Abstract
Ischemic preconditioning (IPC) is induced by exposure to brief durations of transient ischemia, which results in ischemic tolerance to a subsequent longer or lethal period of ischemia. In the present study, the effects of IPC (2 min of transient cerebral ischemia) were examined on immunoreactivity of platelet-derived growth factor (PDGF)-BB and on neuroprotection in the gerbil hippocampal CA1 region following lethal transient cerebral ischemia (LTCI; 5 min of transient cerebral ischemia). IPC was subjected to a 2-min sublethal ischemia and a LTCI was given 5-min transient ischemia. The animals in all of the groups were given recovery times of 1, 2 and 5 days and change in PDGF-BB immunoreactivity was examined as was the neuronal damage/death in the hippocampus induced by LTCI. LTCI induced a significant loss of pyramidal neurons in the hippocampal CA1 region 5 days after LTCI, and significantly decreased PDGF-BB immunoreactivity in the CA1 pyramidal neurons from day 1 after LTCI. Conversely, IPC effectively protected the CA1 pyramidal neurons from LTCI and increased PDGF-BB immunoreactivity in the CA1 pyramidal neurons post-LTCI. In conclusion, the results demonstrated that LTCI significantly altered PDGF-BB immunoreactivity in pyramidal neurons in the hippocampal CA1 region, whereas IPC increased the immunoreactivity. These findings indicated that PDGF-BB may be associated with IPC-mediated neuroprotection.
Keywords: ischemic tolerance, transient ischemic insult, CA1 pyramidal neurons, delayed neuronal death, platelet-derived growth factor
Introduction
Ischemic preconditioning (IPC) is induced by exposure to brief durations of transient ischema, resulting in ischemic tolerance to a subsequent longer or lethal period of transient ischemia (1). Kitagawa et al (2) initially introduced the concept of ischemic tolerance in the brain, and demonstrated that 2 min of transient cerebral ischemia, 1–2 days prior to a subsequent longer or lethal period of transient cerebral ischemia, exerted protective effects against ischemic injury in the gerbil hippocampal CA1 region. Further studies have demonstrated the protective effects of IPC in other animal models, including global and focal cerebral ischemia (1,3–5). Ischemic tolerance has also been demonstrated in human clinical practice; less severe strokes occured in patients that suffered from a prior transient ischemic attack within a short period of time (6,7). IPC-induced neuroprotection is considered a promising target for the development of a potential therapeutic strategy; however, the basic mechanisms underlying IPC-induced neuroprotection remain to be elucidated (1).
Platelet-derived growth factor (PDGF) is a polypeptide that acts as a potent mitogen in several cell types, which consists of two homodimers (AA and BB) and one heterodimer (AB) (8,9). The biological activities of PDGF are mediated through binding to PDGF receptors (PDGFR), designated α and β. The α subunit can bind to either the -A or -B chain, whereas the β subunit can bind only to the-B chain (10–12). PDGF-BB is widely expressed in the central nervous system (13) and is upregulated in neurons following cerebral ischemia in animal models (14–17). Suppression of PDGF-BB mRNA expression enhances N-methyl-D-aspartate (NMDA)-induced excitotoxicity in the neonatal rat brain (18). Conversely, the intraventricular administration of PDGF-BB markedly promotes neuronal survival following cerebral ischemia in rats (19). These findings suggest that PDGF-BB may serve a crucial role in the protection of neurons against cerebral ischemic insults.
To the best of our knowledge, the expression pattern of endogenous PDGF-BB in the IPC-mediated hippocampus, following subsequent transient cerebral ischemia, has not been studied. Therefore, the present study aimed to investigate the effects of IPC on cellular localization and alterations in endogenous PDGF-BB in the IPC-induced hippocampus. The hippocampus is an important structure for the study of neuronal damage and its mechanism following transient cerebral ischemia, and the gerbil is considered a good animal for studying transient cerebral ischemia (20–23).
Materials and methods
Experimental groups and ischemic surgery
As previously described (20), 102 male gerbils were obtained from the Experimental Animal Center, Kangwon National University, Chuncheon, South Korea. The gerbils were 6 months old with a body weight of 65–75 g. The animals were housed in a conventional state under adequate temperature (23°C) and humidity (60%) control with a 12-h light/dark cycle, and provided with free access to water and food. The gerbils were used according to guidelines that are in compliance with the current international laws and policies (24). The present study was approved by the Institutional Animal Care and Use Committee at Kangwon National University (Chuncheon, South Korea; approval no. KW-160802-1).
The gerbils were divided into four groups (n=7 at each time point: 0, 1, 2 and 5 days in each group): i) Sham-operated group, both common carotid arteries were exposed; however, the gerbils were not exposed to ischemia (sham-operation); ii) ischemia-operated group was exposed to 5 min of transient cerebral ischemia (lethal transient cerebral ischemia, LTCI); iii) IPC + sham-operated group was subjected to 2 min sublethal transient ischemia prior to sham-operation; and iv) IPC + ischemia-operated group was subjected to 2 min of sublethal ischemia 1 day prior to 5 min of transient ischemia. The IPC paradigm has been proven to be effective at protecting neurons against ischemic damage in this ischemic model (25). The gerbils in the ischemia-operated and the IPC + ischemia-operated groups were given recovery times of 1, 2 and 5 days, since pyramidal neurons in the hippocampal CA1 region survive until 3 days and begin to die 4–5 days post-LTCI.
Surgery for ischemic insults
IPC and LTCI were developed according to our previously described method (20). Briefly, the experimental animals were anesthetized with a mixture of 2.5% isoflurane in 33% oxygen and 67% nitrous oxide. Ischemia was induced by occluding the bilateral common carotid arteries with non-traumatic aneurysm clips (Yasargil FE 723K; Aesculap AG, Tuttlingen, Germany). After 2 or 5 min of occlusion, the aneurysm clips were removed from the arteries. The body temperature under free-regulating or normothermic (37±0.5°C) conditions was monitored using a rectal temperature probe (TR-100; Fine Science Tools, Foster City, Inc., CA, USA) and was maintained using a thermometric blanket prior to, during and after surgery until the animals completely recovered from anesthesia. Thereafter, the gerbils were maintained in a thermal incubator (temperature, 23°C; humidity, 60%; Mirae Medical Industry, Seoul, South Korea) to maintain the body temperature of animals until they were sacrificed at 1, 2 and 5 days following ischemia.
Spontaneous locomotor activity
In order to elucidate increased hyperactivity following ischemia-reperfusion (I-R), spontaneous locomotor activity was measured, according to a previously published procedure (26). Briefly, gerbils (n=7 at each time point in each group) were maintained in a Plexiglas cage (25×20×12 cm), located inside a soundproof chamber. Locomotor activity was recorded using a Photobeam Activity system-Home Cage (San Diego Instruments, San Diego, CA, USA). Spontaneous locomotor activity was monitored during 1 h, a total of 24 h after I-R and, simultaneously, the number of times each animal stood on its hind legs and the time (in sec) spent exhibiting grooming behavior were recorded. Each animal was observed continuously via a 4×8 photobeam. Scores were generated from live observations, and video sequences were used for subsequent re-analysis.
Tissue processing for histology
According to our previously published procedure (27), the gerbils were deeply anesthetized with pentobarbital sodium (40 mg/kg, i.p; JW Pharmaceutical Co., Ltd., Seoul, South Korea,) and were transcardially perfused with 4% paraformaldehyde. The brains were removed and the tissues cryoprotected by infiltration with 30% sucrose overnight at 4°C. Subsequently, their brains were serially sectioned into 30 µm coronal sections using a cryostat (Leica Microsystems GmbH, Wetzlar, Germany).
Cresyl violet (CV) staining and Fluoro-Jade B (F-J B) histofluorescence
To investigate neuronal damage in the hippocampus following I-R, CV and F-J B histofluorescence staining were performed, as previously described (28). Briefly, for CV staining, the sections were stained with 1.0% (w/v) CV acetate (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and dehydrated. The section were then mounted with Canada balsam (Kanto Chemical Co., Inc., Tokyo, Japan). For F-J B histofluorescence, the sections were immersed in a 0.0004% F-J B (Histochem, Inc., Jefferson, AR, USA) staining solution. After washing, the sections were examined using an epifluorescent microscope (Zeiss Carl, Göttingen, Germany) with blue (450–490 nm) excitation light and a barrier filter.
Immunohistochemistry for neuronal nuclei (NeuN) and PDGF-BB
For immunohistochemical staining, the sections were analyzed according to our previously described procedure (28). The brain sections were blocked with 10% normal goat serum (cat. no. S-1000; Vector Laboratories Inc., Burlingame, CA, USA) in 0.05 M PBS followed by staining with primary mouse anti-NeuN (a neuron-specific soluble nuclear antigen; cat. no. 574597; 1:1,000; EMD Millipore, Billerica, MA, USA) and rabbit anti-PDGF-BB (cat. no. ab21234; 1:200; Abcam, Cambridge, MA, USA) overnight at 4°C. The sections were then incubated with secondary antibodies (cat. no. I-1000; 1:250; Vector Laboratories Inc.) and were developed using Vectastain ABC (Vector Laboratories Inc.). The sections were visualized with 3,3′-diaminobenzidine (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in 0.1 M Tris-HCl buffer. In order to establish the specificity of the immunostaining, a negative control test was carried out with pre-immune serum, instead of primary antibody. The negative control resulted in the absence of immunoreactivity in any structures.
Western blot analysis
To examine alterations in the protein expression levels of PDGF-BB in the hippocampal CA1 region, western blotting was conducted, according to our previously published procedure (28). Briefly, tissues (n=7 at each time point) were homogenized, and the protein concentrations were determined in the supernatants using a Micro Bicinchonininc Acid Protein Assay kit with bovine serum albumin as the standard (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Aliquots containing 20 µg total protein were boiled and loaded onto a 12.5% polyacryamide gel. After electrophoresis, the gels were transferred to nitrocellulose transfer membranes (Pall Corporation, Port Washington, NY, USA). To reduce background staining, the membranes were incubated with 5% non-fat dry milk for 30 min at 4°C prior to incubation with rabbit anti-PDGF-BB (cat. no. ab178409; 1:1,000; Abcam) for 2 hrs at 4°C. Membranes were subsequently exposed to peroxidase-conjugated goat anti-rabbit immunoglobulin G (cat. no. A0545; 1:5,000; Sigma-Aldrich; Merck KgaA). Antibodies were visualized using an enhanced chemiluminescence kit (Pierce; Thermo Fisher Scientific, Inc.). Antibodies against β-actin were used as a loading control (cat. no. ab8227; 1:2,000; Abcam).
Data analysis
For cell counts, as previously described (20), sections were selected according to anatomical landmarks corresponding to anterior-posterior, from −1.4 to −1.8 mm of gerbil brain atlas. The number of NeuN-immunoreactive and F-J B-positive cells was counted in a 200×200 µm square, applied at the approximate center of the CA1 region, including the stratum pyramidale. Cell counts were analyzed as a percentage, with the sham group designated as 100%.
In order to analyze PDGF-BB immunoreactivity, we used our previously published method (28). Briefly, cellular immunoreactivity of PDGF-BB was graded in the hippocampal CA1 and CA3 regions. Digital images were captured using an AxioM1 light microscope (Zeiss Carl) equipped with a digital camera (Axiocam; Zeiss Carl) connected to a PC monitor. Semi-quantification of the immunoreactivity of PDGF-BB was evaluated using digital image analysis software (MetaMorph 4.01; Molecular Devices, LLC, Sunnyvale, CA, USA). The staining intensity of PDGF-BB was evaluated on the basis of optical density (OD), which was obtained following transformation of the mean gray level using the formula: OD = log (256/ mean gray level). The background OD was subtracted from areas adjacent to the measured area. After the background density was subtracted, a ratio of the OD of an image file was calibrated in Adobe Photoshop 8.0 (Adobe Systems, San Jose, CA, USA) and was analyzed as a percentage, with the sham-operated group designated as 100% in NIH Image 1.59 (National Institutes of Health, Bethesda, MD, USA).
Protein expression levels were analyzed, according to our previously published method (29). Briefly, western blots were scanned and semi-quantification was conducted using Scion Image software version 2.0 (Scion Corp., Frederick, MD, USA), which was used to determine relative OD (ROD): A ratio of the ROD was calibrated as %, with the sham-operated group designated as 100 %.
Statistical analysis
All data are presented as the mean ± standard error of the mean. A multiple-sample comparison was applied to test the differences between groups and days. The differences between groups on the same day were assessed using one-way analysis of variance (ANOVA) and Tukey's post hoc test. For analysis of time-dependent differences between the groups, two-way ANOVA was used with a Bonferroni post hoc test. P<0.05 was considered to indicate a statistically significant difference. All experiments were repeated twice.
Results
Spontaneous motor activity
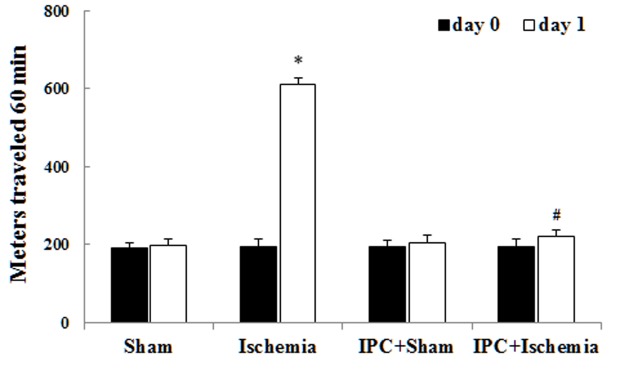
Spontaneous motor activity was detected to investigate the alterations in motor behavior at day 0 (before ischemia) and day 1 (1 day after ischemia; Fig. 1) (30,31). Similar levels of locomotor activity were observed in all experimental groups on day 0. In the ischemia-operated group, locomotor activity was evidently increased compared with that in the sham-operated group 1 day after LTCI. In the IPC + ischemia-operated group, locomotor activity was not significantly increased compared with that in the IPC + sham-operated group 1 day after LTCI. Previous studies have demonstrated that hippocampal neuronal damage leads to locomotor hyperactivity (29,30).
Figure 1.
Locomotor activity in the sham-operated, ischemia-operated, IPC + sham-operated and IPC + ischemia-operated groups at days 0 and 1 after lethal transient cerebral ischemia. Spontaneous locomotor activity is significantly reduced in the IPC + ischemia-operated group compared with in the ischemia-operated group (n=7/group); *P<0.05, vs. the sham-operated group; #P<0.05, vs. the ischemia-operated group. Data are presented as the mean ± standard error of the mean. IPC, ischemic preconditioning.
CV+ cells
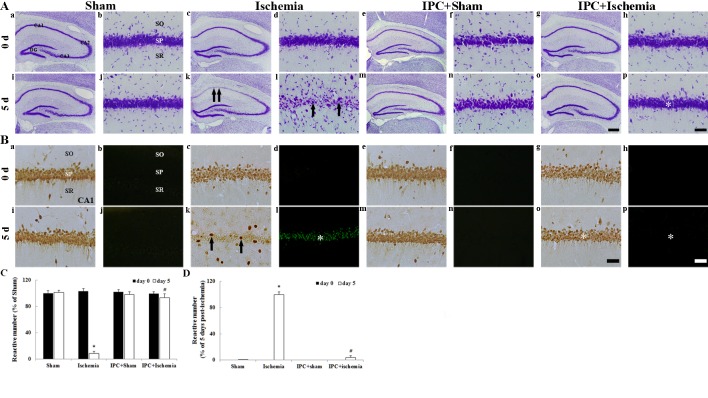
CV staining was used to determine the distribution of all cells in the hippocampus. In all experimental groups on day 0 after LTCI, CV+ cells were easily observed in all subregions of the hippocampus (Fig. 2Aa-Ah). In particular, pyramidal neurons in the stratum pyramidale were identified by their larger and pyramid-like or round shape (Fig. 2Ab, Ad, Af and Ah). In the ischemia-operated group, CV+ pyramidal neurons were markedly decreased in number in the CA1 region, but not in the CA2/3 region, 5 days after LTCI compared with in the sham-operated group (Fig. 2Ak, Al); at a high magnification, damaged CA1 pyramidal neurons were shrunken and contained dark and polygonal nuclei (Fig. 2Al). In the IPC + sham-operated group, the distribution pattern of CV+ cells in the CA1 region was similar to that in the sham-operated group (Fig. 2Am and An). In the IPC + ischemia-operated group, the morphology of CV+ pyramidal neurons in the CA1 region was similar to that in the IPC + sham-operated group (Fig. 2Ao and Ap).
Figure 2.
(A) CV, (B) NeuN and F-J B staining were conducted. (Aa-Ap) CV staining, (Ba, Bc, Be, Bg, Bi, Bk, Bm and Bo) NeuN immunohistochemistry and (Bb, Bd, Bf, Bh, Bj, Bl, Bn and Bp) F-J B histofluorescence staining in the hippocampus of sham-operated, ischemia-operated, IPC + sham-operated and IPC + ischemia-operated groups. In the ischemia-operated group, CV+ cells (arrows in Ak and Al) were damaged in the SP of the CA1 region 5 days after lethal transient cerebral ischemia. In addition, at this time point, few NeuN+ (arrows in Bk) and numerous F-J B+ (asterisk in Bl) cells were detected in the SP. However, the distribution of CV+ (asterisk in Ap), NeuN+ (asterisk in Bo) and F-J B+ (asterisk in Bp) cells in the IPC + ischemia-operated group was similar to that in the sham-operated group. Scale bar: (Aa, Ac, Ae, Ag, Ai, Ak, Am and Ao) 800 µm; (Ab, Ad, Af, Ah, Aj, Al, An, Ap and Ba- Bp) 50 µm. Quantitative analyses of (C) NeuN+ and (D) F-J B+ cells in all groups. Data are presented as the mean ± standard error of the mean. *P<0.05 vs. the sham-operated group; #P<0.05 vs. the ischemia-operated group. SO, stratum oriens; SR, stratum radiatum; SP, stratum pyramidale; IPC, ischemic preconditioning; CV, cresyl violet; NeuN, neuronal nuclei; F-J B, Fluoro-Jade B.
NeuN+ and F-J B+ cells
IPC-mediated neuroprotection in the CA1 region was detected using anti-NeuN immunohistochemistry and F-J B histofluorescence staining (Fig. 2B). In all experimental groups on day 0 after LTCI, pyramidal neurons in the CA1 region were well stained with NeuN (Fig. 2Ba, Bc, Be, Bg and C) and no F-J B+ cells were observed (Fig. 2Bb, Bd, Bf, Bh and D). In the ischemia-operated group, a significant loss of NeuN+ neurons, alongside numerous F-J B+ cells, was observed in the stratum pyramidale of the CA1 region 5 days after LTCI (Fig. 2Bk, C and D).
At day 5 following LTCI, in the IPC + sham-operated group, the pattern of NeuN+ CA1 pyramidal neurons was similar to that in the sham-operated group (Fig. 2Bm and C) and no F-J B+ cells were detected (Fig. 2Bn and D). The distribution of NeuN+ and F-J B+ cells in the stratum pyramidale of the IPC + ischemia-operated group was similar to that in the IPC + sham-operated group (Fig. 2Bo, Bp, C and D).
PDGF-BB immunoreactivity: CA1 region
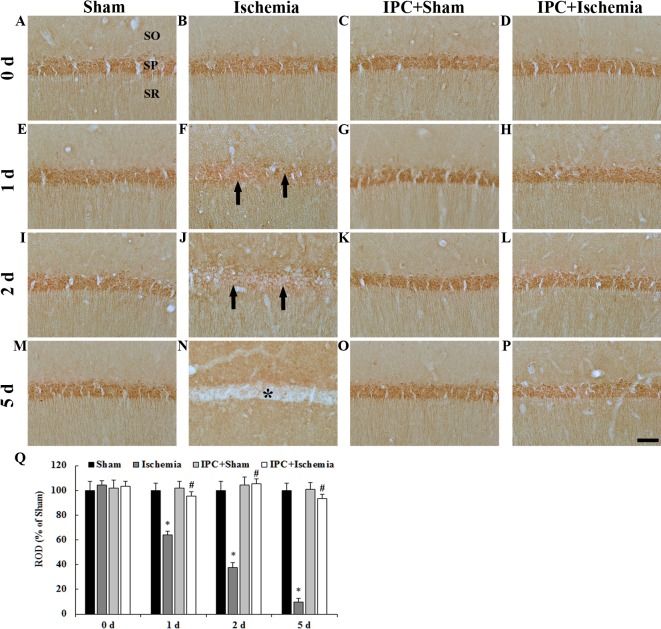
PDGF-BB immunoreactivity in the CA1 region was detected by immunohistochemistry (Fig. 3). PDGF-BB immunoreactivity was easily detected in pyramidal neurons in the stratum pyramidale of the CA1 region in the sham-operated groups (Fig. 3A, E, I, M and Q). In the ischemia-operated group, PDGF-BB immunoreactivity began to be decreased in the CA1 pyramidal neurons from 1 day after LTCI and was hardly observed in CA1 pyramidal neurons 5 days after LTCI (Fig. 3B, F, J, N and Q).
Figure 3.
(A-P) PDGF-BB immunohistochemistry in the CA1 region of the (A, E, I and M) sham-operated, (B, F, J and N) ischemia-operated, (C, G, K and O) IPC + sham-operated and (D, H, L and P) IPC + ischemia-operated groups. In the ischemia-operated group, PDGF-BB immunoreactivity was markedly decreased in the SP (arrows in F, J) at 1 day post-ischemia. PDGF-BB immunoreactivity was barely detected in pyramidal neurons (asterisk in N) of the CA1 region 5 days after LTCI. In the IPC + sham- and IPC + ischemia-operated groups, PDGF-BB immunoreactivity was similar to that in the sham-operated group. Scale bar, 50 µm. (Q) Quantitative analysis of PDGF-BB immunoreactivity in the SP of all groups. The ratio of ROD was calibrated as a %, with the sham-operated group designated as 100%. Data are presented as the mean ± standard error of the mean. *P<0.05 vs. the sham-operated group; #P<0.05 vs. the ischemia-operated group. SO, stratum oriens; SR, stratum radiatum; SP, stratum pyramidale; IPC, ischemic preconditioning; PDGF-BB, platelet-derived growth factor-BB; ROD, relative optical density.
In the IPC + sham-operated group, PDGF-BB immunoreactivity in CA1 pyramidal neurons was similar to that in the sham-operated group (Fig. 3C, G, K, O and Q). In the IPC + ischemia-operated group, PDGF-BB immunoreactivity in CA1 pyramidal neurons was not altered after LTCI compared with in the IPC + sham-operated group (Fig. 3D, H, L, P and Q).
PDGF-BB immunoreactivity: CA3 region
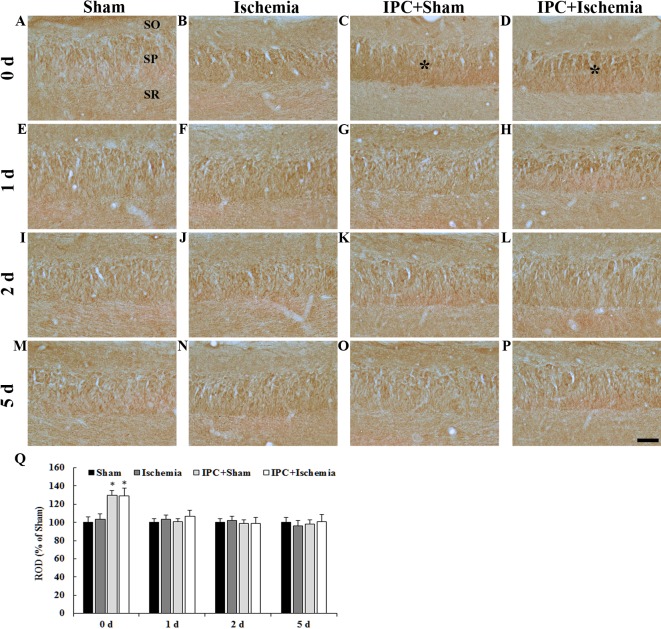
PDGF-BB immunoreactivity in the CA3 region was detected by immunohistochemistry (Fig. 4). In the CA3 region of the sham-operated group, PDGF-BB immunoreactivity was detected in neurons of the stratum pyramidale (Fig. 4A, E, I, M and Q). In the ischemia-operated group, PDGF-BB immunoreactivity was not significantly altered in the stratum pyramidale (Fig. 4B, F, J, N and Q). In the IPC + sham-operated group, PDGF-BB immunoreactivity in the stratum pyramidale was slightly increased 0 days after LTCI compared with in the sham-operated group (Fig. 4C); thereafter, the immunoreactivity was similar to that in the sham-operated group until 5 days post-ischemia (Fig. 4G, K, O and Q). In the IPC + ischemia-operated group, Changes in PDGF-BB immunoreactivity in the stratum pyramidale was similar to that in the IPC + sham-operated group (Fig. 4D, H, L, P and Q).
Figure 4.
(A-P) PDGF-BB immunohistochemistry in the CA3 region of the (A, E, I and M) sham-operated, (B, F, J and N) ischemia-operated, (C, G, K and O) IPC + sham-operated and (D, H, L and P) IPC + ischemia-operated groups. PDGF-BB immunoreactivity was increased in pyramidal neurons of the SP (asterisks in C and D) at day 0 in the IPC + sham-operated and IPC + ischemia-operated groups. Scale bar=50 µm. (Q) Quantitative analysis of PDGF-BB immunoreactivity in the SP of all groups. The ratio of the ROD was calibrated as a %, with the sham-operated group designated as 100%. Data are presented as the mean ± standard error of the mean. *P<0.05 vs. the sham-operated group. SO, stratum oriens; SR, stratum radiatum; SP, stratum pyramidale; IPC, ischemic preconditioning; PDGF-BB, platelet-derived growth factor-BB; ROD, relative optical density.
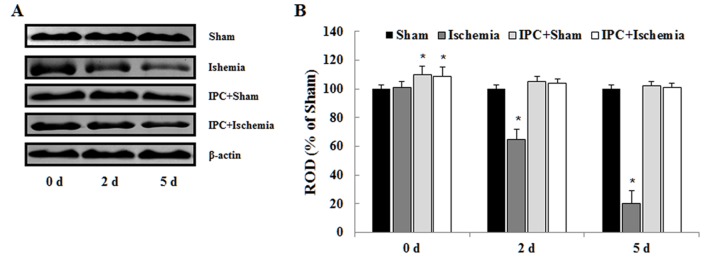
Protein expression levels of PDGF-BB
Western blot analysis indicated that the alterations in PDGF-BB protein expression in the CA1 region post-LTCI were similar to those observed by immunohistochemistry (Fig. 5). In the ischemia-operated group, PDGF-BB protein levels were significantly decreased 2 days after LTCI and the levels were lowest 5 days post-LTCI. In the IPC + sham-operated group, PDGF-BB protein expression was significantly increased compared with in the sham-operated group. In the IPC + ischemia-operated group, PDGF-BB protein levels were not significantly altered following LTCI (Fig. 5).
Figure 5.
(A) Western blot analysis was conducted to detect PDGF-BB protein expression in the CA1 region. PDGF-BB levels were significantly increased in the IPC + sham-operated and IPC + ischemia-operated groups. (B) ROD values are presented as %, with the sham-operated group designated as 100%. Data are presented as the mean ± standard error of the mean. *P<0.05 vs. the sham-operated group. IPC, ischemic preconditioning; PDGF-BB, platelet-derived growth factor-BB; ROD, relative optical density.
Discussion
Transient brain ischemia leads to selective damage/death of pyramidal neurons in the hippocampal CA1 region several days after I-R. This neuronal death is commonly referred to as ‘delayed neuronal death’, since it occurs very slowly for 4–5 days following 5 min of transient brain ischemia, which is a lethal type of ischemia for CA1 pyramidal neurons (27). Conversely, pyramidal neurons in the hippocampal CA3 region are much less vulnerable to ischemic insults (32). The present study examined the delayed neuronal death of CA1 pyramidal neurons using CV histochemistry, NeuN immunohistochemistry and F-J B histofluorescence.
The results of the present study demonstrated that CA1 pyramidal neurons in the IPC-induced gerbil hippocampus did not die following LTCI. IPC, which is induced by exposure to brief durations of transient ischema, does not induce neuronal damage/death in ischemic areas and prevents ischemic injury following a subsequent longer or lethal transient ischemic insult (33). The first description of IPC in the brain was reported by Kitagawa et al (34) in a gerbil model, and similar findings have been reported in rats (35,36) and mice (37). In addition, IPC-mediated neuroprotection has been studied in brain slices (38), as well as in murine cell culture (39). In the present study, brief IPC (2 min of transient ischemia) was used to prevent neuronal death in the hippocampal CA1 region, and this brief IPC stimulus did not induce neuronal damage, as assessed by CV, NeuN and F-J B staining, which is very sensitive to acute neuronal injury (40). The results indicated that CA1 pyramidal neurons exhibited normal features in the IPC-induced gerbil brain 5 days after LTCI. To the best of our knowledge, although IPC provides marked neuroprotection against ischemic brain injury, its underlying mechanisms require further elucidation for the development of therapeutic strategies for the treatment of ischemic stroke, as the molecular mechanisms underlying IPC-induced ischemic tolerance are not fully understood (1).
It is well known that endogenous PDGF-BB is expressed in neurons (13) and its expression is altered after some brain insults (13,14). In particular, it has been reported that PDGF-BB may be considered a potent neuroprotective factor under cerebral ischemic conditions (14,19,41,42). Previous studies have demonstrated that the application of exogenous PDGF-BB protein may prevent neuronal cell death in the CA1 region after global brain ischemia, and may induce infarct tolerance against reversible focal brain ischemia (19,42,43).
Albers et al (44) previously demonstrated that NMDA antagonists may prevent neuronal injury in a gerbil model of transient cerebral ischemia by inhibiting the excitotoxicity induced by NMDA receptors. NMDA-induced excitotoxicity triggers several downstream cascades, including nitrosative and oxidative stress, mitochondrial dysfunction, and protease and phospholipase activation, which culminate in cell death (45). This NMDA-induced excitotoxicity is widely considered to mediate the delayed neuronal death of hippocampal CA1 pyramidal neurons following transient global cerebral ischemia (46). Therefore, inhibition of Ca2+ overload may be a potential mechanism underlying PDGF-mediated neuroprotection (47). Furthermore, the suppression of PDGF-BB mRNA expression has been reported to increase susceptibility of the brain to NMDA-induced excitotoxicity or ischemia (18,48,49). In concordance with these in vivo data, the activation of PDGFR-β by PDGF-BB strongly inhibited NMDA-induced excitotoxicity in cultured hippocampal neurons (50,51). These studies indicated that endogenously synthesized and exogenously applied PDGF-BB may exert neuroprotective effects, and that PDGFR-β expression in neurons may principally mediate this protective effect.
On the basis of these aforementioned studies, the present study compared alterations in PDGF-BB immunoreactivity in the CA1 region between the ischemia-operated and IPC + ischemia-operated groups. PDGF-BB immunoreactivity was significantly decreased in the CA1 pyramidal neurons following LTCI and was barely detected in the neurons 5 days after LTCI; however, IPC maintained PDGF-BB immunoreactivity in the CA1 pyramidal neurons in the IPC + sham- and IPC + ischemia-operated groups. These findings indicated that PDGF-BB may be associated with neuronal damage after ischemic insults and that the regulation of PDGF-BB expression may be affected by PDGFR-β.
In conclusion, the findings of the present study indicated that IPC (2 min of transient cerebral ischemia) increased PDGF-BB expression in the gerbil hippocampal CA1 pyramidal neurons following 5 min of LTCI. The present study provides evidence regarding the mechanism underlying IPC-mediated neuroprotection against transient cerebral ischemic injury.
Acknowledgements
The present study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant no. NRF-2014R1A1A2056105), and the Bio-Synergy Research Project (grant no. NRF-2015M3A9C4076322) of the Ministry of Science, ICT and Future Planning through the National Research Foundation.
References
- 1.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 2.Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K, et al. ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res. 1990;528:21–24. doi: 10.1016/0006-8993(90)90189-I. [DOI] [PubMed] [Google Scholar]
- 3.Kirino T, Tsujita Y, Tamura A. Induced tolerance to ischemia in gerbil hippocampal neurons. J Cereb Blood Flow Metab. 1991;11:299–307. doi: 10.1038/jcbfm.1991.62. [DOI] [PubMed] [Google Scholar]
- 4.Nishi S, Taki W, Uemura Y, Higashi T, Kikuchi H, Kudoh H, Satoh M, Nagata K. Ischemic tolerance due to the induction of HSP70 in a rat ischemic recirculation model. Brain Res. 1993;615:281–288. doi: 10.1016/0006-8993(93)90039-P. [DOI] [PubMed] [Google Scholar]
- 5.Stagliano NE, Perez-Pinzón MA, Moskowitz MA, Huang PL. Focal ischemic preconditioning induces rapid tolerance to middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 1999;19:757–761. doi: 10.1097/00004647-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Weih M, Kallenberg K, Bergk A, Dirnagl U, Harms L, Wernecke KD, Einhäupl KM. Attenuated stroke severity after prodromal TIA: A role for ischemic tolerance in the brain? Stroke. 1999;30:1851–1854. doi: 10.1161/01.STR.30.9.1851. [DOI] [PubMed] [Google Scholar]
- 7.Moncayo J, de Freitas GR, Bogousslavsky J, Altieri M, van Melle G. Do transient ischemic attacks have a neuroprotective effect? Neurology. 2000;54:2089–2094. doi: 10.1212/WNL.54.11.2089. [DOI] [PubMed] [Google Scholar]
- 8.Ross R, Raines EW, Bowen-Pope DF. The biology of platelet-derived growth factor. Cell. 1986;46:155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- 9.Heldin CH, Westermark B. Platelet-derived growth factor: Mechanism of action and possible in vivo function. Cell Regul. 1990;1:555–566. doi: 10.1091/mbc.1.8.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart CE, Forstrom JW, Kelly JD, Seifert RA, Smith RA, Ross R, Murray MJ, Bowen-Pope DF. Two classes of PDGF receptor recognize different isoforms of PDGF. Science. 1988;240:1529–1531. doi: 10.1126/science.2836952. [DOI] [PubMed] [Google Scholar]
- 11.Heldin CH, Bäckström G, Ostman A, Hammacher A, Rönnstrand L, Rubin K, Nistér M, Westermark B. Binding of different dimeric forms of PDGF to human fibroblasts: Evidence for two separate receptor types. EMBO J. 1988;7:1387–1393. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seifert RA, Hart CE, Phillips PE, Forstrom JW, Ross R, Murray MJ, Bowen-Pope DF. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J Biol Chem. 1989;264:8771–8778. [PubMed] [Google Scholar]
- 13.Sasahara M, Fries JW, Raines EW, Gown AM, Westrum LE, Frosch MP, Bonthron DT, Ross R, Collins T. PDGF B-chain in neurons of the central nervous system, posterior pituitary, and in a transgenic model. Cell. 1991;64:217–227. doi: 10.1016/0092-8674(91)90223-L. [DOI] [PubMed] [Google Scholar]
- 14.Iihara K, Sasahara M, Hashimoto N, Uemura Y, Kikuchi H, Hazama F. Ischemia induces the expression of the platelet-derived growth factor-B chain in neurons and brain macrophages in vivo. J Cereb Blood Flow Metab. 1994;14:818–824. doi: 10.1038/jcbfm.1994.102. [DOI] [PubMed] [Google Scholar]
- 15.Iihara K, Sasahara M, Hashimoto N, Hazama F. Induction of platelet-derived growth factor beta-receptor in focal ischemia of rat brain. J Cereb Blood Flow Metab. 1996;16:941–949. doi: 10.1097/00004647-199609000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Krupinski J, Issa R, Bujny T, Slevin M, Kumar P, Kumar S, Kaluza J. A putative role for platelet-derived growth factor in angiogenesis and neuroprotection after ischemic stroke in humans. Stroke. 1997;28:564–573. doi: 10.1161/01.STR.28.3.564. [DOI] [PubMed] [Google Scholar]
- 17.Renner O, Tsimpas A, Kostin S, Valable S, Petit E, Schaper W, Marti HH. Time- and cell type-specific induction of platelet-derived growth factor receptor-beta during cerebral ischemia. Brain Res Mol Brain Res. 2003;113:44–51. doi: 10.1016/S0169-328X(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 18.Egawa-Tsuzuki T, Ohno M, Tanaka N, Takeuchi Y, Uramoto H, Faigle R, Funa K, Ishii Y, Sasahara M. The PDGF B-chain is involved in the ontogenic susceptibility of the developing rat brain to NMDA toxicity. Exp Neurol. 2004;186:89–98. doi: 10.1016/j.expneurol.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Iihara K, Hashimoto N, Tsukahara T, Sakata M, Yanamoto H, Taniguchi T. Platelet-derived growth factor-BB, but not -AA, prevents delayed neuronal death after forebrain ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1097–1106. doi: 10.1097/00004647-199710000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Bae EJ, Chen BH, Yan BC, Shin BN, Cho JH, Kim IH, Ahn JH, Lee JC, Tae HJ, Hong S, et al. Delayed hippocampal neuronal death in young gerbil following transient global cerebral ischemia is related to higher and longer-term expression of p63 in the ischemic hippocampus. Neural Regen Res. 2015;10:944–950. doi: 10.4103/1673-5374.158359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao XY, Wu CF, Yang J, Gao Y, Sun FJ, Wang DX, Wang CH, Lin BC. Effect of arginine vasopressin on the cortex edema in the ischemic stroke of Mongolian gerbils. Neuropeptides. 2015;51:55–62. doi: 10.1016/j.npep.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Lee JC, Kim IH, Park JH, Ahn JH, Cho JH, Cho GS, Tae HJ, Chen BH, Yan BC, Yoo KY, et al. Ischemic preconditioning protects hippocampal pyramidal neurons from transient ischemic injury via the attenuation of oxidative damage through upregulating heme oxygenase-1. Free Radic Biol Med. 2015;79:78–90. doi: 10.1016/j.freeradbiomed.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Lee JC, Park JH, Kim IH, Cho GS, Ahn JH, Tae HJ, Choi SY, Cho JH, Kim DW, Kwon YG, et al. Neuroprotection of ischemic preconditioning is mediated by thioredoxin 2 in the hippocampal CA1 region following a subsequent transient cerebral ischemia. Brain Pathol. 2016;27:276–291. doi: 10.1111/bpa.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Research Council (U.S.), corp-author Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.) and National Academies Press (U.S.): Guide for the care and use of laboratory animals. National Academies Press; Washington, D.C.: 2011. [Google Scholar]
- 25.Nakamura H, Katsumata T, Nishiyama Y, Otori T, Katsura K, Katayama Y. Effect of ischemic preconditioning on cerebral blood flow after subsequent lethal ischemia in gerbils. Life Sci. 2006;78:1713–1719. doi: 10.1016/j.lfs.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Ohk TG, Yoo KY, Park SM, Shin BN, Kim IH, Park JH, Ahn HC, Lee YJ, Kim MJ, Kim TY, et al. Neuronal damage using fluoro-jade B histofluorescence and gliosis in the striatum after various durations of transient cerebral ischemia in gerbils. Neurochem Res. 2012;37:826–834. doi: 10.1007/s11064-011-0678-9. [DOI] [PubMed] [Google Scholar]
- 27.Lee JC, Park JH, Yan BC, Kim IH, Cho GS, Jeoung D, Kwon YG, Kim YM, Lee YL, Shin HC, Won MH. Effects of transient cerebral ischemia on the expression of DNA methyltransferase 1 in the gerbil hippocampal CA1 region. Neurochem Res. 2013;38:74–81. doi: 10.1007/s11064-012-0890-2. [DOI] [PubMed] [Google Scholar]
- 28.Lee JC, Kim IH, Cho GS, Park JH, Ahn JH, Yan BC, Kwon HM, Kim YM, Cheon SH, Cho JH, et al. Ischemic preconditioning-induced neuroprotection against transient cerebral ischemic damage via attenuating ubiquitin aggregation. J Neurol Sci. 2014;336:74–82. doi: 10.1016/j.jns.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Lee CH, Park JH, Choi JH, Yoo KY, Ryu PD, Won MH. Heat shock protein 90 and its cochaperone, p23, are markedly increased in the aged gerbil hippocampus. Exp Gerontol. 2011;46:768–772. doi: 10.1016/j.exger.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Bickford P, Heron C, Young DA, Gerhardt GA, De La Garza R. Impaired acquisition of novel locomotor tasks in aged and norepinephrine-depleted F344 rats. Neurobiol Aging. 1992;13:475–481. doi: 10.1016/0197-4580(92)90075-9. [DOI] [PubMed] [Google Scholar]
- 31.Kuroiwa T, Bonnekoh P, Hossmann KA. Locomotor hyperactivity and hippocampal CA1 injury after transient forebrain ischemia of gerbils. Neurosci Lett. 1991;122:141–144. doi: 10.1016/0304-3940(91)90842-H. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- 33.Lehotský J, Burda J, Danielisová V, Gottlieb M, Kaplán P, Saniová B. Ischemic tolerance: The mechanisms of neuroprotective strategy. Anat Rec (Hoboken) 2009;292:2002–2012. doi: 10.1002/ar.20970. [DOI] [PubMed] [Google Scholar]
- 34.Kitagawa K, Matsumoto M, Kuwabara K, Tagaya M, Ohtsuki T, Hata R, Ueda H, Handa N, Kimura K, Kamada T. ‘Ischemic tolerance’ phenomenon detected in various brain regions. Brain Res. 1991;561:203–211. doi: 10.1016/0006-8993(91)91596-S. [DOI] [PubMed] [Google Scholar]
- 35.Kawahara N, Wang Y, Mukasa A, Furuya K, Shimizu T, Hamakubo T, Aburatani H, Kodama T, Kirino T. Genome-wide gene expression analysis for induced ischemic tolerance and delayed neuronal death following transient global ischemia in rats. J Cereb Blood Flow Metab. 2004;24:212–223. doi: 10.1097/01.WCB.0000106012.33322.A2. [DOI] [PubMed] [Google Scholar]
- 36.Perez-Pinzón MA, Xu GP, Dietrich WD, Rosenthal M, Sick TJ. Rapid preconditioning protects rats against ischemic neuronal damage after 3 but not 7 days of reperfusion following global cerebral ischemia. J Cereb Blood Flow Metab. 1997;17:175–182. doi: 10.1097/00004647-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Atochin DN, Clark J, Demchenko IT, Moskowitz MA, Huang PL. Rapid cerebral ischemic preconditioning in mice deficient in endothelial and neuronal nitric oxide synthases. Stroke. 2003;34:1299–1303. doi: 10.1161/01.STR.0000066870.70976.57. [DOI] [PubMed] [Google Scholar]
- 38.Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Pérez-Pinzón MA. Epsilon PKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J Neurosci. 2003;23:384–391. doi: 10.1523/JNEUROSCI.23-02-00384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grabb MC, Choi DW. Ischemic tolerance in murine cortical cell culture: Critical role for NMDA receptors. J Neurosci. 1999;19:1657–1662. doi: 10.1523/JNEUROSCI.19-05-01657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmued LC, Hopkins KJ. Fluoro-Jade B: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/S0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 41.Kaneko M, Sasahara M, Takayama S, Handa J, Hazama F. Expression of platelet-derived growth factor after transient forebrain ischemia in the gerbil hippocampus. Acta Neuropathol. 1998;95:471–478. doi: 10.1007/s004010050827. [DOI] [PubMed] [Google Scholar]
- 42.Kawabe T, Wen TC, Matsuda S, Ishihara K, Otsuda H, Sakanaka M. Platelet-derived growth factor prevents ischemia-induced neuronal injuries in vivo. Neurosci Res. 1997;29:335–343. doi: 10.1016/S0168-0102(97)00105-3. [DOI] [PubMed] [Google Scholar]
- 43.Sakata M, Yanamoto H, Hashimoto N, Iihara K, Tsukahara T, Taniguchi T, Kikuchi H. Induction of infarct tolerance by platelet-derived growth factor against reversible focal ischemia. Brain Res. 1998;784:250–255. doi: 10.1016/S0006-8993(97)01345-0. [DOI] [PubMed] [Google Scholar]
- 44.Albers GW, Goldberg MP, Choi DW. Do NMDA antagonists prevent neuronal injury? Yes. Arch Neurol. 1992;49:418–420. doi: 10.1001/archneur.1992.00530280112031. [DOI] [PubMed] [Google Scholar]
- 45.Chinopoulos C, Adam-Vizi V. Calcium, mitochondria and oxidative stress in neuronal pathology. Novel aspects of an enduring theme. FEBS J. 2006;273:433–450. doi: 10.1111/j.1742-4658.2005.05103.x. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Funa K, Sasahara M. The roles of PDGF in development and during neurogenesis in the normal and diseased nervous system. J Neuroimmune Pharmacol. 2014;9:168–181. doi: 10.1007/s11481-013-9479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishii Y, Oya T, Zheng L, Gao Z, Kawaguchi M, Sabit H, Matsushima T, Tokunaga A, Ishizawa S, Hori E, et al. Mouse brains deficient in neuronal PDGF receptor-beta develop normally but are vulnerable to injury. J Neurochem. 2006;98:588–600. doi: 10.1111/j.1471-4159.2006.03922.x. [DOI] [PubMed] [Google Scholar]
- 49.Shen J, Ishii Y, Xu G, Dang TC, Hamashima T, Matsushima T, Yamamoto S, Hattori Y, Takatsuru Y, Nabekura J, Sasahara M. PDGFR-β as a positive regulator of tissue repair in a mouse model of focal cerebral ischemia. J Cereb Blood Flow Metab. 2012;32:353–367. doi: 10.1038/jcbfm.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valenzuela CF, Xiong Z, MacDonald JF, Weiner JL, Frazier CJ, Dunwiddie TV, Kazlauskas A, Whiting PJ, Harris RA. Platelet-derived growth factor induces a long-term inhibition of N-methyl-D-aspartate receptor function. J Biol Chem. 1996;271:16151–16159. doi: 10.1074/jbc.271.27.16151. [DOI] [PubMed] [Google Scholar]
- 51.Tseng HC, Dichter MA. Platelet-derived growth factor-BB pretreatment attenuates excitotoxic death in cultured hippocampal neurons. Neurobiol Dis. 2005;19:77–83. doi: 10.1016/j.nbd.2004.11.007. [DOI] [PubMed] [Google Scholar]