Abstract
Overexpression of P-glycoprotein (P-gp) in the brain is an important mechanism involved in drug-resistant epilepsy (DRE). High-mobility group box 1 (HMGB1), an inflammatory cytokine, significantly increases following seizures and may be involved in upregulation of P-gp. However, the underlying mechanisms remain elusive. The aim of the present study was to evaluate the role of HMGB1 and its downstream signaling components, receptor for advanced glycation end-product (RAGE) and nuclear factor-κB (NF-κB), on P-gp expression in rat brains during status epilepticus (SE). Small interfering RNA (siRNA) was administered to rats prior to induction of SE by pilocarpine, to block transcription of the genes encoding HMGB1 and RAGE, respectively. An inhibitor of NF-κB, pyrrolidinedithiocarbamic acid (PDTC), was utilized to inhibit activation of NF-κB. The expression levels of HMGB1, RAGE, phosphorylated-NF-κB p65 (p-p65) and P-gp were detected by western blotting. The relative mRNA expression levels of the genes encoding these proteins were measured using reverse transcription-quantitative polymerase chain reaction and the cellular localization of the proteins was determined by immunofluorescence. Pre-treatment with HMGB1 siRNA reduced the expression levels of RAGE, p-p65 and P-gp. PDTC reduced the expression levels of P-gp. These findings suggested that overexpression of P-gp during seizures may be regulated by HMGB1 via the RAGE/NF-κB signaling pathway, and may be a novel target for treating DRE.
Keywords: rat, status epilepticus, high-mobility group box 1, receptor for advanced glycation end-product, nuclear factor-κB, P-glycoprotein
Introduction
Epilepsy is the most common brain disorder affecting >50 million people worldwide (1). Despite advances in therapies for epilepsy, a third of patients with epilepsy are non-responsive or respond poorly to anti-epilepsy drugs (AEDs) and subsequently develop drug-resistant epilepsy (DRE) (2). There are a number of theories as to how this develops, including the involvement of P-glycoprotein (P-gp) overexpression, which is an efflux pump that is anchored at the blood-brain barrier (3,4). This has been confirmed by brain tissues collected during surgery for DRE (5). P-gp is a multidrug resistance protein and is encoded by MDR1A/B gene. It is primarily distributed at the luminal surface of capillary endothelial cells. However, P-gp can also be expressed in glial cells and neurons. Experiments have demonstrated that P-gp pumps out AEDs from neuronal cells to reduce the concentration of AEDs in brain tissue (6). Inhibiting P-gp expression may improve the efficacy of AEDs in DRE (7,8). Therefore, P-gp is postulated to be a novel clinical target for the treatment of multidrug refractory epilepsy (9). However, the exact underlying mechanisms of P-gp overexpression and its regulatory pathways in DRE are not fully understood.
The immunity and inflammatory processes affecting P-gp expression in epilepsy have been established (10–12). Seizures may trigger multidimensional local inflammatory reactions in brain (13) that are involved in the generation and propagation of epileptic activity. High-mobility group box 1 (HMGB1), a ubiquitous chromatin component, is passively released by stressed or necrotic cells, and is overexpressed in epileptic brains. The interaction between HMGB1 and Toll-like receptor 4 (TLR4) stimulates the innate immune system and inflammation in tissues (14,15). Our previous study demonstrated that P-gp may be downregulated by inhibition of TLR4 or HMGB1 during kainic acid (KA)-induced seizures in rat brains (16). Receptor for advanced glycation end-product (RAGE), another important ligand of HMGB1, has key role in innate immune activation and seizures (17). However, it is unknown whether RAGE activation contributes to the upregulation of P-gp following seizures. Nuclear factor-κB (NF-κB) is a downstream signaling molecule of RAGE, and is a ubiquitous transcription factor that serves a role in the regulation of immune response and inflammation (18). Our previous study suggested that silencing of inhibitor of NF-κB kinase subunit β (IKKβ), which regulates the activity of NF-κB, reduced the expression levels of P-gp and reduced seizures (19). HMGB1 binds to RAGE to stimulate the biological effects of NF-κB. RAGE/NF-κB is known to be a regulator of inflammation (20,21). The present study investigated whether HMGB1 binds to RAGE, leading to activation of NF-κB and enhanced transcription of MDR1A/B and P-gp expression during brain seizures. The results of the present study may provide a molecular mechanism and a novel target for the treatment of DRE.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats (age, 6–7 weeks; weight, 200–250 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and were housed under controlled standard conditions at 65±5% humidity and 21±2°C, a 12 h light/dark cycle and with food and water available. The rats were housed under these conditions for one week prior to treatment. Experimental procedures were approved by the Animal Ethics Committee of Nanjing Medical University, according to international standards.
Experimental groups
Animals were divided randomly into 6 groups: Sham group (treated with saline as a control, n=21); EP group (treated with pilocarpine; n=78); small interfering RNA (siRNA) group (treated with pilocarpine plus siRNA; n=24); pyrrolidinedithiocarbamic acid (PDTC) group (treated only with PDTC; n=12); EP + PDTC group (treated with pilocarpine plus PDTC; n=12); scrambled group (treated with pilocarpine plus scrambled siRNA; n=4).
Surgery for intracerebroventricular (ICV) injection
The purpose of the operation was to inject siRNA. SD rats were anesthetized with 10% chloral hydrate (3 ml/kg) and placed on stereotactic apparatus. A stainless steel single guide cannula (PlasticsOne Inc., Roanoke, VA, USA) was implanted into the right lateral ventricle with coordinates of 0.8 mm posterior to the bregma, 1.5 mm lateral to the midline and 4.5 mm depth to the surface of the skull (22). The cannula was fixed with dental cement (Heraeus Kulzer GmbH, Wehrheim, Germany).
Pilocarpine-induced status epilepticus (SE)
Rats were administrated with lithium chloride (127 mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) by intraperitoneal (IP) injection 24 h before pilocarpine treatment. Methyl scopolamine (1 mg/kg, IP; Sigma-Aldrich) was delivered 30 min before pilocarpine to reduce the peripheral effects and to enhance survival. A single dose of pilocarpine (30 mg/kg, IP) (Sigma-Aldrich) was administered. Subsequently, rats received repeated injections of pilocarpine (10 mg/kg, IP) every 30 min until they developed convulsive seizures. The maximum number of pilocarpine injections was 5 per animal (23). Diazepam (10 mg/kg; Sigma-Aldrich) was utilized to terminate seizure activity 90 min after the onset of SE. Severity of the convulsions were evaluated by Racine's classification and denoted the following stages: 0, behavioral arrest; 1, face clonus; 2, head nodding; 3, forelimb clonus; 4, forelimb clonus and rearing, 5, forelimb clonus with rearing and falling (24). Animals classified as less than Racine's stage 4 were excluded from the experiment. The sham groups were injected with normal saline instead of pilocarpine.
siRNA micro-injection and PDTC administration
siRNAs targeting HMGB1 and RAGE mRNA were designed and synthesized by Shanghai GenePhama Co., Ltd. (Shanghai, China). siRNA sequences are listed in Table I. As described previously, the rats of the siRNA group were treated with 10 µl siRNA (5 µmol/l) (25) by a microinjection pump via a pre-implanted cannula 30 min prior to pilocarpine injection. Following each injection, the needle was left in place for 5 min to allow for drug diffusion. The sham group was treated with 10 µl saline. The Scrambled group was administrated with 10 µl scrambled siRNA (5 µmol/l) and served as the negative control.
Table I.
siRNA sequences.
| siRNA | Sequence (5′-3′) |
|---|---|
| HMGB1-F | GGAAGACGAAGAUGAAGAATT |
| HMGB1-R | UUCUUCAUCUUCGUCUUCCTT |
| RAGE-F | GCCGGAAAUUGUGAAUCCUTT |
| RAGE-R | AGGAUUCACAAUUUCCGGCTT |
| Negative control-F | UUCUCCGAACGUGUCACGUTT |
| Negative control-R | ACGUGACACGUUCGGAGAATT |
siRNA, small interfering RNA; F, forward; R, reverse; bp, base pair; HMGB1, high-mobility group box 1 gene; RAGE, receptor for advanced glycation end-product.
Rats in the EP + PDTC group were treated with PDTC (100 mg/kg, IP; BioVision, Inc., Milpitas, CA, USA) at 14.5 h and 15 min prior to the first pilocarpine injection and at 90 min after onset of SE. A further two injections of PDTC at the same dose was administrated 5 h after termination of SE and 23.5 h after the first injection of pilocarpine (26). The sham group was treated with the same volume of saline and the PDTC group was treated IP with 100 mg/kg PDTC alone.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the hippocampus of the rats using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol. The concentration and purity of the RNA was measured spectrophotometrically at 260 and 280 nm. Total RNA (500 ng) was subjected to DNase I digestion (Takara Bio, Inc., Otsu, Japan), and was subsequently utilized for cDNA synthesis using the PrimeScript™ RT Master Mix (Takara Bio, Inc.). qPCR was then performed using the SYBR® Premix Ex Taq™ kit (Takara Bio, Inc.) with 1 µl 0.5 µM forward and reverse primers (final concentration, 0.5 µm; Sangon Biotech Co., Ltd., Shanghai, China; Table II) and 8 µl diluted cDNA on a 7300 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). Cycling conditions were as follows: Initial predenaturation step at 95°C for 30 sec, followed by 40 cycles of denaturation at 95°C for 5 sec and annealing at 60°C for 31 sec. The relative mRNA expression of the target gene was normalized to GAPDH and was calculated using the 2−ΔΔCq method (27).
Table II.
Primers used for reverse transcription-quantitative polymerase chain reaction.
| Primer | Sequence (5′-3′) | Product size (bp) |
|---|---|---|
| HMGB1-F | CTGATGCAGCTTATACGAAG | 20 |
| HMGB1-R | TCAGGTAAGGAGCAGAACAT | 20 |
| RAGE-F | GAATCCTCCCCAATGGTTCA | 20 |
| RAGE-R | GCCCGACACCGGAAAGT | 17 |
| MDR1A-F | GAGTGAAAAGGTCGTCCAGGAAGCG | 25 |
| MDR1A-R | TCTCGCATGGTCACAGTTCATGAGC | 25 |
| MDR1B-F | CCCAAAGTGACACTGGTGCCTCTG | 24 |
| MDR1B-R | GCCTGGAGCCCATAGCCCCTTTA | 24 |
| GAPDH-F | ATGACTCTACCCACGGCAAG | 20 |
| GAPDH-R | TACTCAGCACCAGCATCACC | 20 |
F, forward; R, reverse; bp, base pair; HMGB1, high-mobility group box 1 gene; RAGE, receptor for advanced glycation end-product gene; MDR1A and MDR1B, P-glycoprotein gene.
Western blot analysis
Total protein was extracted from the hippocampus of rats using a protein extraction kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). Samples were homogenized and centrifuged at 14,000 × g for 15 min, and the supernatant was harvested and diluted to 6.7 µg/µl in SDS/bromophenol blue loading buffer, prior to heating for 5 min at 100°C. Samples were loaded onto 10% gels, separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). Membranes were blocked in 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) at room temperature for 2 h and subsequently incubated at 4°C overnight with the following primary antibodies: Rabbit anti-HMGB1 (1:1,000; catalog no. ab18256; Abcam, Cambridge, UK); rabbit anti-RAGE (1:1,000; catalog no. ab3611; Abcam); mouse anti-P-gp (1:50, catalog no. 517310; Calbiochem; EMD Millipore); rabbit anti-phosphorylated-NF-κB p65 (p-p65) (1:1,000, catalog no. 3033; Cell Signaling Technology, Inc., Danvers, MA, USA). Following incubation with horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (1:5,000; catalog no. ZB230; ZSGB-BIO, Beijing, China) or anti-mouse secondary antibodies (1:5,000; catalog no. ZB2305; ZSGB-BIO) for 1.5 h at room temperature, proteins were detected using electrochemiluminescence (catalog no. WBKLS0500; EMD Millipore) according to the manufacturer's instructions, with Quantity One software (version 4.62; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The optical density of each sample was measured using Scion Image software (version 1.47; Scion Corporation, Frederick, MD, USA). The optical density of each sample was normalized to the mouse anti-β-actin (1:500; catalog no. BM0627; Wuhan Boster Biological Technology, Ltd., Wuhan, China) loading control.
Immunofluorescence
Rat brains were fixed in 4% paraformaldehyde following trans-cardiac perfusion overnight at 4°C, and subsequently soaked in 20 and 30% sucrose for 2 to 3 days, respectively. The brains were then embedded into optimal cutting temperature compound (catalog no. C1400; Applygen Technologies, Inc., Beijing, China). Sections (8-µm thick) were prepared using a vibrating slicer and staining was performed using a standard procedure (28). Briefly, sections were blocked in 0.01 mol/l serum (either rabbit or mouse according to the secondary antibody used; Wuhan Boster Biological Technology, Ltd.) at room temperature for 2 h. The redundant serum was removed then sections were incubated with the following primary antibodies: Rabbit anti-HMGB1 (1:500; catalog no. ab18256; Abcam), rabbit anti-RAGE (1:200; catalog no. ab3611; Abcam) and mouse anti-P-gp (1:50; catalog no. 517310; EMD Millipore) at 4°C overnight. Goat anti-rabbit FITC-conjugated IgG (1:1,000 PBS dilution; catalog no. BA1105; Wuhan Boster Biological Technology, Ltd.) or goat anti-mouse cyanine 3-conjugated IgG (1:1,000 PBS dilution; catalog no. BA1101; Wuhan Boster Biological Technology, Ltd.) secondary antibodies were added at room temperature for 1 h, followed by DAPI (catalog no. AR1177; Wuhan Boster Biological Technology, Ltd.) nuclear stain. Sections were visualized using a Leica fluorescence microscope (DM4000; Leica Microsystems GmbH, Wetzlar, Germany) and the Leica QWin version 3 software (Leica Microsystems GmbH) was used for analysis. Different cell types were identified by analyzing cell morphology.
Statistical analysis
Data was analyzed using SPSS software version 13.0 (SPSS, Inc., Chicago, IL, USA) and are presented as the mean ± standard deviation. Significant differences were analyzed using a one-tailed Student's t-test, or by one-way analysis of variance followed by the Least Significant Difference post-hoc test for multiple comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
Seizure behavior
The concentration of pilocarpine (35.50±9.12 mg/kg) utilized for inducing seizures was equal to that of previous experiments (26). Following pilocarpine administration, hypersalivation, piloerection, licking, chromodacryorrhea, swallowing and chewing occurred immediately. Subsequently, clonic movements of forelimbs, head bobbing and motor limbic seizures were observed. The average latency of first seizures (29) and from first pilocarpine injection to SE were 35.02±24.43 and 37.93±24.78 min, respectively. Induction success refers to the percentage of rats who had seizures, however, the level of which may not be suitable for further experiments; this also includes rats who had seizures however, they succumbed within 90 min following SE. Model success refers to the percentage of rats whose seizure level reached the experimental requirements; these rats survived the 90 min period following SE. The rates of induction and model success in rats in the present study were 91.28 and 82.05%, respectively. The rate of failure, the percentage of rats that were not model successful, was 8.21% and the rate of total mortality, which included anesthesia-associated mortality, rats succumbing during the induction of SE and mortality prior to the scheduled time of specimen collection, was 18.46%.
Expression of HMGB1, RAGE, p-p65 and P-gp following SE
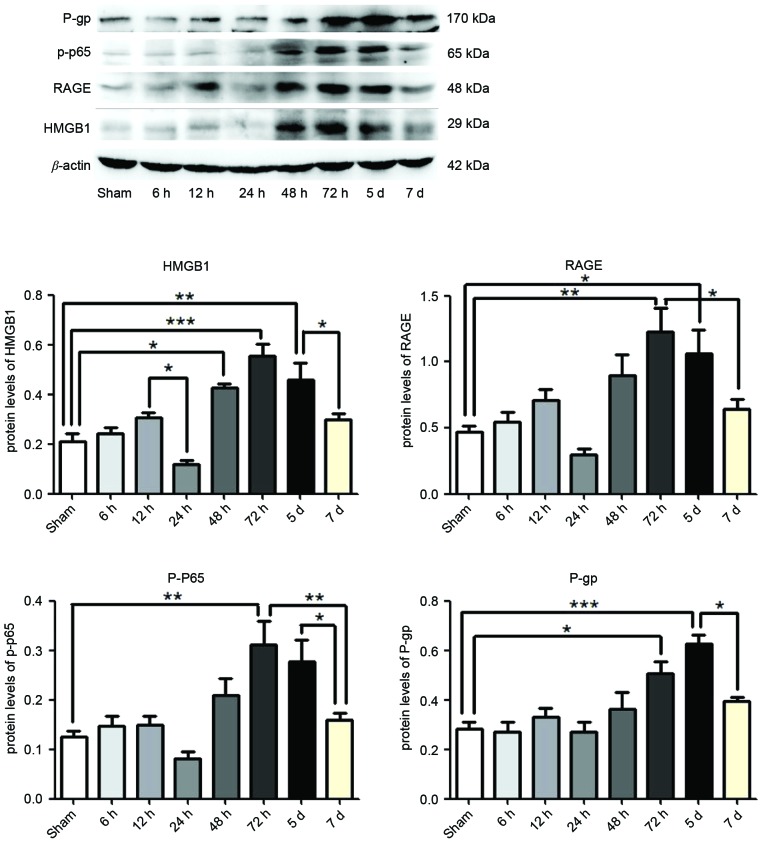
At 6, 12, 24, 48 and 72 h, and 5 and 7 days post-termination of seizures, HMGB1, RAGE, p-p65 and P-gp were measured in the hippocampus of rat brains by western blotting. Compared with the Sham group, the expression levels of HMGB1 were significantly enhanced following 48 h (P<0.05), 72 h (P<0.0001) and 5 days (P<0.01) SE termination, as were the expression levels of RAGE (P<0.01 and P<0.05) and P-gp (P<0.05 and P<0.0001) at 72 h and 5 days respectively, and p-p65 was significantly enhanced following 72 h (P<0.01; Fig. 1). However, the expression levels of HMGB1 were reduced at 24 h and 7 days compared with 12 h and 5 days SE termination, respectively (P<0.05; Fig. 1). The expression levels of RAGE (P<0.05) and p-p65 (P<0.01) were reduced following 7 days compared with 72 h, as were the expression levels of p-p65 and P-gp at 7 days compared with 5 days termination (P<0.05; Fig. 1). The greatest expression of HMGB1, RAGE and p-p65 was observed at 72 h, and declined at later time points (Fig. 1). However, expression levels of P-gp continued to increase at 5 days, which were subsequently reduced by 7 days (Fig. 1).
Figure 1.
Protein expression levels of HMGB1, p-p65, RAGE and P-gp in the hippocampus following status epilepticus. Proteins were measured at 6, 12, 24, 48, 72 h, 5 and 7 days after termination of seizures, by western blotting. β-actin served as the loading control. Densitometry analysis was performed and data are presented as mean ± standard deviation. *P<0.05, **P<0.01 and ***P<0.0001. HMGB1, high-mobility group box 1; RAGE, receptor for advanced glycation end-product; p-p65, phosphorylated-NF-κB p65; P-gp, P-glycoprotein.
HMGB1 siRNA pre-treatment inhibits seizure-induced overexpression of p-p65, RAGE and P-gp
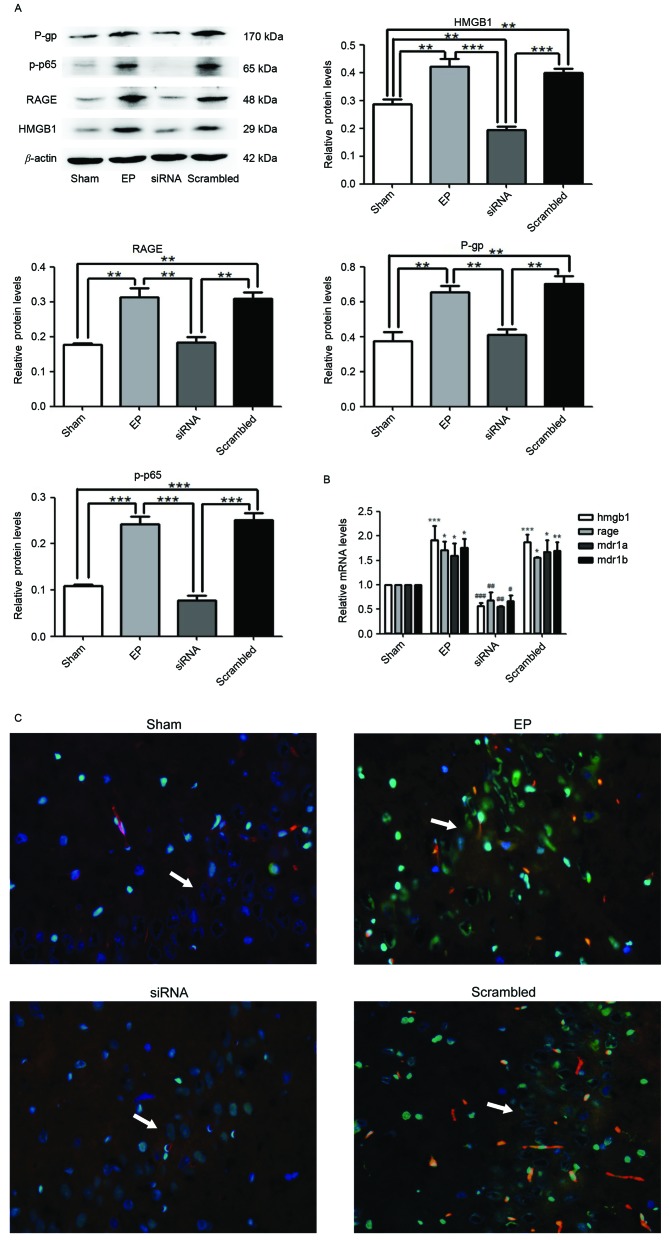
At 30 min prior to IP pilocarpine treatment, HMGB1 siRNA was administered to rats (ICV injection). At 3 days after SE termination, HMGB1, RAGE, p-p65 and P-gp expression levels were measured by western blotting. Protein levels of HMGB1 (P<0.01), RAGE (P<0.01), P-gp (P<0.01) and p-p65 (P<0.0001) in the EP group were significantly greater compared with expression in the sham group (Fig. 2A). Pre-treatment with HMGB1 siRNA significantly reduced the expression levels of all proteins compared with the EP and scrambled groups (P<0.01). The mRNA expression levels of HMGB1, RAGE, MDR1A and MDR1B supported the western blotting data (Fig. 2B). Immunofluorescence staining of tissue sections demonstrated that HMGB1 was localized to the nuclei and cytoplasm of neurons (Fig. 2C). P-gp was primarily localized to blood vessels. HMGB1 and P-gp were expressed in low levels in the Sham group; however, a greater number of cells expressed HMGB1 and P-gp in the hippocampal CA3 region of the EP group. Compared with the EP group, the number of cells expressing HMGB1 and P-gp was reduced in the group treated with HMGB1 siRNA (Fig. 2C).
Figure 2.
HMGB1 siRNA reduces the expression levels of RAGE, p-p65 and P-gp. (A) Protein samples were analyzed 72 h following status epilepticus termination. Densitometry analysis was performed and data are presented as mean ± standard deviation. *P<0.05, **P<0.01, ***P<0.0001. (B) HMGB1, RAGE and MDR1A and MDR1B mRNA expression levels were significantly reduced following treatment with HMGB1 siRNA. *P<0.05, **P<0.01 and ***P<0.0001 vs. Sham; #P<0.05, ##P<0.01 and ###P<0.0001 vs. EP. (C) The localization of HMGB1 and P-gp in the hippocampal CA3 region (indicated by white arrows) of rat brains, as determined by immunofluorescence staining. Blue=nuclei, green=HMGB1 and red=P-gp. Images were captured using ×400 magnification. HMGB1 and P-gp were significantly reduced in the siRNA group compared with the EP and scrambled groups. HMGB1 was localized to the nuclei and cytoplasm. P-gp was primarily localized to blood vessels. P-gp, P-glycoprotein; p-p65, phosphorylated-NF-κB p65; RAGE, receptor for advanced glycation end-product; HMGB1, high-mobility group box 1 gene; MDR1A and MDR1B, P-glycoprotein genes; EP, pilocarpine-induced epilepsy group.
RAGE knockdown attenuates seizure-induced upregulation of p-p65 and P-gp
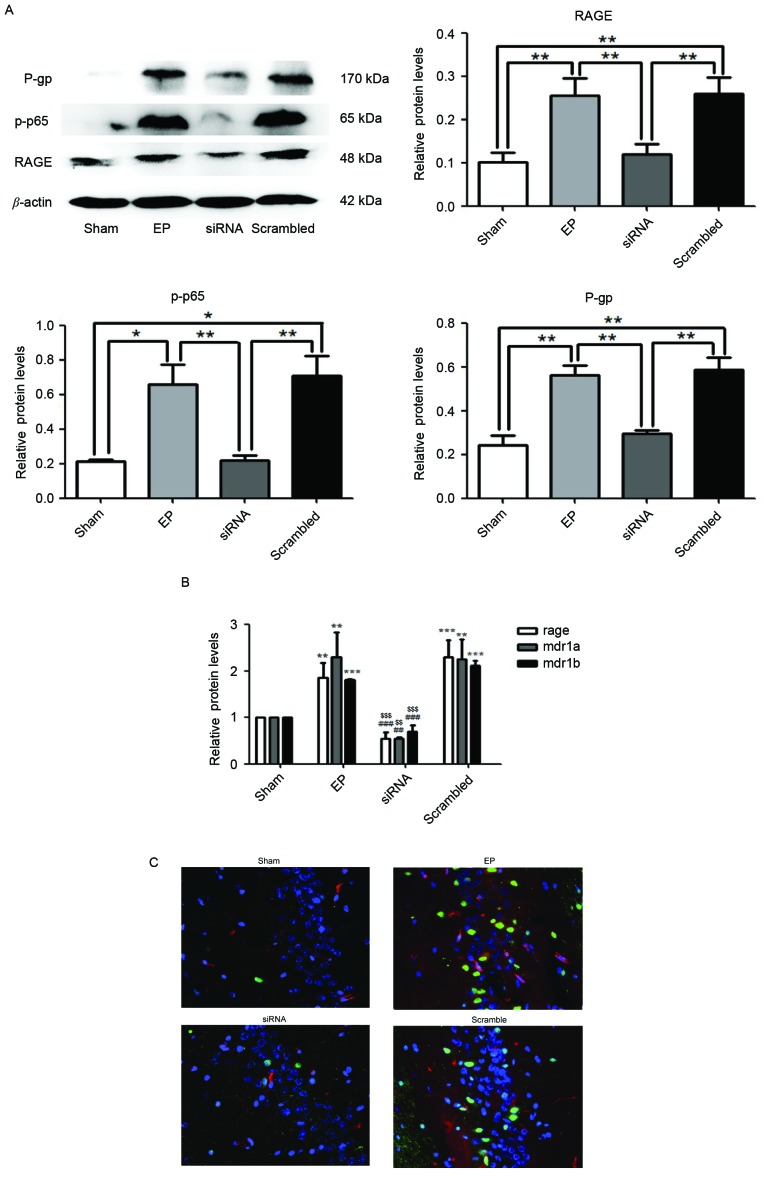
Protein expression levels of RAGE, p-p65 and P-gp were significantly reduced in the siRNA group compared with the EP and scrambled group (P<0.01; Fig. 3A). Similarly, in the group treated with RAGE siRNA, mRNA expression of RAGE, MDR1A and MDR1B were significantly reduced compared with the EP and scrambled groups (Fig. 3B). Immunofluorescence staining demonstrated that RAGE and P-gp were upregulated in the EP group compared with the sham group. Pre-treatment with RAGE siRNA resulted in reduced numbers of P-gp positive cells compared with the scrambled group (Fig. 3C).
Figure 3.
RAGE knockdown reduces seizure-induced p-p65 and P-gp overexpression. Specimens were collected 72 h following status epilepticus termination. (A) RAGE, p-p65 and P-gp were significantly reduced in the siRNA group compared with the EP and scrambled groups, as determined by western blotting. Densitometry analysis was performed and data are presented as the mean ± standard deviation. *P<0.05 and **P<0.01. (B) mRNA expression levels of RAGE and MDR1A/B in the siRNA group were significantly reduced compared with the EP and Scrambled group. **P<0.01 and ***P<0.0001 vs. sham; ##P<0.01 and ###P<0.0001 vs. EP; $$P<0.01 and $$$P<0.0001 vs. Scrambled. (C) Localization of RAGE and P-gp in hippocampal region CA3 of rat brains. Blue=nuclei, green=RAGE and red=P-gp. Images were captured using ×400 magnification. Compared with the EP and scramble groups, the expression levels of RAGE and P-gp in the siRNA group were significantly reduced. RAGE was primarily localized to the nuclei of neurons and P-gp was primarily localized to blood vessels. RAGE, receptor for advanced glycation end-product; p-p65, phosphorylated-NF-κB p65; P-gp, P-glycoprotein; MDR1A and MDR1B, P-glycoprotein gene; RAGE, receptor for advanced glycation end-product; EP, pilocarpine-induced epilepsy group.
Blocking NF-κB activity using PDTC reduces P-gp expression in rat brains following seizures
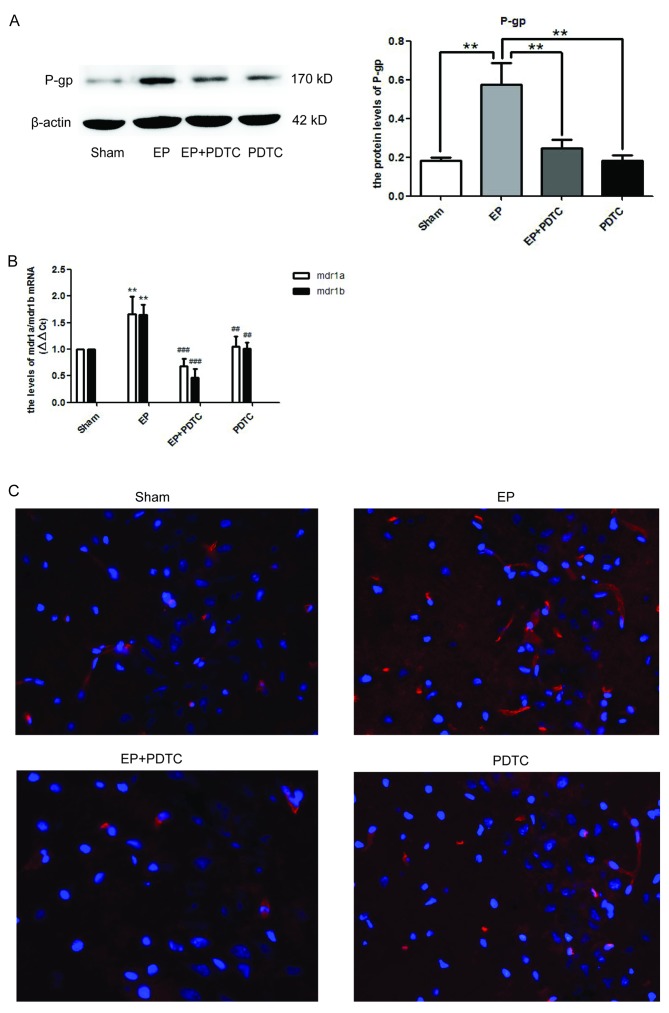
To determine whether NF-κB may regulate the expression of P-gp, PDTC, a specific inhibitor of NF-κB was utilized. As demonstrated in Fig. 4A, 72 h after SE termination, the protein expression levels of P-gp in the EP + PDTC group were significantly reduced compared with the EP group (P<0.01). This was supported by the mRNA expression levels of MDR1A/B (Fig. 4B), and the immunofluorescence staining of P-gp (Fig. 4C).
Figure 4.
Inhibition of NF-κB by PDTC reduces the expression levels of P-gp in epileptic rat brains. (A) P-gp expression levels in each group were detected at 72 h following termination of status epilepticus by western blotting. Densitometry analysis was performed and data are presented as mean ± standard deviation. PDTC significantly reduced the expression levels of P-gp in the EP + PDTC compared with the EP and Sham groups (**P<0.01). However, there was no statistical difference in expression between the EP + PDCT and PDTC groups. (B) mRNA expression levels of MDR1A/B were significantly enhanced in the EP group compared with the sham group, and were significantly reduced in the EP + PDTC compared with the PDTC group. (P<0.01 vs. sham; ##P<0.01 and ###P<0.0001 vs. EP). (C) Expression and localization of P-gp in hippocampal region CA3 of rat brains. Blue=nuclei and red=P-gp. Images were captured using ×400 magnification. Compared with the EP group, P-gp was reduced in the EP + PDTC group and was primarily localized to blood vessels. NF-κB, nuclear factor-κB; PDTC, pyrrolidinedithiocarbamic acid; P-gp, P-glycoprotein; EP, pilocarpine-induced epilepsy group.
Discussion
The possibility that inflammatory processes in the brain contribute to seizures and the establishment of a chronic epileptic foci, is becoming increasingly recognized. Pro-inflammatory cytokines, including interleukin-1β, and danger signals, including HMGB1 and S100β, are overexpressed in human and experimental epileptogenic tissues (30–32). They have proconvulsant activity in various seizure models, possibly by reducing the seizure threshold via functional interactions with classical neurotransmitter systems (33). This demonstrates potential novel targets for therapeutic intervention to epilepsy.
HMGB1 is a nuclear protein that has cytokine-like functions following its extracellular release. It is either passively released from cells undergoing injury or actively secreted by cells under stressful conditions (34). The hyperacetylated form of HMGB1 regulates the transcription of various pro-inflammatory cytokines via binding to TLR4 and RAGE (35,36).
Experimental models of epilepsy suggest that HMGB1-TLR4 signaling may contribute to seizures in humans, and could potentially be targeted to produce anticonvulsant effects in patients currently resistant to drugs (14). In addition, our previous study demonstrated that the susceptibility to seizures may be reduced by inhibition of HMGB1 and TRL4 in KA-induced epilepsy (37).
The present study revealed that the expression levels of HMGB1 were enhanced following SE up to 72 h, which were subsequently reduced again. However, there was a marked decrease in HMGB1 expression at 24 h. Luo et al (38) reported a similar effect in a KA-induced seizure mouse model. Wang et al (39) revealed that HMGB1 was released by macrophages 12 h post-stimulation and this resulted in an intracellular decrease in HMGB1 stores. In addition, HMGB1 secretion was reduced 24 h post-stimulation and resynthesized. It was speculated that brain cells may have similar mechanisms. Proliferated neuroglia cells were demonstrated to be involved in enhanced secretion of HMGB1 24 h post-stimulation (39).
RAGE is a transmembrane receptor of the immunoglobulin superfamily and interacts with ligands following activation of pro-inflammatory and immune responses (40). A previous study suggested that overexpression of RAGE in temporal epilepsy contributes to hyperexcitability in acute and chronic seizures. A potential underlying mechanism may be that HMGB1 activates RAGE, resulting in inflammation in epileptic brains (17).
It is established that HMGB1/RAGE have a role in inflammatory responses (41,42), and their interaction results in downstream activation of the pro-inflammatory transcription factor, NF-κB (43). NF-κB is a key protein that is regulated by RAGE signaling (44). Upon activation, NF-κB translocates to the nucleus and subsequently binds to DNA sequences to stimulate transcription of target genes, including various cytokines, adhesion molecules and the gene encoding RAGE itself (45). In present study, NF-κB expression levels were reduced following knockdown of HMGB1 and RAGE, which suggested that HMGB1/RAGE regulates the transcription of the gene encoding NF-κB.
Our previous study demonstrated that following knockdown of the gene encoding IKKβ, which phosphorylates an inhibitory protein of NF-κB to enable its nuclear migration, expression of the P-gp protein was reduced in a KA-induced epileptic brain. This result concurred with the present study, as inhibition of NF-κB by PDTC following SE termination resulted in reduced expression levels of P-gp. Therefore, it is possible that NF-κB regulates MDR1A/B gene transcription.
In rodents, P-gp is encoded by the MDR1A and MDR1B genes. P-gp encoded by MDR1A serves a role as an efflux pump in cells of the liver, intestine, kidney and blood-brain barrier (46). The mRNA expression levels of MDR1A and MDR1B in the present study were not significantly different. In addition to vascular endothelial cells, nerve cells can express P-gp in the brain following seizures (47). However, the immunofluorescence staining in the present study demonstrated that P-gp was primarily localized to blood vessels, and there was little expression observed in neurons. This may be due to the model utilized and the time in which specimens were observed. van Vliet et al (48) demonstrated that P-gp is observed in glial-like cells in the rat dentate gyrus 1 week after seizures induced by electrical kindling. However, Guo et al (49) reported that P-gp was not expressed in hippocampus neurons 4 weeks after pilocarpine-induced epilepsy in rats. During immunofluorescence testing, specific markers for the different cell types were not applied. This was a limitation of the present study, which could be resolved with specific markers for blood vessels and neurons in future studies.
In conclusion, the present study demonstrated that HMGB1 knockdown may reduce the expression levels of MDR1A/B mRNA and P-gp protein via RAGE/NF-κB inflammatory signaling pathways. This may partly explain the underlying mechanism of P-gp overexpression in pilocarpine-induced SE rat brains. Therefore, targeting the HMGB1/RAGE/NF-κB signaling pathway may be a potential strategy for the treatment of DRE.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81171222). The authors would like to thank Dr Zhang Qiaoquan (Department of Neuropathology, Nanjing Brain Hospital, Jiangsu, China) for their assistance with the immunofluorescence.
Glossary
Abbreviations
- AED
anti-epilepsy drug
- DRE
drug-refractory epilepsy
- EP
pilocarpine-induced epilepsy
- HMGB1
high-mobility group box 1
- NF-κB
nuclear factor-κB
- P-gp
p-glycoprotein
- p-P65
phospho-NF-κB p65
- RAGE
receptor for advanced glycation end-product
- siRNA
small interfering RNA
References
- 1.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: A meta-analytic approach. Epilepsia. 2010;51:883–890. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ni H, Sun Q, Tian T, Feng X, Sun BL. Long-term expression of metabolism-associated genes in the rat hippocampus following recurrent neonatal seizures and its regulation by melatonin. Mol Med Rep. 2015;12:2727–2734. doi: 10.3892/mmr.2015.3691. [DOI] [PubMed] [Google Scholar]
- 3.Stepień KM, Tomaszewska J, Czuczwar SJ. The multidrug transporter P-glycoprotein in pharmacoresistance to antiepileptic drugs. Pharmacol Rep. 2012;64:1011–1019. doi: 10.1016/S1734-1140(12)70900-3. [DOI] [PubMed] [Google Scholar]
- 4.Zhang CB, Kwan P, Zuo Z, Baum L. The transport of antiepileptic drugs by P-glycoprotein. Adv Drug Deliv Rev. 2012;64:930–42. doi: 10.1016/j.addr.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Avemary J, Salvamoser JD, Peraud A, Rémi J, Noachtar S, Fricker G, Potschka H. Dynamic regulation of P-glycoprotein in human brain capillaries. Mol Pharm. 2013;10:3333–3341. doi: 10.1021/mp4001102. [DOI] [PubMed] [Google Scholar]
- 6.Potschka H, Luna-Munguia H. CNS transporters and drug delivery in epilepsy. Curr Pharm Des. 2014;20:1534–1542. doi: 10.2174/13816128113199990461. [DOI] [PubMed] [Google Scholar]
- 7.Bartels AL, de Klerk OL, Kortekaas R, de Vries JJ, Leenders KL. 11C-verapamil to assess P-gp function in human brain during aging, depression and neurodegenerative disease. Curr Top Med Chem. 2010;10:1775–1784. doi: 10.2174/156802610792928059. [DOI] [PubMed] [Google Scholar]
- 8.Holtman L, van Vliet EA, Edelbroek PM, Aronica E, Gorter JA. Cox-2 inhibition can lead to adverse effects in a rat model for temporal lobeepilepsy. Epilepsy Res. 2010;91:49–56. doi: 10.1016/j.eplepsyres.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Hartz AM, Notenboom S, Bauer B. Signaling to P-glycoprotein-A new therapeutic target to treat drug-resistant epilepsy? Drug News Perspect. 2009;22:393–397. doi: 10.1358/dnp.2009.22.7.1401354. [DOI] [PubMed] [Google Scholar]
- 10.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawase A, Norikane S, Okada A, Adachi M, Kato Y, Iwaki M. Distinct alterations in ATP-Binding cassette transporter expression in liver, kidney, small intestine, and brain in adjuvant-induced arthritic rats. J Pharm Sci. 2014;103:2556–2564. doi: 10.1002/jps.24210. [DOI] [PubMed] [Google Scholar]
- 12.Doorduin J, de Vries EF, Dierckx RA, Klein HC. P-glycoprotein activity in the blood-brain barrier is affected by virus-induced neuroinflammation and antipsychotic treatment. Neuropharmacology. 2014;85:548–553. doi: 10.1016/j.neuropharm.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Rojas A, Jiang JX, Ganesh T, Yang MS, Lelutiu N, Gueorguieva P, Dingledine R. Cyclooxygenase-2 in epilepsy. Epilepsia. 2014;55:17–25. doi: 10.1111/epi.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16:413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- 15.Chiavegato A, Zurolo E, Losi G, Aronica E, Carmignoto G. The inflammatory molecules IL-1β and HMGB1 can rapidly enhance focal seizure generation in a brain slice model of temporal lobe epilepsy. Front Cell Neurosci. 2014;8:155. doi: 10.3389/fncel.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Huang XJ, Yu N, Xie Y, Zhang K, Wen F, Liu H, Di Q. HMGB1 contributes to the expression of P-glycoprotein in mouse epileptic brain through Toll-like receptor 4 and receptor for advanced glycation end products. PLoS One. 2015;10:e0140918. doi: 10.1371/journal.pone.0140918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iori V, Maroso M, Rizzi M, Iyer AM, Vertemara R, Carli M, Agresti A, Antonelli A, Bianchi ME, Aronica E, et al. Receptor for advanced glycation endproducts is upregulated in temporal lobe epilepsy and contributes to experimental seizures. Neurobiol Dis. 2013;58:102–114. doi: 10.1016/j.nbd.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Roberts DJ, Goralski KB. A critical overview of the influence of inflammation and infection on P-glycoprotein expression and activity in the brain. Expert Opin Drug Metab Toxicol. 2008;4:1245–1264. doi: 10.1517/17425255.4.10.1245. [DOI] [PubMed] [Google Scholar]
- 19.Yu N, Liu H, Zhang YF, Su LY, Liu XH, Li LC, Hao JB, Huang XJ, Di Q. Effects of brain IKKβ gene silencing by small interfering RNA on P-Glycoprotein expression and brain damage in the rat kainic acid-induced seizure model. CNS Neurol Disord Drug Targets. 2014;13:661–672. doi: 10.2174/18715273113129990106. [DOI] [PubMed] [Google Scholar]
- 20.Angelo MF, Aguirre A, Avilés Reyes RX, Villarreal A, Lukin J, Melendez M, Vanasco V, Barker P, Alvarez S, Epstein A, et al. The proinflammatory RAGE/NF-κB pathway is involved in neuronal damage and reactive gliosis in a model of sleep apnea by intermittent hypoxia. PLoS One. 2014;9:e107901. doi: 10.1371/journal.pone.0107901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batkulwar KB, Bansode SB, Patil GV, Godbole RK, Kazi RS, Chinnathambi S, Shanmugam D, Kulkarni MJ. Investigation of phosphoproteome in RAGE signaling. Proteomics. 2015;15:245–259. doi: 10.1002/pmic.201400169. [DOI] [PubMed] [Google Scholar]
- 22.Senn C, Hangartner C, Moes S, Guerini D, Hofbauer KG. Central administration of small interfering RNAs in rats: A comparison with antisenseoligonucleotides. Eur J Pharmacol. 2005;522:30–37. doi: 10.1016/j.ejphar.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Sun H, Wu H, Yu X, Zhang G, Zhang R, Zhan S, Wang H, Bu N, Ma X, Li Y. Angiotensin II and its receptor in activated microglia enhanced neuronal loss and cognitive impairment following pilocarpine-induced status epilepticus. Mol Cell Neurosci. 2015;65:58–67. doi: 10.1016/j.mcn.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90176-9. [DOI] [PubMed] [Google Scholar]
- 25.Lei C, Lin S, Zhang C, Tao W, Dong W, Hao Z, Liu M, Wu B. Activation of cerebral recovery by matrix metalloproteinase-9 after intracerebral hemorrhage. Neuroscience. 2013;230:86–93. doi: 10.1016/j.neuroscience.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Soerensen J, Pekcec A, Fuest C, Nickel A, Potschka H. Pyrrolidine dithiocarbamate protects the piriform cortex in the pilocarpine status epilepticus model. Epilepsy Res. 2009;87:177–183. doi: 10.1016/j.eplepsyres.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Cui Z, Wang S, Zhang D. CD93 and GIPC expression and localization during central nervous system inflammation. Neural Regen Res. 2014;9:1995–2001. doi: 10.4103/1673-5374.145383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: Behavioural, eletroencephalographic and neuropathological study. Behav Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- 30.Choy M, Dubé CM, Patterson K, Barnes SR, Maras P, Blood AB, Hasso AN, Obenaus A, Baram TZ. A novel noninvasive predictive epilepsy biomarker with clinical potential. J Neurosci. 2014;34:8672–8684. doi: 10.1523/JNEUROSCI.4806-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanotto C, Abib RT, Batassini C, Tortorelli LS, Biasibetti R, Rodrigues L, Nardin P, Hansen F, Gottfried C, Leite MC, Gonçalves CA. Non-specific inhibitors of aquaporin-4 stimulate S100B secretion in acute hippocampal slices of rats. Brain Res. 2013;1491:14–22. doi: 10.1016/j.brainres.2012.10.065. [DOI] [PubMed] [Google Scholar]
- 32.Kołosowska K, Maciejak P, Szyndler J, Turzyńska D, Sobolewska A, Płaźnik A. The role of interleukin-1β in the pentylenetetrazole-induced kindling of seizures, in the rat hippocampus. Eur J Pharmacol. 2014;731:31–37. doi: 10.1016/j.ejphar.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Vezzani A. Epilepsy and inflammation in the brain: Overview and pathophysiology. Epilepsy Curr. 2014;14(1 Suppl):S3–S7. doi: 10.5698/1535-7511-14.s2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang H, Antoine DJ, Andersson U, Tracey KJ. The many faces of HMGB1: Molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol. 2013;93:865–873. doi: 10.1189/jlb.1212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maroso M, Balosso S, Ravizza T, Liu J, Bianchi ME, Vezzani A. Interleukin-1 type 1 receptor/Toll-like receptor signalling in epilepsy: The importance of IL-1beta and high-mobility group box 1. J Intern Med. 2011;270:319–326. doi: 10.1111/j.1365-2796.2011.02431.x. [DOI] [PubMed] [Google Scholar]
- 36.Huebener P, Pradere JP, Hernandez C, Gwak GY, Caviglia JM, Mu X, Loike JD, Jenkins RE, Antoine DJ, Schwabe RF. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest. 2015;125:539–550. doi: 10.1172/JCI76887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang XJ, Hao JB, Di Q, Yu N, Zhang YF, Wen F, Chen Y. High mobility group protein B1 contributes to the expression of P-glycoprotein in hippocampus of epileptic rats. J Nanjing Med University (natural science) 2014;8:1029–1033. [Google Scholar]
- 38.Luo LD, Jin YC, Kim ID, Lee JK. Glycyrrhizin suppresses HMGB1 inductions in the hippocampus and subsequent accumulation in serum of a kainic acid-induced seizure mouse mode. Cell Mol Neurobiol. 2014;34:987–997. doi: 10.1007/s10571-014-0075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMGB-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 40.Ibrahim ZA, Armour CL, Phipps S, Sukkar MB. RAGE and TLRs: Relatives, friends or neighbours? Mol Immunol. 2013;56:739–744. doi: 10.1016/j.molimm.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Xie J, Méndez JD, Méndez-Valenzuela V, Aguilar-Hernández MM. Cellular signalling of the receptor for advanced glycation end products (RAGE) Cell Signal. 2013;25:2185–2197. doi: 10.1016/j.cellsig.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Mazarati A, Maroso M, Iori V, Vezzani A, Carli M. High-mobility group box-1 impairs memory in mice through both toll-like receptor 4 and Receptor for Advanced Glycation End Products. Exp Neurol. 2011;232:143–148. doi: 10.1016/j.expneurol.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174:7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 44.Kalea AZ, Reiniger N, Yang H, Arriero M, Schmidt AM, Hudson BI. Alternative splicing of the murine receptor for advanced glycation end-products (RAGE) gene. FASEB J. 2009;23:1766–1774. doi: 10.1096/fj.08-117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl) 2005;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 46.Borst P, Schinkel AH. P-glycoprotein ABCB1: A major player in drug handling by mammals. J Clin Invest. 2013;123:4131–4133. doi: 10.1172/JCI70430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aronica E, Sisodiya SM, Gorter JA. Cerebral expression of drug transporters in epilepsy. Adv Drug Deliv Rev. 2012;64:919–929. doi: 10.1016/j.addr.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 48.van Vliet E, Aronica E, Redeker S, Marchi N, Rizzi M, Vezzani A, Gorter J. Selective and persistent upregulation of mdr1b mRNA and P-glycoprotein in the parahippocampal cortex of chronic epileptic rats. Epilepsy Res. 2004;60:203–213. doi: 10.1016/j.eplepsyres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Guo Y, Jiang L. Drug transporters are altered in brain, liver and kidney of rats with chronic epilepsy induced by lithium-pilocarpine. Neurol Res. 2010;32:106–112. doi: 10.1179/174313209X408954. [DOI] [PubMed] [Google Scholar]