Highlights
-
•
Gastroblastoma is a rare gastric biphasic tumor with both epithelial and mesenchymal components.
-
•
To the best of our knowledge only eight cases have been reported in the English literature till date.
-
•
Surgical excision remains the mainstay of treatment.
Keywords: Casereport, Gastroblastoma, Gastrectomy
Abstract
Introduction
Gastroblastoma is a rare gastric biphasic tumor with both epithelial and mesenchymal components. To the best of our knowledge only eight cases have been reported in the English literature till date.
Presentation of case
We report a case of a 29-year-old female, hospitalized for epigastric pain with poor general condition. An upper gastrointestinal endoscopy showed a polypoid mass in the stomach near the gastric cardia suspicious of gastrointestinal stromal tumor. The patient underwent atypical proximal gastrectomy with splenectomy. Detailed histopathological examination of the resected specimen revealed the diagnosis of gastroblastoma. After six months, the patient developed loco-regional recurrence for which surgical debulking was performed.
Discussion
Gastroblastoma is predominantly seen in young adults with non-specific complaints. They appear as submucosal lesion in the stomach mimicking gastrointestinal stromal tumor. Preoperative diagnosis is often difficult. Surgical resection remains the mainstay of treatment. On histology, they consist of mesenchymal component which stain positively for vimentin and CD10 and epithelial component which is positive for cytokeratin on immunohistochemistry.
Conclusion
Gastroblastoma is a malignant tumor with risk of local recurrence after curative resection.
1. Introduction
Gastroblastoma is a recently described gastric tumor characterized by presence of both epithelial and mesenchymal components. This biphasic tumor was first reported to be a distinct entity by Miettinen et al. in 2009 [1]. Till date, only eight cases have been reported in English literature. Due to the rarity of this disease, the etiopathogenesis, malignant potential and appropriate treatment for this disease remains unknown.
We report a case of gastroblastoma with aggressive behavior in a young lady who developed local recurrence within six months of curative treatment. Additionally, we performed a literature review so as to provide better understanding of the clinico-pathological characteristics, therapeutic approaches and prognosis of this rare type of gastric tumor. This case has been reported in line with the SCARE criteria [2].
2. Case description
A 29-year-old lady presented with complaints of epigastric pain for eight months. The pain was dull aching in nature, paroxysmal, unrelated to food and without radiation. Patient also had a single episode of hematemesis two days prior to the admission.
Upper gastrointestinal endoscopy revealed the presence of a 7-cm polypoid submucosal lesion near the gastric cardia on the posterior wall of the stomach towards the greater curvature. The lesion was located about 30 cm from the incisors. Endoscopic biopsy was inconclusive. Laboratory tests showed hypochromic microcytic anemia (Hb − 6 g/dl, MCV − 66,7 fL, MCHC − 27 g/dl). Liver and renal function tests were normal. Serum CEA level was normal and serum CA 19-9 level was 12 U/ml.
Abdominal CT scan showed the presence of a solid-cystic tumor of 7 cm arising from the posterior wall of the stomach close to the gastric cardia (Fig. 1).
Fig. 1.
Abdominal CT scan showing the presence of a cystic tumor of 7 cm with a component tissue developing at the expense of the stomach, with enhancement after contrast injection.
Intra operatively, the tumor was located on the posterior gastric wall occupying the proximal stomach with encroachment of the splenic hilum. Atypical partial gastrectomy with splenectomy was performed due to the extension to the splenic hilum. Postoperative recovery was uneventful.
On macroscopic examination, the tumor was 7 × 4 ×4 cm in size. On cut section, there were focal areas of necrosis and hemorrhage. The tumor showed infiltration of all the layers of the gastric wall with invasion of the splenic hilum. Six enlarged lymph nodes were found at the splenic hilum and two enlarged lymph nodes were located at the gastric fundus.
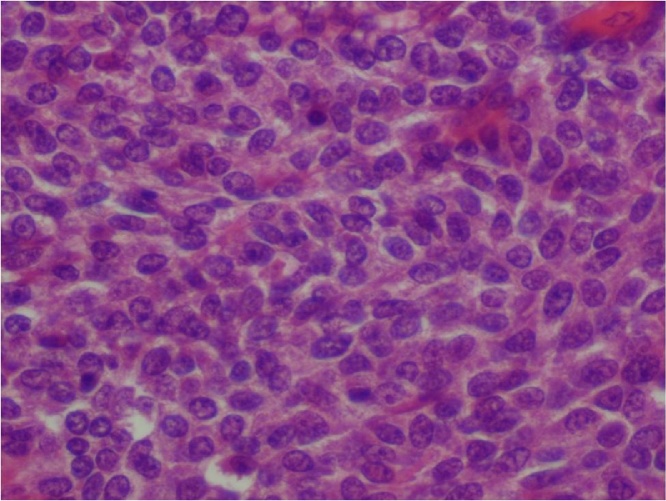
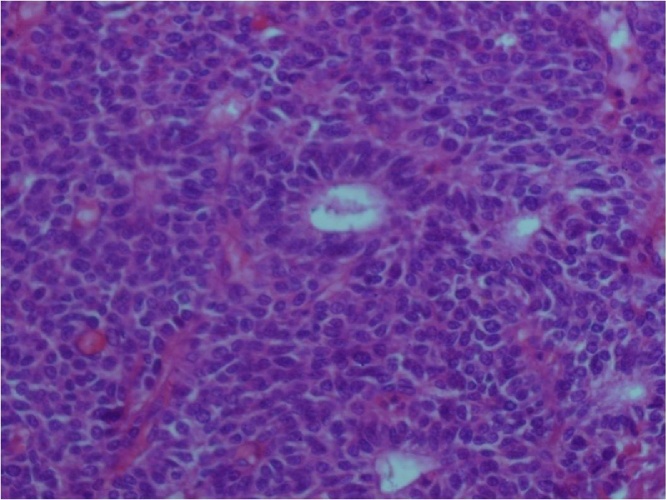
On microscopic examination, the tumor was predominantly located in the submucosa with focal invasion of the mucosa, muscular layer, serosa and splenic hilar fat at places. The tumor consisted of two components − mesenchymal and epithelial (Fig. 2). The predominant component was mesenchymal, consisting of ovoid cells arranged in layers, with regular nuclei and scanty cytoplasm (Fig. 3). There were 21 mitoses per 10 high power fields. The epithelial component was focally distributed, consisting of lightly-reduced glands, lined by cuboidal to columnar cells with atypical hyper-chromatic nuclei and infrequent mitosis (Fig. 4). Both components were of blastematic immature type. Peri-tumoral vascular emboli were present. Two of the six nodes of the splenic hilum were metastatic while both of the lymph nodes at the fundus were free of tumor.
Fig. 2.
Tumor consisted of mesenchymal component made of oval cells arranged in cords, tubules and epithelial component arranged in glands (hematoxylin eosin X 200).
Fig. 3.
Mesenchymal component consisted of oval cells with scant cytoplasm and monomorphic nuclei (HE X 400).
Fig. 4.
Epithelial component showed glands lined by cubo-cylindrical cells (HE X 400).
On immunohistochemistry, the tumor was uniformly positive for vimentin, CD 99 (Mic 2) and focally positive for CD 10 (Fig. 5). Staining with anti-cytokeratin antibodies, anti-chromogranin, anti-synaptophysin and anti-C-kit was negative. Based on the above histopathological findings, final diagnosis of gastroblastoma was made.
Fig. 5.
Immunohistochemistry: positivity of neoplastic cells with vimentin (A), CD99 (B) and focal positivity with CD10 (C) (X 200).
At six months of follow up, patient developed loco-regional recurrence in the retro-gastric area for which surgical debulking was performed. However, the patient died one month after debulking due to massive pulmonary embolism.
3. Discussion
Gastroblastoma is a very rare epithelio-mesenchymal biphasic gastric tumor. The etiopathogenesis of this tumor is not very clear, however, it is believed to develop from a totipotent stem cell [3]. It predominantly occurs in young adults with a mean age of 22 years [1]. Among the eight previously reported cases, there were six males and two females. Similar to these cases, our patient was a young girl in her third decade of life. Clinically, the symptoms are nonspecific and dominated by atypical epigastric pain, impaired general condition, and abdominal mass if the tumor is of large size [4]. In two previously reported cases, gastroblastoma manifested as gastrointestinal bleed similar to that reported in the present case (Table 1).
Table 1.
Clinical presentation and treatment of different cases published in the literature.
| Sr. No. | Author | Age | Sex | Clinical features | Location | Size | Lymph nodal/distant metastases | Treatment | Follow up (months) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Miettinen et al. [1] | 30 | Male | Anemia, fatigue, and abdominal mass | Gastric antrum | 15*12 cm | Absent | Antrectomy followed by radiation therapy | 168 | No recurrence |
| 2 | Miettinen et al. [1] | 27 | Female | Abdominal pain and mass | Greater curvature, gastric body | 6*4*3,5 cm | Absent | Partial gastrectomy | 60 | No recurrence |
| 3 | Miettinen et al. [1] | 19 | Male | Abdominal pain and mass | Greater curvature, gastric body |
5*4*2,5 cm | Absent | Subtotal gastrectomy | 36 | No recurrence |
| 4 | Shin et al. [3] | 9 | Male | Abdominal pain and mass | Gastric antrum | 9*6,5 cm | Absent | Distal gastrectomy | 9 | No recurrence |
| 5 | Wey et al. [6] | 28 | Male | Constipation and abdominal mass | Distal stomach | 3,8*3,3*2,5 cm | Present | Neoadjuvant chemotherapy followed by partial gastrectomy | 3 | Clinically stable. No new lesions |
| 6 | Yang Yang Ma et al. [4] | 12 | Male | Intermittent blood in stool and abdominal mass | Gastric antrum | 4,5*2,5*2,5 cm | Absent | Subtotal gastrectomy | 8 | No recurrence |
| 7 | Teresa Fernandes et al. [8] | 19 | Female | Abdominal pain and mass | Gastric antrum | 10.5 cm | Absent | Partial distal gastrectomy with lymphadenectomy | 20 | No recurrence |
| 8 | Na Zheng et al. [9] | 12 | Male | Bloody stool | – | 7 cm | Absent | Subtotal gastrectomy | 8 | No recurrence |
| 9 | Our case report | 29 | Female | Epigastric pain and hematemesis | Greater curvature, gastric body |
7 cm | Present | Partial gastrectomy with splenectomy | 6 | Recurrence |
On upper gastrointestinal endoscopy, it appears as a bulging mass lifting the gastric mucosa [5]. The overlying gastric mucosa may be normal or ulcerated. If the tumor is developing exoluminally, the tumor may not be visible on endoscopy and even deep-seated biopsies do not allow the diagnosis [5]. Endoscopic ultrasound is recommended in such cases to visualize the tumor and perform deep biopsies safely [5]. This aspect was noted in our observation. Out of the eight reported cases, gastroblastoma was located in the antrum in five patients, along the greater curvature in two cases and in one patient the location was not specified. In our case the tumor was present along the greater curvature in the proximal stomach.
Surgical resection with clear margins has been the preferred treatment of choice. Like gastrointestinal stromal tumors, laparoscopic approach may be useful for small tumors less than 5 cm located in the anterior surface of the stomach away from the gastroesophageal junction. Gastroblastoms are lymphophilic tumors indicating the importance of a lymph node dissection [1]. In our case, this dissection was not performed because of the pre-operative diagnosis of a stromal tumor.
Diagnostic confirmation is possible only by the histopathological examination. Macroscopically, they can have varied size ranging from 3.8 to 15 cm. The tumor consistency is variable. The cut surface often shows areas of hemorrhage or necrosis as seen in the present case [1]. It can appear as ulcerated, polypoidal, intramural or exophytic lesion [1].
In our case, the tumor was predominantly located in the submucosa with focal areas of infiltration in to the gastric mucosa leading to ulcerations. The tumor also seeped through the muscular and serosal layer to involve the splenic hilum.
Histologically, gastroblastoma shows biphasic cell proliferation involving mesenchymal and epithelial cells. The epithelial component is organized mainly in nests, cords and tubules composed of glands with reduced light, carpeted in places by the cubo-cylindrical cells, the hyper-chromatic nuclei and the slightly eosinophilic cytoplasm, and discrete nucleoli. In some cells, the nucleus and cytoplasm were condensed and clearly bordered by separate cells. The nuclei of glandular structures were dark and elongated [1]. The mesenchymal cells were arranged in short fascicles or layers formed by oval cells with scant cytoplasm with inconspicuous nucleoli and regular nuclei. No signs of structural differentiation such as fences or nuclear vacuolation were noted. Both components showed blastemal immature appearance.
Gastroblastoma variably combines both cell components, in fact, the mesenchymal component is often predominantly observed in 4 cases in the literature [4].
The mitotic index varied from 0 to 30.
Immunohistochemically, as per the original description by Miettinen M et al. [1], mesenchymal cells express vimentin and CD10 while epithelial cells express cytokeratins. However, subsequent case studies on gastroblastoma have reported positivity for additional markers as summarized in Table 2. In the present case, the mesenchymal cells showed positivity for vimentin but negative reaction for CD 10. and epithelial cells express some CD vimentin with CD10 positive response of mesenchymal and epithelial cells for CD 99. The tumor showed negative reaction for markers of gastrointestinal stromal tumors (CD117), neuroendocrine tumors (synaptophysin, chromogranin), synovial sarcoma, and leiomyosarcoma.
Table 2.
Immune-histochemical profile of the different cases published in the literature.
| Immunohistochemical marker | Miettinen et al. [1] |
Shin et al. [3] |
Wey et al. [6] |
Yangyang Ma [4] |
Fernandes et al. [8] |
Our case |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mesenchymal element | Epithelial element | Mesenchymal element | Epithelial element | Mesenchymal element | Epithelial element | Mesenchymal element | Epithelial element | Mesenchymal element | Epithelial element | Mesenchymal element | Epithelial element | |
| Vimentin | + | – | + | – | + | – | + | – | + | – | + | – |
| CD10 | + | – | + | – | + | + | + | – | + | + | + | + |
| Pancytokeratin | – | + | – | + | + | – | + | – | + | |||
| LMWCK | + | – | + | + | – | + | ||||||
| CK7 | – | + | + | – | – | |||||||
| CK20 | – | – | – | – | – | – | ||||||
| EMA | – | – | + | – | – | – | – | – | ||||
| SMA | – | – | – | – | – | – | – | – | – | |||
| Desmin | – | – | – | – | – | – | – | – | – | |||
| C-kit (CD117) | – | – | – | + | – | + | – | – | – | – | – | – |
| DOG-1 | – | – | – | – | – | – | ||||||
| CD56 | + | + | + | + | + | – | + | + | – | – | ||
| S-100 | – | – | – | – | – | – | – | – | – | |||
| SYN | – | – | – | – | – | – | – | – | ||||
| CgA | – | – | – | – | – | – | – | – | – | – | ||
| NSE | – | – | – | – | – | – | ||||||
| Calretinin | – | – | – | – | – | – | – | – | – | |||
| P63 | – | – | – | – | – | – | ||||||
| CD34 | – | – | – | – | – | – | – | – | ||||
| CD99 | – | – | – | – | + | + | ||||||
| CDX2 | – | – | – | – | ||||||||
| Inhibin | – | – | – | – | – | – | ||||||
The differential diagnosis includes inflammatory myofibroblastic tumors, teratoma, gastrointestinal stromal tumor (GIST), synovial sarcoma, and carcinosarcoma. The literature review shows that lymph node metastases have been reported in one case, as in our patient who developed local and distant lymph node metastases [4]. Furthermore, Wey et al. [6] have reported a case of gastroblastome with lymph node metastasis and at distance. No standard therapy has been established for this tumor. The intra-peritoneal chemotherapy could reduce the loco-regional recurrence and peritoneal dissemination [7]. One reported case had received neo-adjuvant chemotherapy but without response [6]. Another one had received post-operative radiotherapy [1].
Gastroblastoma appears to have low malignant potential as recurrence after curative resection has not been reported in the literature. Our case is the first report of local recurrence after curative resection. The follow-up periods were from 3 months to 14 years. No cases of recurrence were reported during the reported monitoring period. The prognosis depends on several parameters including the size of the tumor, the degree of parietal invasion, mitotic index, and lymph node invasion [7].
Gastroblastoma seem to have a low malignant potential. The average survival was 48.3 months [4], with a range from 3 months to 14 years. In our case, the survival was 7 months.
4. Conclusion
Gastroblastomas is a distinct clinico-pathological entity due to its clinical, radiological, histopathological and immunohistochemical profile. Our case is the first reported case in Tunisia and the ninth observation described in the literature. More data are needed to understand the biological and clinical behavior of the tumor. Despite the development of the diagnostic, morphological, immune-histochemical and anatomo-pathological techniques, the diagnosis is often difficult.
Conflict of interest
The authors have no conflict of interest to declare.
Funding
This study was not funded by any organization or institution.
Ethical approval
This case study was approved by the Ethics Committee of Hopital Fattouma Bourguiba.
Consent
Written informed consent was obtained from the patient
Authors’ contributions
Study concept or design − OT, HA,
Data collection − HA, TK, OT
Data interpretation − MA, HA, RG
Literature review − HA, RS, AZ,
Drafting of the paper − HA, RG, TK
Editing of the paper − HA, TK
Guarantor
Omar Toumi
Houssem ammar
Acknowledgement
None
Contributor Information
Omar Toumi, Email: toumi.amor@rns.tn.
Houssem Ammar, Email: hosshoss24@hotmail.fr.
Ibtissem Korbi, Email: ibtissemkorbi@gmail.com.
Mariem Ayed, Email: mariemayed@hotmail.com.
Rahul Gupta, Email: rahul.g.85@gmail.com.
Mohamed Nasr, Email: mohamednasr@yahoo.com.
Randa Salem, Email: randasa@yahoo.com.
Rim Hadhri, Email: rym_hadhri@yahoo.fr.
Sonia Zayed, Email: soniazayed@yahoo.fr.
Faouzi Noomen, Email: faouzinoomen@yahoo.fr.
Abdelfatteh Zakhama, Email: Hamdi@yahoo.com, http://mailto:hosshoss24@hotmail.fr.
Khadija Zouari, Email: khadija.zouari@rns.tn.
References
- 1.Miettinen M., Dow N., Lasota J., Sobin L.H. A distinctive novel epitheliomesenchymal biphasic tumor of the stomach in young adults (gastroblastoma): a series of 3 cases. Am. J. Surg. Pathol. 2009;33:1370–1377. doi: 10.1097/pas.0b013e3181a6a792. [DOI] [PubMed] [Google Scholar]
- 2.Shin D.H., ILee J.H., Kang H.J., Choi K.U., Kim J.Y., Park D.Y., Lee C.H. Novel epitheliomesenchymal biphasic stomach tumor in a 9-year-old: morphological, ultrastructural and immunohistochemical findings. J. Clin. Pathol. 2010;63:270–274. doi: 10.1136/jcp.2009.074104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agha R.A., Fowler A.J., Saeta A., Barai I., Rajmohan S., Orgill D.P. for the SCARE Group: the SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Ma Y., Zheng J., Zhu H., Dong K., Zheng S., Xiao X. Gastroblastoma in a 12-year-old chinese boy. Int. J. Clin. Exp. Pathol. 2014;7:3380–3384. [PMC free article] [PubMed] [Google Scholar]
- 5.Chak A., Canto M.I., Rosch T., Dittler H.J., Hawes R.H., Tio T.L. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest. Endosc. 1997;45:468–473. doi: 10.1016/s0016-5107(97)70175-5. [DOI] [PubMed] [Google Scholar]
- 6.Wey E.A., Britton A.J., Sferra J.J., Kasunic T., Pepe L.R., Appelman H.D. Gastroblastoma in a 28-year-old man with nodal metastasis: proof of the malignant potential. Arch. Pathol. Lab. Med. 2012;136:961–964. doi: 10.5858/arpa.2011-0372-CR. [DOI] [PubMed] [Google Scholar]
- 7.Msika S., Benhamiche A.M., Jouve J.L., Rat P., Faivre J. Prognostic factors after curative resection for gastric cancer. A population-based study. Eur. J. Cancer. 2000;36:390–396. doi: 10.1016/s0959-8049(99)00308-1. [DOI] [PubMed] [Google Scholar]
- 8.Fernandes T., Silva R., Devesa V., Lopes J.M., Carneiro F., Viamonte B. AIRP best cases in radiologic-pathologic correlation: gastroblastoma: a rare biphasic gastric tumor. Radiographics. 2014;34:1929–1933. doi: 10.1148/rg.347130103. [DOI] [PubMed] [Google Scholar]
- 9.Zheng N., Xiao X.M., Dong K.R., Chen L., Ma Y.Y., Li K. Primary gastric tumors in infants and children: 15 cases of 20-year report. J. Cancer Res. Clin. Oncol. 2016;142:1061–1067. doi: 10.1007/s00432-015-2103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]