Abstract
The nonvitamin K antagonist oral anticoagulants (NOACs), also referred to as direct oral anticoagulants (DOACs), dabigatran, apixaban, edoxaban, and rivaroxaban, have emerged as effective alternatives to vitamin K antagonists (VKAs) across several indications, including the prevention of stroke and systemic embolism (SSE) in patients with atrial fibrillation (AF) and the treatment of venous thromboembolism (VTE). Their use in patients with renal impairment is of particular importance, given the prevalence of renal dysfunction in the indicated populations and the impact of renal function on the metabolism of the NOACs. This publication reviews the pharmacokinetic/pharmacodynamic properties of the NOACs and clinical trial results for patients with renal impairment within the AF and VTE indications. Pharmacokinetic/pharmacodynamic data show the NOACs are dependent on renal clearance to varying extents. Relative to VKAs, the efficacy and safety of the NOACs is preserved in patients with moderate renal impairment. The dosing recommendations for patients with renal impairment differ depending on the NOAC, whereby some of the NOACs require dose reductions based solely on renal function, while others require consideration of additional criteria. However, despite these specific dosing recommendations, emerging real-world evidence suggests patients are not being dosed appropriately, indicating a possible knowledge gap. Adherence to recommended dosing algorithms has implications on the optimal efficacy and safety of the NOACs. To this end, renal function should be assessed in patients on a NOAC, as worsening of renal function may warrant change in the dose of a NOAC or change in oral anticoagulant.
Keywords: anticoagulation, NOAC, nonvalvular atrial fibrillation, nonvitamin K antagonist oral anticoagulant, renal impairment, venous thromboembolism
Introduction
The nonvitamin K antagonist oral anticoagulants [NOACs, also referred to as direct oral anticoagulants (DOACs)], dabigatran, apixaban, edoxaban and rivaroxaban have emerged as effective alternatives to warfarin. Their improved pharmacologic properties allow for fixed dosing with a rapid onset of action and fewer drug interactions. They also eliminate the need for regular coagulation monitoring.
Clinical trial data and guideline recommendations, in addition to the pharmacological advances, have positioned the NOACs as favourable options in their approved indications.1–5 The approved indications vary depending on NOAC and country (Supplemental Table S1), but currently span the prevention of stroke and systemic embolism (SSE) in patients with atrial fibrillation (AF), the prevention of venous thromboembolic events (VTEs) in patients who have undergone elective hip or knee-replacement surgery, the treatment of VTE and prevention of recurrent deep vein thrombosis (DVT) and pulmonary embolism (PE), and in some jurisdictions outside of Canada, the prevention of atherothrombotic events following an acute coronary syndrome.6–10 Although the NOACs overcome several limitations of warfarin, warfarin remains the preferred oral anticoagulant in patients with mechanical prosthetic valves, rheumatic mitral stenosis or patients with an estimated glomerular filtration rate between 15 and 30 ml/min/1.73 m2. 1
The efficacy and safety of the NOACs in patients with renal impairment is of particular importance, given the high prevalence of renal impairment. AF occurs two to three times more often in patients with chronic kidney disease (CKD) compared with those with normal renal function, 11 and these patients have a greater risk of thromboembolic events and bleeding.12–14 The prevalence of VTE in patients with CKD is not well characterized, although recent trials have shown patients with CKD are at an increased risk for VTE.15–17 In addition, in patients with VTE, impaired renal function is associated with an increased risk of PE and bleeding. 18
The elimination process differs between the NOACs and involves varying contributions from renal, fecal, biliary and hepatic routes. All of the NOACs are, to some extent, excreted by the kidney. Due to the dependence on renal clearance, the elimination of the NOACs is reduced in patients with impaired renal function, potentially impacting efficacy and increasing bleeding risk.6–9 This paper reviews the pharmacokinetic/pharmacodynamic and clinical trial data for the NOACs in patients with renal impairment within the main phase III AF and VTE trials, and provides an overview of the regulatory recommendations and clinical considerations for the NOACs in patients with varying renal function.
Pharmacokinetic/pharmacodynamic properties
The pharmacokinetic/pharmacodynamic properties for the direct thrombin inhibitor, dabigatran, and the Factor Xa inhibitors, apixaban, edoxaban and rivaroxaban are outlined in Table 1. The NOACs have predictable pharmacologic profiles, with shorter half-lives and a rapid onset of action compared with warfarin.6–9,19 However, differences between the pharmacologic profiles of the NOACs exist, and are discussed below.
Table 1.
Pharmacokinetic/pharmacodynamic properties of the NOACs.
| Characteristic | Dabigatran4,8 | Apixaban5,6 | Edoxaban2,9 | Rivaroxaban3,7 |
|---|---|---|---|---|
| Target | Factor IIa | Factor Xa | Factor Xa | Factor Xa |
| Prodrug | Yes | No | No | No |
| Dosing | BID | BID | OD | OD |
| Bioavailability, % | 6.5 | 50 | 62 | 80–100 * |
| Half-life, hours | 12–14 | 8–15 | 9–11 | 5–13 |
| Renal clearance (unchanged bioavailable drug) | 85% | ~27% | 50% | ~33% |
| Cmax, hours | 1–2 | 3–4 | 1–2 | 2–4 |
| Drug interactions | P-gp inhibitors | Strong inhibitors of CYP3A4 and P-gp |
P-gp inhibitors | Strong inhibitors of CYP3A4 and P-gp |
When the 15 mg and 20 mg doses are taken with food. BID, twice daily; Cmax, maximum concentration; OD, once daily; P-gp, P-glycoprotein.
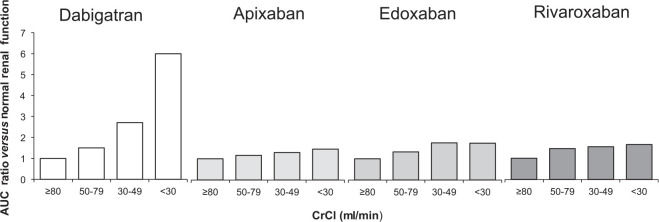
The NOACs are at least partially eliminated by the kidneys. Dabigatran and edoxaban have the greatest dependence on renal elimination (85% and 50%, respectively),8,9 whereas ~27% and ~33% of apixaban and rivaroxaban, respectively, are renally cleared as unchanged drug (Table 1).6,7 Consequently, altered renal elimination of the NOACs impacts drug exposure. Increased drug exposure is observed with declining renal function, with dabigatran being impacted to the greatest extent (Figure 1).
Figure 1.
Area under the curve (AUC) accumulation with declining renal function. Plasma concentrations for the nonvitamin K antagonist oral anticoagulant, reported as AUC, for patients with renal impairment as compared with patients with normal renal function (⩾80 ml/min).6–9
AUC, area under the curve; CrCl, creatinine clearance.
Impact of renal function on nonvitamin K antagonist oral anticoagulant dosing
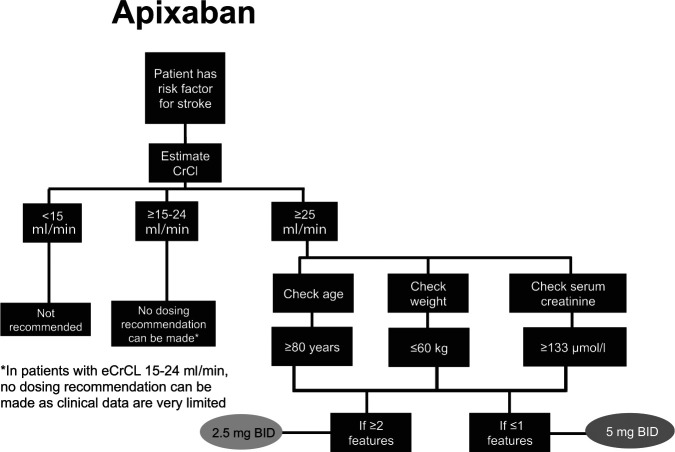
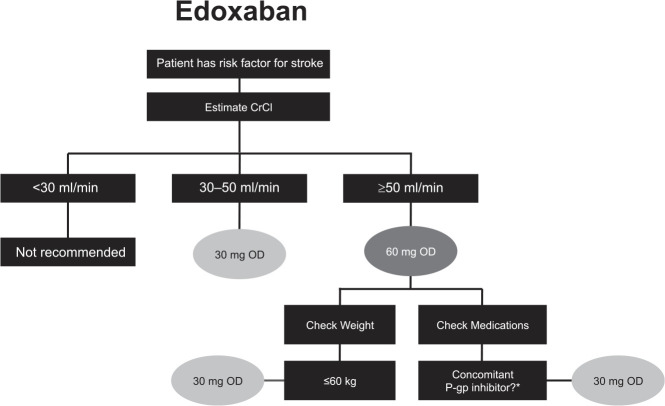
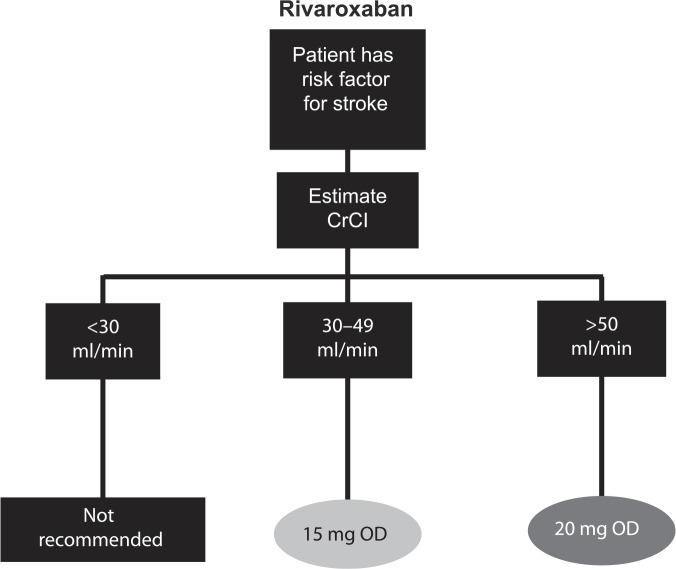
The NOACs were investigated at various doses in phase III clinical trials, which has led to approved dose recommendations in major jurisdictions (Supplemental Tables S2 and S3). Phase III clinical trials for apixaban and edoxaban in patients with AF included renal function, among other factors in their dosing criteria; rivaroxaban is the only NOAC that prospectively studied a reduced dose based solely on renal function.2,3,5 Dose reductions based on renal function were not studied in phase III trials for dabigatran in patients with AF. See Figures 2–5 for Health Canada dosing algorithms for dabigatran, apixaban, edoxaban and rivaroxaban according to renal function.
Figure 2.
Health Canada dosing algorithm for dabigatran according to renal function. 8
BID, twice daily; CrCl, creatinine clearance.
Figure 3.
Health Canada dosing algorithm for apixaban according to renal function. 6
*In patients with estimated creatinine clearance (eCrCl) 15–24 ml/min, no dosing recommendation can be made, as clinical data are very limited.
Figure 4.
Health Canada dosing algorithm for edoxaban according to renal function. 9
*Except amiodarone and verapamil.
CrCl, creatinine clearance; OD, once daily; P-gp, P-glycoprotein.
Figure 5.
Health Canada dosing algorithm for rivaroxaban according to renal function. 7
CrCl, creatinine clearance; OD, once daily.
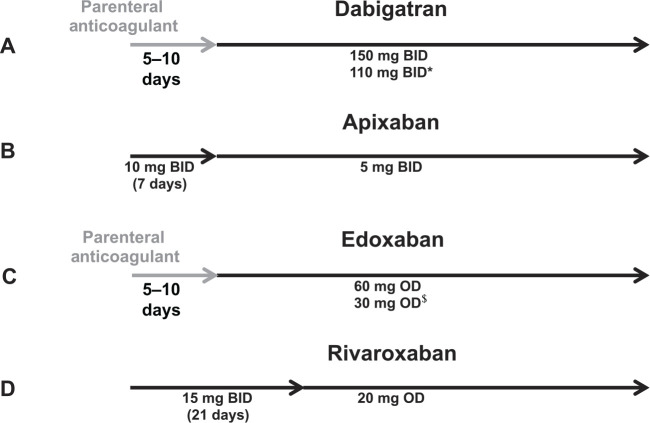
For the treatment of acute VTE, dose adjustments were not necessary for patients with renal impairment receiving dabigatran, apixaban or rivaroxaban, and therefore, dose reductions were not included in the design of the phase III clinical trials (Figure 6).20–24 For rivaroxaban, this decision was based on dose-finding studies, which suggested that doses up to 40 mg/day did not appear to increase the risk of bleeding.25–27 Edoxaban was the only NOAC studied at a reduced dose for patients with renal impairment in the phase III trials for the VTE treatment indication and dose reductions are recommended for patients with creatinine clearance (CrCl) between 30 and 50 ml/min.9,28 As per the clinical trial designs, dabigatran and edoxaban require at least a 5-day lead-in with low-molecular weight or unfractionated heparin (median duration: 9 days in RECOVER; 7 days in HOKUSAI). This is reflected in the approved dosing regimens for dabigatran and edoxaban (Figure 6).
Figure 6.
Health Canada dosing regimens for the treatment of venous thromboembolic events and prevention of deep vein thrombosis and pulmonary embolism. Depicted are the Health Canada approved dosing regimens for (A) dabigatran, (B) apixaban, (C) edoxaban and (D) rivaroxaban.6–9 *Dabigatran 110 mg twice a day (BID) should be considered for patients ⩾ 80 years, or those at higher risk of bleeding (⩾75 years with ⩾1 risk factor for bleeding). $Edoxaban 30 mg once a day (OD) is recommended for moderate renal impairment (creatinine clearance 30–50 ml/min), weight ⩽ 60 kg, or concomitant use of P-glycoprotein inhibitors (except amiodarone and verapamil).
Nonvitamin K antagonist oral anticoagulant in patients with impaired renal function: what we’ve learned from clinical trials
Atrial fibrillation
The efficacy and safety of the NOACs in patients with AF and moderate renal impairment were investigated in secondary analyses of the pivotal trials.29–32 An overview of the baseline characteristics for patients with moderate renal impairment is shown in Table 2. Patients with impaired renal function in the RE-LY and ARISTOTLE trials were primarily composed of moderate-risk populations (mean CHADS2 score of 2.6 in ARISTOTLE), while patients in the ENGAGE AF trial were moderate-to-high risk (mean CHADS2 score of 3.1) and patients in the ROCKET AF trial were generally older and at higher risk of stroke (mean CHADS2 score of 3.7) (Figure 7). Of patients with moderate renal impairment, 50%, 22%, 100% and 100% received the lower dose of dabigatran, apixaban, edoxaban and rivaroxaban, respectively. Although only a few patients with severe renal impairment (CrCl <30 ml/min) were enrolled in the phase III trials (e.g. only 136 patients of the 18,201 patients in the ARISTOTLE trial had severe renal impairment and received apixaban), jurisdictions outside of Canada allow the use of NOACs in patients with a CrCl as low as 15 ml/min. However, the Canadian Cardiovascular Society (CCS) guidelines for the management of AF do not recommend the use of NOACs in this vulnerable patient population, as the evidence for patients with CrCl <30 ml/min comes from pharmacokinetic studies in a limited number of patients.1,33
Table 2.
Clinical trial characteristics for patients with atrial fibrillation and moderate renal impairment.
| Trial | Dabigatran4,30 |
Apixaban5,31 |
Edoxaban2,9,32 |
Rivaroxaban3,29 |
|
|---|---|---|---|---|---|
| RE-LY | ARISTOTLE | ENGAGE AF | ROCKET AF | ||
| Trial Design | Open label, blind to dabigatran dose | Double blind | Double blind | Double blind | |
| Length of trial, years | 2.0 | 1.8 | 2.8 | 1.9 | |
| Renal dose considerations | None; patients randomized to dabigatran dose | 50% dose reduction (2.5 mg BID) if 2/3 criteria met: age ⩾
80 weight ⩽ 60 kg creatinine ⩾ 133 μmol/l (1.5 mg/dl) |
50% dose reduction (30 mg OD) if CrCl 30–49 ml/min | 25% dose reduction (15 mg OD) if CrCl 30–49 ml/min | |
| Number of patients in phase III clinical trial | 18,113 | 18,201 | 21,105 | 14,264 | |
| Number of patients with CrCl <50 ml/min | 3374 | 3017 | 2740 * | 2950 $ | |
| Riva $ ‡ | Warfarin $ ‡ | ||||
| Age, years | 75.2 | 77.6 | 79 * | 79 | 79 |
| CHADS2 score, mean | Not reported | 2.6 | 3.1 * | 3.7 | 3.7 |
| Heart failure, % | 32.6 | 32.7 | 55 * | 66.0 | 65.3 |
| Hypertension, % | 85.6 | 84.9 | 92 * | 91.7 | 92.1 |
| Diabetes, % | 29.1 | 21.1 | 28 * | 31.8 | 33.3 |
| Previous stroke or TIA, % | 20.1 | 25.1 | 30 * | 50.1 | 49.1 |
Baseline characteristics reported for patients with moderate renal impairment receiving high dose edoxaban (60 mg daily or dose reduced to 30 mg daily) or warfarin; $CrCl <30–49 ml/min; ‡Baseline characteristics reported for rivaroxaban 15 mg and warfarin arms individually. AF, atrial fibrillation; BID, twice daily; CHADS2, score for estimating risk of stroke; CrCl, creatinine clearance; OD, once daily; TIA, transischemic attack.
Figure 7.
Breakdown of CHADS2 scores in nonvitamin K antagonist oral anticoagulant pivotal trials and renal subanalyses. The breakdown of patients according to CHADS2 score in ARISTOTLE, RE-LY, ENGAGE AF and ROCKET AF are depicted according to (a) overall trial population2–5 and (b) patients with moderate renal impairment (30–49 ml/min).29–32 *Mean CHADS2 score not reported for dabigatran according to renal function.
AF, atrial fibrillation; CHADS2, score for estimating risk of stroke.
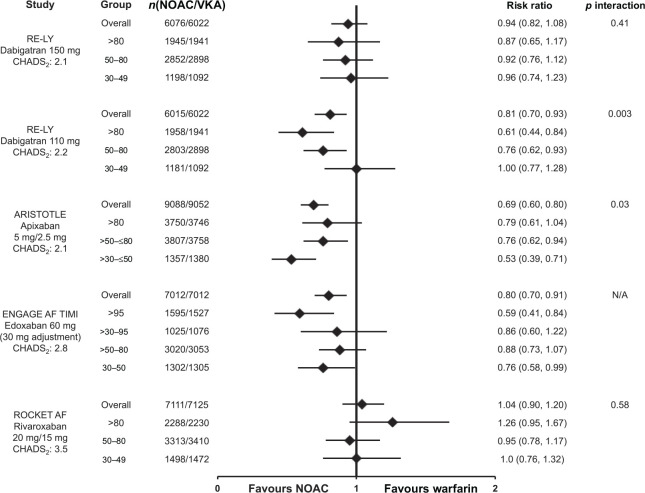
Differences in trial design and populations make indirect comparisons of the efficacy and safety of the NOACs inappropriate, however, across all treatment arms, stroke rates increased with declining renal function (Supplemental Table S4).29–32,34 Figure 8 shows the risk of SSE (primary efficacy endpoint, intention-to-treat analysis [ITT]) in patients with moderate renal impairment. Renal impairment had no significant effect on the risk of SSE (p value for interaction was nonsignificant), suggesting the NOACs, at the doses tested, performed just as well as dose-adjusted warfarin in patients with moderate renal impairment,29–32 consistent with overall trial results.2–5
Figure 8.
Stroke and systemic embolism in atrial fibrillation patients by renal function. Risk of stroke and systemic embolism for the nonvitamin K antagonist oral anticoagulant (dabigatran, 30 apixaban,6,31 edoxaban, 35 and rivaroxaban 29 ) versus warfarin according to renal function subgroups and overall trial populations. Data are reported for the intention-to-treat populations. Mean CHADS2 scores are reported for the respective nonvitamin K antagonist oral anticoagulant arm from the phase III pivotal trials.
AF, atrial fibrillation; CHADS2, score for estimating risk of stroke; NOAC, nonvitamin K antagonist oral anticoagulant; VKA, vitamin K antagonist.
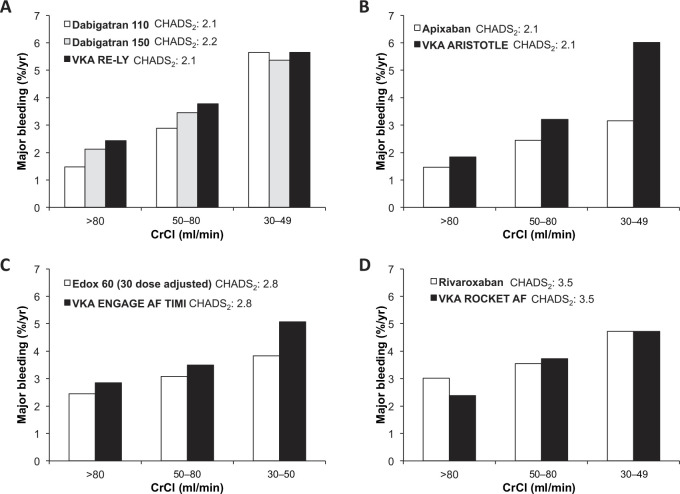
In comparison with warfarin, the pattern of major bleeding with dabigatran, edoxaban, or rivaroxaban was similar in patients with normal renal function and those with renal dysfunction (Figure 9).29,30,32,35 An interaction was observed for major bleeding with apixaban (interaction p value = 0.030); where patients with moderate renal impairment had a nominally significant greater reduction in major bleeding [hazard ration (HR): 0.53; 95% confidence interval (CI): 0.39–0.71], as compared with differences between apixaban and warfarin in patients with normal renal function (HR: 0.79; 95% CI: 0.61–1.04) and mild renal impairment (HR: 0.76; 95% CI: 0.62–0.94). 6 It is not known whether the observed benefit with apixaban is related to the molecule, to chance, other confounders, or possibly to a higher-than-expected bleeding rate with warfarin in the moderate renal subgroup of this trial (Figure 10B).
Figure 9.
Major bleeding in atrial fibrillation patients by renal function. Risk of major bleeding for the nonvitamin K antagonist oral anticoagulants (dabigatran, 8 apixaban, 6 edoxaban 35 and rivaroxaban 7 ) versus warfarin according to renal function subgroups and overall trial populations. Data are reported for the safety populations on treatment, except for dabigatran which only reported data for the randomized set. Mean CHADS2 scores are reported for the respective nonvitamin K antagonist oral anticoagulant arm from the phase III pivotal trials.
AF, atrial fibrillation; CHADS2, score for estimating risk of stroke; NOAC, nonvitamin K antagonist oral anticoagulant; VKA, vitamin K antagonist.
Figure 10.
Major bleeding in atrial fibrillation patients according to renal function for nonvitamin K antagonist oral anticoagulant and warfarin arms of the phase III trials. Major bleeding event rates (%/year) according to renal function subgroups from the phase III pivotal trials for (a) dabigatran, (b) apixaban, (c) edoxaban and (d) rivaroxaban.6–8, 35 Mean CHADS2 scores are reported for the respective arm from the phase III pivotal trials.
AF, atrial fibrillation; CHADS2, score for estimating risk of stroke; CrCl, creatinine clearance; VKA, vitamin K antagonist.
There are limited clinical data on the use of the NOACs in patients with AF on dialysis, as these patients were not included in the phase III clinical trials. Thus, the use of the NOACs in patients on dialysis are not recommended. Recently, pharmacokinetic studies have shown that apixaban 5 mg BID and rivaroxaban 10 mg OD results in similar drug exposure in patients on dialysis when compared with healthy controls.36,37 A pharmacokinetic study investigating a single dose of rivaroxaban 15 mg OD in patients on chronic dialysis found changes in pharmacokinetic and pharmacodynamic parameters to be comparable with changes observed in patients with moderate-to-severe renal impairment who were not undergoing dialysis. 38 Additionally, a single 15 mg dose of edoxaban in patients with end-stage renal disease receiving dialysis was found to be well tolerated. 39 Dialysis only slightly decreased the total edoxaban exposure when compared with the same patients off dialysis, suggesting an additional dose of edoxaban after dialysis may not be required. 39 These results are encouraging and support future clinical trials investigating the efficacy and safety of apixaban, edoxaban and rivaroxaban in patients on dialysis. To our knowledge, similar pharmacokinetic studies have not been performed for dabigatran. It should be emphasized that there are no clinical data for the NOACs in patients on dialysis and the use of NOACs in this population is not recommended. 1
Venous thromboembolic events
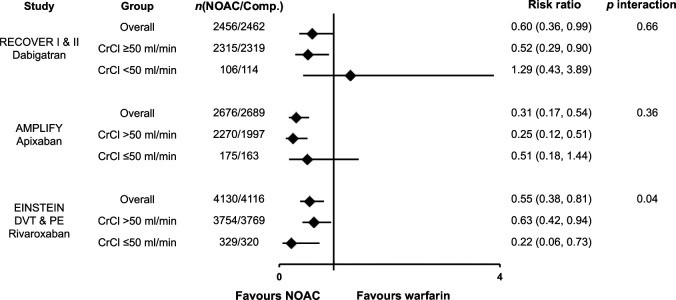
Efficacy, as measured by the risk of VTE recurrence in patients with renal impairment is shown in Figure 11. Overall, the NOACs showed similar efficacy as compared with warfarin for the prevention of VTE recurrence regardless of kidney function. 40
Figure 11.
Venous thromboembolic event recurrence or death related to venous thromboembolic event by renal function. Efficacy outcomes of recurrent venous thromboembolic event or death related to venous thromboembolic event for dabigatran, apixaban, edoxaban and rivaroxaban versus warfarin by renal function subgroups as reported by Geldhof et al. 40 Data are reported as risk ratios for nonvitamin K antagonist oral anticoagulant versus warfarin.
Comp, comparator arm; CrCl, creatinine clearance; DVT, deep vein thrombosis; NOAC, nonvitamin K antagonist oral anticoagulant; PE, pulmonary embolism.
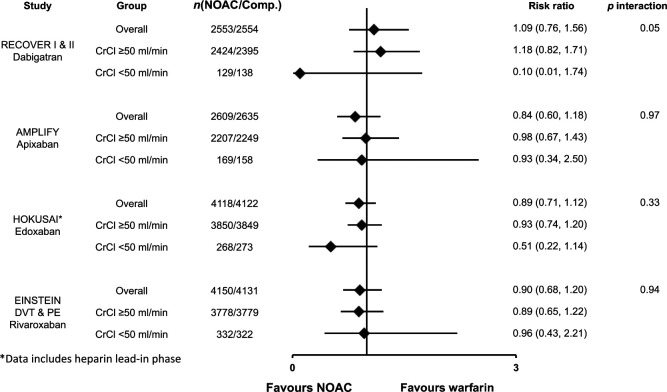
The risk of major bleeding for dabigatran, apixaban and rivaroxaban in patients with varying renal function is shown in Figure 12 (data for edoxaban were not reported). 40 Compared with warfarin, all three drugs led to less major bleeding in the overall population, as well as in the subgroups of renal function.
Figure 12.
Major bleeding in venous thromboembolic event patients by renal function. Major bleeding data for dabigatran, apixaban and rivaroxaban according to renal function subgroups as reported by Geldhof et al. 40 Data are reported as risk ratios of nonvitamin K antagonist oral anticoagulant versus warfarin. The definition of major bleeding was similar in all included trials and conformed to the International Society on Thrombosis and Haemostasis guidelines. Major bleeding data for edoxaban by renal function was not available at the time of publication.
Comp, comparator arm; CNOAC, nonvitamin K antagonist oral anticoagulant; CrCl, creatinine clearance; DVT, deep vein thrombosis; PE, pulmonary embolism.
Clinical considerations
Clinical trial results have demonstrated the efficacy and safety of NOACs in patients with renal impairment and their approved labels provide guidance on dosing. The Health Canada dosing recommendations for dabigatran, apixaban, edoxaban and rivaroxaban in patients with AF and renal impairment are shown in Figures 2–5. The CCS guidelines for the management of AF recommend NOACs not be used when CrCl is <30 ml/min. 1 Due to the dependence of dabigatran on renal clearance, the CCS guidelines suggest apixaban or rivaroxaban may be preferred for patients with moderate renal impairment. 1 For the treatment of VTE and prevention of recurrent DVT and PE, dose adjustments in patients with moderate renal impairment are not required for dabigatran, apixaban or rivaroxaban. Dosing should follow the approved regimens (Figure 6).
The dose recommendations from Health Canada are similar to the FDA for patients with renal impairment, however the FDA has extrapolated data from the clinical trials and approved a low dose of dabigatran (75 mg BID), apixaban (5.0 mg BID or 2.5 mg BID) and rivaroxaban (15 mg OD) for patients with AF and a CrCl of 15–30 ml/min.41–43 The low dose of dabigatran was not tested in phase III clinical trials and was approved based on pharmacokinetic modeling, comparing pharmacokinetic data from RE-LY with data from a small study of subjects with compromised renal function. 44 Health Canada and the US Food and Drug Administration (FDA) have made similar dosing recommendations for apixaban, edoxaban and rivaroxaban in the treatment of VTE (Figure 6 and Supplemental Table S3). No dabigatran dose reductions are recommended by the FDA for this indication.
Another notable difference between the Health Canada and FDA recommendations is seen with edoxaban. Edoxaban was given a black box warning by the FDA for reduced efficacy in AF patients with CrCl >95 ml/min and the FDA recommended the use of a different anticoagulant in this population. 35 This black box warning was not included in the Health Canada label for edoxaban. This recommendation by the FDA was based on the fact these patients had higher rates of ischemic stroke (Supplemental Table S4) owing to the 40% reduction in edoxaban blood levels in patients with normal renal function compared with patients with a CrCl between 50 and 80 ml/min.34,35 Similar trends were seen across the other Factor Xa inhibitors; that is, relative to warfarin, rates of SSE are numerically higher in patients with normal renal function compared with patients with impaired renal function treated with apixaban (HR: 1.35 versus 0.87) and rivaroxaban (HR: 1.07 versus 0.82) (Supplemental Table S4). 34 These data highlight the importance of adhering to approved dosing regimens in order to obtain the efficacy benefits seen in the phase III randomized controlled trials.
Emerging real-world evidence and global prescription data suggest patients with AF are not being dosed appropriately.45,46 An analysis of the SPRINT AF registry assessed the prevalence of Canadian patients eligible for the reduced dose of apixaban or rivaroxaban. This analysis suggested that more patients are being prescribed a reduced dose of the Factor Xa inhibitors than those who met eligibility criteria as defined in the phase III clinical trials. 45 It is possible these results reflect dosing decisions based on other clinical considerations, where patients with a higher comorbid burden, who are more likely to have worse outcomes, may be receiving the lower dose even if they do not meet the recommended dosing criteria. Recent real-world evidence reported on outcomes in patients receiving lower doses of the NOACs without a ‘renal indication’ for dose reduction. 47 The reasons why the lower NOAC dose was prescribed are not known. The low dose of apixaban was associated with a 4.87-times higher risk of stroke compared with the higher dose. 47 There was no difference in rate of major bleeding associated with use of the low dose. This association was not seen for rivaroxaban or dabigatran.
Studies have shown physicians may place more value on the avoidance of bleeding and less value on the avoidance of stroke than patients at high risk of AF. Whereas the opposite perspective is seen with patients who place a higher value on the avoidance of stroke. 48 This more cautious perspective may further explain why some patients at higher risk of bleeding are inappropriately prescribed a lower dose, thus potentially putting them at higher risk of stroke due to insufficient anticoagulation. 46 However, even if the recommended dose of a NOAC is prescribed, issues with adherence may result in the patient not receiving the proper dose. A recent survey found approximately one-third of patients taking a twice daily OAC only took their medication once a day. 49 These patients were 4.5 to 5 times more likely to not adhere to their dosing regimen when compared with patients receiving once daily medications. The impact of these findings would be further compounded if patients were inappropriately prescribed the lower dose. Underdosing may have important implications, as rates of SSE, major bleeding and mortality are generally higher for patients receiving the lower versus higher NOAC dose.6–9
The impact of inappropriate dosing highlights the importance of monitoring renal function and ensuring NOACs are used appropriately. Patients receiving a NOAC should have their renal function monitored at least once per year.1,6–9,33 Medication assessments should be performed to identify concomitant medications that may alter renal function or renal excretion of NOACs. As in patients with normal renal function, treatment of patients with renal impairment should be individualized, weighing the benefits of oral anticoagulant treatment against the risk of bleeding.
Conclusion
Patients with renal impairment have an increased risk of thrombosis and bleeding, emphasizing the importance of assessing the efficacy and safety of NOACs in this population. Elimination of NOACs is altered in patients with impaired renal function due to varying dependence on renal clearance. Increased drug exposure, observed with declining renal function, may put patients at a higher risk of SSE, VTE and bleeding. The dosing recommendations for patients with renal impairment differ depending on the NOAC, with some of the NOACs requiring dose reductions based solely on renal function and others taking additional criteria into consideration. Rivaroxaban is the only NOAC that was prospectively studied with a reduced dose for patients with moderate renal impairment. When adhering to approved dosing regimens for patients with renal impairment, the NOACs are a safe and effective option for the prevention of SSE in patients with AF and the treatment of VTE. However, real-world evidence suggests patients are not being dosed appropriately and that dose adjustments are being based on clinical considerations outside approved dosing criteria. Inappropriate dose reductions may have important implications, as patients who are insufficiently anticoagulated would be at a higher risk of stroke or thromboembolism.
Supplementary Material
Acknowledgments
The authors thank MEDUCOM Health Inc. for providing editorial and logistical support. The authors had complete editorial control, take full responsibility for the content of this publication, and approve the final article.
Footnotes
Funding: Publication of this article was supported by Bayer Inc., who provided financial support to MEDUCOM Health Inc. for costs associated with logistics of manuscript preparation and submission.
Conflict of interest statement: AGT provided consultation to Bayer Inc. and received speaking honoraria from Bayer Inc., Janssen Inc., Boehringer Ingelheim Ltd., Bristol-Myers Squibb and Pfizer. AC and DP are employed by Bayer Inc., which markets rivaroxaban.
Contributor Information
Alexander G.G. Turpie, McMaster University, Suite 802, 19 Brant Street, Toronto, ON, M5V 2L2, Canada.
Daniel Purdham, Bayer Inc., Mississauga, ON, Canada.
Antonio Ciaccia, Bayer Inc., Mississauga, ON, Canada.
References
- 1. Verma A, Cairns JA, Mitchell LB, et al. 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol 2014; 30: 1114–1130. [DOI] [PubMed] [Google Scholar]
- 2. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093–2104. [DOI] [PubMed] [Google Scholar]
- 3. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 4. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 5. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992. [DOI] [PubMed] [Google Scholar]
- 6. Eliquis Health Canada Product Monograph. Montreal, QC:Bristol-Myers Squibb Canada. 16 June 2016. [Google Scholar]
- 7. Xarelto Health Canada Product Monograph. Mississauga, ON:Bayer Inc. 20 July 2015. [Google Scholar]
- 8. Pradaxa Health Canada Product Monograph.Burlington, ON:Boehringer Ingelheim Canada Ltd.11 August 2016. [Google Scholar]
- 9. Lixiana Health Canada Product Monograph. Laval, QC:Servier Canada Inc. 12 January 2017. [Google Scholar]
- 10. Xarelto EMA Summary of Product Characteristics. Bayer Pharma AG. 10 June 2016. [Google Scholar]
- 11. Soliman EZ, Prineas RJ, Go AS, et al. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC). Am Heart J 2010; 159: 1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Go AS, Fang MC, Udaltsova N, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation 2009; 119: 1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138: 1093–1100. [DOI] [PubMed] [Google Scholar]
- 14. Barrios V, Gorriz JL. Atrial fibrillation and chronic kidney disease: focus on rivaroxaban. J Comp Eff Res 2015; 4: 651–664. [DOI] [PubMed] [Google Scholar]
- 15. Wattanakit K, Cushman M. Chronic kidney disease and venous thromboembolism: epidemiology and mechanisms. Curr Opin Pulm Med 2009; 15: 408–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wattanakit K, Cushman M, Stehman-Breen C, et al. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol 2008; 19: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ocak G, Lijfering WM, Verduijn M, et al. Risk of venous thrombosis in patients with chronic kidney disease: identification of high-risk groups. J Thromb Haemost 2013; 11: 627–633. [DOI] [PubMed] [Google Scholar]
- 18. Monreal M, Falga C, Valle R, et al. Venous thromboembolism in patients with renal insufficiency: findings from the RIETE Registry. Am J Med 2006; 119: 1073–1079. [DOI] [PubMed] [Google Scholar]
- 19. Coumadin Health Canada Product Monograph. Montreal: Bristol-Myers Squibb Canada. 20 May 2016. [Google Scholar]
- 20. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013; 369: 799–808. [DOI] [PubMed] [Google Scholar]
- 21. Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363: 2499–2510. [DOI] [PubMed] [Google Scholar]
- 22. Buller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366: 1287–1297. [DOI] [PubMed] [Google Scholar]
- 23. Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014; 129: 764–772. [DOI] [PubMed] [Google Scholar]
- 24. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361: 2342–2352. [DOI] [PubMed] [Google Scholar]
- 25. Bauersachs RM, Lensing AW, Prins MH, et al. Rivaroxaban versus enoxaparin/vitamin K antagonist therapy in patients with venous thromboembolism and renal impairment. Thromb J 2014; 12: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buller HR, Lensing AW, Prins MH, et al. A dose-ranging study evaluating once-daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein-DVT Dose-Ranging Study. Blood 2008; 112: 2242–2247. [DOI] [PubMed] [Google Scholar]
- 27. Agnelli G, Gallus A, Goldhaber SZ, et al. Treatment of proximal deep-vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY 59-7939): the ODIXa-DVT (Oral Direct Factor Xa Inhibitor BAY 59-7939 in Patients With Acute Symptomatic Deep-Vein Thrombosis) study. Circulation 2007; 116: 180–187. [DOI] [PubMed] [Google Scholar]
- 28. Hokusai-VTE Investigators, Büller HR, Décousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369: 1406–1415. [DOI] [PubMed] [Google Scholar]
- 29. Fox KA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J 2011; 32: 2387–2394. [DOI] [PubMed] [Google Scholar]
- 30. Hijazi Z, Hohnloser SH, Oldgren J, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation 2014; 129: 961–970. [DOI] [PubMed] [Google Scholar]
- 31. Hohnloser SH, Hijazi Z, Thomas L, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J 2012; 33: 2821–2830. [DOI] [PubMed] [Google Scholar]
- 32. Bohula EA, Giugliano RP, Ruff CT, et al. Impact of renal function on outcomes with edoxaban in the ENGAGE AF-TIMI 48 trial. Circulation 2016; 134: 24–36. [DOI] [PubMed] [Google Scholar]
- 33. Macle L, Cairns JA, Andrade JG, et al. The 2014 Atrial Fibrillation Guidelines Companion: A Practical Approach to the Use of the Canadian Cardiovascular Society Guidelines. Can J Cardiol 2015; 31: 1207–1218. [DOI] [PubMed] [Google Scholar]
- 34. US Food and Drug Administration. Cardiovascular and Renal Drugs Advisory Committee. Edoxaban NDA 206316. Statistical Considerations, ENGAGE AF Trial. 2014. [Google Scholar]
- 35. Savaysa US Food and Drug Administration Prescribing Information. Parsippany, NJ: Daiichi Sankyo, Inc. September, 2015. [Google Scholar]
- 36. Wang X, Tirucherai G, Marbury TC, et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. J Clin Pharmacol. Epub ahead of print 22 December 2015. doi: 10.1002/jcph.628. [DOI] [PubMed] [Google Scholar]
- 37. De Vriese AS, Caluwe R, Bailleul E, et al. Dose-finding study of rivaroxaban in hemodialysis patients. Am J Kidney Dis 2015; 66: 91–98. [DOI] [PubMed] [Google Scholar]
- 38. Dias C, Moore KT, Murphy J, et al. Pharmacokinetics, pharmacodynamics, and safety of single-dose rivaroxaban in chronic hemodialysis. Am J Nephrol 2016; 43: 229–236. [DOI] [PubMed] [Google Scholar]
- 39. Parasrampuria DA, Marbury T, Matsushima N, et al. Pharmacokinetics, safety, and tolerability of edoxaban in end-stage renal disease subjects undergoing haemodialysis. Thromb Haemost 2015; 113: 719–727. [DOI] [PubMed] [Google Scholar]
- 40. Geldhof V, Vandenbriele C, Verhamme P, et al. Venous thromboembolism in the elderly: efficacy and safety of non-VKA oral anticoagulants. Thromb J 2014; 12: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eliquis US Food and Drug Administration prescribing information. Bristol-Myers Squibb Company. July 2016. [Google Scholar]
- 42. Xarelto US Food and Drug Administration prescribing information. Bayer Pharma AG. January 2015. [Google Scholar]
- 43. Pradaxa US Food and Drug Administration prescribing information. Boehringer Ingelheim Pharmaceuticals, Inc. November 2015. [Google Scholar]
- 44. Centre for Drug Evaluation and Research. Summary Review (Dabigatran). 2010. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022512Orig1s000SumR.pdf (last updated 19 October 2010).
- 45. Gupta M, Singh N, Tsigoulis M, et al. Underuse of full dose factor Xa inhibition in atrial fibrillation: insight from the SPRINT-AF registry. J Am Coll Cardiol 2015; 65: A348. [Google Scholar]
- 46. Weitz JI, Eikelboom JW. Appropriate apixaban dosing: prescribers take note. JAMA Cardiol 2016; 1: 635–636. [DOI] [PubMed] [Google Scholar]
- 47. Yao X, Shah N, Sangaralingham L, et al. Deviation from dosing labels of non-vitamin K antagonist oral anticoagulants may be associated with reduced effectiveness or safety. Paper presented at 2016 American Heart Association (AHA) Scientific Sessions, 15 November 2016; New Orleans, LA. Abstract 17709. [Google Scholar]
- 48. Devereaux PJ, Anderson DR, Gardner MJ, et al. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. BMJ 2001; 323: 1218–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Andrade JG, Krahn AD, Skanes AC, et al. Values and preferences of physicians and patients with nonvalvular atrial fibrillation who receive oral anticoagulation therapy for stroke prevention. Can J Cardiol 2016; 32: 747–753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.