Abstract
Bone homeostasis requires a balance between the bone formation of osteoblasts and bone resorption of osteoclasts to maintain ideal bone mass and bone quality. An imbalance in bone remodeling processes results in bone metabolic disorders such as osteoporosis. Hydrogen sulfide (H2S), a gasotransmitter, has attracted the focus of many researchers due to its multiple physiological functions. It has been implicated in anti-inflammatory, vasodilatory, angiogenic, cytoprotective, anti-oxidative and anti-apoptotic mechanisms. H2S has also been shown to exert osteoprotective activity through its anti-inflammatory and anti-oxidative effects. However, the underlying molecular mechanisms by which H2S mitigates bone diseases are not completely understood. Experimental evidence suggests that H2S may regulate signaling pathways by directly influencing a gene in the cascade or interacting with some other gasotransmitter (carbon monoxide or nitric oxide) or both. MicroRNAs (miRNAs) are short non-coding RNAs which regulate gene expression by targeting, binding and suppressing mRNAs; thus controlling cell fate. Certainly, bone remodeling is also regulated by miRNAs expression and has been reported in many studies. MicroRNAs also regulate H2S biosynthesis. The inter-regulation of microRNAs and H2S opens a new possibility for exploring the H2S-microRNA crosstalk in bone diseases. However, the relationship between miRNAs, bone development, and H2S is still not well explained. This review focuses on miRNAs and their roles in regulating bone remodeling and possible mechanisms behind H2S mediated bone loss inhibition, H2S-miRNAs crosstalk in relation to the pathophysiology of bone remodeling, and future perspectives for miRNA-H2S as a therapeutic agent for bone diseases.
Keywords: Osteoporosis, Bone remodeling, Osteoblast, Osteoclast, Hydrogen sulfide
1. Introduction
Bone is dynamic tissue and its constant rebuilding occurs via the combined action of “osteoblasts” that generate bone and “osteoclasts” that reabsorb it. Bone metabolic disorder increased bone resorption and decreased bone formation, always lead to low bone mass, deteriorated bone structure, and increased bone fractures, which are responsible for calling osteoporosis a “silent disease”. It is mostly prominent in postmenopausal individuals due to the cessation of ovarian function and estrogen deficiency [1]. Hydrogen sulfide (H2S) is a gas transmitter released endogenously by mammalian cells. It has been found to be a protective agent against oxidative stress and inflammatory response [2]. It is well known that oxidative stress and inflammatory factors can induce an imbalance in osteoblast and osteoclast activity. Recent studies suggest that H2S is also involved in the process of bone remodeling by increasing bone formation, and thereby preventing the trabecular bone loss in ovariectomized (OVX) mice through Wnt signaling [3]. MicroRNAs (miRNAs) are small, single-stranded noncoding RNAs that regulate gene expression through the binding and inhibition of target mRNAs. Several miRNAs modulate bone development through their regulation of osteoblasts and osteoclasts, suggesting a contribution to bone formation, resorption, and bone remodeling. miR-133 and miR-135 collectively suppress the transcriptional activity of Runx-2 and Smad5 [4] whereas miR-21, miR-155 and miR-223 are involved in the differentiation of osteoclasts [5]. In this review, we focus on regulation mediated by miRNAs and physiological effects of H2S during bone remodeling. We also hypothesize the possible molecular mechanisms during the H2S mediated inhibition process of bone loss. Lastly, we discuss miRNA therapy and potential solutions to bone-loss disorders. In summary, this review evaluates how future investigations could be used in miRNA biology to understand, prevent, and treat bone loss and to explore the in-depth osteoprotective mechanisms of H2S.
2. MiRNAs: treatment and main problems
miRNAs are short (approximately 22 nucleotide bases or less), single-stranded noncoding RNA molecules which act to inhibit the expression of target mRNAs (in Fig. 1). Biogenesis of canonical microRNAs (miRNAs) involves multiple steps: nuclear processing of primary miRNA (pre-miRNA) by DROSHA, nuclear export of precursor miRNA (pre-miRNA) by Exportin 5 (XPO5), and cytoplasmic processing of pre-miRNA by DICER, however, DROSHA and DICER are essential for the miRNA maturation during the canonical miRNA pathway, but XPO5 can be complemented by alternative mechanisms [6]. In some pathological conditions, there has a dysregulation of this miRNA processing machinery components, such as the expression levels of Drosha and Dicer are down-regulated in ovarian cancer and neuroblastomas, while Exportin-5 is also down-regulated in bladder cancer [7]. When these matured miRNAs are processed and expressed, they bind to partially complementary sites in target mRNAs, leading to mRNA degradation or protein translation interference causing translational repression, mRNA destabilization, and/or mRNA cleavage for post-transcriptional regulation of protein synthesis [8,9]. Furthermore, this interaction between the miRNA and target mRNA resulted in decreased target protein levels without affecting the stability of the mRNA. This profile of a significant reduction in protein level without a proportionate reduction in target mRNA levels became a hallmark of miRNA function [10]. miRNAs serve important regulatory roles in various developmental, physiological, and pathological conditions, such as cell function and differentiation, tumorigenesis and viral infection. More than 1000 miRNAs were encoded in the human genome and regulate up to 60% of human genes. A single gene can be targeted by a cluster of miRNAs, and a single miRNA also can target many protein-coding genes.
Fig. 1.
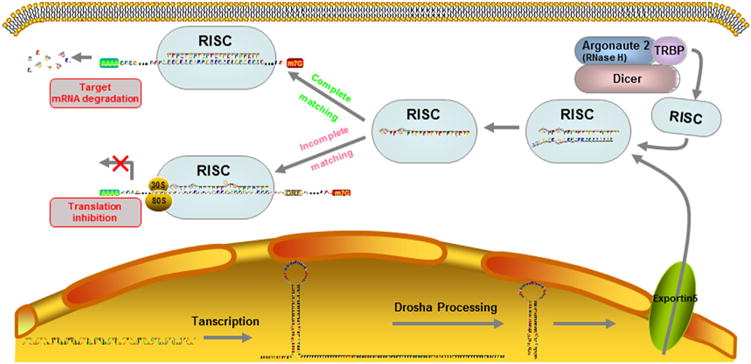
The biogenesis of miRNAs. Pri-miRNAs are transcribed from intergenic genes and processed into a pre-miRNA by the Drosha/DGCR8 complex, creating a 70–80 nts, hairpin-looped molecule, which is then shuttled out of the nucleus via the exportin-5 mediated transportation. Cytoplasmatic digestion of the pre-miRNA is facilitated by Dicer, resulting in double-stranded mature miRNAs. Mature miRNAs modulate gene expression by RNA-induced silencing complex (RISC) which composed by the dicer, argonaute 2, and TRBP. If miRNAs perfectly base pairing with sequences in the target mRNAs, mRNA will deadenylation and decapping and then cleavage; alternatively, miRNAs could repressing the transcription of target mRNAs when the miRNAs incomplete matching with target mRNAs.
The usage of miRNA has numerous advantages for cell behavior controlling when paralleled to other nucleic acid-based approaches, however the main hindrances in miRNA therapy is that they have coulomb repulsion and not able to easily cross the cell membrane to exert their effects, which caused by the negatively charged of miRNAs (their mimics or inhibitors) and also cell membrane, so “naked” miRNAs always are degraded rapidly in vivo [11]. How to deliver miRNAs across the cell membrane to the cytoplasm and then lead specific mRNA degradation or translational inhibition become more and more important. Although oligonucleotides can be delivered into the cytoplasm, the limitations also obviously. Transfection can deliver small RNAs into cytoplasm but still have some obvious limitations, such as electroporation is not practical in vivo, immune responses to viral vectors are of concern; nano-carrier is less toxic than viral vectors, however, their efficiency is still low and also unstable. So promoting transfection efficiency and decreasing the toxicity of nano-carriers is of up-most importance in establishing effective miRNA treatment, meanwhile, elevating oligonucleotides stability also played a pivotal role in oligonucleotide therapeutics developments. Zhang et al. designed a hyper branched polymer (HP) vector for miRNA delivery, they using polyethylene glycol (PEG) chains and molecular weight cationic poly-ethylenimine (PEI) attached to the outer shell, then miR-26a self-assembled into the nano-sized spherical shell sandwiched between the inner and outer hydrophilic PEG layers and can be delivered steadily and efficiently [12]. Another non-toxic, arginine-rich, CPP peptide (VSRRRRRRGGRRRR) were developed and called low molecular weight protamine (LMWP), also be used for miRNA-29b delivery in the application of bone regeneration [13]. Both this two miRNA delivery system offer plausible strategies for miRNA therapy because of their high transfection efficiency and negligible toxicity.
3. miRNAs regulation during the bone remodeling
Bone homeostasis is altered dramatically in physiological and pathological conditions, including injury and metabolic diseases. miRNAs are important regulators of gene expression, also could as a possible biomarker and potential novel therapeutic targets to against bone disorders, such as bone fracture healing and osteoporosis treatment. However, the distinct regulatory roles of individual miRNAs in skeletal development and osteogenic differentiation have not been well characterized, so we summarized regulation effects during bone remodeling which shown in Table 1 and Fig. 2. As we know, mechanical stimulation plays a pivotal role during the process of skeletal development. Deficient of mechanical stimulation will produce a rapid bone loss, while tension force and fluid shear stress could affect bone remodeling and also miRNA changes. 9 miRNAs have been identified as core miRNAs of tension force-induced bone formation [14]. Fluid shear stress (FSS) across the surface of bone cells is another potent regulator of bone cell behavior, enhances cell proliferation and osteogenic differentiation. One hour of FSS at 12dyn/cm2 could induce actin stress fiber formation and rearrangement, up-regulate osteogenic differentiation, meanwhile decrease the expression levels of miR-20a, −21, −19b, −34a, −34c, −140, and −200b [15]. Microgravity during spaceflight which has no mechanical stimulation could be the main cause of bone loss, and the molecular mechanism may relate to miR-132-3p which suppress Ep300 protein expression and in turn decreases the activity and acetylation of Runx-2, then inhibit osteoblast differentiation [16].
Table 1.
miRNAs profile in the process of bone remodeling.
| miRNA | Target | Main Mechanism | Cell line/animal model | |
|---|---|---|---|---|
| miR-17 | BMP-2 | Enhanced miR-17 expression inhibits osteogenic pathways through the repression of BMP-2 [19] | mesenchymal progenitor cell | |
| Smurf1 | Down-regulation of miR-17 activates Smurf1 expression and inhibits its osteogenic differentiation [77] | PDLSCs | ||
| miR-20a | PPARγ Bambi Crim1 |
Promotes osteogenic differentiation of hMSCs by up-regulation of BMP/Runx-2 signaling [18] | hMSCs | |
| miR-21 | TGFBR2 | Inhibits cell proliferation and collagen synthesis by targeting TGFβ pathway [78] | palatal mesenchymal cells (PMCs) | |
| PDCD4 | miR-21 expression in BM-MSCs increases osteoblast differentiation [79] | BM-MSCs | ||
| BMPRII | Down-regulates BMPRII protein level [80] | PC3 and Lncap cells | ||
| Fas ligand | Down-regulation of miR-21 could contribute to osteoclast apoptosis via target gene (Fas-L) [81] | osteoclasts | ||
| PLAP-1 | Upregulates PLAP-1 protein levels during osteogenic differentiation [82] | PDLCs | ||
| ACVR2B | Induces osteogenic differentiation of PDLSCs by regulating ACVR2B protein expression [83] | PDLCs | ||
| Spry1 | miR-21 promotes MSC osteogenesis by repressing Spry1 and promotes bone formation by blocking TNFα in OVX mice [79] | MSCs/OVX mice | ||
| miR-23a | Runx-2 | Represses Runx2 in mature osteocytes [84] | Osteocytes | |
| Fas | Lowered or depleted miR-23a significantly enhances TNF-α induced MC3T3-E1 apoptosis [85] | MC3T3-E1 | ||
| miR-26a | GSK-3β | Targets Gsk-3β to activate the osteoblastic activity of endogenous stem cells in osteoporotic mice [12] | mouse MSCs | |
| SMAD1 | Inhibition of miR-26a could increase SMAD1 transcription, up-regulate bone marker genes and thus enhance osteoblast differentiation [86] | C57BL/6 mice with critical-sized bone defects hADSCs | ||
| miR-27 | Apc | Promotes osteoblast differentiation through modulation of Wnt signaling [87] | hFOB1.19 cells | |
| miR-29a | SPARC | Inhibits osteoclast formation and increases the pro-osteoblast gene Runx2, related with Wnt signaling and phosphorylation of ERK1/2 and AKT [88] | MSCs; bone marrow macrophages | |
| miR-29b | COL1A1, COL3A1 | Positive regulator of osteoblast differentiation [89] | HOBs | |
| HDAC4, TGFβ3, AcvR2A, CTNNBIP1, DUSP2 | Promotes osteogenesis and increases levels of osteogenic markers (Runx2, ALP, and bone extracellular matrix protein) [13] | rat and mouse cells | ||
| miR-29c | SPARC | Represses synthesis of osteonectin and Dkk-1, increases osteoblastic differentiation [90] | MC3T3-E1; Primary osteoblasts | |
| miR-31 | Osterix | miR-31 decreases osterix and its targets genes (BGLAP and COL1A1) levels, inhibits osteoblastic differentiation [91] | MCF-7, SaOS2, MG-63, U2OS | |
| miR-34a | EphA5 | Promotes cell migration and condensation on collagen substrate [92] | Chicken limb mesenchymal cells | |
| miR-34c | Notch1, | miR-34c inhibits osteoblast differentiation and increases osteoclastogenesis in vitro, contributes to aged-related osteoporosis in vivo [75] | C2C12 cells | |
| Notch2, Jag1 Runx-2 | Impedes osteoblast differentiation through inhibition of Runx2 protein expression [93] | MC3T3-E1 | ||
| miR-93 | Osterix/Sp7 | miR-93 reduces Sp7 protein expression and attenuates osteoblast mineralization [94] | primary mouse osteoblasts | |
| miR-101 | PLAP-1 | Targets and inhibits PLAP-1 transcription and suppresses the osteogenic differentiation process of PDLCs [82] | Primary human PDLC | |
| miR-103-3p | Cav1.2 protein | Inhibits MC3T3-E1 proliferation mainly by suppressing the expression of Cav1.2 protein [95] | MC3T3-E1 | |
| miR-126-3p | SOST | Inhibits SOST protein production and promotes the mineralization through WNT signaling [96] | TMOBb cells | |
| miR-127 | Inhibition of miR-127 could enhance osteoblastic differentiation, osteocyte-like morphological changes, and survival [20] | UAMS-32 cells MLO-Y4 cells |
||
| miRNA-132-3p | Ep300 | Decreases Ep300 protein expression, suppresses the activity and acetylation of Runx2, inhibits osteoblast differentiation and induces bone loss [16] | hind limb unloading rats; primary rat osteoblasts | |
| miR-133 | Runx-2 | Down-regulates Runx-2 expression, an inhibitor of bone remodeling [97] | C2C12 mesenchymal cells | |
| miR-135 | Smad5 | Down-regulates Smad5 expression, an inhibitor of bone remodeling [97] | C2C12 mesenchymal cells | |
| miR-136 | Upregulated in OVX rats, miR-136 precursor suppresses osteoblastic differentiation while its inhibitors enhance osteocyte-like morphological changes and survival [20] | UAMS-32 cells MLO-Y4 cells |
||
| miR-138 | FAK | miR-138 expression suppresses FAK translation and then suppression of MSC differentiation into osteoblasts and reduced the formation of aberrant bone in mice [98] | hMSCs | |
| miR-140 | HDAC4/ADA | Accumulates in cartilage and down-regulated in Osteoarthritis [99] | Mouse embryos | |
| MTS5 BMP-2 |
Induces osteogenic gene expression [100] | Primary chondrocytes | ||
| miR-141 | Dlx5 | miR-141 modulates BMP-2-induced pre-osteoblast differentiation through the repression of Dlx5 [101] | MC3T3-E1 cells | |
| miR-145-5p | Osterix/Sp7 | Inhibits the osteogenic differentiation of C2C12 and MC3T3-E1 cells and odontoblast differentiation during tooth development [102] | C2C12 cells, MC3T3-E1 cells; odontoblast | |
| miR-148b | miRNA-148b expression results in a rapid and robust induction of ALP activity and calcium deposition [17] | hMSCs | ||
| miR-155 | SMAD2 | Targets BMP-2 signaling and dampens TGFβ signaling, inhibits osteoclast differentiation and enhances osteocytes differentiation [103] | THP-1, HeLa cell, RAW264.7, KUSA-A1, hMSCs | |
| miR-182 | FoxO1 | Increases cell apoptosis and hinders osteoblast proliferation and differentiation by inhibiting the expression of FoxO1 [104] | C3H10T1/2; MC3T3-E1 | |
| miR-199a | BMP-2 Dlx5 |
miR-199a increases after BMP4 stimulation of human primary pulmonary artery smooth muscle cells (PASMCs) [105] | PASMCs MC3T3-E1 |
|
| miR-200a | miR-200a modulates BMP-2-induced pre-osteoblast differentiation through the translational repression of Dlx5 [101] | |||
| miR-200b | Zeb1, Zeb2 Smad2, Snail |
Induces cell migration and palatal fusion [106] Induces palatogenesis and palate fusion [107] |
Mouse palate Mammalian palate |
|
| miR-204 | Runx-2 | Inhibits osteoblastic differentiation and stimulates adipogenesis of mesenchymal progenitor cells [19] | ST2, C2C12, C3H10T1/2, hMSCs | |
| miR-206 | Connexin 43 | Inhibits osteogenesis in mice by targeting Cx43, and is upregulated in OVX rats [108] | transgenic mice with miR-206 | |
| miR-210 | ACVR1B | Positive regulator of osteoblastic differentiation by inhibiting the TGF-β/activin signaling pathway through inhibition of AcvR1b [109] | bone marrow derived ST2 stromal cells | |
| miR-211 | Runx-2 | Down-regulation of RUNX2 antagonizes osteogenesis and promotes adipogenesis [19] | BMSCs | |
| miR-214 | ATF4 | miR-214 directly targets ATF4 to inhibit osteoblast activity and inhibits bone formation in ovariectomized and hind limb-unloaded mice [110] | aged patients with fractures, OVX or hind limb mice | |
| miR-218 | Runx-2 | Decreases RUNX2 expression in undifferentiated PDLSC, DPSC, GSC, and BMSCs [111] | PDLSC, DPSC, GSC, BMSCs | |
| miR-223 | NFI-A; M-CSFR | miR-223 plays an essential role during osteoclast differentiation [112] | RAW 264.7 cells | |
| miR-320a | CTNNB1 | Regulates RUNX2 and LEPR, over-expressed in the osteoporotic samples and expressed in primary osteoblasts [113] | trabecular bone from osteoporotic women; Human osteoblast | |
| miR-378 | GalNT-7 | Upregulated in OVX rats and inhibits osteoblastic differentiation by modulating nephronectin expression [20] | OVX mice; MC3T3-E1, Hek293 cells | |
| miR-424-5p | miR-424-5p was found to be downregulated in tension force-induced osteogenesis in PDLCs and human mesenchymal stromal cells [14] | PDLC; hMSCs | ||
| miR-483-5p | IGF2 | Over-expressed in osteoporotic samples and expressed in primary osteoblasts [113] | trabecular bone from osteoporotic women; Human osteoblast | |
| miR-637 | Osterix/Sp7 | miR-637 is repressed during osteogenesis and increased during adipogenesis [114] | BM-MSCs lineage | |
| miR-2861 | HADC5 | Loss of miR-2861 leads to increased expression of the miR-2861 target HDAC5 (suppressor ofRUNX2) [115] | ST2 stromal cells | |
| Let-7 | HMGA2 | Let-7 represses HMGA2 expression, promotes osteogenesis and suppresses adipogenesis of MSCs in vitro, and promotes ectopic bone formation in vivo [116] | MSCs |
Fig. 2.
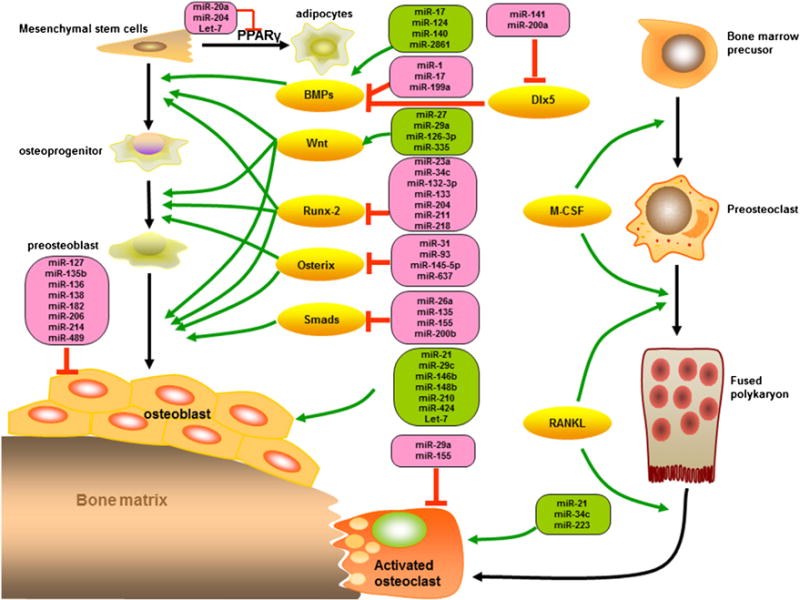
miRNAs regulate the key signal molecules during the osteoblast differentiation and osteoclastogenesis. PPARγ: peroxisome proliferator-activated receptor gamma; Dlx5: distal-less homeobox 5; BMPs: bone morphogenetic proteins; M-CSF: macrophage-colony stimulating factor; RANKL: receptor activator of NF-κB ligand.
MSCs are intensively studied because they exhibit unique biological properties in vivo that can be exploited for the treatment of many pathological conditions, most notably bone disease, and degenerative illnesses. MSCs are the main source of osteoblasts, chondrocytes, and adipocytes, all these phenotypes affects stabilize of bone microenvironment and bone health. MSCs could isolate from bone marrow, umbilical cord blood, muscle and adipose tissue etc., also miRNAs controls the differentiation of MSCs and then promise for the skeletal tissue related cell-based therapies. Hence the investigation of miRNA expression in osteogenic differentiation of MSCs and osteoblasts may offer opportunities for the clarification of osteogenesis. Down-regulate of miR-27a, miR-489 and up-regulate miR-148b are essential for regulating osteogenesis in human MSCs cells [17]. BMPs/Runx2 signaling plays a crucial role in the osteogenesis of MSCs, and Osterix which acts downstream to Runx2 is essential for embryonic osteoblast differentiation and bone formation. Whereas PPARγ, activated by C/EBPs, stimulates adipogenesis of MSCs and prohibits osteogenesis. miR-20a was proved as a positive regulator of bone formation, such as elevated BMPs, Runx2, Osterix, osteocalcin, and osteopontin, whereas decrease adipocyte markers PPARγ and osteoblast antagonist, Bambi and Crim1 [18]. MiR-204 and its homolog miR-211 also were reported as an important endogenous negative regulator of Runx2 in mesenchymal progenitor cell lines and bone marrow-derived mesenchymal stem cells (BM-MSCs) [19].
Ovariectomy (OVX)-induced bone loss is the gold standard to mimic postmenopausal osteoporosis (PMOP) and also in osteoporotic fracture researches. 8 miRNAs were identified (miR-127, −133a, −133a*, −133b, −136, −206, −378, −378*) upregulated but miR-204 was downregulated in OVX mice; they also confirmed that miR-127 and –136 as negative regulators for bone formation in UAMS-32 and MLO-Y4 cells in vitro [20]. Meanwhile, miR-21, miR-23a, miR-24, miR-25, miR-100 and miR-125b are upregulated in osteoporotic fracture patients in a clinical study [21]. Remarkably, although the regulated miRNAs in vitro, in mice or human, are not a coincidence, all studies confirmed that miRNAs involved in osteoporotic physiopathology.
Biomaterials have been widely used in several bone regeneration procedures during oral and orthopedic surgery, such as Bio-Oss (Geistlich) and Peptide-15 (P-15), miRNAs also were confirmed involve in these process. 9 miRNAs (miR-423, miR-492, miR-191, miR-23a, miR-377, miR-494, miR-214, miR-193b, miR-320) up-regulated and 4 miRNAs (miR-27a, miR-24, miR-188, let-7c) down-regulated when osteoblast-like cell line (MG63) exposed to Bio-Oss [22]. Peptide-15 (P-15), an analog of the cell-binding domain of collagen that can alter osteoblast activity to promote bone formation in vitro, up-regulated 11 miRNAs but down-regulated 6 miRNAs when supplemented into MG-63 cell line [23]. Although so many miRNAs have been identified related to osteoporosis, only miR-21, miR-133a and miR-146a can be detected in plasma. Lower miR-21 and higher miR-133a levels in plasma of osteopenia and osteoporosis than normal patients, and can be used as sensitive plasma biomarkers for clinical PMOP diagnosis [24].
4. H2S formation, oxidation, toxicity, and physiological functions
4.1. H2S formation, metabolism and its toxicity
Hydrogen sulfide (H2S) is a colorless, flammable and water-soluble gas characterized by a peculiar smell of rotten eggs. Its toxic actions have been well established long back, but it was proposed as an endogenously generated modulator until recent years. H2S have been known as the third ‘Gasotransmitter’ in addition to nitric oxide and carbon monoxide. Because H2S is unstable in solution and easily oxidized in the presence of oxygen, so almost 50% of H2S usually gets lost from open cell culture wells within 5 min and difficult to make the precise measurement of H2S concentration [25]. Endogenously, H2S is mainly produced from L-cysteine by these enzymes; cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE), cysteine aminotransferase (CAT) and 3-mercapto-pyruvate sulfurtransferase (3-MST) (in Fig. 3). CBS catalyzes the condensation of homocysteine (Hcy) and serine, giving rise to cystathionine while CSE catalyzes the conversion of cystathionine to cysteine (Cys). While CAT catalyzes the reaction between L-cysteine and α-ketoglutarate, leading to the synthesis of 3-mercaptopyruvate and L-glutamate, 3-MST transfers sulfur from 3-mercaptopyruvate to sulfurous acid, pyruvate, and thiosulfate. Subsequently, thiosulfate is reduced to H2S and glutathione disulfide in the presence of reduced glutathione [26].
Fig. 3.
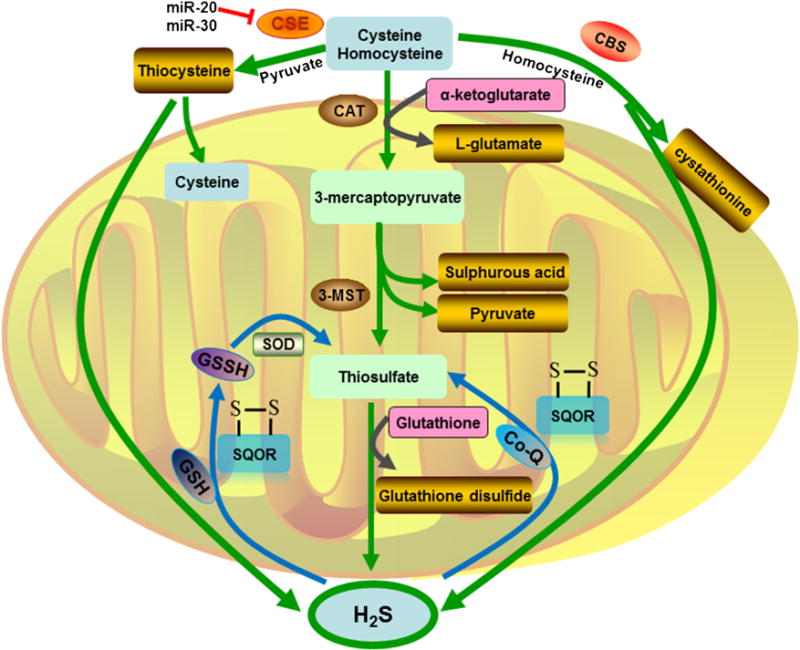
Sulfide metabolism and transsulfuration pathways. Cystathionine β (CBS) and cystathionine γ-lyase (CSE) govern the flow of sulfur through homocysteine, cystathionine, and cysteine for the generating H2S in the cytosol. While the third way for H2S production via cysteine aminotransferase (CAT) and 3-mercaptopyruvate sulfurtransferase (3-MST). sulfide quinone oxidoreductase (SQOR) oxidizes H2S to glutathione persulfide (GSSH) with GSH as the electron acceptor or directly to thiosulfate (SO32−) using co-enzyme Q (Co-Q) as the electron acceptor. Both the production and oxidation of H2S were processed in the mitochondrion.
Quinone oxidoreductase (SQOR) is an ancient flavoprotein of the disulfide oxidoreductase family that is present in nearly all domains of life (from archaea to humans but not plants), and classified into six types (types I–VI) based on its sequence and structural analyses [27]. SQOR proteins with a molecular mass of about 50 kDa, and associated with the prokaryotic cytoplasmic membrane or the mitochondrial inner membrane [28]. SQOR avidly consume sulfide as a fuel in the mitochondria isolated from mouse kidneys, liver, heart and brain [29,30], so it is a key enzyme during the metabolism of H2S to maintain the sulfide homeostasis and bioenergetics. SQOR in mitochondrial catalyzes a two-electron oxidation of H2S to sulfane sulfur using coenzyme Qas the electron acceptor and the sulfane sulfur produced in the SQOR reaction is a metabolic precursor of substrates for better-characterized down-stream enzymes, such as sulfite oxidase [31]. Human SQOR can use multiple thiophilic acceptors, including sulfide, sulfite, and glutathione, to form as products, hydrodisulfide, thiosulfate, and glutathione persulfide, respectively [32]. Moreover, H2S is also consumed by mitochondrial oxidation mediated by sulfide quinone reductase-like protein (SQRDL)-the vertebrate homolog of SQOR [29,30]. Jin et al. found that overexpression of the SQRDL I264T variant in the preosteoblast MC3T3-E1 cells significantly increased osteogenic differentiation and mineralization, whereas the SQRDL wild type had no effect or a negative effect on osteoblast differentiation. In addition, overexpression of the SQRDL I264T variant also did not affect osteoclastic differentiation of the primary-cultured monocytes. They believed the functional role of the H2S-catalyzing enzyme SQRDL I264T nsSNP may be a significant susceptibility variant for osteoporosis in Korean postmenopausal women that is involved in osteoblast differentiation [33].
H2S has long been considered as a toxic pollutant, but recent studies suggest H2S has multiple biological actions, although therapeutic and toxic effects of H2S depend on the inhaled concentrations. After exposure to 20–100 ppm of H2S, eye irritation, respiratory tract irritation and headache may occur, approximately 500ppm may cause unconsciousness, collapsing and pulmonary edema, higher than 700 ppm will cause loss of consciousness (syncope), paralysis of the respiratory system and may lead to death [34]. In general, H2S in blood and tissues is physiologically beneficial under 1 mM, however, 20 to 300mM in blood also been reported as a safety range [35].
4.2. H2S donors
Sodium hydrogen sulfide (NaHS) and sodium sulfide (Na2S) are the two most commonly used sources of H2S. They are easily soluble in water and cost efficient too, however, the limitation of NaHS and Na2S is that they have very short half-lives. There also have some slow and steady release sulfide donors such as GYY4137, SG1002, AP39 and S-propargyl-cysteine can be adopted as potential H2S supplement options in the future [36]. ATB-346 [2-(6-methoxynapthalen-2-yl)-propionic acid 4-thiocarbamoyl phenyl ester], an H2S-releasing donor with COX inhibition effects, offers superior anti-inflammatory and anti-nociceptive activity, meanwhile, ATB-346 also has better chondroprotective effects than naproxen but without detrimental effects [37]. Diallyl disulfide and diallyl trisulfide are organosulfur compounds found in members of allium species such as garlic (Allium sativum), onion (Allium cepa), chives (Allium schoenoprasum) etc., that act as H2S donors and also have antioxidant, anti-inflammatory, cytoprotective, and cardioprotectionproperties [38]. H2S-releasing diclofenac derivatives (ACS15 and ACS32) inhibit osteoclast formation and its activity subsequently prevents the process of osteolysis, can be a potential candidate for the clinical treatment of osteolytic bone disease [39]. These donors mentioned above with continuous, low level and steady H2S release, can be ideal as a physiological mediator and potentially therapeutic tool in future clinical studies.
4.3. Physiological functions of H2S
H2S can reduce the levels of IL-1β, IL-6 and tumor necrosis factor-alpha (TNF-α) in the serum of myocardial ischemia rat model, and also decrease the expression of intercellular adhesion molecule-1 (ICAM-1) and nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) to exert anti-inflammatory effects [40]. H2S also exerts chondroprotective effects by markedly decrease the level of IL-1 β and inhibit the activation of the extracellular signal-regulated kinase (ERK)/IκBa/NF-κB pathways [41]. Na2S supplementation significantly reduced the production of TNF-α and IL-8 in human U937 monocytes which induced by high glucose via activation of phosphatidylinositol-3,4,5-trisphosphate (PIP3)/AMP-activated protein kinase (AMPK)/peroxisome proliferator activated receptor-γ (PPAR-γ) signaling [42]. An anti-inflammatory consequence of exogenous H2S also been found in chemical hypoxia-stimulated PC12 cells, which is partially due to inhibition of reactive oxygen species (ROS)/p38MAPK/inducible nitric oxide synthase (iNOS) pathway [43].
Oxidative stress is a form of cellular injury caused by excessive formation of ROS, such as superoxide anion (O2−), hydroxyl radical (OH−), peroxynitrite (ONOO−) and hydrogen peroxide (H2O2), that leads to an imbalance between pro-oxidant and antioxidant systems. While H2S can act as an antioxidant gasotransmitter and thereby protectant against oxidative stress in smoking rats via PI3 K/Akt-dependent activation of Nrf2 signaling [44]. H2S could readily scavenge the production of free radicals (O2−, H2O2, and ONOO−) which induced by Hcy in rat VSMCs cultured in vitro [45] and also enhance AP-1 binding activity with the SIRT3 promoter then upregulate SIRT3 expression to reduce oxidant-provoked vascular endothelial dysfunction [46]. In additional, H2S inhibits mitochondrial ROS production via the sulfhydration of Cys-59 residue, which in turn prevents the phosphorylation of p66Shc and activation of mitochondrial redox signaling [47]. Collectively, these findings recommend that H2S is competent of preventing and scavenging the ROS and consequently strengthening the endogenous antioxidant system, while future studies still are required to promote the prospective therapeutic benefits of the antioxidant properties of H2S.
High salt and high glucose always induce cell apoptosis, while H2S can reverse these processes and protect the cell from apoptosis. High-salt treatment could increase generation of oxygen free radicals, decrease mitochondrial membrane potential, activate cytoplasmic caspase-9 and caspase-3, and then induced vascular endothelial cell (VEC) apoptosis, however, H2S markedly reversed oxidative stress and also mitochondria-related VEC apoptosis [48]. S-propargyl-cysteine (SPRC), a novel donor of H2S, activated Nrf2 via CSE and Akt pathways, and up-regulated expression of antioxidant enzyme superoxide dismutase (SOD), then extraordinarily attenuated ROS generation and apoptosis in H9C2 cells which induced by high glucose [49]. Diallyl trisulfide (another H2S donor) increased CSE expression and reduced apoptosis in H9C2 cells, via a mechanism involving IGF1R/pAkt signaling and modulation of ROS-mediated enzyme expression [38]. H2S exert an anti-apoptotic effects in the myocardium of smoking rats by inhibiting JNK and p38 MAPK pathways and activating PI3 K/Akt signaling [50], meanwhile, H2S attenuated p38 phosphorylation, decreased IL-6 secretion and showed protective effects against oxygen-glucose deprivation/reoxygenation (OGD/R)-induced cell death in PC12 cells [51].
H2S rapidly moves through cell membranes without involving any specific transporter/receptor and promotes a number of cellular signals that exert a classical pleiotropic physiological profile with anti-inflammatory, vasodilatory, angiogenic, cytoprotective, and anti-oxidative and anti-apoptotic action which we have mentioned above (shown in Fig. 4).
Fig. 4.
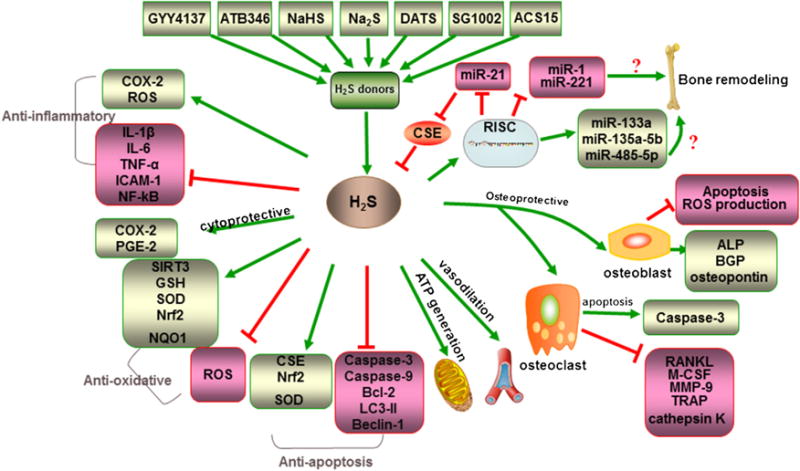
Physiological functions of H2S. Including anti-inflammatory, cytoprotective, anti-oxidative, anti-apoptosis, ATP generation, vasodilatation, and osteoprotective effects, even about crosstalk of miRNAs and H2S. During its osteoprotective processing, H2S stimulate the osteogenic differentiation markers (ALP, BGP, osteopontin) but inhibit osteoblast apoptosis, whereas, H2S could promote apoptosis of osteoclast, and inhibit osteoclast differentiation and maturation.
5. Osteoprotective effects of H2S
H2S as an endogenous gasotransmitter not only provides anti-inflammatory, anti-oxidative and anti-apoptotic effects but also associates closely with skeletal and bone development, so the osteoprotective effects of H2S have appealed many researchers’ attention in recent years. Oxidative damage is a significant contributor to the morphological and functional changes in the development of osteoporosis. S-DCF derivatives ACS15 and ACS32 as H2S donors could inhibit RANKL-induced osteoclast formation and resorption and caused caspase-3 activation and apoptosis in mature osteoclasts which depended on IKK/NFκB inhibition and then inhibit bone loss [39]. MMPs function is critical for bone development and regeneration, so alterations in MMPs function may also modify bone quality, for instance increasing of H2S (16 μM to 40 μM) could decrease the MMPs expression and osteoclasts activity [52]. H2S has been proved as a potential therapeutic reagents for the treatment of periodontal and inflammatory bone diseases, including prohibited cytotoxicity and osteoclastic differentiation in mouse bone marrow cells (such as decreasing RANKL, TRAP, M-CSF, MMP-9 and cathepsin K mRNA levels) and also recovered osteoblastic differentiation (including ALP, osteopontin, and osteocalcin, and mineralized nodule formation) in human periodontal ligament cell (hPDLC) model which stimulated by nicotine and periodontopathogens [53]. H2S always have duplex protective effects during the bone remodeling as mentioned above, not only inhibit the bone absorption of osteoclastic cells, but also make more bone formation of osteoblastic cells. Bone marrow mesenchymal stem cells (BM-MSCs) are the original source of osteoblasts for bone formation, while H2S deficiency causes decreased intracellular Ca2+ influx and then downregulates PKC/ERK-mediated Wnt/β-catenin signaling which controls osteogenic differentiation of BM-MSCs [54]. In hMSCs GYY4137 treatment has shown to increase murine osteo blastogenesis by H2S-induced activation of Wnt signaling, through increased production of the Wnt ligands (Wnt16, Wnt2b, Wnt6, and Wnt10b) in the bone marrow, thus inducing osteogenic differentiation in hMSCs [3]. H2S also increased the viability and reduced apoptosis of MC3T3-E1 osteoblastic cells caused by H2O2 and also stimulated osteoblast differentiation by enhancing both transcription and activity of alkaline phosphatase and osteocalcin, it demonstrated that H2S protects osteoblastic cells against oxidative stress via an MAPK (p38 and ERK1/2)-dependent mechanism [55]. H2S inhibit dexamethasone (Dex)-induced viability reduction and cell apoptosis in MC3T3-E1 cells, via activated AMP-activated protein kinase (AMPK) signaling and inhibited ROS production [56].
An H2S-releasing derivative like ATB-346 treatment resulted in significant inhibition of bone defects and other histological characteristics [57]. OVX are known as a golden standard to simulate the PMOP and always accompanied with a lower H2S level, while GYY4137 could normalize serum H2S and increase bone formation and then completely prevented OVX-induced trabecular bone loss. Although many studies have proved the osteoprotective effects of H2S, however, there have some contradictory results regarding the calcification of osteoblasts and bone resorption of osteoclasts. Three different doses (14, 28 and 70 μM/kg/day) of NaHS were administered systemically but shown no effects on alveolar bone loss in a rat model [58]. NaHS application caused a transient increase of osteoclast proliferation and differentiation with up-regulation of RANKL, TNF-α, and NF-kB expression, suggesting that H2S may contribute to alveolar bone resorption through RANKL expression [59], and similar results also were obtained in GYY4137 treatment [60]. In addition, H2S also was found to inhibit calcium deposition in the extracellular matrix and to suppress the induction of the genes (alkaline phosphatase, osteocalcin, and Cbfa1) involved in osteoblastic transformation and mineralization of vascular smooth muscle cells [61].
Osteoarthritis (OA) is another kind of bone disease with mechanical abnormalities by involving degradation of joints, including articular cartilage and subchondral bone. While H2S exhibited chondroprotective effects in clinical patients via activated the extracellular signal-regulated kinase (ERK)/IκBa/NF-κB pathway and MAPK and PI3 K/Akt pathways, meanwhile, H2S inhibited the expression of COX-2, iNOS, IL-6, IL-8 and reduce the production of MMPs (MMP-2, MMP-13, and MMP-14), PGE2 and NO, then relieve symptoms of patients who suffering from OA [41].
6. Effects of H2S on miRNA expression
Although the physiological functions of H2S have been reported extensively in the literature, only a few publications document that miRNAs may regulate enzymes that control H2S biosynthesis and H2S also have a regulatory effect on miRNAs.
6.1. miRNAs regulate H2S biosynthesis
Gene expression of CSE which produces endogenous H2S is controlled by miR-21, miR-22 and miR-30 [62]. Upregulated miR-30 family members downregulate the CSE level in the infarct and border zones following myocardial infarction (MI) in rat hearts [63]. Silencing of miR-30 in vivo confirmed that miR-30 targets CSE and inhibition of miR-30 could protect the MI heart by upregulating CSE expression [63]. We know that estrogen (E2) can rebalance the bone formation and bone resorption, and then inhibit the bone loss in PMOP patient, it was called hormone replacement therapy (HRT), meanwhile, Estrogen also induces CSE expression through estrogen receptor alpha (ERa), which upregulates transcription of specificity protein-1 (SP1), then binds to the promoter region of the CSE gene to stimulate CSE transcription and enhance H2S biosynthesis [64]. In the same study, deficient estrogen increased levels of miR-22 which inhibit the transcription of SP1 and CSE, then decrease the H2S level, but all these effects were normalized by E2 supplement and miR-22 mimic inhibition [64]. Although there have no direct study reported miR-21, miR-22 and miR-30 change H2S level in bone-related research, the above results already suggested that all these three miRNAs downregulate CSE expression to decrease H2S production, could be the novel targets for bone disease treatment.
6.2. H2S regulates miRNAs
miRNAs can regulate the biosynthesis of H2S, and H2S also can influence the expression of miRNAs. It was demonstrated that NaHS and Na2S released H2S can up-regulate miR-133a level in cultured cardiomyocytes in vitro and exhibited cardioprotective effects in cardiomyocytes hypertrophy [65,66]. These studies suggest that H2S supplementation could provide insight on H2S-mediated cardioprotection in the failing heart. miR-1 have been reported as a pro-apoptotic marker and directly suppresses anti-apoptotic proteins including Bcl-2, HSP 60, and HSP 70, however, H2S decreases miR-1 mediated apoptosis in myocardial I/R [67]. miR-221 always is upregulated in patients with coronary artery disease, while DATS, as an H2S donor, subsequently downregulates miR-221 in a dose-dependent manner, so H2S supplementation could be a potential therapeutic strategy for coronary artery diseases by reducing miR-221 levels [68]. Rho-associated protein kinase 2 (ROCK2) is activated to promote neurodegeneration during the progression of Parkinson’s disease, while H2S increase the expression of miR-135a-5p which targeted the 3′-UTR of ROCK2 mRNA to inhibit its translation in neuronal cells, and then played neuroprotective effects in the neural injury of Parkinson’s disease [69]. Another study also confirmed the neuroprotective of H2S, while the molecular mechanism is that H2S enhance the expression of miR-485-5p which can prohibit the level of tumor necrosis factor receptor type 1-associated DEATH domain protein (TRADD), thus protecting against the apoptosis of neuronal cells [70]. Anyway, above studies have proved that H2S also regulated the expression of miRNAs and then exert its cardioprotective, anti-apoptotic and neuroprotective effects in vitro and in vivo.
6.3. Cross-talk of H2S and miRNA
Inter-regulation of miRNAs and H2S opens a new avenue for exploring the H2S-microRNA cross-talk in many kinds of diseases. An example of H2S-miRNA cross-talk is that H2S downregulates miR-21 to mitigate phenylephrine-induced cardiomyocyte hypertrophy [66] and miR-21 targets SP1 to decrease CSE transcription and H2S production [71]. Although some studies represented that miR-21 may have a negative impact as it reduces H2S levels, the beneficial effects of miR-21 were also observed in cardiac cells, including inhibition of apoptosis and protection of cardiomyocytes from H2O2 damage [72]. Na2S, an H2S donor, induced miR-21 in primary cardiomyocytes and heart tissue, attenuated inflammation activity, inhibited apoptosis and necrosis in cardiomyocytes in vitro. It also reduced myocardial infarct size after ischemia/reperfusion (I/R) injury in vivo [73]. Conversely, these protective effects were lacking after silencing miR-21 expression [73]. These findings put forward that the cross-talk of H2S-miRNA may diverge and have diverse roles in different diseases.
7. Discussion and future research directions
H2S and miRNA are two relatively new research areas during the bone research, but the molecular mechanism still not well clarified. Major view of researchers is that H2S have osteoprotective effects during the bone remodeling, including decreased MMPs level in bone tissue homogenates [52] and inhibited bone loss in OVX-induced osteoporotic mice [3], while H2S deficiency can cause aberrant intracellular Ca2+ influx and decreased Ca2+ flux downregulates PKC/ERK-mediated Wnt/β-catenin signaling which controls osteogenic differentiation of BM-MSCs [54]. However, contrast results also represented that H2S supplementation increased alveolar bone resorption through RANKL expression and osteoclast differentiation, so the detailed mechanism of H2S is still unknown for such contrary results in bone remodeling. Whether the in conformity results related to the difference in bone type (long bone and alveolar bone) or difference in animal model and cells still needs to be more explored.
In addition, our previous research findings have also proven that Hcy, a sulfur-containing amino acid and also the H2S precursor, can induce bone loss, but in most studies, H2S shown prohibits bone loss. It has been already well demonstrated that Hcy and H2S play opposite roles during endothelial dysfunction and hepatic microcirculation diseases, so how about the metabolic cycle of Hcy and H2S working during bone remodeling is still unclear? The exact molecular mechanisms remain uncertain and need to be continued study. MiR-21, miR-22, and miR-30 are demonstrated to modulate CSE gene expression and then regulate H2S production, there is still a lack of knowledge as for whether other miRNAs are involved in H2S modulations. Meanwhile, we have summarized that H2S regulated the miRNAs in Part 6.2, such as miR-221, miR-1, miR-133a, miRNA-135a-5p and miRNA-485-5p, but all these results were obtained from other disease research, whether these miRNAs related to bone disease? Some reports also found that downregulation of miR-221 can trigger osteogenic differentiation of in human stem cells [74], downregulation of miR-1 during BMP-2-induced osteogenesis [75], miR-133a was upregulated in osteoblast-like periodontal ligament stem cells and inhibit osteoclastogenesis in circulating monocytes [76], miRNA-135a-5p and miRNA-485-5p also involved in the process of bone remodeling. However, whether H2S regulate above miRNAs and then maintain the bone health still need more evidence.
In summary, we have reviewed that many kinds of miRNAs are involved in the process of bone remodeling, have different targets and that H2S inhibits bone loss in OVX animal model. Despite this, there is no study elucidating why H2S inhibits bone loss and what kinds of miRNAs are mediating this process. We have summarized miR-17 and miR-140 target BMP-2, miR-26a and miR-155 target SMADs, miR-23a, miR-34c and miR-204 target Runx-2 while miR-31 and miR-93 target Osterix, all these miRNAs found to regulate one of the most significant pathways during bone development, the BMP-2/SMADs/Runx-2/Osterix pathway; although H2S already been proved can increase the expression of Runx-2 and Osterix [3,55], whether H2S is involved in this process or these miRNAs regulate H2S production and then modulate bone remodeling still remains ambiguous and needs additional exploration. Lastly, two upstream regulators of miRNAs, long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs) are emerging and bring a great prospect in the field of bone research studies. So potential research, exploring the cross-talk among lncRNAs, circRNAs, miRNAs, and H2S could elucidate novel regulatory mechanisms for bone turnover and thereby provide new strategies for the treatment of bone disorders.
Acknowledgments
The authors are grateful to Dr. Jyoti Behera, and Dr. Mohammed Nuru, for their help in critical English editing. Financial support from National Institute of health grant: AR-067667 and HL-107640-N. Tyagi and S.C. Tyagi is greatly acknowledged.
Footnotes
Conflict of interest
The authors have declared no conflict of interest.
References
- 1.Larsson S, Fazzalari NL. Anti-osteoporosis therapy and fracture healing. Arch Orthop Trauma Surg. 2014;134(2):291–29. doi: 10.1007/s00402-012-1558-8. [DOI] [PubMed] [Google Scholar]
- 2.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322(5901):587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grassi F, Malik Tyagi A, Calvert JW, Gambari L, Walker LD, Yu M, Robinson J, Li JY, Lisignoli G, Vaccaro C, Adams J, Pacifici R. Hydrogen Sulfide Is a Novel Regulator of Bone Formation Implicated in the Bone Loss Induced by Estrogen Deficiency. J Bone Miner Res: Official J Am Soc Bone Miner Res. 2016;31(5):949–963. doi: 10.1002/jbmr.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacool Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O’Brien CA, Manolagas SC. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol. 2010;24(2):323–334. doi: 10.1210/me.2009-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim YK, Kim B, Kim VN. Re-evaluation of the roles of DROSHA, export in 5, and DICER in microRNA biogenesis. Proc Natl Acad Sci U S A. 2016;113(13):E1881–9. doi: 10.1073/pnas.1602532113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J, Zhao J, Zhu W, Qi D, Wang L, Sun J, Wang B, Ma X, Dai Q, Yu X. MicroRNA biogenesis pathway genes polymorphisms and cancer risk: a systematic review and meta-analysis. PeerJ. 2016;4:e2706. doi: 10.7717/peerj.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin EC, Qureshi AT, Dasa V, Freitas MA, Gimble JM, Davis TA. MicroRNA regulation of stem cell differentiation and diseases of the bone and adipose tissue: Perspectives on miRNA biogenesis and cellular transcriptome. Biochimie. 2016;124:98–111. doi: 10.1016/j.biochi.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Yao S. MicroRNA biogenesis and their functions in regulating stem cell potency and differentiation. Biol Proced Online. 2016;18:8. doi: 10.1186/s12575-016-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felekkis K, Touvana E, Stefanou C, Deltas C. microRNAs: a newly described class of encoded molecules that play a role in health and disease. Hippokratia. 2010;14(4):236–240. [PMC free article] [PubMed] [Google Scholar]
- 11.Verma IM. Medicine. Gene therapy that works. Science. 2013;341(6148):853–855. doi: 10.1126/science.1242551. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Li Y, Chen YE, Chen J, Ma PX. Cell-free 3D scaffold with two-stage delivery of miRNA-26a to regenerate critical-sized bone defects. Nat Commun. 2016;7:10376. doi: 10.1038/ncomms10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh JS, Lee JY, Choi YS, Chung CP, Park YJ. Peptide-mediated intracellular delivery of miRNA-29b for osteogenic stem cell differentiation. Biomaterials. 2013;34(17):4347–4359. doi: 10.1016/j.biomaterials.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 14.Chang M, Lin H, Luo M, Wang J, Han G. Integrated miRNA and mRNA expression profiling of tension force-induced bone formation in periodontal ligament cells, In vitro cellular & developmental biology. Animal. 2015;51(8):797–80. doi: 10.1007/s11626-015-9892-0. [DOI] [PubMed] [Google Scholar]
- 15.Mai ZH, Peng ZL, Zhang JL, Chen L, Liang HY, Cai B, Ai H. miRNA expression profile during fluid shear stress-induced osteogenic differentiation in MC3T3-E1 cells. Chin Med J (Engl) 2013;126(8):1544–1550. [PubMed] [Google Scholar]
- 16.Hu Z, Wang Y, Sun Z, Wang H, Zhou H, Zhang L, Zhang S, Cao X. miRNA-132-3p inhibits osteoblast differentiation by targeting Ep300 in simulated microgravity. Sci Rep. 2015;5:18655. doi: 10.1038/srep18655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoolmeesters A, Eklund T, Leake D, Vermeulen A, Smith Q, Force Aldred S, Fedorov Y. Functional profiling reveals critical role for miRNA in differentiation of human mesenchymal stem cells. PLoS One. 2009;4(5):e5605. doi: 10.1371/journal.pone.0005605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang JF, Fu WM, He ML, Xie WD, Lv Q, Wan G, Li G, Wang H, Lu G, Hu X, Jiang S, Li JN, Lin MC, Zhang YO, Kung HF. MiRNA-20a promotes osteogenic differentiation of human mesenchymal stem cells by coregulating BMP signaling. RNA Biol. 2011;8(5):829–838. doi: 10.4161/rna.8.5.16043. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Zhao L, Xing L, Chen D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010;28(2):357–364. doi: 10.1002/stem.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An JH, Ohn JH, Song JA, Yang JY, Park H, Choi HJ, Kim SW, Kim SY, Park WY, Shin CS. Changes of microRNA profile and microRNA-mRNA regulatory network in bones of ovariectomized mice. J Bone Miner Res. 2014;29(3):644–656. doi: 10.1002/jbmr.2060. [DOI] [PubMed] [Google Scholar]
- 21.Seeliger C, Karpinski K, Haug AT, Vester H, Schmitt A, Bauer JS, van Griensven M. Five freely circulating miRNAs and bone tissue miRNAs are associated with osteoporotic fractures. J Bone Miner Res. 2014;29(8):1718–1728. doi: 10.1002/jbmr.2175. [DOI] [PubMed] [Google Scholar]
- 22.Palmieri A, Pezzetti F, Brunelli G, Martinelli M, Lo Muzio L, Scarano A, Scapoli L, Arlotti M, Guerzoni L, Carinci F. Anorganic bovine bone (Bio-Oss) regulates miRNA of osteoblast-like cells. Int J Periodontics Restorative Dent. 2010;30(1):83–87. [PubMed] [Google Scholar]
- 23.Palmieri A, Pezzetti F, Brunelli G, Martinelli M, Lo Muzio L, Scarano A, Degidi M, Piattelli A, Carinci F. Peptide-15 changes miRNA expression in osteoblast-like cells. Implant Dent. 2008;17(1):100–108. doi: 10.1097/ID.0b013e318166d182. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Wang Z, Fu Q, Zhang J. Plasma miRNA levels correlate with sensitivity to bone mineral density in postmenopausal osteoporosis patients. Biomarkers. 2014;19(7):553–556. doi: 10.3109/1354750X.2014.935957. [DOI] [PubMed] [Google Scholar]
- 25.DeLeon ER, Stoy GF, Olson KR. Passive loss of hydrogen sulfide in biological experiments. Anal Biochem. 2012;421(1):203–207. doi: 10.1016/j.ab.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids. 2011;41(1):113–121. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- 27.Shuman KE, Hanson TE. A sulfide:quinone oxidoreductase from Chlorobaculum tepidum displays unusual kinetic properties. FEMS Microbiol Lett. 2016;363(12) doi: 10.1093/femsle/fnw100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lencina AM, Ding Z, Schurig-Briccio LA, Gennis RB. Characterization of the Type III sulfide:quinone oxidoreductase from Caldivirga maquilingensis and its membrane binding. Biochim Biophys Acta. 2013;1827(3):266–275. doi: 10.1016/j.bbabio.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lagoutte E, Mimoun S, Andriamihaja M, Chaumontet C, Blachier F, Bouillaud F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim Biophys Acta. 2010;1797(8):1500–1511. doi: 10.1016/j.bbabio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Ackermann M, Kubitza M, Hauska G, Pina AL. The vertebrate homologue of sulfide-quinone reductase in mammalian mitochondria. Cell Tissue Res. 2014;358(3):779–792. doi: 10.1007/s00441-014-1983-9. [DOI] [PubMed] [Google Scholar]
- 31.Jackson MR, Melideo SL, Jorns MS. Human sulfide:quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry. 2012;51(34):6804–6815. doi: 10.1021/bi300778t. [DOI] [PubMed] [Google Scholar]
- 32.Mishanina TV, Yadav PK, Ballou DP, Banerjee R. Transient kinetic analysis of hydrogen sulfide oxidation catalyzed by human sulfide quinone oxidoreductase. J Biol Chem. 2015;290(41):25072–25080. doi: 10.1074/jbc.M115.682369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin HS, Kim J, Park S, Park E, Kim BY, Choi VN, Yoo YH, Kim BT, Jeong SY. Association of the I264T variant in the sulfide quinone reductase-like (SQRDL) gene with osteoporosis in Korean postmenopausal women. PLoS One. 2015;10(8):e0135285. doi: 10.1371/journal.pone.0135285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dongo E, Hornyak I, Benko Z, Kiss L. The cardioprotective potential of hydrogen sulfide in myocardial ischemia/reperfusion injury (review) Acta Physiol Hung. 2011;98(4):369–381. doi: 10.1556/APhysiol.98.2011.4.1. [DOI] [PubMed] [Google Scholar]
- 35.Kashfi K, Olson KR. Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem Pharmacol. 2013;85(5):689–703. doi: 10.1016/j.bcp.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hackfort BT, Mishra PK. Emerging role of hydrogen sulfide-microRNA cross-talk in cardiovasculardiseases. American J Physiol Heart Circ Physiol ( 2016 doi: 10.1152/ajpheart.00660.2015. ajpheart 00660 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dief AE, Mostafa DK, Sharara GM, Zeitoun TH. Hydrogen sulfide releasing naproxen offers better anti-inflammatory and chondroprotective effect relative to naproxen in a rat model of zymosan induced arthritis. Eur Rev Med Pharmacol Sci. 2015;19(8):1537–1546. [PubMed] [Google Scholar]
- 38.Tsai CY, Wen SY, Shibu MA, Yang YC, Peng H, Wang B, Wei YM, Chang HY, Lee CY, Huang CY, Kuo WW. Diallyl trisulfide protects against high glucose-induced cardiac apoptosis by stimulating the production of cystathionine gamma-lyase-derived hydrogen sulfide. Int J Cardiol. 2015;195:300–310. doi: 10.1016/j.ijcard.2015.05.111. [DOI] [PubMed] [Google Scholar]
- 39.Frantzias J, Logan JG, Mollat P, Sparatore A, Del Soldato P, Ralston SH, Idris AI. Hydrogen sulphide-releasing diclofenac derivatives inhibit breast cancer-induced osteoclastogenesis in vitro and prevent osteolysis ex vivo. Br J Pharmacol. 2012;165(6):1914–1925. doi: 10.1111/j.1476-5381.2011.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu F, Liu GJ, Liu N, Zhang G, Zhang JX, Li LF. Effect of hydrogen sulfide on inflammatory cytokines in acute myocardial ischemia injury in rats. Exp Ther Med. 2015;9(3):1068–1074. doi: 10.3892/etm.2015.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ha C, Tian S, Sun K, Wang D, Lv J, Wang Y. Hydrogen sulfide attenuates IL-1beta-induced inflammatory signaling and dysfunction of osteoarthritic chondrocytes. Int J Mol Med. 2015;35(6):1657–1666. doi: 10.3892/ijmm.2015.2183. [DOI] [PubMed] [Google Scholar]
- 42.Manna P, Jain SK. L-cysteine and hydrogen sulfide increase PIP3 and AMPK/PPARgamma expression and decrease ROS and vascular inflammation markers in high glucose treated human U937 monocytes. J Cell Biochem. 2013;114(10):2334–2345. doi: 10.1002/jcb.24578. [DOI] [PubMed] [Google Scholar]
- 43.Yu XH, Cui LB, Wu K, Zheng XL, Cayabyab FS, Chen ZW, Tang CK. Hydrogen sulfide as a potent cardiovascular protective agent. Clin Chim Acta. 2014;437:78–87. doi: 10.1016/j.cca.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Zhou X, Zhao L, Mao J, Huang J, Chen J. Antioxidant effects of hydrogen sulfide on left ventricular remodeling in smoking rats are mediated via PI3 K/Akt-dependent activation of Nrf2. Toxicol Sci. 2015;144(1):197–203. doi: 10.1093/toxsci/kfu272. [DOI] [PubMed] [Google Scholar]
- 45.Yan SK, Chang T, Wang H, Wu L, Wang R, Meng QH. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;351(2):485–49. doi: 10.1016/j.bbrc.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 46.Xie L, Feng H, Li S, Meng G, Liu S, Tang X, Ma Y, Han Y, Xiao Y, Gu Y, Shao Y, Park CM, Xian M, Huang Y, Ferro A, Wang R, Moore PK, Wang H, Ji Y. SIRT3 mediates the antioxidant effect of hydrogen sulfide in endothelial cells. Antioxid Redox Signaling. 2016;24(6):329–343. doi: 10.1089/ars.2015.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie ZZ, Shi MM, Xie L, Wu ZY, Li G, Hua F, Bian JS. Sulfhydration of p66Shc at cysteine59 mediates the antioxidant effect of hydrogen sulfide. Antioxid Redox Signaling. 2014;21(18):2531–2542. doi: 10.1089/ars.2013.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zong Y, Huang Y, Chen S, Zhu M, Chen Q, Feng S, Sun Y, Zhang Q, Tang C, Du J, Jin H. Downregulation of endogenous hydrogen sulfide pathway is involved in mitochondrion-related endothelial cell apoptosis induced by high salt. Oxid Med Cell Longevity. 2015;2015:754670. doi: 10.1155/2015/754670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang H, Mao Y, Tan B, Luo S, Zhu Y. The protective effects of endogenous hydrogen sulfide modulator, S-propargyl-cysteine, on high glucose-induced apoptosis in cardiomyocytes: a novel mechanism mediated by the activation of Nrf2. Eur J Pharmacol. 2015;761:135–143. doi: 10.1016/j.ejphar.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Zhou X, An G, Chen J. Hydrogen sulfide improves left ventricular function in smoking rats via regulation of apoptosis and autophagy. Apoptosis. 2014;19(6):998–1005. doi: 10.1007/s10495-014-0978-z. [DOI] [PubMed] [Google Scholar]
- 51.Li C, Liu Y, Tang P, Liu P, Hou C, Zhang X, Chen L, Zhang L, Gu C. Hydrogen sulfide prevents OGD/R-induced apoptosis by suppressing the phosphorylation of p38 and secretion of IL-6 in PC12 cells. Neuroreport. 2016;27(4):230–234. doi: 10.1097/WNR.0000000000000522. [DOI] [PubMed] [Google Scholar]
- 52.Vacek TP, Qipshidze N, Tyagi SC. Hydrogen sulfide and sodium nitroprusside compete to activate/deactivate MMPs in bone tissue homogenates. Vasc Health Risk Manage. 2013;9:117–123. doi: 10.2147/VHRM.S39508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee SK, Chung JH, Choi SC, Auh QS, Lee YM, Lee SI, Kim EC. Sodium hydrogen sulfide inhibits nicotine and lipopolysaccharide-induced osteoclastic differentiation and reversed osteoblastic differentiation in human periodontal ligament cells. J Cell Biochem. 2013;114(5):1183–1193. doi: 10.1002/jcb.24461. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y, Yang R, Liu X, Zhou Y, Qu C, Kikuiri T, Wang S, Zandi E, Du J, Ambudkar IS, Shi S. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca(2+) channel sulfhydration. Cell Stem Cell. 2014;15(1):66–78. doi: 10.1016/j.stem.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu ZS, Wang XY, Xiao DM, Hu LF, Lu M, Wu ZY, Bian JS. Hydrogen sulfide protects MC3T3-E1 osteoblastic cells against H2O2-induced oxidative damage-implications for the treatment of osteoporosis. Free Radic Biol Med. 2011;50(10):1314–1323. doi: 10.1016/j.freeradbiomed.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 56.Yang M, Huang Y, Chen J, Chen YL, Ma JJ, Shi PH. Activation of AMPK participates hydrogen sulfide-induced cyto-protective effect against dexamethasone in osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun. 2014;454(1):42–47. doi: 10.1016/j.bbrc.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 57.Herrera BS, Coimbra LS, da Silva AR, Teixeira SA, Costa SK, Wallace JL, Spolidorio LC, Muscara MN. The H2S-releasing naproxen derivativeATB-346, inhibits alveolar bone loss and inflammation in rats with ligature-induced periodontitis. Med Gas Res. 2015;5:4. doi: 10.1186/s13618-015-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toker H, Balci Yuce H, Goze F, Ozdemir H, Akpinar A, Bostanci V. The effects of hydrogen sulphide on alveolar bone loss in periodontitis. Minerva Stomatol. 2014;63(4):103–110. [PubMed] [Google Scholar]
- 59.Irie K, Ekuni D, Yamamoto T, Morita M, Yaegaki K, Ii H, Imai T. A single application of hydrogen sulphide induces a transient osteoclast differentiation with RANKL expression in the rat model. Arch Oral Biol. 2009;54(8):723–729. doi: 10.1016/j.archoralbio.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 60.Kurabayashi M. Hydrogen sulfide: a new regulator of osteoclastogenesis? Arteriosclerosis Thrombosis Vasc Biol. 2014;34(3):471–473. doi: 10.1161/ATVBAHA.114.303072. [DOI] [PubMed] [Google Scholar]
- 61.Zavaczki E, Jeney V, Agarwal A, Zarjou A, Oros M, Katko M, Varga Z, Balla G, Balla J. Hydrogen sulfide inhibits the calcification and osteoblastic differentiation of vascular smooth muscle cells. Kidney Int. 2011;80(7):731–739. doi: 10.1038/ki.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hackfort BT, Mishra PK. Emerging role of hydrogen sulfide-microRNA crosstalk in cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2016;310(7):H802–12. doi: 10.1152/ajpheart.00660.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen Y, Shen Z, Miao L, Xin X, Lin S, Zhu Y, Guo W, Zhu YZ. miRNA-30 family inhibition protects against cardiac ischemic injury by regulating cystathionine-gamma-lyase expression. Antioxid Redox Signaling. 2015;22(3):224–240. doi: 10.1089/ars.2014.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L, Tang ZP, Zhao W, Cong BH, Lu JQ, Tang XL, Li XH, Zhu XY, Ni X. MiR-22/Sp-1 links estrogens with the up-regulation of cystathionine gamma-lyase in myocardium, which contributes to estrogenic cardioprotection against oxidative stress. Endocrinology. 2015;156(6):2124–2137. doi: 10.1210/en.2014-1362. [DOI] [PubMed] [Google Scholar]
- 65.Kesherwani V, Nandi SS, Sharawat SK, Shahshahan HR, Mishra PK. Hydrogen sulfide mitigates homocysteine-mediated pathological remodeling by inducing miR-133a in cardiomyocytes. Mol Cell Biochem. 2015;404(1–2):241–250. doi: 10.1007/s11010-015-2383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu J, Hao DD, Zhang JS, Zhu YC. Hydrogen sulphide inhibits cardiomyocyte hypertrophy by up-regulating miR-133a. Biochem Biophys Res Commun. 2011;413(2):342–347. doi: 10.1016/j.bbrc.2011.08.101. [DOI] [PubMed] [Google Scholar]
- 67.Kang B, Hong J, Xiao J, Zhu X, Ni X, Zhang Y, He B, Wang Z. Involvement of miR-1 in the protective effect of hydrogen sulfide against cardiomyocyte apoptosis induced by ischemia/reperfusion. Mol Biol Rep. 2014;41(10):6845–6853. doi: 10.1007/s11033-014-3570-2. [DOI] [PubMed] [Google Scholar]
- 68.Chiang EP, Chiu SC, Pai MH, Wang YC, Wang FY, Kuo YH, Tang FY. Organosulfur garlic compounds induce neovasculogenesis in human endothelial progenitor cells through a modulation of MicroRNA 221 and the PI3-K/Akt signaling pathways. J Agric Food Chem. 2013;61(20):4839–4849. doi: 10.1021/jf304951p. [DOI] [PubMed] [Google Scholar]
- 69.Liu Y, Liao S, Quan H, Lin Y, Li J, Yang Q. Involvement of microRNA-135a-5p in the protective effects of hydrogen sulfide against Parkinson’s disease. Cell Physiol Biochem. 2016;40(1–2):18–26. doi: 10.1159/000452521. [DOI] [PubMed] [Google Scholar]
- 70.Chen Z, Zhang Z, Zhang D, Li H, Sun Z. Hydrogen sulfide protects against TNF-alpha induced neuronal cell apoptosis through miR-485-5p/TRADD signaling. Biochem Biophys Res Commun. 2016;478(3):1304–1309. doi: 10.1016/j.bbrc.2016.08.116. [DOI] [PubMed] [Google Scholar]
- 71.Yang G, Pei Y, Cao Q, Wang R. MicroRNA-21 represses human cystathionine gamma-lyase expression by targeting at specificity protein-1 in smooth muscle cells. J Cell Physiol. 2012;227(9):3192–3200. doi: 10.1002/jcp.24006. [DOI] [PubMed] [Google Scholar]
- 72.Qin Y, Yu Y, Dong H, Bian X, Guo X, Dong S. MicroRNA 21 inhibits left ventricular remodeling in the early phase of rat model with ischemia-reperfusion injury by suppressing cell apoptosis. Int J Med Sci. 2012;9(6):413–423. doi: 10.7150/ijms.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Toldo S, Das A, Mezzaroma E, Chau VQ, Marchetti C, Durrant D, Samidurai A, Van Tassell BW, Yin C, Ockaili RA, Vigneshwar N, Mukhopadhyay ND, Kukreja RC, Abbate A, Salloum FN. Induction of microRNA-21 with exogenous hydrogen sulfide attenuates myocardial ischemic and inflammatory injury in mice. Circ Cardiovasc Genet. 2014;7(3):311–320. doi: 10.1161/CIRCGENETICS.113.000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bakhshandeh B, Hafizi M, Ghaemi N, Soleimani M. Down-regulation of miRNA-221 triggers osteogenic differentiation in human stem cells. Biotechnol Lett. 2012;34(8):1579–1587. doi: 10.1007/s10529-012-0934-3. [DOI] [PubMed] [Google Scholar]
- 75.Bae Y, Yang T, Zeng HC, Campeau PM, Chen Y, Bertin T, Dawson BC, Munivez E, Tao J, Lee BH. miRNA-34c regulates Notch signaling during bone development. Hum Mol Genet. 2012;21(13):2991–3000. doi: 10.1093/hmg/dds129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Li L, Moore BT, Peng XH, Fang X, Lappe JM, Recker RR, Xiao P. MiR-133a in human circulating monocytes: a potential biomarker associated with postmenopausal osteoporosis. PLoS One. 2012;7(4):e34641. doi: 10.1371/journal.pone.0034641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Y, Liu W, Hu C, Xue Z, Wang G, Ding B, Luo H, Tang L, Kong X, Chen X, Liu N, Ding Y, Jin Y. MiR-17 modulates osteogenic differentiation through a coherent feed-forward loop in mesenchymal stem cells isolated from periodontal ligaments of patients with periodontitis. Stem cells. 2011;29(11):1804–1816. doi: 10.1002/stem.728. [DOI] [PubMed] [Google Scholar]
- 78.Li L, Shi JY, Zhu GQ, Shi B. MiR-17-92 cluster regulates cell proliferation and collagen synthesis by targeting TGFB pathway in mouse palatal mesenchymal cells. J Cell Biochem. 2012;113(4):1235–1244. doi: 10.1002/jcb.23457. [DOI] [PubMed] [Google Scholar]
- 79.Yang N, Wang G, Hu C, Shi Y, Liao L, Shi S, Cai Y, Cheng S, Wang X, Liu Y, Tang L, Ding Y, Jin Y. Tumor necrosis factor alpha suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner Res. 2013;28(3):559–573. doi: 10.1002/jbmr.1798. [DOI] [PubMed] [Google Scholar]
- 80.Qin W, Zhao B, Shi Y, Yao C, Jin L, Jin Y. BMPRII is a direct target of miR-21. Acta Biochim Biophys Sin. 2009;41(7):618–623. doi: 10.1093/abbs/gmp049. [DOI] [PubMed] [Google Scholar]
- 81.Sugatani T, Hruska KA. Down-regulation of miR-21 biogenesis by estrogen action contributes to osteoclastic apoptosis. J Cell Biochem. 2013;114(6):1217–1222. doi: 10.1002/jcb.24471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li C, Li C, Yue J, Huang X, Chen M, Gao J, Wu B. miR-21 and miR-101 regulate PLAP-1 expression in periodontal ligament cells. Mol Med Rep. 2012;5(5):1340–1346. doi: 10.3892/mmr.2012.797. [DOI] [PubMed] [Google Scholar]
- 83.Wei F, Liu D, Feng C, Zhang F, Yang S, Hu Y, Ding G, Wang S. microRNA-21 mediates stretch-induced osteogenic differentiation in human periodontal ligament stem cells. Stem Cells Dev. 2015;24(3):312–319. doi: 10.1089/scd.2014.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He J, Zhang JF, Yi C, Lv Q, Xie WD, Li JN, Wan G, Cui K, Kung HF, Yang J, Yang BB, Zhang Y. miRNA-mediated functional changes through co-regulating function related genes. PLoS One. 2010;5(10):e13558. doi: 10.1371/journal.pone.0013558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dong J, Cui X, Jiang Z, Sun J. MicroRNA-23a modulates tumor necrosis factor-alpha-induced osteoblasts apoptosis by directly targeting Fas. J Cell Biochem. 2013;114(12):2738–2745. doi: 10.1002/jcb.24622. [DOI] [PubMed] [Google Scholar]
- 86.Luzi E, Marini F, Sala SC, Tognarini I, Galli G, Brandi ML. Osteogenic differentiation of human adipose tissue-derived stem cells is modulated by the miR-26a targeting of the SMAD1 transcription factor. J Bone Miner Res. 2008;23(2):287–295. doi: 10.1359/jbmr.071011. [DOI] [PubMed] [Google Scholar]
- 87.Wang T, Xu Z. miR-27 promotes osteoblast differentiation by modulating Wnt signaling. Biochem Biophys Res Commun. 2010;402(2):186–189. doi: 10.1016/j.bbrc.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 88.Wang FS, Chuang PC, Lin CL, Chen MW, Ke HJ, Chang YH, Chen YS, Wu SL, Ko JY. MicroRNA-29a protects against glucocorticoid-induced bone loss and fragility in rats by orchestrating bone acquisition and resorption. Arthritis Rheum. 2013;65(6):1530–1540. doi: 10.1002/art.37948. [DOI] [PubMed] [Google Scholar]
- 89.Laxman N, Rubin CJ, Mallmin H, Nilsson O, Pastinen T, Grundberg E, Kindmark A. Global miRNA expression and correlation with mRNA levels in primary human bone cells. RNA. 2015;21(8):1433–1443. doi: 10.1261/rna.049148.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kapinas K, Kessler CB, Delany AM. miR-29 suppression of osteonectin in osteoblasts: regulation during differentiation and by canonical Wnt signaling. J Cell Biochem. 2009;108(1):216–224. doi: 10.1002/jcb.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baglio SR, Devescovi V, Granchi D, Baldini N. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals osterix regulation by miR-31. Gene. 2013;527(1):321–331. doi: 10.1016/j.gene.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 92.Kim D, Song J, Kim S, Chun CH, Jin EJ. MicroRNA-34a regulates migration of chondroblast and IL-1beta-induced degeneration of chondrocytes by targeting EphA5. Biochem Biophys Res Commun. 2011;415(4):551–557. doi: 10.1016/j.bbrc.2011.10.087. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y, Xie RL, Croce CM, Stein JL, Lian JB, van Wijnen AJ, Stein GS. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc Natl Acad Sci U S A. 2011;108(24):9863–9868. doi: 10.1073/pnas.1018493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang L, Cheng P, Chen C, He HB, Xie GQ, Zhou HD, Xie H, Wu XP, Luo XH. miR-93/Sp7 function loop mediates osteoblast mineralization. J Bone Miner Res. 2012;27(7):1598–1606. doi: 10.1002/jbmr.1621. [DOI] [PubMed] [Google Scholar]
- 95.Sun Z, Cao X, Hu Z, Zhang L, Wang H, Zhou H, Li D, Zhang S, Xie M. MiR-103 inhibits osteoblast proliferation mainly through suppressing Cav1.2 expression in simulated microgravity. Bone. 2015;76:121–128. doi: 10.1016/j.bone.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 96.Johnson MG, Kristianto J, Yuan B, Konicke K, Blank R. Big endothelin changes the cellular miRNA environment in TMOb osteoblasts and increases mineralization. Connect Tissue Res. 2014;55(Suppl 1):113–116. doi: 10.3109/03008207.2014.923866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, Lian JB, Stein GS. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci U S A. 2008;105(37):13906–13911. doi: 10.1073/pnas.0804438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eskildsen T, Taipaleenmaki H, Stenvang J, Abdallah BM, Ditzel N, Nossent AY, Bak M, Kauppinen S, Kassem M. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc Natl Acad Sci U S A. 2011;108(15):6139–6144. doi: 10.1073/pnas.1016758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakamura Y, He X, Kato H, Wakitani S, Kobayashi T, Watanabe S, Iida A, Tahara H, Warman ML, Watanapokasin R, Postlethwait JH. Sox9 is upstream of microRNA-140 in cartilage. Appl Biochem Biotechnol. 2012;166(1):64–71. doi: 10.1007/s12010-011-9404-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nicolas FE, Pais H, Schwach F, Lindow M, Kauppinen S, Moulton V, Dalmay T. mRNA expression profiling reveals conserved and non-conserved miR-140 targets. RNA Biol. 2011;8(4):607–615. doi: 10.4161/rna.8.4.15390. [DOI] [PubMed] [Google Scholar]
- 101.Itoh T, Nozawa Y, Akao Y. MicroRNA-141 and -200a are involved in bone morphogenetic protein-2-induced mouse pre-osteoblast differentiation by targeting distal-less homeobox 5. J Biol Chem. 2009;284(29):19272–19279. doi: 10.1074/jbc.M109.014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu H, Lin H, Zhang L, Sun Q, Yuan G, Zhang L, Chen S, Chen Z. miR-145 and miR-143 regulate odontoblast differentiation through targeting Klf4 and Osx genes in a feedback loop. J Biol Chem. 2013;288(13):9261–9271. doi: 10.1074/jbc.M112.433730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eguchi T, Watanabe K, Hara ES, Ono M, Kuboki T, Calderwood SK. OstemiR: a novel panel of microRNA biomarkers in osteoblastic and osteocytic differentiation from mesencymal stem cells. PLoS One. 2013;8(3):e58796. doi: 10.1371/journal.pone.0058796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim KM, Park SJ, Jung SH, Kim EJ, Jogeswar G, Ajita J, Rhee Y, Kim CH, Lim SK. miR-182 is a negative regulator of osteoblast proliferation, differentiation, and skeletogenesis through targeting FoxO1. J Bone Miner Res. 2012;27(8):1669–1679. doi: 10.1002/jbmr.1604. [DOI] [PubMed] [Google Scholar]
- 105.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454(7200):56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shin JO, Nakagawa E, Kim EJ, Cho KW, Lee JM, Cho SW, Jung HS. miR-200b regulates cell migration via Zeb family during mouse palate development. Histochem Cell Biol. 2012;137(4):459–470. doi: 10.1007/s00418-012-0915-6. [DOI] [PubMed] [Google Scholar]
- 107.Chen Y, Ge W, Xu L, Qu C, Zhu M, Zhang W, Xiao Y. miR-200b is involved in intestinal fibrosis of Crohn’s disease. Int J Mol Med. 2012;29(4):601–606. doi: 10.3892/ijmm.2012.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Inose H, Ochi H, Kimura A, Fujita K, Xu R, Sato S, Iwasaki M, Sunamura S, Takeuchi Y, Fukumoto S, Saito K, Nakamura T, Siomi H, Ito H, Arai Y, Shinomiya K, Takeda S. A microRNA regulatory mechanism of osteoblast differentiation. Proc Natl Acad Sci U S A. 2009;106(49):20794–20799. doi: 10.1073/pnas.0909311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mizuno Y, Tokuzawa Y, Ninomiya Y, Yagi K, Yatsuka-Kanesaki Y, Suda T, Fukuda T, Katagiri T, Kondoh Y, Amemiya T, Tashiro H, Okazaki Y. miR-210 promotes osteoblastic differentiation through inhibition of AcvR1b. FEBS Lett. 2009;583(13):2263–2268. doi: 10.1016/j.febslet.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 110.Wang X, Guo B, Li Q, Peng J, Yang Z, Wang A, Li D, Hou Z, Lv K, Kan G, Cao H, Wu H, Song J, Pan X, Sun Q, Ling S, Li Y, Zhu M, Zhang P, Peng S, Xie X, Tang T, Hong A, Bian Z, Bai Y, Lu A, Li Y, He F, Zhang G, Li Y. miR-214 targets ATF4 to inhibit bone formation. Nat Med. 2013;19(1):93–100. doi: 10.1038/nm.3026. [DOI] [PubMed] [Google Scholar]
- 111.Gay I, Cavender A, Peto D, Sun Z, Speer A, Cao H, Amendt BA. Differentiation of human dental stem cells reveals a role for microRNA-218. J Periodontal Res. 2014;49(1):110–120. doi: 10.1111/jre.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sugatani T, Hruska KA. Impaired micro-RNA pathways diminish osteoclast differentiation and function. J Biol Chem. 2009;284(7):4667–4678. doi: 10.1074/jbc.M805777200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De-Ugarte L, Yoskovitz G, Balcells S, Guerri-Fernandez R, Martinez-Diaz S, Mellibovsky L, Urreizti R, Nogues X, Grinberg D, Garcia-Giralt N, Diez-Perez A. MiRNA profiling of whole trabecular bone: identification of osteoporosis-related changes in MiRNAs in human hip bones. BMC Med Genomics. 2015;8:75. doi: 10.1186/s12920-015-0149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang JF, Fu WM, He ML, Wang H, Wang WM, Yu SC, Bian XW, Zhou J, Lin MC, Lu G, Poon WS, Kung HF. MiR-637 maintains the balance between adipocytes and osteoblasts by directly targeting Osterix. Mol Biol Cell. 2011;22(21):3955–3961. doi: 10.1091/mbc.E11-04-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li H, Xie H, Liu W, Hu R, Huang B, Tan YF, Xu K, Sheng ZF, Zhou HD, Wu XP, Luo XH. A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J Clin Invest. 2009;119(12):3666–3677. doi: 10.1172/JCI39832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wei J, Li H, Wang S, Li T, Fan J, Liang X, Li J, Han Q, Zhu L, Fan L, Zhao RC. let-7 enhances osteogenesis and bone formation while repressing adipogenesis of human stromal/mesenchymal stem cells by regulating HMGA2. Stem Cells Dev. 2014;23(13):1452–1463. doi: 10.1089/scd.2013.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]


