes. The mRNA expression levels of PRAC, EDN3, SOSTDC1, KLK12, KLK13, KLK5, KLK6, OLIG2, GBX2, MUC16, CCL1 and HTR1D were examined by reverse transcription-quantitative polymerase chain reaction in the 27 pairs of cancer and matched normal samples. *P<0.05, **P<0.01 and ***P<0.001 vs. normal. PRAC, PRAC1 small nuclear protein; EDN3, endothelin 3; SOSTDC1, sclerostin domain containing 1; KLK, kallikrein related peptidase; GBX2, gastrulation brain homeobox 2; MUC16, mucin 16; CCL1, C-C motif chemokine ligand 1; HTR1D, 5-hydroxytryptamine receptor 1D; OLIG2, oligodendrocyte transcription factor 2.
Functional enrichment analysis of DEGs
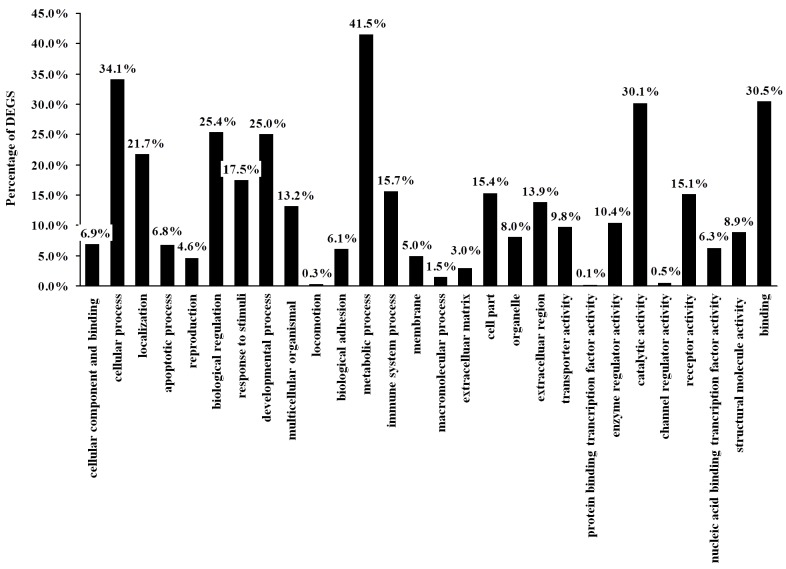
Next, GO analysis of DEGs was performed to obtain a more comprehensive understanding of gene-related biological functions. All DEGs were divided into three major categories based on GO annotations: Biological processes, cellular components and molecular functions. The present study identified that the 236 DEGS were classified into 28 functional categories (Table III); 13 in biological processes, 9 in molecular functions and 6 in cellular components including reproduction, cellular processes and metabolic processes (P<0.05; Fig. 3).
Table III.
Detailed list of significant DEGs by functional categories from GO analysis.
| Functional category | Significant DEGs |
|---|---|
| Binding (GO:0005488) | EDN3, CXCL5, GBX2, LRRTM4, TMPRSS11D, WFDC5, NR0B1, VWA3B, CELSR3, INHBA, ONECUT2, OLIG2, PROK1, TMPRSS11B, KLK10, KLK7, PTGDS, F10, IL1β, CCL1, ISL1, SPINK5, GDF6, FOXD3, CRNN, KLK11, TMPRSS11A, KLK9, TMPRSS11F, TMPRSS11E, HOXB13, CCL14, S100A12, IGF2BP2, IL24, CCL18, KLK12, KLK5, BNIPL, TMPRSS2, TNNI3, ESM1, FOXD1, CAMP, SPINK7, PRSS27, WISP2, PGLYRP3, CNGB1, EN2, UPK1A, KLK8, DSG1, PPP1R3C, DAPL1, PGLYRP4, CD1E, TRIO, SIM2, GAS2L3, SALL4 |
| Catalytic activity (GO:0003824) | TMPRSS11D, WFDC5, VWA3B, OLIG2, TMPRSS11B, KLK10, KLK7, PTGDS, F10, KLK11, TMPRSS11F, TMPRSS11E, IGF2BP2, KLK12, KLK5, TMPRSS2, CAMP, SPINK7, PRSS27, KLK8, PPP1R3C, DAPL1, TRIO |
| Enzyme regulator activity | TMPRSS11D, WFDC5, VWA3B, OLIG2, TMPRSS11B, KLK10, KLK7, PTGDS, F10, |
| (GO:0030234) | KLK11, TMPRSS11F, TMPRSS11E, IGF2BP2, KLK12, KLK5, TMPRSS2, CAMP, SPINK7, PRSS27, KLK8, PPP1R3C, DAPL1, TRIO |
| Nucleic acid binding transcription factor activity (GO:0001071) | GBX2, NR0B1, ONECUT2, OLIG2, ISL1, FOXD3, HOXB13, FOXD1, EN2, SIM2, SALL4 |
| Receptor activity (GO:0004872) | LRRTM4, TMPRSS11D, NR0B1, CELSR3, TMPRSS11B, KLK10, KLK7, F10, KLK11, TMPRSS11A KLK9, TMPRSS11F, TMPRSS11E, KLK12, KLK5, TMPRSS2, PRSS27, UPK1A, KLK8 |
| Structural molecule activity (GO:0005198) | TNNI3, GAS2L3 |
| Transporter activity (GO:0005215) | CNGB1 |
| Apoptotic process (GO:0006915) | TMPRSS11D, INHBA, TMPRSS11B, GDF6, KLK11, TMPRSS11A, KLK9 TMPRSS11F, TMPRSS11E, IGF2BP2, PGL, YRP3, DAPL1, PGLYRP4 |
| Biological adhesion (GO:0022610) | LRRTM4, CELSR3, WISP2, UPK1A |
| Biological regulation (GO:0065007) | EDN3, CXCL5, GBX2, LRRTM4, MPRSS11D, WFDC5, NR0B1, VWA3B |
| INHBA, ONECUT2, OLIG2, TMPRSS11B, KLK10, KLK7, F10, IL1B, ISL1, GDF6, FOXD3, KLK11, TMPRSS11A, KLK9, TMPRSS11F, TMPRSS11E, HOXB13, IL24, KLK12, KLK5, TMPRSS2, FOXD1, CAMP SPINK7, PRSS27, WISP2, CNGB1, EN2, KLK8, PPP1R3C, DAPL1, TRIO, SIM2, SALL4 | |
| Cellular component organization or biogenesis (GO:0071840) | CELSR3 |
| Cellular process (GO:0009987) | EDN3, CXCL5, LRRTM4, CELSR3, INHBA, ONECUT2, PTGDS, IL1B CCL1, GDF6, FOXD3, CRNN, CCL14, S100A12, IGF2BP2, IL24, CCL18, BNIPL, FOXD1, WISP2, CNGB1, UPK1A, DSG1, DAPL1, TRIO, GAS2L3 |
| Developmental process (GO:0032502) | GBX2, LRRTM4, TMPRSS11D, NR0B1, CELSR3, INHBA, ONECUT2, OLIG2, PROK1, TMPRSS11B, ISL1, GDF6, KLK11, TMPRSS11A, KLK9, TMPRSS11F, TMPRSS11E, HOXB13, IGF2BP2, BNIPL, TMPRSS2, TNNI3, WISP2, PGL, YRP3, EN2, DAPL1, PGLYRP4, SIM2, GAS2L3 |
| Immune system process (GO:0002376) | CXCL5, TMPRSS11D, TMPRSS11B, KLK10, KLK7, PTGDS, F10, KLK11, TMPRSS11A, KLK9, TMPRSS11F, TMPRSS11E, IGF2BP2, KLK12, KLK5, TMPRSS2, PRSS27, CNGB1, KLK8 |
| Localization (GO:0051179) | CXCL5, TMPRSS11D, TMPRSS11B, KLK10, KLK7, PTGDS, F10, KLK11, TMPRSS11A, KLK9, TMPRSS11F, TMPRSS11E, IGF2BP2, KLK12, KLK5, TMPRSS2, PRSS27, CNGB1, KLK8 |
| Locomotion (GO:0040011) | CXCL5 |
| metabolic process (GO:0008152) | GBX2, TMPRSS11D, WFDC5, NR0B1, VWA3B INHBA, ONECUT2, OLIG2, TMPRSS11B, KLK10, KLK7, PTGDS, F10, ISL1, GDF6, FOXD3 CRNN, KLK11, TMPRSS11A, KLK9, TMPRSS11F, TMPRSS11E, HOXB13, S100A12, IGF2BP2, KLK12, KLK5, TMPRSS2, FOXD1, CAMP, SPINK7 PRSS27, PGLYRP3, EN2, KLK8, PPP1R3C, DAPL1 PGLYRP4, TRIO, SIM2. SALL4 |
| Multicellular organismal process (GO:0032501) | TMPRSS11D, ONECUT2, PROK1, TMPRSS11B, KLK11, TMPRSS11A, KLK9, TMPRSS11F, TMPRSS11E, HOXB13, IGF2BP2, TMPRSS2, TNNI3, UPK1A, TRIO |
| Reproduction (GO:0000003) | F10, HOXB13, TMPRSS2, PRSS27, UPK1A |
| Response to stimulus (GO:0050896) | CXCL5, TMPRSS11D, INHBA, TMPRSS11B, KLK10, KLK7, F10, IL1β, CCL1, GDF6, KLK11, TMPRSS11A, KLK9, TMPRSS11F, TMPRSS11E, CCL14, S100A12, IL24, CCL18, KLK12, KLK5, TMPRSS2, CAMP, PRSS27, WISP2, CNGB1, UPK1A, KLK8, TRIO |
| Cell part (GO:0044464) | PGLYRP4, TRIO, SIM2, SALL4 |
| Extracellular matrix (GO:0031012) | LRRTM4, WISP2 |
| Extracellular region (GO:0005576) | CXCL5, LRRTM4, TMPRSS11D, INHBA, TMPRSS11B, KLK10, KLK7, F10, IL1β, GDF6, KLK11, TMPRSS11A, KLK9, TMPRSS11F, TMPRSS11E, IL24, KLK12, KLK5, TMPRSS2, PRSS27, WISP2, KLK8 |
| Macromolecular complex (GO:0032991) | IGF2BP2 |
| Membrane (GO:0016020) | CNGB1, DSG1, CD1E |
| Organelle (GO:0043226) | ONECUT2, FOXD3, TNNI3, FOXD1 |
DEGs, differentially expressed genes; GO, gene ontology.
Figure 3.
Functional annotation of the significant DEGs. The 236 significant DEGs were categorized by Gene Ontology analysis into 28 functional categories, according to the relevant biological functions of cellular components, molecular functions and biological progresses. Numbers above each column represent the % of total DEGs involved in each functional category. DEGs, differentially expressed genes.
Pathway analysis of DEGs
To understand the functional pathways of significant DEGs, pathway analysis was performed. The 236 significant DEGs (188 with pathway annotation) were involved in numerous key pathways in cancer, including cytokine-cytokine receptor interactions, metabolism of xenobiotics by cytochrome P450 and retinol metabolism (Table IV). A total of 10 significant pathways was detected. The cytokine-cytokine receptor pathway includes numerous chemokines which can link inflammation to cancer. Cytochrome P450 members, which have been linked to carcinogenesis, were also detected. The DEGs involved in these pathways may serve important roles in cervical cancer.
Table IV.
KEGG pathway analysis of significant DEGs.
| KEGG pathway | Number of DEGs involved in pathway | % of total DEGs involved in pathway | P-value | DEGs |
|---|---|---|---|---|
| Arachidonic acid metabolism | 8 | 4.26 | 1.8083×10−5 | ALOX12, TMEM242, CYP2B7P, CYP2C18, CYP4F22, PLA2G2F, PLA2G4F, PTGDS |
| Cytokine-cytokine receptor interaction | 11 | 5.85 | 0.0007 | CCL1, CCL14, CCL18, CSF2, CXCL14, CXCL5, EGFR, GDF6, IL-1β, IL24, INHBA |
| Staphylococcus aureus infection | 7 | 3.72 | 0.0008 | DSG1, KRT13, KRT14, KRT16P1, KRT16P2, KRT16P3, KRT32, |
| Pathogenic Escherichia coli infection | 9 | 4.79 | 0.0009 | CCDC178, CLDN8, KRT13, KRT14, KRT16P1, KRT16P2, KRT16P3, KRT32, TUBA3D |
| Rheumatoid arthritis | 5 | 2.66 | 0.0085 | CCL18, CSF2, CXCL5, IL-1β, MMP3 |
| Linoleic acid metabolism | 3 | 1.6 | 0.0137 | CYP2C18, PLA2G2F, PLA2G4F |
| Metabolism of xenobiotics by cytochrome P450 | 4 | 2.13 | 0.0139 | CYP2B7P, CYP2C18, CYP2F1, GSTM5 |
| Retinol metabolism | 4 | 2.13 | 0.0139 | CYP2B7P, CYP2C18, RDH12, SDR9C7 |
| PPAR signaling pathway | 5 | 2.66 | 0.0153 | C1QL1, COL10A1, FABP12, FABP4, FABP5 |
| Cytosolic DNA-sensing pathway | 3 | 1.6 | 0.0470 | AIM2, HCG22, IL-1β |
KEGG, Kyoto Encyclopedia of genes and genomes; DEGs, differentially expressed genes.
Discussion
Tumor initiation and progression is a multiple pathway, complicated process, comprising of various dynamic changes in the genome. These genetic alternations contribute to the malignant transformation of cells from normal to cancerous, in addition to tumor progression and metastasis. Malignant cells can acquire favorable genotypes through various biological processes; genetic mutations, epigenetic modifications and non-mutational regulation of gene expression (17). Therefore, genome instability and genetic mutation can be regarded as a hallmark of cancer. With the development of molecular biology and genetics, biologists have gained an improved view of the landscape of the cancer genome by utilizing novel methods including high-throughput genome sequencing. The present study provided a comprehensive transcriptome analysis of cervical squamous cancer tissue with matched normal tissues by RNA-Seq. First, a basic analysis of the sequencing data was performed to detect levels of DEGs. Then, advanced analysis was conducted to give insights into the biological functions and pathways that involved the DEGs. Thus, the present study may aid in discovering novel diagnostic and therapeutic targets for cervical cancer.
The present RNA-Seq analysis acquired ~290 million reads, a number which is adequate for transcriptome sequencing. Furthermore, the genome map rate of sequencing reads was ~85%, indicating that the sequencing process met the criteria of the initial quality control for RNA-Seq techniques (18). These data suggested that the quality of the experimental method and data processing were sufficient.
The significant DEGs were further analysed to confirm whether the sequencing results were consistent with previous research. Matrix metalloproteinase (MMP)3 has been reported to be upregulated in cervical cancer by microarray analysis and by immunohistochemistry (19,20). MMP3 has also been reported to exhibit higher expression and enzyme activity in breast cancer cells that metastatic to the brain and during epithelial-to-mesenchymal transition (EMT) (21). The data from the present study revealed that MMP3 (Table II) was one of the overexpressed DEGs identified in three different cervical cancer samples compared with normal tissues, which is supported by previous studies (19,20). SIX homeobox 1 (SIX1) protein is a transcription factor regulating cell proliferation, apoptosis and organogenesis (22) and has been reported to serve an important role in various human diseases, including cancer. SIX1 is reported to be overexpressed in breast cancer and to promote EMT and metastasis through transforming growth factor (TGF)-β signaling (23). SIX1 also acts as a master regulator of the cervical cancer initiation progression; overexpression of SIX1 promotes DNA replication and anchorage-independent growth of cervical cancer cells (24). Furthermore, high expression of SIX1 in cervical cancers enhances vascular endothelial growth factor C expression by inhibiting TGF-β signaling, thus promoting lymphangiogenesis and lymph node metastasis (25). The RNA-Seq data demonstrated that SIX1 was significantly overexpressed in cervical cancer samples with an average log2 change 6.478514 [P<0.05; the reads per kilobase per million mapped reads data of the tumor and normal samples were compared using the empirical Bayes hierarchical model (26)]. Taken together, gene expression patterns in the present study were highly consistent with previous studies indicating that the RNA-Seq data was valid.
In the present study, numerous novel DEGs were also identified in cervical cancer. GBX2 is a homeobox gene that is overexpressed in prostate cancer (27). Overexpression of GBX2 stimulates expression of interleukin (IL)-6 at the transcription level, through binding to an ATTA motif within the promoter of the IL-6 gene, to promote malignant growth of prostate cancer cells (28). However, the function of GBX2 in other types of cancers has yet to be studied. The results of the present study revealed that GBX2 was significantly upregulated in cervical cancer samples compared with normal tissues. Further studies are required to illuminate the role of GBX2 in cervical cancer. HTR1D belongs to the serotonin receptor family. Knockdown of HTR1D expression in pancreatic cancer cells by small interfering RNAs inhibits cell proliferation and invasion (29). In addition, inhibition of HTR1D suppresses the activity of urokinase plasminogen activator receptor/MMP-2 signaling and integrin/Src/protein tyrosine kinase 2-mediated signaling, in addition to EMT master regulators zinc finger E-box-binding homeobox 1 and Snail family transcriptional repressor 1 (29). EDN signaling serves an important role in cell differentiation, proliferation and migration. EDN signaling is also involved in carcinogenesis, through regulating cell survival and invasiveness (30). Epigenetic activation of EDN-3 through hypermethylation of the EDN3 promoter has been reported in human colon and breast cancer, indicating that EDN3 is a tumor suppressor (31,32). SOSTDC1 has been reported to be downregulated in thyroid cancer cells, breast cancer and in adult and pediatric renal tumors (33–35). SOSTDC1 is a critical regulator of extracellular matrix, through modulating Wnt family member 3A, bone morphogenetic protein (BMP)-2 and BMP-7 signaling in breast cancer cells (34). Serine proteases of the kallikrein family are implicated in various human diseases, including cancer. The expression of KLK family members varies in different types of cancer. Higher expression of KLK13 in breast cancer is associated with improved prognosis, indicating that KLK13 may be a tumor suppressor in breast (36). KLK6 is highly expressed in human non-small cell lung cancer and promotes cell growth and proliferation (37). KLK5 and KLK12 are downregulated in human breast cancer and higher KLK5 and KLK12 expression are associated with improved prognosis (38,39). The data from the present study revealed that GBX2 and HTR1D were overexpressed in cervical cancer tissues, while EDN3, SOSTDC1, KLK5, KLK6, KLK12 and KLK13 were downregulated in cervical cancer tissues, compared with normal tissues. Further functional studies will need to be performed to elucidate the role of these novel DEGs in cervical cancer.
In conclusion, the present study provided a comprehensive transcriptome landscape of cervical cancer and identified novel DEGs in cervical cancer tissues compared with matched normal tissues. These novel genes may be useful for improved understanding of the molecular mechanisms of cervical cancer pathogenesis and potential identification of novel biomarkers and therapeutic targets in the future.
Acknowledgements
This work was supported by the Fujian Province Science and Technology Plan Key Project (grant no. 2013Y0030) and the National Clinical Key Specialty Construction Program of China.
References
- 1.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F. WHO international agency for research on cancer: Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. doi: 10.1016/S1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 4.Arteaga CL, Baselga J. Impact of genomics on personalized cancer medicine. Clin Cancer Res. 2012;18:612–618. doi: 10.1158/1078-0432.CCR-11-2019. [DOI] [PubMed] [Google Scholar]
- 5.Ozsolak F, Milos PM. RNA sequencing: Advances, challenges and opportunities. Nat Rev Genet. 2011;12:87–98. doi: 10.1038/nrg2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Gerstein M, Snyder M. RNA-Seq: A revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagalakshmi U, Wang Z, Waern K, Shou C, Raha D, Gerstein M, Snyder M. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science. 2008;320:1344–1349. doi: 10.1126/science.1158441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sultan M, Schulz MH, Richard H, Magen A, Klingenhoff A, Scherf M, Seifert M, Borodina T, Soldatov A, Parkhomchuk D, et al. A global view of gene activity and alternative splicing by deep sequencing of the human transcriptome. Science. 2008;321:956–960. doi: 10.1126/science.1160342. [DOI] [PubMed] [Google Scholar]
- 9.Stephens PJ, Tarpey PS, Davies H, van Loo P, Greenman C, Wedge DC, Nik-Zainal S, Martin S, Varela I, Bignell GR, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–404. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y, Wang X, Wu F, Huang R, Xue F, Liang G, Tao M, Cai P, Huang Y. Transcriptome profiling of the cancer, adjacent non-tumor and distant normal tissues from a colorectal cancer patient by deep sequencing. PLoS One. 2012;7:e41001. doi: 10.1371/journal.pone.0041001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Lv L, Jin Y, Zhou Y, Jin J, Ma Z, Ren Z. Deep sequencing of transcriptome profiling of GSTM2 knock-down in swine testis cells. Sci Rep. 2016;6:38254. doi: 10.1038/srep38254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Audic S, Claverie JM. The significance of digital gene expression profiles. Genome Res. 1997;7:986–995. doi: 10.1101/gr.7.10.986. [DOI] [PubMed] [Google Scholar]
- 14.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Statist. 2001;29:1165–1188. [Google Scholar]
- 15.Abdi H. Encyclopedia of Measurement and Statistics. Thousand Oaks, CA: Sage; 2007. ‘Bonferroni and Sidak corrections for multiple comparisons’. [Google Scholar]
- 16.Meijer DH, Kane MF, Mehta S, Liu H, Harrington E, Taylor CM, Stiles CD, Rowitch DH. Separated at birth? The functional and molecular divergence of OLIG1 and OLIG2. Nat Rev Neurosci. 2012;13:819–831. doi: 10.1038/nrn3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Conesa A, Madrigal P, Tarazona S, Gomez-Cabrero D, Cervera A, McPherson A, Szcześniak MW, Gaffney DJ, Elo LL, Zhang X, Mortazavi A. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016;17:181. doi: 10.1186/s13059-016-0881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajkumar T, Sabitha K, Vijayalakshmi N, Shirley S, Bose MV, Gopal G, Selvaluxmy G. Identification and validation of genes involved in cervical tumourigenesis. BMC Cancer. 2011;11:80. doi: 10.1186/1471-2407-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagemann T, Bozanovic T, Hooper S, Ljubic A, Slettenaar VI, Wilson JL, Singh N, Gayther SA, Shepherd JH, Van Trappen PO. Molecular profiling of cervical cancer progression. Br J Cancer. 2007;96:321–328. doi: 10.1038/sj.bjc.6603543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu W, Ren Z, Li P, Yu D, Chen J, Huang R, Liu H. Six1: A critical transcription factor in tumorigenesis. Int J Cancer. 2015;136:1245–1253. doi: 10.1002/ijc.28755. [DOI] [PubMed] [Google Scholar]
- 23.Micalizzi DS, Christensen KL, Jedlicka P, Coletta RD, Barón AE, Harrell JC, Horwitz KB, Billheimer D, Heichman KA, Welm AL, et al. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-beta signaling. J Clin Invest. 2009;119:2678–2690. doi: 10.1172/JCI37815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu D, Zhang XX, Xi BX, Wan DY, Li L, Zhou J, Wang W, Ma D, Wang H, Gao QL. Sine oculis homeobox homolog 1 promotes DNA replication and cell proliferation in cervical cancer. Int J Oncol. 2014;45:1232–1240. doi: 10.3892/ijo.2014.2510. [DOI] [PubMed] [Google Scholar]
- 25.Liu D, Li L, Zhang XX, Wan DY, Xi BX, Hu Z, Ding WC, Zhu D, Wang XL, Wang W, et al. SIX1 promotes tumor lymphangiogenesis by coordinating TGFβ signals that increase expression of VEGF-C. Cancer Res. 2014;74:5597–5607. doi: 10.1158/0008-5472.CAN-13-3598. [DOI] [PubMed] [Google Scholar]
- 26.Leng N, Dawson JA, Thomson JA, Ruotti V, Rissman AI, Smits BM, Haag JD, Gould MN, Stewart RM, Kendziorski C. EBSeq: An empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics. 2013;29:1035–1043. doi: 10.1093/bioinformatics/btt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao AC, Isaacs JT. Expression of homeobox gene-GBX2 in human prostatic cancer cells. Prostate. 1996;29:395–398. doi: 10.1002/(SICI)1097-0045(199612)29:6<395::AID-PROS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 28.Gao AC, Lou W, Isaacs JT. Enhanced GBX2 expression stimulates growth of human prostate cancer cells via transcriptional up-regulation of the interleukin 6 gene. Clin Cancer Res. 2000;6:493–497. [PubMed] [Google Scholar]
- 29.Gurbuz N, Ashour AA, Alpay SN, Ozpolat B. Down-regulation of 5-HT1B and 5-HT1D receptors inhibits proliferation, clonogenicity and invasion of human pancreatic cancer cells. PLoS One. 2014;9:e110067. doi: 10.1371/journal.pone.0110067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawanabe Y, Nauli SM. Endothelin. Cell Mol Life Sci. 2011;68:195–203. doi: 10.1007/s00018-010-0518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiesmann F, Veeck J, Galm O, Hartmann A, Esteller M, Knüchel R, Dahl E. Frequent loss of endothelin-3 (EDN3) expression due to epigenetic inactivation in human breast cancer. Breast Cancer Res. 2009;11:R34. doi: 10.1186/bcr2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang R, Löhr CV, Fischer K, Dashwood WM, Greenwood JA, Ho E, Williams DE, Ashktorab H, Dashwood MR, Dashwood RH. Epigenetic inactivation of endothelin-2 and endothelin-3 in colon cancer. Int J Cancer. 2013;132:1004–1012. doi: 10.1002/ijc.27762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang W, Guan H, He X, Ke W, Xu L, Liu L, Xiao H, Li Y. Down-regulation of SOSTDC1 promotes thyroid cancer cell proliferation via regulating cyclin A2 and cyclin E2. Oncotarget. 2015;6:31780–31791. doi: 10.18632/oncotarget.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clausen KA, Blish KR, Birse CE, Triplette MA, Kute TE, Russell GB, D'Agostino RB, Jr, Miller LD, Torti FM, Torti SV. SOSTDC1 differentially modulates Smad and beta-catenin activation and is down-regulated in breast cancer. Breast Cancer Res Treat. 2011;129:737–746. doi: 10.1007/s10549-010-1261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blish KR, Clausen KA, Hawkins GA, Garvin AJ, Willingham MC, Turner JC, Torti FM, Torti SV. Loss of heterozygosity and SOSTDC1 in adult and pediatric renal tumors. J Exp Clin Cancer Res. 2010;29:147. doi: 10.1186/1756-9966-29-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang A, Yousef GM, Scorilas A, Grass L, Sismondi P, Ponzone R, Diamandis EP. Human kallikrein gene 13 (KLK13) expression by quantitative RT-PCR: An independent indicator of favourable prognosis in breast cancer. Br J Cancer. 2002;86:1457–1464. doi: 10.1038/sj.bjc.6600283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nathalie HV, Chris P, Serge G, Catherine C, Benjamin B, Claire B, Christelle P, Briollais L, Pascale R, Marie-Lise J, Yves C. High kallikrein-related peptidase 6 in non-small cell lung cancer cells: An indicator of tumour proliferation and poor prognosis. J Cell Mol Med. 2009;13:4014–4022. doi: 10.1111/j.1582-4934.2009.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avgeris M, Papachristopoulou G, Polychronis A, Scorilas A. Down-regulation of kallikrein-related peptidase 5 (KLK5) expression in breast cancer patients: A biomarker for the differential diagnosis of breast lesions. Clin Proteomics. 2011;8:5. doi: 10.1186/1559-0275-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talieri M, Devetzi M, Scorilas A, Pappa E, Tsapralis N, Missitzis I, Ardavanis A. Human kallikrein-related peptidase 12 (KLK12) splice variants expression in breast cancer and their clinical impact. Tumour Biol. 2012;33:1075–1084. doi: 10.1007/s13277-012-0347-x. [DOI] [PubMed] [Google Scholar]