Abstract
Laryngeal cancers are mostly squamous cell carcinomas. Although targeting radio-resistant cancer cells is important for improving the treatmental efficiency, the signaling pathway- and therapeutic strategy-related to laryngeal carcinoma still require further study. Galangin is an active pharmacological ingredient, isolated from propolis and Alpinia officinarum Hance, and has been reported to have anticancer and anti-oxidative properties through regulation of cell cycle, resulting in angiogenesis, apoptosis, invasion and migration without triggering any toxicity in normal cells. PI3K/AKT and p38 are important signaling pathways to modulate cancer cell apoptosis and proliferation through caspase-3, NF-κB and mTOR signal pathways. Autophagy is also enhanced by activating LC3s and Beclin 1. In the present study, galangin was found to suppress laryngeal cancer cell proliferation. Also, flow cytometry, immunohistochemical and western blot analysis indicated that cell apoptosis was induced for galangin administration, promoting caspase-3 expression through regulating PI3K/AKT/NF-κB. Furthermore, galangin inhibited laryngeal cancer cell proliferation, related to p38 inactivation by galangin treatment. Additionally, mTOR activation regulated by PI3K/AKT was reduced by galangin, suppressing cancer cell transcription and proliferation. Our data also indicated that the tumor volume and weight in nude mice were reduced for galangin use in vivo accompanied by Ki-67 decrease and TUNEL increase in tumor tissues. Together, our data indicated that galangin has a potential role in suppressing human laryngeal cancer via inhibiting tumor cell proliferation, activating apoptosis and autophagy, which were regulated by p38 and AKT/NF-κB/mTOR pathways, providing a therapeutic strategy for human laryngeal cancer treatment.
Keywords: laryngeal cancer, galangin, caspase-3, AKT/NF-κB/mTOR
Introduction
Laryngeal cancer consists mostly of squamous cell carcinomas (also known as the larynx or laryngeal carcinoma), indicating their origin from human skin of larynx (1,2). According to GLOBOCAN 2012, there were an estimated 156,877 new cases and 83,376 deaths in the world, and the adjusted incidence and mortality rates were 2.1/100,000 and 1.1/100,000, respectively (3). Cancer could develop in any part of the larynx according to a previous study. However, the cure rate presently is influenced by the tumor location (4). The origin of laryngeal cancer is a specialised area, which needs the coordinated expertise of ear, nose and throat surgeons and oncologists (5,6). The laryngeal cancer symptoms rely on the tumor size and location. Specific treatment has a close relationship with the type, location, as well as the stage of laryngeal cancer (7,8). Treatment includes radiotherapy, surgery and chemotherapy, alone or in combination. However, a patient affected severely may need a laryngectomy, the total or the partial remove of the vocal cords. In 2013, data indicated that laryngeal cancer has led to a large number of deaths since 1990 (9). Furthermore, the survival rate of patients with laryngeal cancer has decreased compared with the survival rate of patients with all other types of cancers as a whole (4). Hence, finding effective and safe therapeutic strategy is urgenyly needed to improve cancer treatments. It has been indicated that the treatment of a variety of drugs targeting different signaling pathways can provide effective strategy for various cancer cell mutations, and postpone cancer adaptation procedure subsequently.
Galangin (Fig. 1A) is a member of the flavonol class of flavonoids, which occurs at high concentrations in the rhizome of Alpinia officinarum Hance, as well as in propolis, applied in China for centuries as a spice and a traditional Chinese medicine for various diseases (10,11). As a potential scavenger of free radicals, including singlet oxygen and superoxide anion, galangin has various bioactivities and influences many cellular processes (12). In addition to its anti-mutagenic, anti-oxidant, as well as anti-inflammatory functions, galangin has been reported to possess antitumor role in a number of in vitro and in vivo systems, including melanoma, hepatoma and leukaemia (13,14). Furthermore, a previous study suggested that galangin possesses therapeutic potential as an antitumor agent for liver cancer (15). Galangin induced apoptosis by suppressing tumor cell migration, promoting caspase-3 and increasing ROS production (16). However, the effect of galangin on human laryngeal cancer progression has not been investigated yet. In the present study, we investigated the anticancer effects of galangin on two types of human laryngeal cancer cells.
Figure 1.
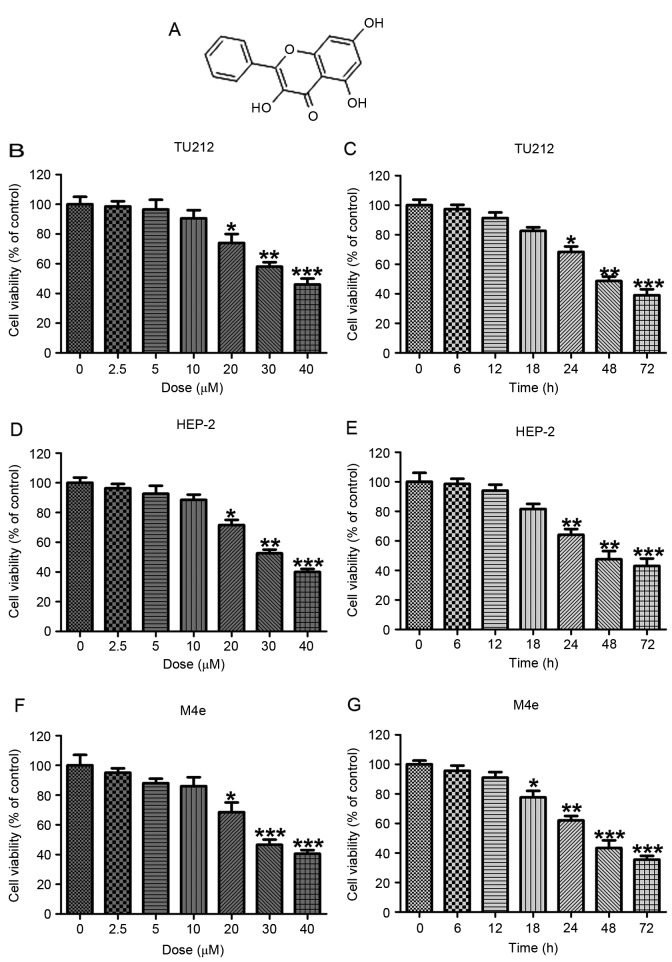
The human laryngeal carcinoma cell viability was calculated by MTT analysis. (A) The chemical structure of galangin. (B) The human laryngeal carcinoma TU212 cells were treated with different concentrations of galangin (0–40 µM) for 24 h, and the cell viability was calculated by MTT. (C) TU2I2 cells were treated with 10 µM galangin for the indicated time. The cell viability was evaluated by MTT assays. (D) The human laryngeal carcinoma HEP-2 cells were treated with different concentrations of galangin (0–40 µM) for 24 h, and then the cell viability was calculated by MTT. (E) HEP-2 cells were treated with 10 µM galangin for the indicated time. The cell viability was evaluated by MTT assays. (F) The human laryngeal carcinoma M4e cells were treated with different concentrations of galangin (0–40 µM) for 24 h, and the cell viability was calculated by MTT. (G) M4e cells were treated with 10 µM galangin for the indicated time. The cell viability was evaluated by MTT assays. All analysis were conducted in triplicate, and the results are the mean ± SEM of three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 (compared to the control/Con).
Apoptosis is a distinct genetic and biochemical pathway of cell death necessary for cell growth, development and maintenance of homeostasis in organisms. Caspase-9 was activated with the elevated level of cleaved caspase-9, which in turn cleaved caspase-3 and ultimately induced apoptosis (17,18). Autophagy is a cellular process of catabolic degradation in which damaged, dysfunctional, or superfluous organelles and proteins are sequestered, engulfed, and recycled to maintain cellular metabolism, viability and homeostasis (19,20). The mTOR kinase, which is activated by signaling pathway originating from growth factors, plays a critical role in regulating autophagy progression (21). There are diverse signaling pathways implicated in the regulation of mTOR signaling, including positive regulation of mTOR (PI3K/Akt and p38 MAPK signaling) suppressing autophagy (22,23). The PI3K/Akt signaling pathway is implicated in cell migration and invasion, which induces the expression of NF-κB transcription factor, resulting in cancer cell proliferation (24).
This study is the first time that galangin was used to treat human laryngeal cancer TU212 and HEP-2 cells, to prove if galangin had a potential effect on inhibiting laryngeal cancer progression in the future. In the present study, TU212 and HEP-2 cells, exposed to galangin, suppressed cancer cell proliferation, migration and invasion. Caspase-3, PI3K/AKT and p38 signaling pathways were investigated. These results indicated that galangin performed its anticancer effect on human laryngeal cancer possibly via inactivating PI3K/AKT- and p38-signaling pathways.
Materials and methods
Cells and treatment
Human laryngeal cancer cell lines, TU212 and M4e, human normal larynx epithelial HBE cells, and human normal liver HHL-5 cells, were purchased from the American Type Culture Collection (ATCC; Manassas, VA USA). The mouse normal larynx epithelial RTE cells, and human laryngeal cancer HEP-2 cells were purchased from the Nanjing KeyGen Biotech, Co., Ltd. (Nanjing, China). TU212, HEP-2 and RTE cells were routinely cultured in RPMI-1640 medium (Gibco, Carlsbad, CA, USA), containing 10% fetal bovine serum (FBS; Gibco), 1% penicillin/streptomycin. The cell lines M4e, HBE and HHL-5 were cultured in Dulbeccos modified Eagles medium (DMEM; Gibco) supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. All cells were kept in a humidified atmosphere with 5% CO2 and 95% humidity at 37°C in an incubator. Galangin (>98% purity), purchased from the Hangzhou DayangChem, Co., Ltd., (Hangzhou, China) was used for the treatment of human laryngeal cancer dissolved in dimethyl sulfoxide (DMSO) and then stored at −20°C for experimental treatment use. The final DMSO concentration in cells is <0.1% (v/v) in each treatment.
Cell viability analysis
In order to calculate the growth inhibitory role of galangin in different cell lines, ~1×103 cells/well were seeded in plates (Corning Inc., Corning, NY, USA) with the respectively complete growth media. The following day, the cells were treated with different concentrations of galangin for different time as shown in the figures and incubated at 37°C. Then, cell viability was calculated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) analysis at 570 nm.
Wound healing analysis
TU212 and HEP-2 cells were seeded in 60-mM dishes and incubated until confluence. After a 3-h cell pre-treatment with 50 µM mytomicin C, wounds were created by scratching cell sheets with a sterile 200-µl pipette tip. The culture medium was replaced with fresh medium containing either DMSO or galangin. The images of a specific position on the scratched areas were taken by an inverted microscope (Leica Microsystems, Wetzlar, Germany) using a ×10 objective every 24 h. The wound widths were assessed and the relative wound widths were evaluated.
Cancer cell migration and invasion
TU212 and HEP-2 cells were seeded into the upper chamber of a Transwell insert pre-coated with 5 µg/ml fibronectin for migration or a BD™ Matrigel invasion chamber for invasion. Medium with 10% serum was put in the lower chamber as a chemo-attractant, and cells were then incubated for 4 h of migration invasion. Non-migratory and non-invasive cancer cells were removed from the upper chamber using a cotton bud. The cells on the lower insert surface were stained with Diff-Quick. Cells were finally calculated as the number of cells observed in five different microscope fields of three independent inserts.
Apoptosis analysis
Apoptosis assay of tissue samples was determined by TUNEL used an In Situ Cell Death Detection kit, fluorescein (Roche Applied Science, Indianapolis, IN, USA) following the manufacturers protocol. Tumor tissue sections were counterstained with hematoxylin. Then, the number of TUNEL-positive cells was evaluated under a microscope. The number of apoptosis cells were counted by the ratio of apoptotic cells to the total cells. The experiment was conducted three times independently for each cell line.
TU212 and HEP-2 cell apoptosis was determined by flow cytometric (FCM) analysis
The tumor cells were harvested, and then washed three times with chilled phosphate-buffered saline (PBS), stained with Annexin V-FITC and propidium iodide (PI) diluted in the binding buffer, and tested by FACSCalibur FCM (BD Biosciences, San Jose, CA, USA) for 15-min incubation at the room temperature in the dark. Fluorescence was then detected at an excitation wavelength of 480 nm through 530 nm FL-1 and 585 nm FL-2 filters. The apoptotic cells were then quantified.
Western blot analysis
For western blot analysis, the cancer cells of TU212 and HEP-2 were washed with chilled PBS and lysed on ice in modified RIPA buffer, containing 50 mM Tris-HCl pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM Na3VO4, and 1 mM NaF, with protease inhibitors (100 µM phenylmethylsulfonyl fluoride, 10 µg/ml leupeptin, 10 µg/ml pepstatin and 2 mM EDTA). The lysates were centrifuged at 12,000 × g for 20 min at 4°C and the supernatant fractions were collected. The proteins were separated by SDS-PAGE electrophoresis and transferred to Immobilon-P membranes. The specific proteins were detected using an enhanced chemiluminescence (ECL) western blotting kit according to the manufacturers instructions. The primary antibodies including PI3K, Bcl-2, Bax, caspase-9, caspase-3, PARP, LC3, Beclin 1, Ras, Raf, p38, AKT, p-AKT, NF-κB, p-NF-κB and GAPDH. GAPDH was used as the loading control. The gray value of the western blotting band was anlyzed by ImageJ software (Version 1.4.2b, Mac OS X; National Institutes of Health, Bethesda, MD, USA USA), representing the specific protein expression levels.
Nude mouse xenograft tumor assay
The 6-week-old SPF male BALB/c nude mice, weighed 20–25 g, and were obtained from Vital River Laboratory Animals Co., Ltd. (Beijing, China). Before the experiments, all mice were required to adapt to the environment for a week. They were housed in a specific pathogen-free, temperature and humidity-controlled environment (25±2°C, 50±2% humidity) with a standard 12-h light/12-h dark cycle with food and water in their cages. All procedures were in accordance with the Regulations of Experimental Animal Administration issued by the Ministry of Science and Technology of China. The mice were randomly divided into 4 groups: the control (Con) group, the galangin group (10 mg/kg), the galangin group (20 mg/kg) and the galangin group (30 mg/kg). Subsequently, 150 µl of the TU212 cell suspension (containing 2×107 cells) was injected subcutaneously into the right axilla area of the mice. Ten days before the cancer cell inoculation, the treatment groups were gavaged with galangin every day and observed for the growth of tumors at 42 days. The control mice were only injected with the equal volume of TU212 cells. The growth of tumors were determined after the mice were sacrificed, and stored for the following experiments.
Histopathological examination of tissues
Histopathological evaluation was performed on mice that were collected. Samples were fixed with 10% buffered formalin, imbedded in paraffin and sliced. Samples were subjected to immunohistochemical staining (Ki-67) according to CST Technology Co. Introduction and performed by Shanghai Zhenda Biotechnology, Co., Ltd. (Shanghai, China).
Immunofluorescence assay
TU212 and HEP-2 cells were cultured on sterilized glass coverslips overnight and treated with galangin for 24 h. After being fixed with 4% paraformaldehyde solution and blocked with 4% BSA in PBS, cells on coverslips were incubated with TSC1, LC3II and Bax primary antibody and anti-rabbit secondary antibody conjugated with Alex Flour 555. Images were captured with a fluorescence microscope (Olympus, Tokyo, Japan).
Statistical analysis
Data were expressed as mean ± SEM from three or more experiments. Treated cells and the corresponding controls were compared using GraphPad Prism (version 6.0; GraphPad Software, Inc., La Jolla, CA, USA) by a one-way ANOVA with Dunns least significant difference tests. Differences between groups were considered significant at P<0.05.
Results
Galangin inhibits human laryngeal carcinoma cell viability without toxicity on normal cells
The human laryngeal carcinoma cell viability treated by galangin was calculated. As shown in Fig. 1B, TU212 cells were treated with various concentrations of galangin for 24 h, then the MTT analysis was used to calculate cell viability. With the increasing of galangin concentration, we found that TU212 cell viability was reduced, especially at >10 µM, showing significant difference compared to the Con ones. Additionally, after the TU212 cells were treated with 10 µM galangin for different time as indicated, we found that the cell viability was reduced in a time-dependent manner (Fig. 2C). Furthermore, in human laryngeal carcinoma HEP-2 cells, the cell viability was decreased with the upregulation of galangin concentrations, especially at >10 µM (Fig. 1D). Also, HEP-2 cell viability was decreased by 10 µM galangin administration for different times. In addition, significant difference was observed for over 24 h (Fig. 1E). Finally, M4e cells were also included to confirm the suppressive role of galangin in human laryngeal carcinoma. From Fig. 1F, shows that the M4e cell viability was downregulated for galangin treatment, which was comparable to the Con ones at >20 µM. Similarly, the cell viability was lower by 10 µM galangin treatment for different times (Fig. 1G). The data indicated that galangin indeed has a potential value in reducing human laryngeal carcinoma cell proliferation in a dose- and time-dependent manner.
Figure 2.
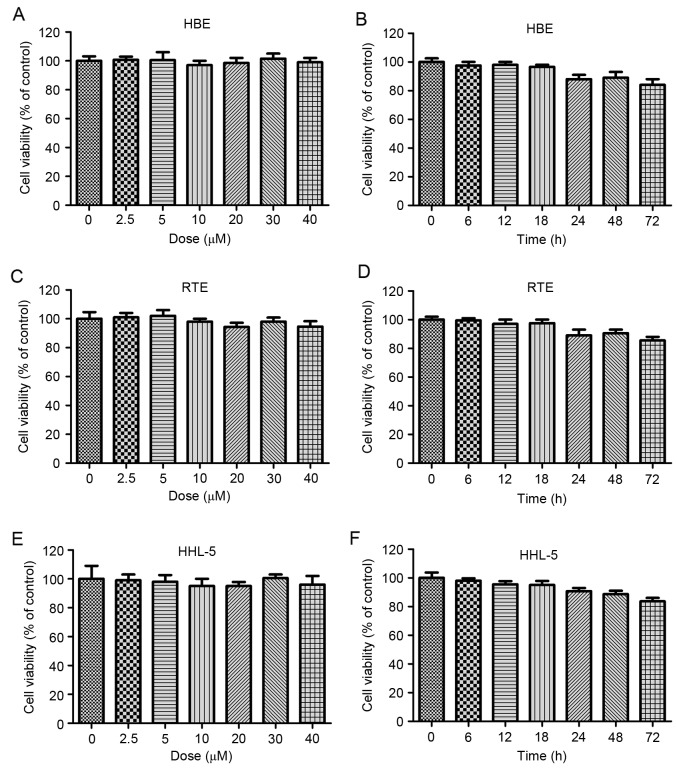
Galangin shows no toxicity on normal cells. (A) The human larynx epithelial HBE cells were treated with different concentrations of galangin, ranged from 0 to 40 µM, for 24 h, and then the cell viability was calculated by MTT. (B) HBE cells were treated with 40 µM galangin for the indicated time. The cell viability was evaluated by MTT assays. (C) The mouse larynx epithelial RTE cells were treated with different concentrations of galangin, ranged from 0 to 40 µM, for 24 h, and the cell viability was calculated by MTT. (D) RTE cells were treated with 40 µM galangin for the indicated time. The cell viability was evaluated by MTT assays. (E) The human normal liver HHL-5 cells were treated with different concentrations of galangin, ranged from 0 to 40 µM, for 24 h, and then the cell viability was calculated by MTT. (F) HHL-5 cells were treated with 40 µM galangin for the indicated time. The cell viability was evaluated by MTT assays. All analysis were conducted in triplicate, and the results are the mean ± SEM of three independent experiments.
Galangin has been proved to be toxic for human laryngeal carcinoma cells, and further study was needed to calculate its effects on normal cells. As shown in Fig. 2A and B, no significant difference was observed in human normal larynx epithelial HBE cells, between the various groups treated under different concentrations of galangin for different times. In line with the results above, in the mouse larynx epithelial cells, no significant difference was observed after galangin treatment under different conditions (Fig. 2C and D). The human normal liver HHL-5 cells were included to measure the toxicity of galangin on normal cells. As shown in Fig. 2E and F, galangin treatment under different conditions did not alter the cell viability of HHL-5. The results indicated that galangin shows no toxicity to normal cells, providing its effectivity in human laryngeal carcinoma treatment in future.
Galangin promotes laryngeal carcinoma cell morphology alteration and suppresses migration, invasion as well as proliferation
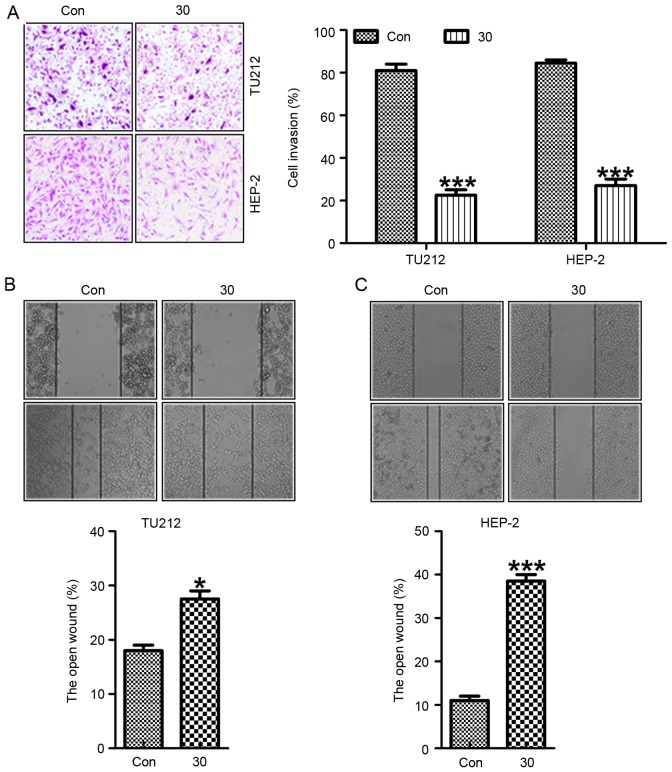
Exposure of TU212 and HEP-2 cells to galangin at different concentrations resulted in necrotic morphological changes and a downregulation in the percentage of viable cells, which was dose-dependent (Fig. 3A). Pretreatment with galangin considerably increased the inhibition of migrated cancer cells (Fig. 3B and C). In addition, we determined the invasion of TU212 and HEP-2 cells treated with galangin at the indicated dose. Fig. 4A shows that the invasion of TU212 and HEP-2 cells was markedly downregulated for galangin tretament compared to the control ones. In addition, images were taken at 0 and 24 h after galangin treatment as shown in Fig 4B and C. TU212 and HEP-2 cells treated with galangin for 24 h indicated that galangin suppressed the cancer cell migration, which was comparable to the control group in the absence of galangin. These results suggested that galangin suppressed TU212 and HEP-2 cell proliferation.
Figure 3.
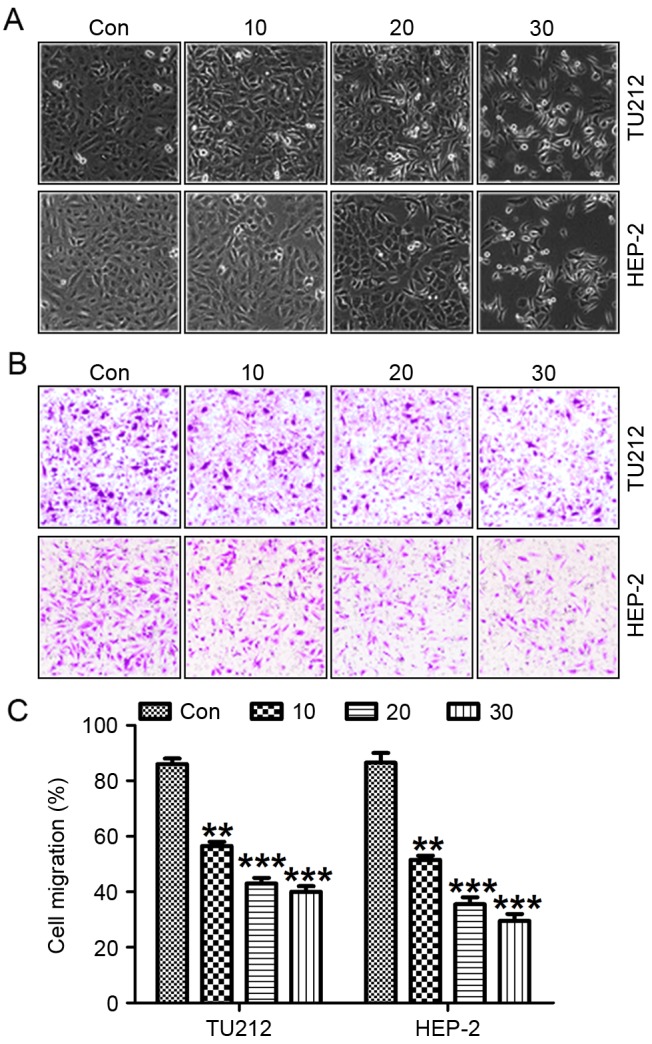
Galangin promotes laryngeal carcinoma cell morphology alteration and migration suppression. (A) Galangin induced morphological changes in human laryngeal carcinoma cells of TU212 and HEP-2. (B) The representative images of migrated cancer cells are shown. (C) Galangin reduced the number of migrated TU212 and HEP-2 cells under different conditions. All analysis was conducted in triplicate, and the results are mean ± SEM of three independent experiments. **P<0.01 and ***P<0.001 (compared to the control/Con).
Figure 4.
Galangin inhibits the laryngeal carcinoma cell invasion and proliferation. (A) TU212 and HEP-2 cells were treated with different concentrations of galangin, and then the invasive cells were calculated, which is shown in the left panel. After the scratching, (B) TU212 and (C) HEP-2 cells were treated with galangin at the indicated concentration for 24 h. Images were then acquired at 0 and 24 h under the microscope. The black lines represent the areas lacking the migratory cells. Representative pictures from three independent experiments are shown. All analysis was conducted in triplicate, and the results are mean ± SEM of three independent experiments. *P<0.05; ***P<0.001 (compared to the control/Con).
Galangin induces apoptosis in human laryngeal carcinoma cells of TU212 and HEP-2
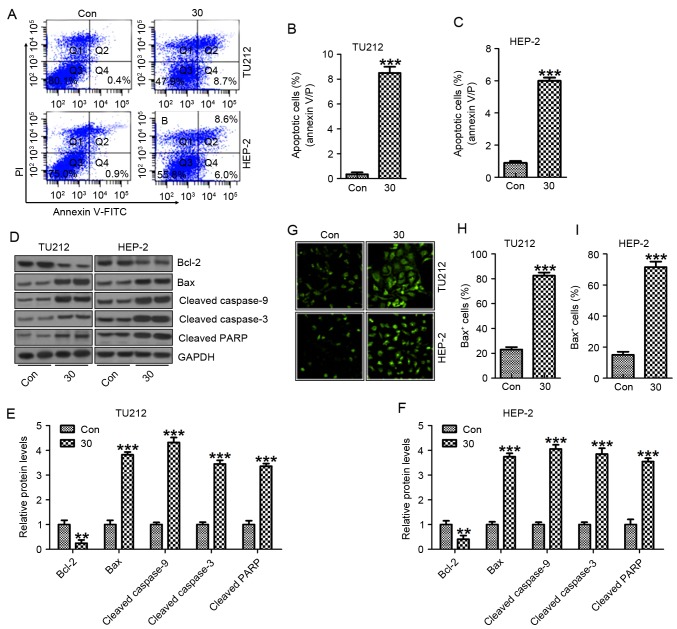
In this regard, we attempted to explore the mechanism of galangin on TU212 and HEP-2 cell growth and progression. The data from flow cytometric assay clearly showed that galangin induced apoptosis in TU212 cancer cells (Fig. 5A and B) and HEP-2 (Fig. 5A and C). In apoptosis process, mitochondrial outer membrane permeabilization is known as a ‘point-of-no-return’ and is closely regulated by Bcl-2 family proteins, especially Bax activation (25). Galangin apparently promoted Bax protein expresssion and pre-treatment with galangin significantly suppressed Bcl-2 protein levels (Fig. 5D-F). The results from western blot analysis showed that galangin enhanced activation of cleaved caspase-3 and caspase-9, resulting in increased PARP cleavage in TU212 (Fig. 5D and E) and HEP-2 cells (Fig. 5D and F). Furthermore, Bax activation was confirmed to be increased for galangin treatment in TU212 (Fig. 5G and H) and HEP-2 cells (Fig. 5G and I). These results suggested that galangin treatment-triggered apoptosis was modulated through caspase activation.
Figure 5.
Galangin induces apoptosis in human laryngeal carcinoma TU212 and HEP-2 cells. (A) Flow cytometric assays were applied to determine the number of apoptotic TU212 and HEP-2 cells. (B) The quantification of TU212 apoptosis levels is shown. (C) The quantification of HEP-2 apoptosis levels was shown. (D) The signaling pathway, leading to apoptosis, was analyzed by western blot analysis in TU212 and HEP-2 cells. Protein levels of Bcl-2, Bax, cleaved caspase-9, cleaved caspase-3 and cleaved PARP were evaluated. (E) Preotein levels of Bcl-2, Bax, cleaved caspase-9, cleaved caspase-3 and cleaved PARP in TU212 cells were quantified. (F) Protein levels of Bcl-2, Bax, cleaved caspase-9, cleaved caspase-3 and cleaved PARP in HEP-2 cells were quantified. (G) Immunofluorescence assays were carried out to determine Bax positive TU212 and HEP-2 cells. The quantification of Bax positive (H) TU212 and (I) HEP-2 cells are displayed. The analysis was conducted in triplicate, and the results exhibit the mean ± SEM of three independent experiments. **P<0.01 and ***P<0.001 (compared to the control/Con).
Galangin induces human laryngeal carcinoma cell death through autophagy regulation
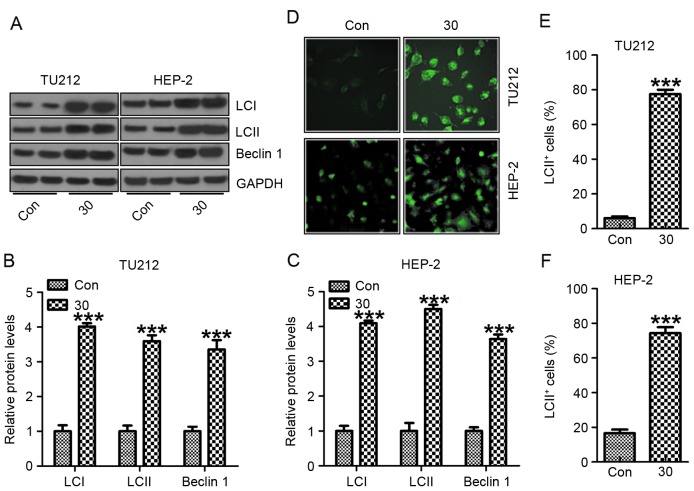
LCI, LC3II and Beclin 1 are required for the autophagy-mediated elimination of unfolded ubiquinated long half-life proteins (26). As shown in Fig. 6A, western blot analysis was carried out to reveal a considerable increase in LC3I, LC3II and Beclin 1 expression induced by galangin exposure compared to the control group without galangin treatment in TU212 (Fig. 6B) and HEP-2 (Fig. 6C) cells. The increased LC3I, LC3II and Beclin 1 further suggested that galangin stimulated autophagy and cell death in human laryngeal carcinoma (27). Similarly, in Fig. 6D-F, immunofluorescent assays further evidenced that LC3II was highly induced in galangin treatment. The data above indicate that galangin could result in human laryngeal carcinoma cell death, contributing to tumor suppression.
Figure 6.
Galangin induces human laryngeal carcinoma cell death through autophagy regulation. (A) LCI, LC3II and Beclin 1 protein levels were measured through western blot analysis. The quantification of LCI, LC3II and Beclin 1 in (B) TU212 and (C) HEP-2 cells is shown. (D) Immunofluorescence assays were carried out to determine LCII positive TU212 and HEP-2 cells. The quantification of LCII positive cells (E) TU212 and (F) HEP-2 cells is shown. The analysis was conducted in triplicate, and the results are the mean ± SEM of three independent experiments. **P<0.01 and ***P<0.001 (compared to the control/Con).
Galangin inhibits human laryngeal carcinoma TU212 and HEP-2 cell proliferation via p38 and AKT/NF-κB suppression
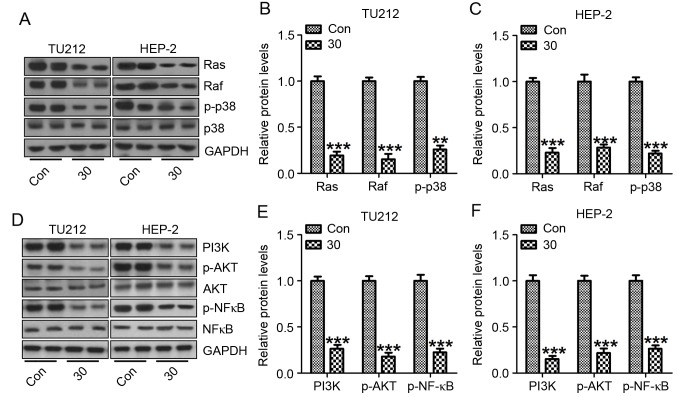
To further explore the potential molecular mechanism involved in galangin-induced TU212 and HEP-2 cell death, the protein expression of Raf, Ras and p38 were calculated. The results showed that Raf, Ras and p-p38 was highly expressed in the control group without galangin treatment in both TU212 (Fig. 7A and B) and HEP-2 cells (Fig. 7A and C). Importantly, galangin supplementation partially reversed the overexpression of Raf, Ras and p-p38. Enhancement of p38 activation plays an important role in modulating tumor migration, invasion as well as metastasis in some cancer cases (28). Altogether, galangin treatment-attenuated TU212 and HEP-2 cell progression partially relied on p38 signaling pathway.
Figure 7.
Galangin inhibits human laryngeal carcinoma TU212 and HEP-2 cell proliferation via p38 and AKT/NF-κB suppression. (A) Signals of Ras, Raf and p-p38 were determined by western blot analysis. (B) The quantification of Ras, Raf and p-p38 in TU212 cells. (C) The quantification of Ras, Raf and p-p38 in HEP-2 cells. (D) Signals of PI3K, p-AKT and NF-κB were measured by western blot assays. The quantification of PI3K, p-AKT and NF-κB in (E) TU212, and (F) HEP-2 cells following immunoblot analysis. The analysis was conducted in triplicate, and the results are the mean ± SEM of three independent experiments. **P<0.01 and ***P<0.001 (compared to the control/Con).
NF-κB is a pleiotropic transcription factor, which is related to various biological processes, including inflammation, apoptosis as well as autophogy (29). NF-κB activation has been detected in >50% of tumors, regulated by PI3K/AKT signaling pathway (30). In this study, PI3K/AKT was expressed highly in the cancer cells without galangin treatment, which was downregulated after galangin administration in both TU212 (Fig. 7D and E) and HEP-2 (Fig. 7D and F) cells. These results indicated that galangin might play important roles in TU212 and HEP-2 cells as an antitumor agent inhibiting cancer cell proliferation by targeting p38 and NF-κB.
Galangin impedes human laryngeal carcinoma TU212 and HEP-2 cell proliferation via suppressing mTOR
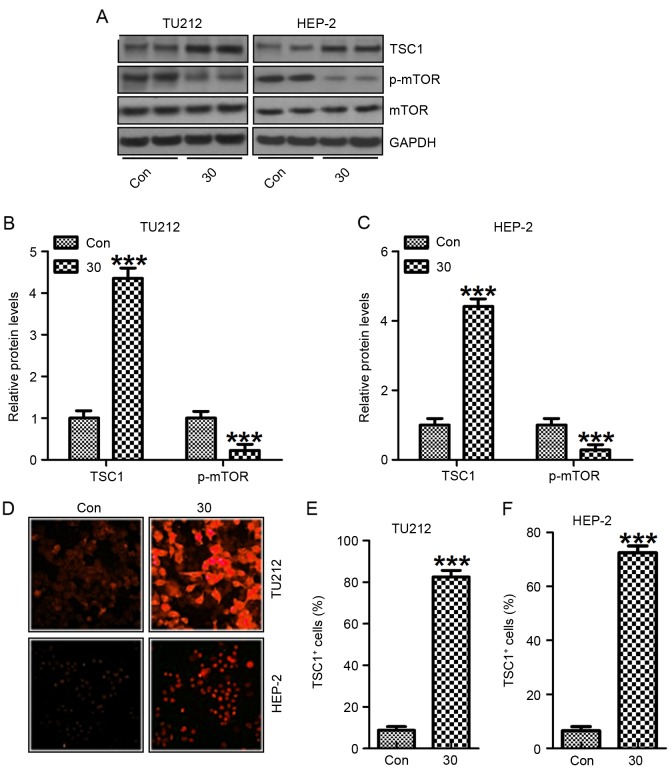
Finally, suppressing the PI3K-Akt-mTOR signaling pathway has been considered as an essential molecular mechanism causing tumor suppression (31). In this regard, we found that TSC1, an inhibitor of mTOR activation, was upregulated after the treatment with galangin. On the contrary, mTOR phosphorylated levels were reduced in TU212 and HEP-2 cells (Fig. 8A-C). Also, immunofluorescent analysis was performed to explore TSC1 levels in both TU212 (Fig. 8D and E) and HEP-2 (Fig. 8D and F) cells. TSC1 were highly expressed for galangin treatment in comparison to the control ones. Collectively, these results indicated that galangin suppressed human laryngeal carcinoma cell growth via mTOR signaling pathway.
Figure 8.
Galangin impedes human laryngeal carcinoma TU212 and HEP-2 cell proliferation via suppressing mTOR. (A) Western blot analysis was carried out to evaluate TSC1, p-mTOR and mTOR expression in TU212 and HEP-2 cells. TSC1 and p-mTOR protein levels in (B) TU212 and (C) HEP-2 cells were quantified after western blot assays. (D) Immunofluorescence assays were carried out to determine TSC1 positive cells in TU212 and HEP-2 cells. The quantification of TSC1 positive cells in (E) TU212 and (F) HEP-2 cells is shown. The analysis was conducted in triplicate, and the results are the mean ± SEM of three independent experiments. ***P<0.001 (compared to the control/Con).
Galangin promotes human laryngeal carcinoma growth inhibition in a xenograft tumor model in vivo
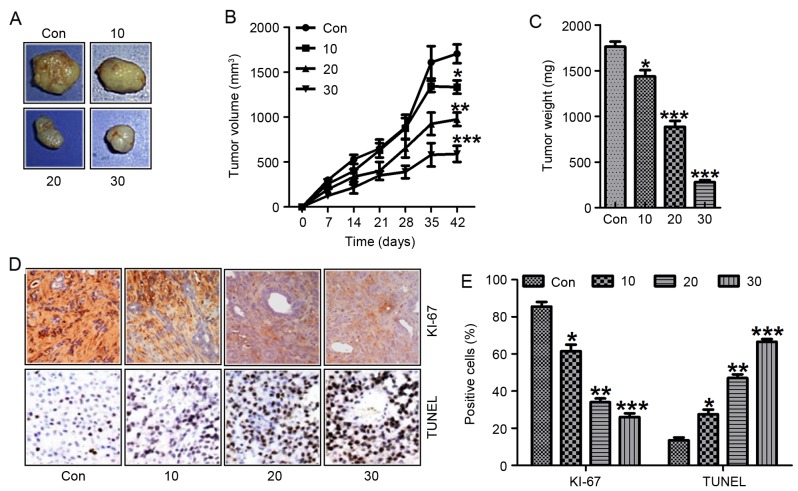
To confirm the enhanced galangin-regulated inhibition of human laryngeal carcinoma growth, we analyzed the effects of galangin treatment on tumorigenicity in vivo using a TU212 xenograft mouse model. After administration with galangin for 42 days, both the tumor volume and weight were inhibited (Fig. 9A-C). Additionally, reduction of tumor Ki-67 (Fig. 9D and E) and upregulation of TUNEL (Fig. 9D and E) through IHC assays were also noted in the galangin-treated group in a dose-dependent manner. These results indicated that galangin could promote suppression of xenografted human laryngeal carcinoma cell growth in vivo, which was in line with the data in vitro.
Figure 9.
Galangin promotes human laryngeal carcinoma growth inhibition in a xenograft tumor model in vivo. (A) The tumor images are shown. (B) Tumor volumes were measured at different times. (C) Tumor weight was calculated at different treat-times. (D) Upper panel, the Ki-67 positive cells in a human laryngeal carcinoma sample were evaluated via immunohistochemical analysis. Lower panel, TUNEL positive cells in a tumor sample were assessed via immunohistochemical analysis. (E) The quantification of Ki-67 and TUNEL positive cells. The analysis was conducted in triplicate, and the results are the mean ± SEM of three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 (compared to the control/Con).
Discussion
Human laryngeal cancer is known as one of the most common tumors of the head and neck region in the world (1,2,32). Patients suffering from laryngeal cancer show a poor survival rate with poor advance during the last decades (33). Thus, more effective treatments are needed to be explored for preventing laryngeal cancer. Natural compounds show an essential role in cancer and other disease prevention and treatment worldwide (4). Galangin is known as an anti-tumor agent, which is effective in preventing a broad range of tumors, such as liver, breast, as well as lung cancer (14–16,34). Galangin has been reported to perform its role in inhibiting tumor growth through apoptosis regulation (35). However, whether galangin could be a potential compound for human laryngeal cancer prevention is not known. Thus, galangin was used here to provide possible therapeutic strategy for suppressing human laryngeal cancer. Notably, it is the first study to explore the molecular mechanisms of galangin-triggered human laryngeal cancer cell death. In our study, we found that exposure of galangin into TU212 and HEP-2 cells could partially suppress cancer cell proliferation, invasion and migration. Autophagy is a regulated process of degradation and recycling of cellular constituents; the process is important in organelle turnover and the bioenergetics management of starvation (36). During autophagy, the precursor form of LC3 is post-modified into LC3-I and LC3-II (37,38). LC3-I is localized in the cytosol, and LC3-II is a membrane-associated and a key hallmark for autophagosome formation (39). LC3-II can be used to estimate the abundance of autophagosomes before they are destroyed through fusion with lysosomes (40). Here, augmented LC3II was found after galangin treatment, indicating that the autophagy was induced. Obviously in in vivo study, the tumor size and weight was reduced for galangin administration. The results suggested that galangin has potential, to be developed as a therapeutic strategy for human laryngeal carcinoma.
PI3K/AKT signal pathway is crucial for regulating various cell activities, such as proliferation, cell growth, survival, chemotaxis, the inflammatory response and apoptosis (41). Enhanced activation of PI3K/AKT pathway is linked to the development and progression of many cancers, as well as resistance to chemotherapy (42). A previous study reported that inactivation of PI3K/AKT signaling pathway is involved in the success of chemotherapy-caused apoptosis in some cancer cells (43,44). In the present study, PI3K was reduced in galangin treatment, subsequently downregulating the protein levels of phosphorylated AKT, being in line with previous results, indicating that galangin might perform its role in suppressing human laryngeal carcinoma through PI3K/AKT signaling pathway inactivation. Apoptosis constitutes a fundamental intrinsic mechanism of tumor suppression, as the resistance of apoptosis is a well-established hallmark of cancer (45,46). It is known that Bcl-2 protein is also a key regulator for apoptosis and its tumorigenic potential is supported by the finding of overexpression of Bcl-2 in various types of tumor, which is related to the activation of AKT (47). AKT is a key player in regulating cell signals that are important for cell death and survival. Activation of the AKT pathway promotes cell survival and is involved in the upregulation of Bcl-2 (48,49). In this study, we found that with the altered trend of AKT expressed levels, Bcl-2 was reduced in galangin treatment. The caspase-3 and PARP cleavage were highly improved, contributing to the death of TU212 and HEP-2 cells and leading to cell apoptosis and cell death due to galangin tretament. The results above suggested that galangin suppressed human laryngeal cancer development and progression through apoptosis induction.
NF-κB takes part in the information transfer process, including tissue damage, apoptosis, as well as stress, tumor suppression and cell differentiation, thus, it is an important nuclear transcription factor (50,51). NF-κB in different tumors or cancers is modulated by PI3K/AKT pathway (52). P-AKT activates IκB kinase (IKKα), leading to inhibition of NF-κB degradation by IκB, allowing NF-κB to be transferred into the nucleus from the cytoplasm, where it activates its target genes and promotes cell survival (53). In this study, NF-κB was markedly activated accompanied by AKT phosphorylation in the absence of galangin treatment. However, galangin administration downregulated NF-κB phosphorylated activity, leading to the upregulation of caspase-9, caspase-3 and PARP cleavage, indicating that galangin could inhibit human laryngeal cancer via AKT-mediated NF-κB signaling pathway. In addition, PI3K/AKT pathway is of great importance for the cell proliferation. One of the best-known downstream substrates of PI3K/AKT is the mammalian target of rapamycin (mTOR), inducing mammalian autophagy significantly, regulating protein translation (54). Previously it was indicated that the activation of PI3K-AKT-mTOR signal pathway may be involved in autophagy suppression (55). Impeding PI3K-AKT-mTOR signal pathway has been indicated to be effective for various cancers treatment (56,57). In our investigation, here the phosphorylated mTOR in high expression was suppressed in TU212 and HEP-2 cells for galangin administration, and in contrast, TSC1, an inhibitor of mTOR, was upregulated for galangin. The results illustrated that galangin prevented human laryngeal cancer through inhibiting laryngeal cancer cells proliferation and inducing autophagy via PI3K/AKT-regulated mTOR activity.
Collectively, our findings above demonstrated that galangin prevented human laryngeal cancer proliferation, invasion and migration by PI3K/AKT and p38 suppression, resulting in caspase activation, NF-κB dephosphorylation as well as mTOR inactivation with reduced Ki-67 expression and enhanced TUNEL levels. The present study indicated that the use of the dietary compound galangin might be a potential therapeutic strategy for human laryngeal carcinoma treatment.
References
- 1.Zhang Y, Chen Y, Yu J, Liu G, Huang Z. Integrated transcriptome analysis reveals miRNA-mRNA crosstalk in laryngeal squamous cell carcinoma. Genomics. 2014;104:249–256. doi: 10.1016/j.ygeno.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Liu M, Wu H, Liu T, Li Y, Wang F, Wan H, Li X, Tang H. Regulation of the cell cycle gene, BTG2, by miR-21 in human laryngeal carcinoma. Cell Res. 2009;19:828–837. doi: 10.1038/cr.2009.72. [DOI] [PubMed] [Google Scholar]
- 3.van Dijk BA, Karim-Kos HE, Coebergh JW, Marres HA, de Vries E. Progress against laryngeal cancer in The Netherlands between 1989 and 2010. Int J Cancer. 2014;134:674–681. doi: 10.1002/ijc.28388. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman HT, Porter K, Karnell LH, Cooper JS, Weber RS, Langer CJ, Ang KK, Gay G, Stewart A, Robinson RA. Laryngeal cancer in the United States: Changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116:1–13. doi: 10.1097/01.mlg.0000236095.97947.26. (Suppl 111) [DOI] [PubMed] [Google Scholar]
- 5.Ouyang D, Liu TR, Liu XW, Chen YF, Wang J, Su X, Yang AK. Combined hyoid bone flap in laryngeal reconstruction after extensive partial laryngectomy for laryngeal cancer. Eur Arch Otorhinolaryngol. 2013;270:1455–1462. doi: 10.1007/s00405-012-2147-8. [DOI] [PubMed] [Google Scholar]
- 6.Diab S, Pascoe J, Shahriar M, Read D, Kinde H, Moore J, Odani J, Uzal F. Study of laryngopharyngeal pathology in Thoroughbred horses in southern California. Equine Vet J. 2009;41:903–907. doi: 10.2746/042516409X448968. [DOI] [PubMed] [Google Scholar]
- 7.Silver CE, Beitler JJ, Shaha AR, Rinaldo A, Ferlito A. Current trends in initial management of laryngeal cancer: The declining use of open surgery. Eur Arch Otorhinolaryngol. 2009;266:1333–1352. doi: 10.1007/s00405-009-1028-2. [DOI] [PubMed] [Google Scholar]
- 8.Ulualp SO. Mapping regional laryngopharyngeal mechanoreceptor response. Clin Exp Otorhinolaryngol. 2014;7:319–323. doi: 10.3342/ceo.2014.7.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang SY, Lu ZM, Chen LS, Luo XN, Ge PJ, Song XH, Chen SH, Wu YL. Supracricoid partial laryngectomy cricohyoidoepiglottopexy (SCPL-CHEP) versus vertical partial laryngectomy for the treatment of glottic carcinoma. Eur Arch Otorhinolaryngol. 2013;270:1027–1034. doi: 10.1007/s00405-012-2241-y. [DOI] [PubMed] [Google Scholar]
- 10.Zhang HT, Luo H, Wu J, Lan LB, Fan DH, Zhu KD, Chen XY, Wen M, Liu HM. Galangin induces apoptosis of hepatocellular carcinoma cells via the mitochondrial pathway. World J Gastroenterol. 2010;16:3377–3384. doi: 10.3748/wjg.v16.i27.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capasso R, Mascolo N. Inhibitory effect of the plant flavonoid galangin on rat vas deferens in vitro. Life Sci. 2003;72:2993–3001. doi: 10.1016/S0024-3205(03)00232-7. [DOI] [PubMed] [Google Scholar]
- 12.Kim DA, Jeon YK, Nam MJ. Galangin induces apoptosis in gastric cancer cells via regulation of ubiquitin carboxy-terminal hydrolase isozyme L1 and glutathione S-transferase P. Food Chem Toxicol. 2012;50:684–688. doi: 10.1016/j.fct.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Tang B, Huang Q, Hua Z. Galangin inhibits tumor growth and metastasis of B16F10 melanoma. J Cell Biochem. 2013;114:152–161. doi: 10.1002/jcb.24312. [DOI] [PubMed] [Google Scholar]
- 14.Heo MY, Sohn SJ, Au WW. Anti-genotoxicity of galangin as a cancer chemopreventive agent candidate. Mutat Res. 2001;488:135–150. doi: 10.1016/S1383-5742(01)00054-0. [DOI] [PubMed] [Google Scholar]
- 15.Agati G, Azzarello E, Pollastri S, Tattini M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012;196:67–76. doi: 10.1016/j.plantsci.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother Res. 2015;29:323–331. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- 17.Schultz DR, Harrington WJ., Jr Apoptosis: Programmed cell death at a molecular level. Semin Arthritis Rheum. 2003;32:345–369. doi: 10.1053/sarh.2003.50005. [DOI] [PubMed] [Google Scholar]
- 18.Tomita T. Cleaved caspase-3 immunocytochemical staining for pancreatic islets and pancreatic endocrine tumors: A potential marker for biological malignancy. Islets. 2010;2:82–88. doi: 10.4161/isl.2.2.10807. [DOI] [PubMed] [Google Scholar]
- 19.McCormick J, Knight RA, Barry SP, Scarabelli TM, Abounit K, Latchman DS, Stephanou A. Autophagy in the stress-induced myocardium. Front Biosci (Elite Ed) 2012;4:2131–2141. doi: 10.2741/e530. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Zhang Q, Tian R, Wang Q, Zhao JJ, Iglehart JD, Wang ZC, Richardson AL. Lysosomal transmembrane protein LAPTM4B promotes autophagy and tolerance to metabolic stress in cancer cells. Cancer Res. 2011;71:7481–7489. doi: 10.1158/0008-5472.CAN-11-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z, Antalek M, Nguyen L, Li X, Tian X, Le A, Zi X. The effect of gartanin, a naturally occurring xanthone in mangosteen juice, on the mTOR pathway, autophagy, apoptosis, and the growth of human urinary bladder cancer cell lines. Nutr Cancer. 2013;65:68–77. doi: 10.1080/01635581.2013.785011. (Suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling YH, Aracil M, Zou Y, Yuan Z, Lu B, Jimeno J, Cuervo AM, Perez-Soler R. PM02734 (elisidepsin) induces caspase-independent cell death associated with features of autophagy, inhibition of the Akt/mTOR signaling pathway, and activation of death-associated protein kinase. Clin Cancer Res. 2011;17:5353–5366. doi: 10.1158/1078-0432.CCR-10-1948. [DOI] [PubMed] [Google Scholar]
- 23.Zhai B, Hu F, Jiang X, Xu J, Zhao D, Liu B, Pan S, Dong X, Tan G, Wei Z, et al. Inhibition of Akt reverses the acquired resistance to sorafenib by switching protective autophagy to autophagic cell death in hepatocellular carcinoma. Mol Cancer Ther. 2014;13:1589–1598. doi: 10.1158/1535-7163.MCT-13-1043. [DOI] [PubMed] [Google Scholar]
- 24.Graham TR, Odero-Marah VA, Chung LW, Agrawal KC, Davis R, Abdel-Mageed AB. PI3K/Akt-dependent transcriptional regulation and activation of BMP-2-Smad signaling by NF-kappaB in metastatic prostate cancer cells. Prostate. 2009;69:168–180. doi: 10.1002/pros.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- 26.Hale AN, Ledbetter DJ, Gawriluk TR, Rucker EB., III Autophagy: Regulation and role in development. Autophagy. 2013;9:951–972. doi: 10.4161/auto.24273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C, Han X, Zhang H, Wu J, Li B. The interplay between autophagy and apoptosis induced by tanshinone IIA in prostate cancer cells. Tumour Biol. 2016;37:7667–7674. doi: 10.1007/s13277-015-4602-9. [DOI] [PubMed] [Google Scholar]
- 28.Platanias LC. Map kinase signaling pathways and hematologic malignancies. Blood. 2003;101:4667–4679. doi: 10.1182/blood-2002-12-3647. [DOI] [PubMed] [Google Scholar]
- 29.Rauch BH, Weber A, Braun M, Zimmermann N, Schrör K. PDGF-induced Akt phosphorylation does not activate NF-kappa B in human vascular smooth muscle cells and fibroblasts. FEBS Lett. 2000;481:3–7. doi: 10.1016/S0014-5793(00)01957-8. [DOI] [PubMed] [Google Scholar]
- 30.Ryu HJ, Kim JE, Yeo SI, Kang TC. p65/RelA-Ser529 NF-κB subunit phosphorylation induces autophagic astroglial death (Clasmatodendrosis) following status epilepticus. Cell Mol Neurobiol. 2011;31:1071–1078. doi: 10.1007/s10571-011-9706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samuels Y, Velculescu VE. Oncogenic mutations of PIK3CA in human cancers. Cell Cycle. 2004;3:1221–1224. doi: 10.4161/cc.3.10.1164. [DOI] [PubMed] [Google Scholar]
- 32.Chen K, Song F, He M, Li H, Qian B, Zhang W, Wei Q, Hao X. Trends in head and neck cancer incidence in Tianjin, China, between 1981 and 2002. Head Neck. 2009;31:175–182. doi: 10.1002/hed.20946. [DOI] [PubMed] [Google Scholar]
- 33.Li XY, Guo X, Feng S, Li XT, Wei HQ, Yang HA, Ren Z, Jiang XJ. Relationship between a family history of malignancy and the incidence of laryngeal carcinoma in the Liaoning province of China. Clin Otolaryngol. 2009;34:127–131. doi: 10.1111/j.1749-4486.2008.01840.x. [DOI] [PubMed] [Google Scholar]
- 34.Li S, Chou G, Hseu Y, Yang H, Kwan H, Yu Z. Isolation of anticancer constituents from flos genkwa (Daphne genkwa Sieb.et Zucc.) through bioassay-guided procedures. Chem Cent J. 2013;7:159. doi: 10.1186/1752-153X-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee LT, Huang YT, Hwang JJ, Lee AY, Ke FC, Huang CJ, Kandaswami C, Lee PP, Lee MT. Transinactivation of the epidermal growth factor receptor tyrosine kinase and focal adhesion kinase phosphorylation by dietary flavonoids: Effect on invasive potential of human carcinoma cells. Biochem Pharmacol. 2004;67:2103–2114. doi: 10.1016/j.bcp.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 36.Khandelwal VK, Mitrofan LM, Hyttinen JM, Chaudhari KR, Buccione R, Kaarniranta K, Ravingerová T, Mönkkönen J. Oxidative stress plays an important role in zoledronic acid-induced autophagy. Physiol Res. 2014;63:S601–S612. doi: 10.33549/physiolres.932934. (Suppl 4) [DOI] [PubMed] [Google Scholar]
- 37.McLeland CB, Rodriguez J, Stern ST. Autophagy monitoring assay: Qualitative analysis of MAP LC3-I to II conversion by immunoblot. Methods Mol Biol. 2011;697:199–206. doi: 10.1007/978-1-60327-198-1_21. [DOI] [PubMed] [Google Scholar]
- 38.Giménez-Xavier P, Francisco R, Platini F, Pérez R, Ambrosio S. LC3-I conversion to LC3-II does not necessarily result in complete autophagy. Int J Mol Med. 2008;22:781–785. [PubMed] [Google Scholar]
- 39.Kroemer G, Levine B. Autophagic cell death: The story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reggiori F, Monastyrska I, Verheije MH, Calì T, Ulasli M, Bianchi S, Bernasconi R, de Haan CA, Molinari M. Coronaviruses Hijack the LC3-I-positive EDEMosomes, ER-derived vesicles exporting short-lived ERAD regulators, for replication. Cell Host Microbe. 2010;7:500–508. doi: 10.1016/j.chom.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saini KS, Loi S, de Azambuja E, Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE, Piccart-Gebhart MJ. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev. 2013;39:935–946. doi: 10.1016/j.ctrv.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Han L, Yang Y, Yue X, Huang K, Liu X, Pu P, Jiang H, Yan W, Jiang T, Kang C. Inactivation of PI3K/AKT signaling inhibits glioma cell growth through modulation of β-catenin-mediated transcription. Brain Res. 2010;1366:9–17. doi: 10.1016/j.brainres.2010.09.097. [DOI] [PubMed] [Google Scholar]
- 43.Lee J, Zhang G, Wu X, Xu F, Zhou J, Zhang X. Growth inhibitory effect of dihydroartemisinin on Bcr/Abl+ chronic myeloid leukemia K562 cells involve AKT, ERK and NF-κB modulation. J Cancer Res Clin Oncol. 2012;138:2095–2102. doi: 10.1007/s00432-012-1292-1. [DOI] [PubMed] [Google Scholar]
- 44.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 45.Noble P, Vyas M, Al-Attar A, Durrant S, Scholefield J, Durrant L. High levels of cleaved caspase-3 in colorectal tumour stroma predict good survival. Br J Cancer. 2013;108:2097–2105. doi: 10.1038/bjc.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu K, Liu PC, Liu R, Wu X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med Sci Monit Basic Res. 2015;21:15–20. doi: 10.12659/MSMBR.893327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheppard KE, Cullinane C, Hannan KM, Wall M, Chan J, Barber F, Foo J, Cameron D, Neilsen A, Ng P, et al. Synergistic inhibition of ovarian cancer cell growth by combining selective PI3K/mTOR and RAS/ERK pathway inhibitors. Eur J Cancer. 2013;49:3936–3944. doi: 10.1016/j.ejca.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Kim KW, Moretti L, Mitchell LR, Jung DK, Lu B. Combined Bcl-2/mammalian target of rapamycin inhibition leads to enhanced radiosensitization via induction of apoptosis and autophagy in non-small cell lung tumor xenograft model. Clin Cancer Res. 2009;15:6096–6105. doi: 10.1158/1078-0432.CCR-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levine B, Sinha S, Kroemer G. Bcl-2 family members: Dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Go EK, Jung KJ, Kim JY, Yu BP, Chung HY. Betaine suppresses proinflammatory signaling during aging: The involvement of nuclear factor-kappaB via nuclear factor-inducing kinase/IkappaB kinase and mitogen-activated protein kinases. J Gerontol A Biol Sci Med Sci. 2005;60:1252–1264. doi: 10.1093/gerona/60.10.1252. [DOI] [PubMed] [Google Scholar]
- 51.Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaiz-Pena GN, Kamat AA, Spannuth WA, Gershenson DM, Lutgendorf SK, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13:3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 52.Jayasooriya RG, Choi YH, Moon SK, Kim WJ, Kim GY. Methanol extract of Hydroclathrus clathratus suppresses matrix metalloproteinase-9 in T24 bladder carcinoma cells by suppressing the NF-κB and MAPK pathways. Oncol Rep. 2012;27:541–546. doi: 10.3892/or.2011.1501. [DOI] [PubMed] [Google Scholar]
- 53.Li W, Wang H, Kuang CY, Zhu JK, Yu Y, Qin ZX, Liu J, Huang L. An essential role for the Id1/PI3K/Akt/NF-κB/survivin signalling pathway in promoting the proliferation of endothelial progenitor cells in vitro. Mol Cell Biochem. 2012;363:135–145. doi: 10.1007/s11010-011-1166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 55.Sun CH, Chang YH, Pan CC. Activation of the PI3K/Akt/mTOR pathway correlates with tumour progression and reduced survival in patients with urothelial carcinoma of the urinary bladder. Histopathology. 2011;58:1054–1063. doi: 10.1111/j.1365-2559.2011.03856.x. [DOI] [PubMed] [Google Scholar]
- 56.Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Bäsecke J, Libra M, Stivala F, Milella M, Tafuri A, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008;22:686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 57.Zhang P, Pang X, Tu Y. Thioredoxin-interacting protein as a common regulation target for multiple drugs in clinical therapy/application. Cancer Transl Med. 2015;1:26–30. doi: 10.4103/2395-3977.151488. [DOI] [Google Scholar]