Abstract
Purpose
To investigate the cause of imaging artifacts observed during gadoxetic acid–enhanced arterial phase imaging of the liver.
Materials and Methods
This HIPAA-compliant study was approved by the institutional review board. Data were collected prospectively at two sites (site A, United States; site B, Japan) from patients undergoing contrast material–enhanced MR imaging with gadoxetic acid (site A, n = 154, dose = 0.05 mmol/kg; site B, n = 130, 0.025 mmol/kg) or gadobenate dimeglumine (only site A, n = 1666) from January 2014 to September 2014 at site A and from November 2014 to January 2015 at site B. Detailed comparisons between the two agents were made in the patients with dynamic liver acquisitions (n = 372) and age-, sex-, and baseline oxygen saturation (Spo2)-matched pairs (n = 130) at site A. Acquired data included self-reported dyspnea after contrast agent injection, Spo2, and breath-hold fidelity monitored with respiratory bellows.
Results
Self-reported dyspnea was more frequent with gadoxetic acid than with gadobenate dimeglumine (site A, 6.5% [10 of 154] vs 0.1% [two of 1666], P < .001; site B, 1.5% [two of 130]). In the matched-pair comparison, gadoxetic acid, as compared with gadobenate dimeglumine, had higher breath-hold failure rates (site A, 34.6% [45 of 130] vs 11.7% [15 of 130], P < .0001; site B, 16.2% [21 of 130]) and more severe artifacts during arterial phase imaging (site A, 7.7% [10 of 130] vs 0% [none of 130], P < .001; site B, 2.3% [three of 130]). Severe imaging artifacts in patients who received gadoxetic acid were significantly associated with male sex (P = .023), body mass index (P = .021), and breath-hold failure (P < .001) but not with dyspnea or Spo2 decrease.
Conclusion
Severe motion-related artifacts in the arterial phase of gadoxetic acid–enhanced liver MR imaging are associated with breath-hold failure but not with subjective feelings of dyspnea or a substantial decrease in blood Spo2. Subjective feelings of dyspnea are not necessarily associated with imaging artifacts. The phenomenon, albeit at a lower rate, was confirmed at a second site in Japan.
Gadoxetic acid is a useful liver-specific magnetic resonance (MR) imaging contrast agent (1–3) for liver metastasis (4–6), hepatocellular carcinoma (7,8), and other diseases (9). For the diagnosis of hypervascular lesions with gadoxetic acid (10,11), the arterial phase is essential (12–14). An increasingly recognized side effect of gadoxetic acid has been reported and is known as acute transient dyspnea (15) or transient severe motion during arterial phase imaging (16). Previous reports revealed a high incidence (5%– 18%) of severe image degradation during gadoxetic acid–enhanced arterial phase imaging (15,17,18). Furthermore, approximately 14% of patients self-reported transient dyspnea after the administration of gadoxetic acid. Interestingly, the published reports of this phenomenon are from the United States, and the prevalence in other populations is unknown. Anecdotally, gadoxetic acid–related transient dyspnea has not been observed frequently in Japanese populations (19).
Although it is conceivable that a subjective feeling of dyspnea may cause diaphragmatic motion that in turn leads to image degradation, other reasons for artifacts are difficult to exclude. A potential confounder of previous studies investigating gadoxetic acid–related image degradation is an imaging artifact related to mismatching of the k-space acquisition with the bolus because of rapid injection of gadoxetic acid (20–23), which may be difficult to differentiate from respiratory motion–related artifacts. However, breath-hold fidelity can be assessed directly with prospective respiratory monitoring by using bellows and may help determine whether respiratory motion is associated with image artifacts. Furthermore, peripheral oxygen saturation (Spo2) measurement may help elucidate whether transient hypoxia is related to this phenomenon. We hypothesized that prospective objective measurements of breath-hold fidelity and oxygen saturation performed in conjunction with assessment of self-reported transient dyspnea may clarify the association between gadoxetic acid administration and image degradation during the arterial phase.
Therefore, the purpose of our study was to investigate the association of imaging artifacts observed during gadoxetic acid–enhanced arterial phase imaging of the liver.
Materials and Methods
All data were collected from January to September 2014 at site A and from November 2014 to January 2015 at site B. On the basis of recent reports (15) and anecdotal experience, we performed an internal quality assurance/quality improvement study to examine the effects of contrast material injections on subjective dyspnea and breath-hold failure during MR imaging examinations (University of Wisconsin, Madison, Wis) as part of an internal quality assurance project. Subsequently, the institutional review board allowed us to use these data for this Health Insurance Portability and Accountability Act–compliant study, for which the need to obtain written informed consent was waived. Therefore, the data in this study were acquired prospectively, but they were analyzed retrospectively.
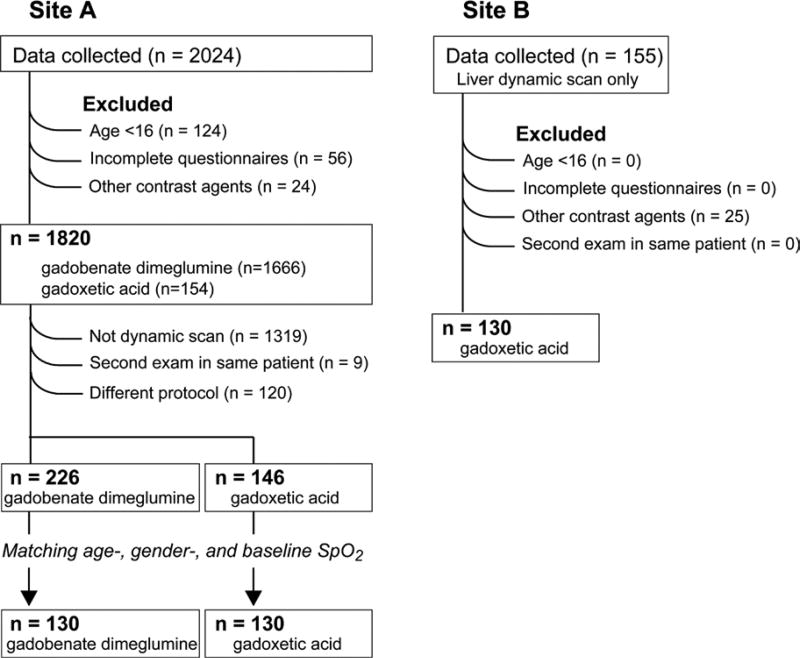
A total of 2024 data collection sheets were collected. Patients younger than 16 years of age (n = 124) were excluded out of concern that younger subjects may self-report subjective dyspnea in a different manner than adult patients. Other data collection sheets were excluded because they were incomplete (n = 56) or were used with other contrast agents that are infrequently used at site A (n = 24), leaving a total of 1820 patients imaged by using gadoxetic acid (n = 154) and gadobenate dimeglumine (n = 1666) (Table 1, Fig 1) who were used for the study of adverse reactions after contrast agent injection. For the detailed analyses of dynamic liver imaging data, we excluded the examinations that did not include dynamic acquisitions (n = 1319), examinations that were the second examination in the same patient (n = 9), and examinations performed with different dynamic protocols (n = 120). After the exclusion process, 372 patients were left for unmatched comparison (Tables 2, 3). For further fair comparison, we randomly selected the patients from the 372 patients as much as possible for whom an age-, sex-, and baseline Spo2 level–matched pair was available (n = 130 for each contrast agent; Tables 2, 3). Statistical power was calculated as follows: According to the previous report, the prevalence of severe artifact due to transient motion was 10% and 0.5% (16) for gadoxetic acid and gadobenate dimeglumine, respectively. With an a of .05 and 130 subjects for each group, the estimated statistical power was 0.96. Among the patients who underwent dynamic contrast-enhanced liver imaging, 130 age- and sex-matched pairs were selected for further analyses. During the age-matching, the pairs were selected from the same decade.
Table 1.
Patient Demographic Data, Spo2 and Self-Reported Adverse Effects
| Parameter | Gadoxetic Acid (n = 154) |
Gadobenate Dimeglumine (n = 1666) |
P Value |
|---|---|---|---|
| No. of female patients | 102 (66.2) | 916 (55.0) | .007 |
| Age (y)* | 52.0 ± 15.2 | 52.3 ± 17.0 | .826 |
| Oxygen administration† | 8 (5.2) | 153 (9.2) | .074 |
| Spo2 at baseline (%) | 96.8 ± 2.1 | 95.9 ± 2.7 | <.001 |
| Self-report of adverse effect | |||
| Total | 14 (9.1) | 41 (2.5) | .001 |
| Nausea | 2 (1.3) | 16 (1.0) | .660 |
| Vomiting | 0 | 6 (0.4) | .999 |
| Allergic like | 0 | 6 (0.4) | .999 |
| Dyspnea | 10 (6.5) | 2 (0.1) | <.001 |
| Other | 2 (1.3) | 11 (0.7) | .302 |
Note.—Unless otherwise specified, data are numbers of patients, with percentages in parentheses. Spo2 was obtained by using peripheral pulse oximetry.
Data are means ± standard deviations.
Number of patients in whom a nasal cannula was used for the administration of oxygen during the MR imaging examination.
Figure 1.
Study flowchart shows inclusion and exclusion of patients.
Table 2.
Demographic Data of Age- and Sex-matched Patient Pairs
| Site A, Unmatched
|
Site A, Matched
|
|||||
|---|---|---|---|---|---|---|
| Parameter | Gadoxetic Acid | Gadobenate Dimeglumine |
P Value | Gadoxetic Acid | Gadobenate Dimeglumine |
P Value |
| No. of patients | 146 | 226 | 130 | 130 | ||
|
| ||||||
| No. of female patients | 110 | 94 | .003 | 81 (62.3) | 81 (62.3) | .999 |
|
| ||||||
| Age (y)* | 52.0 ± 15.0 | 56.1 ± 14.6 | <.001 | 51.9 ± 15.1 | 52.2 ± 15.7 | .891 |
|
| ||||||
| Spo2 at baseline (%) | 96.8 ± 2.1 | 96.1 ± 2.5 | <.001 | 96.8 ± 2.1 | 96.5 ± 2.1 | .124 |
|
| ||||||
| Race | .918 | .843 | ||||
|
| ||||||
| White | 120 (82.2) | 182 (80.5) | 108 (83.1) | 100 (78.1) | ||
|
| ||||||
| African American | 4 (2.7) | 4 (1.8) | 4 (3.1) | 4 (3.1) | ||
|
| ||||||
| American Indian | 2 (1.4) | 2 (0.9) | 2 (1.5) | 2 (1.5) | ||
|
| ||||||
| Hispanic/Latino | 1 (0.7) | 1 (0.7) | 1 (0.8) | 1 (0.8) | ||
|
| ||||||
| Asian | 3 (2.1) | 7 (3.1) | 3 (2.3) | 4 (3.1) | ||
|
| ||||||
| Decline to state/other | 16 (11.0) | 30 (13.3) | 12 (9.2) | 19 (14.6) | ||
|
| ||||||
| Anxiety | 17 (11.6) | 29 (12.8) | .733 | 16 (12.4) | 18 (13.9) | .731 |
|
| ||||||
| Ascites | 5 (3.4) | 8 (3.6) | .947 | 5 (3.9) | 4 (3.1) | .999 |
|
| ||||||
| Allergy | ||||||
|
| ||||||
| Total | 83 (56.9) | 129 (57.1) | .965 | 75 (57.7) | 74 (56.9) | .900 |
|
| ||||||
| Allergy to GBCAs | 2 (1.4) | 0 (0) | .153 | 1 (0.8) | 0 | .999 |
|
| ||||||
| Allergy to something other than GBCAs | 83 (56.9) | 129 (57.1) | .965 | 75 (57.7) | 74 (56.9) | .900 |
|
| ||||||
| Cirrhosis | 7 (4.8) | 66 (29.2) | <.001 | 7 (5.4) | 36 (27.7) | <.001 |
|
| ||||||
| Lung disease | ||||||
|
| ||||||
| Total | 20 (13.7) | 25 (11.1) | .449 | 17 (13.1) | 14 (10.1) | .566 |
|
| ||||||
| Asthma | 13 (8.9) | 14 (6.2) | .330 | 11 (8.5) | 8 (6.2) | .474 |
|
| ||||||
| COPD | 5 (3.4) | 7 (3.1) | .862 | 4 (3.1) | 4 (3.1) | .999 |
|
| ||||||
| Restrictive lung disease | 2 (1.4) | 0 | .053 | 2 (1.5) | 0 | 498 |
|
| ||||||
| Pneumonia | 1 (0.7) | 2 (0.9) | .999 | 1 (0.8) | 1 (0.8) | .999 |
|
| ||||||
| Lung surgery | 0 | 2 (1.5) | .498 | 0 | 2 (1.5) | .498 |
|
| ||||||
| Pleural effusion | 4 (2.7) | 3 (1.3) | .440 | 4 (3.1) | 1 (0.8) | .370 |
|
| ||||||
| Cardiac disease | ||||||
|
| ||||||
| Total | 24 (16.4) | 49 (21.7) | .210 | 21 (16.2) | 27 (20.8) | .337 |
|
| ||||||
| Myocardial infarction | 5 (3.4) | 5 (2.2) | .523 | 5 (3.9) | 2 (1.5) | .447 |
|
| ||||||
| Congenital heart disease | 1 (0.7) | 3 (1.3) | .999 | 1 (0.8) | 3 (2.3) | .622 |
|
| ||||||
| Heart disease not otherwise specified | 20 (13.7) | 46 (20.4) | .096 | 17 (13.1) | 25 (19.2) | .177 |
Note.—Unless otherwise stated, data are numbers of patients, with percentages in parentheses. COPD = chronic obstructive pulmonary disease, GBCAs = gadolinium-based contrast agents.
Data are means ± standard deviations.
Table 3.
Self-reported Adverse Reactions, Breath-holding Failures, and Image Artifacts in Age- and Sex-matched Patient Pairs
| Site A, Unmatched
|
Site A, Matched
|
||||||
|---|---|---|---|---|---|---|---|
| Parameter | Gadoxetic Acid (n = 146) |
Gadobenate Dimeglumine (n = 226) |
P Value | Gadoxetic Acid (n = 130) |
Gadobenate Dimeglumine (n = 130) |
P Value | Site B: Gadoxetic Acid (n = 130) |
| Self-report of adverse effect | 14 (9.6) | 8 (3.5) | .017 | 10 (7.7) | 6 (4.6) | .300 | 4 (3.1) |
|
| |||||||
| Nausea | 2 (1.4) | 5 (2.2) | .709 | 2 (1.5) | 4 (3.1) | .684 | 0 |
|
| |||||||
| Vomiting | 0 | 0 | … | 0 | 0 | … | 0 |
|
| |||||||
| Allergy like | 0 | 0 | … | 0 | 0 | … | 0 |
|
| |||||||
| Dyspnea | 10 (6.9) | 0 | <.001 | 8 (6.2) | 0 | .007 | 2 (1.5) |
|
| |||||||
| Other | 2 (1.4) | 3 (1.3) | .999 | 0 | 2 (1.5) | .498 | 2 (1.5) |
|
| |||||||
| Substantial decrease in Spo2 | |||||||
|
| |||||||
| Precontrast | 5 (3.4) | 16 (7.1) | .101 | 5 (3.9) | 12 (9.2) | .130 | 2 (1.5) |
|
| |||||||
| Arterial phase | 12 (8.2) | 20 (8.9) | .853 | 11 (8.5) | 14 (10.9) | .514 | 3 (2.3) |
|
| |||||||
| Only arterial phase | 10 (6.9) | 12 (5.3) | .542 | 9 (6.9) | 8 (6.2) | .802 | 3 (2.3) |
|
| |||||||
| Breath-holding failure | |||||||
|
| |||||||
| Precontrast | 18 (12.3) | 16 (7.1) | .093 | 14 (10.8) | 9 (7.0) | .282 | 1 (0.8) |
|
| |||||||
| Arterial phase | 52 (35.6) | 24 (10.7) | <.001 | 45 (34.6) | 15 (11.7) | <.001 | 21 (16.2) |
|
| |||||||
| Only arterial phase | 40 (27.4) | 10 (4.4) | <.001 | 36 (27.7) | 8 (6.2) | <.001 | 20 (15.4) |
|
| |||||||
| Substantial image artifacts | |||||||
|
| |||||||
| Precontrast | 1 (0.7) | 4 (1.8) | .919 | 1 (0.8) | 3 (2.3) | .622 | 4 (3.1) |
|
| |||||||
| Arterial phase | 33 (22.6) | 7 (3.1) | <.001 | 29 (22.3) | 4 (3.1) | <.001 | 20 (15.4) |
|
| |||||||
| Only arterial phase | 32 (21.9) | 7 (3.1) | <.001 | 28 (21.5) | 4 (3.1) | <.001 | 17 (13.1) |
|
| |||||||
| Severe image artifacts | |||||||
|
| |||||||
| Precontrast | 0 | 0 | … | 0 | 0 | … | 0 |
|
| |||||||
| Arterial phase | 11 (7.5) | 1 (0.4) | <.001 | 10 (7.7) | 0 | <.001 | 3 (2.3) |
|
| |||||||
| Only arterial phase | 11 (7.5) | 1 (0.4) | <.001 | 10 (7.7) | 0 | <.001 | 3 (2.3) |
Note.—Data are numbers of patients, with percentages in parentheses.
Patient demographic data, including age, sex, race, documented anxiety, cirrhosis, body mass index (BMI), and any history of atopy or allergies to gadolinium-based contrast agents, were recorded from electronic health records. Ascites and pleural effusions, which are known risk factors for dyspnea, were identified through the blinded image quality assessment described below.
After completing the data collection at site A, we prospectively recorded the same data at a second site (site B, University of Yamanashi, Chuo-shi, Yamanashi, Japan) to reveal whether the results obtained at site A would be reproducible in a site in Japan. This part of the study was approved by the institutional review board of the second institution. Informed consent was verbally obtained. A total of 155 data collection forms were collected. After excluding 25 forms for contrast agents other than gadoxetic acid, we included 130 forms from 130 patients who underwent gadoxetic acid–enhanced MR imaging.
Clinical MR imaging technologists at both sites recorded the following items by using a standardized data collection sheet:
Spo2 measured by using a peripheral pulse oximeter (Veris, Medrad, Pittsburg, Pa or Expression, Invivo, Gainesville, Fla at site A; 8600FO Pulse Oximeter, Nomin Medical, Plymouth, Minn at site B). Spo2 (as a percentage was recorded before contrast agent injection during free breathing and during breath-hold acquisitions (precontrast and arterial phase after contrast agent injection). The Spo2 was monitored during the entire acquisition time, and the lowest value for each imaging phase was recorded.
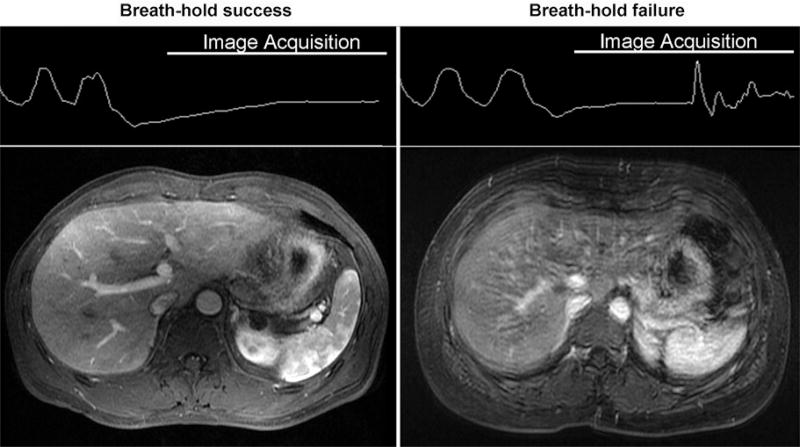
Breath-hold fidelity assessed by using respiratory bellows monitors for precontrast and arterial phase acquisitions. Breath hold was classified as successful or failed. Breath-hold success was defined by a straight or slowly varying trace during the entire image acquisition. Breath-hold failure was recognized as the onset of sudden pronounced oscillations, such as in the example of the bellows tracing in Figure 2.
Requirement for nasal oxygenation during the examination.
Any adverse effects self-reported by the patient. Adverse effects were categorized as nausea, vomiting, allergic-like reactions (rash, sneezing, itchiness, throat tightness) (24), difficulty in holding breath (transient dyspnea), and others.
Figure 2.
Respiratory bellows were used to monitor the success of breath holding. Breath-hold success was defined as a straight or slowly varying trace during image acquisition. Breath-holding failure was recognized as the occurrence of sudden oscillations in bellows tracing in this figure. The example images were obtained in a 50-year-old man (success) and a 67-year-old man (failure).
To avoid biasing patients toward reporting a specific adverse event, care was taken to avoid leading patients by intentionally asking questions regarding adverse events. Rather, only adverse events that were self-reported were recorded.
Contrast Agents
At site A, gadobenate dimeglumine (Multihance; Bracco Diagnostics, Princeton, NJ) was administered at a dose of 0.1 mmol per kilogram of body weight at a rate of 2 mL/sec, followed by a 50mL saline flush. Gadoxetic acid (Eovist/ Primovist; Bayer Healthcare Pharmaceuticals, Wayne, NJ) was administered at a dose of 0.05 mmol/kg at a rate of 2 mL/sec, followed by a 50-mL saline flush. A dose of 0.05 mmol/kg gadoxetic acid is used as the standard of care at site A. This is based on previous data (25–27) that demonstrated superior contrast agent performance during the arterial phase. It should be noted that the concentration of gadoxetic acid (0.25 M) was half that of gadobenate dimeglumine (0.5 M); therefore, the volume of the two injections was identical (0.2 mL/kg). The choice of gadoxetic acid or gadobenate dimeglumine was based on a standardized protocol at site A in which gadobenate dimeglumine is used primarily in patients with cirrhosis, whereas gadoxetic acid is used for assessment of liver disease in patients without cirrhosis. However, seven patients with cirrhosis were imaged with gadoxetic acid because assessment of biliary disorder was the purpose of the MR imaging examinations (Table 3).
At site B, gadoxetic acid was administered at a dose of 0.025 mmol/kg at a rate of 1 mL/sec, followed by a saline flush of 20 mL.
MR Imaging
At site A, MR imaging was performed with one of the following MR systems: at 1.5 T (Optima-MR450w or Signa-HDxt; GE Healthcare, Waukesha, Wis) with an eight- or 12-channel phased-array coil or at 3.0 T (Discovery-MR750 or Discovery-MR750w; GE Healthcare) with a 12- or 32-channel phased-array coil. Dynamic contrast–material enhanced T1-weighted MR imaging was performed by using a three-dimensional spoiled gradient-echo acquisition with spectrally selective intermittent fat inversion (LAVA) with true in-plane spatial resolution of 1.1–1.5 × 1.9–2.0 mm (1.5 T) and 1.3 × 1.6–1.7 mm (3.0 T) with 5 mm (1.5 T) and 3.4 mm (3.0 T) section thicknesses. Image acquisition time was approximately 22 seconds (1.5 T) or 20 seconds (3.0 T).
At site B, MR imaging was performed with either a 1.5-T MR system (Signa-HDxt; GE Healthcare) with an eight-channel phased-array coil or a 3.0-T MR system (Discovery-MR750w; GE Healthcare) with a 32-channel phased-array coil. Dynamic MR imaging was performed with LAVA with a real spatial resolution of 1.1–1.2 × 2.1–2.4 mm (1.5 T) and 1.1–1.2 × 1.8–2.0 mm (3.0 T) with 5 mm (1.5 T) and 4 mm (3.0 T) section thicknesses. Image acquisition time was approximately 16 seconds at both 1.5 T and 3.0 T. Detailed MR parameters are in Table E1 (online).
Image Quality Assessment
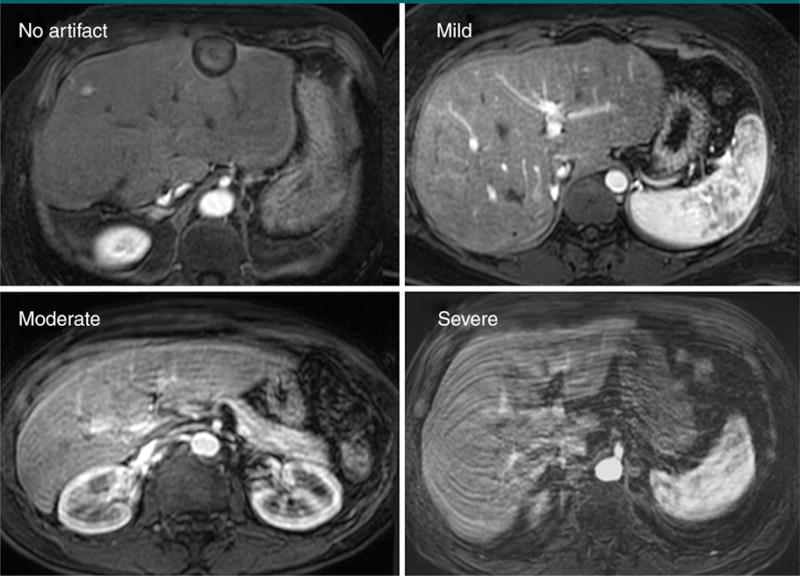
Visual assessment of the precontrast and arterial phase images was performed to grade image quality. Two radiologists trained in abdominal imaging (P.B. and U.M. for the data at site A; K.S. and U.M. for the data at site B) evaluated the severity of motion-related artifacts in consensus by using a four-point scale of no artifact; mild artifact, not interfering diagnostic assessment; moderate artifact affecting diagnostic assessment; and severe artifact, rendering image nondiagnostic (Fig 3). Moderate and severe artifacts were defined as substantial artifacts. Readers were blinded to the results of breath-hold fidelity and to self-reported adverse effects.
Figure 3.
Examples of image quality grading. The grade of artifacts was assessed by using a four-point scale. Moderate or severe artifacts were combined and classified as “substantial” artifacts. The example images were obtained in a 67-year-old woman (no artifact), a 63-year-old man (mild artifact), a 50-year-old woman (moderate artifact), and a 33-year-old man (severe artifact).
Statistical Analyses
A substantial decrease in Spo2 was defined as a decrease of 4 or more percentage points in paired Spo2 measurements. See Figure E1 (online) for details on the choice of threshold. The Spo2 during the breath hold (precontrast and arterial phase imaging) was compared with that during free breathing before contrast agent injection.
Multivariate logistic analysis was used to find the factors that were associated with substantial and severe artifacts. In this analysis, independent variables included age, sex, BMI, self-reported dyspnea, breath-hold failure, and decrease in Spo2.
Results with gadoxetic acid and gadobenate dimeglumine were compared by using Student t tests for numeric factors and χ2 tests or the Fisher exact test (if the expectation of the event was < 5) for categoric data.
All statistical analyses were perfomed with JMP, version 11.1 (SAS Institute, Cary, NC). P < .05 was considered to indicate a significant difference.
Results
Self-reported Adverse Effects in All Patients at Site A
At site A, adverse effects were self-reported in 14 (9.1%) of 154 patients with gadoxetic acid and in 41 (2.5%) of 1666 patients with gadobenate dimeglumine. All adverse effects were self-limited, and none required treatment. The incidence of self-reported dyspnea was significantly higher for gadoxetic acid than for gadobenate dimeglumine (6.5% [10 of 154] vs 0.1% [two of 1666], P < .001) (Table 1). All of the specific comments from individual patients who self-reported dyspnea are listed in Table E2 (online).
Comparison in Patients with Dynamic Liver MR Imaging at Site A
After we performed age-, sex-, and baseline Spo2 level–matching to arrive at cohorts of 130 patients each, with the exception of incidence of cirrhosis, there was no significant difference in the demographics and other risk factors between the patients who received gadoxetic acid and those who received gadobenate dimeglumine (Table 2).
There was no significant difference in the prevalence of substantial decrease in Spo2 level during the arterial phase between patients who received gadoxetic acid and those who received gadobenate dimeglumine (unmatched patients, 8.2% [12 of 146] vs 8.9% [20 of 226]; matched patients, 8.5% [11 of 130] vs 10.9% [14 of 130]; P = .514) (Table 3).
Breath-hold failure rates in the arterial phase were significantly more frequent with gadoxetic acid (unmatched, 35.6% [52 of 146]; matched, 34.6% [45 of 130]) than with gadobenate dimeglumine (unmatched, 10.7% [24 of 226]; matched, 11.7% [15 of 130]; P < .001). The rate of breath-hold failure only in the arterial phase but not in precontrast acquisitions was also significantly higher with gadoxetic acid than with gadobenate dimeglumine (unmatched, 27.4% [40 of 146] vs 4.4% [10 of 226]; matched, 27.7% [36 of 130] vs 6.2% [eight of 130]; P < .001) (Table 3, Fig 1).
Both rates of substantial imaging artifacts (unmatched, 22.6% [33 of 146] vs 3.1% [seven of 226], P < .001; matched, 22.3% [29 of 130] vs 3.1% [four of 130]; P < .001) and severe imaging artifacts (unmatched, 7.5% [11 of 146] vs 0.4% [one of 226]; P < .01; 7.7% [10 of 130] vs 0% [none of 130] for severe artifacts; P < .001) in the arterial phase were significantly more frequent with gadoxetic acid than with gadobenate dimeglumine.
We compared the prevalence of substantial decrease in Spo2 levels in patients who underwent gadoxetic acid–enhanced dynamic liver MR imaging. No significant difference was observed between patients who had substantial artifacts in the arterial phase (12.1% [four of 33]) and those who did not (7.1% [eight of 113]; P = .469) with gadoxetic acid, as well as with gadobenate dimeglumine (14.3% [one of seven] vs 8.7% [19 of 218]; P = .484).
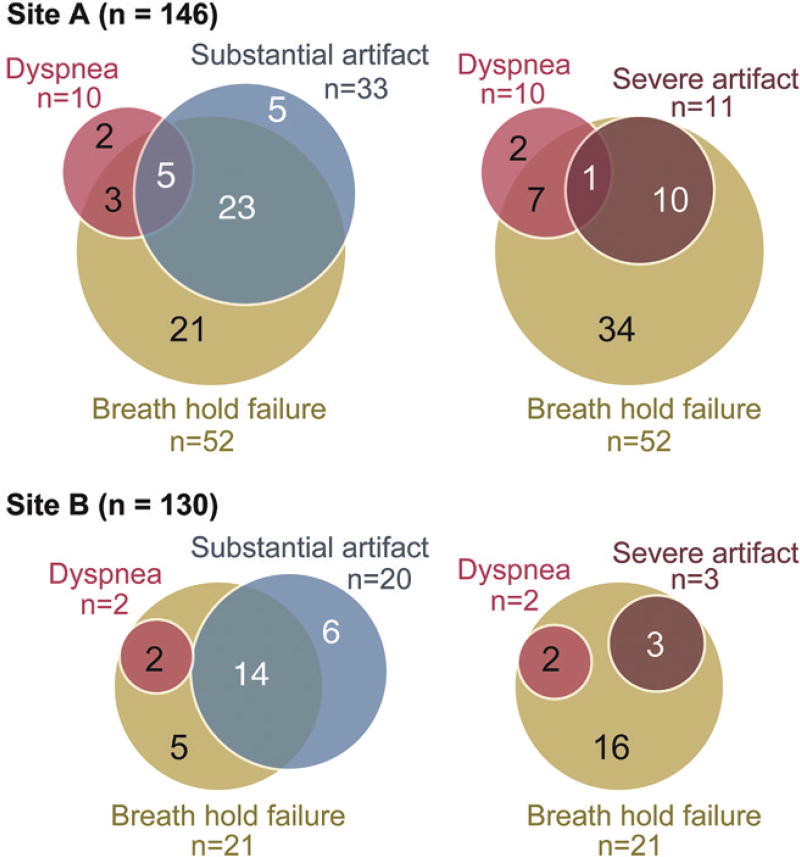
Gadoxetic Acid Administration and Image Degradation
Among the patients who received gadoxetic acid, substantial artifacts on arterial phase images were frequently associated with breath-holding failure: 28 patients failed breath holding among those who had substantial artifacts on gadoxetic acid–enhanced arterial phase images (n = 33) and six of seven for gadobenate dimeglumine (Fig 4). Severe imaging artifacts were observed only in patients who received gadoxetic acid and who failed breath holding during the arterial phase (Table 3, Fig 4). Among the 10 patients who self-reported dyspnea, only one had severe imaging artifacts.
Figure 4.
Diagram shows overlap of recorded breath-holding failures, image artifacts, and self-reported dyspnea for both sites. Substantial artifacts were frequently associated with breath-holding failure (determined by using a respiratory bellows) at both site A (24 of 29) and site B (14 of 20). Severe artifacts were always observed in patients with failed breath holding during the examination. Self-report of dyspnea was associated with breath-hold failure (six of eight at site A and two of two at site B). Interestingly, self-report of dyspnea was not necessarily associated with imaging artifacts.
Multivariate analysis revealed that breath-hold failure was significantly associated with substantial artifacts (P < .001) and severe artifacts (P < .001). In addition to breath-hold failure, male sex (P = .023) and higher BMI (P = .021) were associated with severe artifacts.
Results from Site B
At site B, adverse effects were self-reported in four of 130 patients who received gadoxetic acid. All adverse effects were self-limited. Two patients complained about dyspnea (1.5% [two of 130]) (Table 2, Table 3). Two other patients reported a warm sensation and abdominal discomfort. A substantial decrease in Spo2 levels was observed in three patients (2.3%) during arterial phase imaging. Breath-holding failure occurred during the arterial phase in 21 patients (16.2%). Substantial and severe artifacts on arterial phase images were observed in 20 (15.4%) and three (2.3%) of 130 patients, respectively.
Compared with site A, a similar association was observed among self-reported dyspnea, breath-hold failure, and substantial and severe artifacts on arterial phase images (Fig 4). Substantial artifacts were frequently associated with breath-hold failure (70% [14 of 20]). All three patients with severe artifacts failed breath holding during arterial phase imaging. Two patients with self-reported dyspnea had neither substantial nor severe artifacts.
Discussion
In this study, we confirmed that severe motion-related artifacts at arterial phase liver imaging are more frequently observed with gadoxetic acid than with gadobenate dimeglumine. By assessing breath-hold fidelity with respiratory bellows, we demonstrated that imaging artifacts in the arterial phase of gadoxetic acid–enhanced imaging are predominantly associated with breath-hold failure. Subjective feelings of dyspnea were closely associated with breath-hold failure but did not necessarily lead to imaging artifacts. We confirmed this relationship at two institutions, in the United States and Japan. We did not find any differences in the rate of Spo2 decrease between the patients who received gadoxetic acid and those who received gadobenate dimeglumine. Interestingly, higher BMI was an independent risk factor for severe artifacts during the arterial phase.
The prevalence of gadoxetic acid– related transient subjective dyspnea was reported to be 14% (15). Our results support these findings, except with a lower prevalence of subjective dyspnea with gadoxetic acid in our study (6% at site A and 2% at site B). We also confirmed previous observations that clinically substantial and severe artifacts in the arterial phase are more frequently observed with gadoxetic acid than with gadobenate dimeglumine (16,17). Using respiratory bellows, we were able to provide direct confirmation that these artifacts were associated with breath-hold failure during the examination, particularly for the patients with severe artifacts. Interestingly, in some patients, substantial imaging artifacts were observed despite successful breath holding. Although we attempted to monitor breath-holding failures using respiratory bellows, motion that was not observable with respiratory bellows may still exist. It should be noted that the same volume and injection rate were used for both gadoxetic acid and gadobenate dimeglumine at site A. This ensured that any potential mismatches in the contrast agent bolus and the k-space acquisition, which can result in truncation/ringing artifacts, were essentially identical for the two contrast agents (21), although the relaxivities of the contrast agents were not equal.
Interestingly, on the basis of the respiratory bellows data, more patients failed breath holding than self-reported subjective dyspnea. After receiving gadoxetic acid, 44 patients at site A and 19 patients at site B failed their breath hold without self-reported dyspnea. Failed breath holding without self-reported dyspnea was also observed in 24 patients who received gadobenate dimeglumine. That might suggest that patients were not aware of their difficulty or that patients were too stoic to report their discomfort. Although a specifically designed study is warranted to elucidate this phenomenon, we speculate that this unreported poor breath holding after contrast agent injection may be a common event, but with variable presentation, influenced in part by the type of contrast agent.
Previous reports (28) have revealed that a higher contrast agent dose is a potential risk factor for respiratory motion–related artifacts in gadoxetic acid–enhanced arterial phase imaging. Consistent with that report, we observed a higher prevalence of subjective dyspnea, imaging artifacts, and breath-hold failure at site A with 0.05 mmol/ kg than at site B with 0.025 mmol/kg. In addition, the injection rate was twice as high at site A (2 vs 1 mL/sec). This notion is further supported by our risk analysis, in which a higher BMI was a risk factor for severe imaging artifacts. Indeed, in our study, patients with higher BMIs typically received a higher contrast agent dose because we use a body weight–tailored dose determination. Because heavier patients are more likely to have a higher degree of adiposity, this may have led to unexpected overdosing in patients with high BMIs— more overdosing than might have occured with the calculation of dose on the basis of expected lean body mass (ie, the concept of lean body mass reflects the fact that the vascular volume does not necessarily parallel with body weight in patients with high BMIs) (29).
Slightly shorter acquisition times at site B may also explain the lower rate of motion-related artifact and breath-holding failures at site B than at site A. Indeed, a recent study (16) revealed that, with the use of multiphasic arterial phase acquisitions, the imaging artifacts more likely occur at the end of the breath hold, suggesting that shorter acquisitions may help reduce image artifacts related to transient dyspnea.
Our results have several clinical implications. In combination with the results of previous studies, the results of our study suggest that shortening image acquisition may improve image quality after gadoxetic acid injection (16). Another implication is that the use of a lower dose and consideration for lean body mass–tailored dosing may also help avoid this phenomenon.
Our study had some important limitations. First, the MR imaging technologists at both sites were not blinded to the type of contrast agent used in the examination. Although the potential bias from this lack of blinding is likely to be minimal, future studies with a double-blinded randomized placebo-controlled design would be helpful. In addition, although an age- and sex-matched analysis was performed, the nonrandomized fashion of contrast agent administration may have caused population-related biases, which could only be controlled with intraindividual comparisons (17). Especially, the choice of contrast agents at site A was based on the clinical diagnosis, which meant that patients with cirrhosis typically received gadobenate dimeglumine. Interestingly, a previous study (15) found the reverse tendency—that is, patients with cirrhosis more frequently received gadoxetic acid than gadobenate dimeglumine. Nevertheless, their results were the same as ours—the use of gadoxetic acid resulted in a higher prevalence of transient motion-related artifacts. Also, we could not compare the results between sites A and B, because multiple factors were different between the two sites, including patient demographic data, MR imaging parameters, and contrast agent dose. Nonstandard dosing at site A was another limitation. We would expect a future study designed for looking into the effect of dose on this phenomenon. Finally, the multiple regression analyses could have been overfitting because of a small number of positive cases with six dependent variables. The results should be assessed with caution.
In conclusion, severe motion-related artifacts in the arterial phase of gadoxetic acid–enhanced liver MR imaging are associated with breath-hold failure but not with subjective feelings of dyspnea or a substantial decrease in blood oxygen saturation. Subjective feelings of dyspnea are not necessarily associated with breath-hold failure that leads to imaging artifacts.
Supplementary Material
Advances in Knowledge.
-
■
Self-reported dyspnea (eight [6.2%] vs 0 of 130), breath-hold failure (45 [34%] vs 15 [11.7%] of 130), and substantial artifacts in arterial phase imaging (29 [22.3%] vs four [3.1%] of 130) were significantly more frequent with gadoxetic acid than with gadobenate dimeglumine (P < .007).
-
■
Respiratory monitoring with bellows confirmed that substantial imaging artifacts were associated with breath-hold failure in 70% (14 of 20) to 85% (28 of 33) of patients.
-
■
Self-reported dyspnea was closely associated with breath-hold failure (eight [80%] of 10 at site A and two [100%] of two at site B) but does not necessarily relate to severe imaging artifacts (one [10%] of 10 at site A and none of two at site B).
-
■
The same phenomenon was observed, but at a lower incidence rate, at a second site in Japan; at site B, self-reported dyspnea was observed in two (1.5%) of 130 patients; breath-hold failure, in 21 patients (16.2%); and severe artifacts in arterial phase imaging in three patients (2.3%).
Implication for Patient Care.
-
■
Radiologists and MR imaging technologists should be aware of the imaging artifacts observed in gadoxetic acid–enhanced arterial phase imaging, which are associated with breath-hold failure during the examination.
Acknowledgments
The authors acknowledge the assistance of Ashley Ganser for tabulating the data collected in this study. The authors also thank GE Healthcare and Bracco Diagnostics, which provide research support to the University of Wisconsin.
Supported by GE Healthcare and Bracco Diagnostics.
Abbreviations
- BMI
body mass index
- Spo2
oxygen saturation
Footnotes
Author contributions:
Guarantors of integrity of entire study, U.M., S.B.R.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; manuscript final version approval, all authors; agrees to ensure any questions related to the work are appropriately resolved, all authors; literature research, U.M., P.B., S.B.R.; clinical studies, all authors; statistical analysis, U.M., S.B.R.; and manuscript editing, U.M., P.B., C.A.B., S.B.R.
Disclosures of Conflicts of Interest: U.M. disclosed no relevant relationships. P. B. disclosed no relevant relationships. C.A.B. disclosed no relevant relationships. K.S. disclosed no relevant relationships. S.B.R. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution receives research support from Bracco Diagnostics and GE Healthcare. Other relationships: disclosed no relevant relationships.
Online supplemental material is available for this article.
References
- 1.Frydrychowicz A, Lubner MG, Brown JJ, et al. Hepatobiliary MR imaging with gadolinium-based contrast agents. J Magn Reson Imaging. 2012;35(3):492–511. doi: 10.1002/jmri.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zech CJ, Bartolozzi C, Bioulac-Sage P, et al. Consensus report of the Fifth International Forum for Liver MRI. AJR Am J Roentgenol. 2013;201(1):97–107. doi: 10.2214/AJR.12.9491. [DOI] [PubMed] [Google Scholar]
- 3.Sirlin CB, Hussain H, Jonas E, et al. Consensus report from the 6th International Forum for Liver MRI using gadoxetic acid. J Magn Reson Imaging. 2014;40(3):516–529. doi: 10.1002/jmri.24419. [DOI] [PubMed] [Google Scholar]
- 4.Armbruster M, Zech CJ, Sourbron S, et al. Diagnostic accuracy of dynamic gadoxetic-acid-enhanced MRI and PET/CT compared in patients with liver metastases from neuroendocrine neoplasms. J Magn Reson Imaging. 2014;40(2):457–466. doi: 10.1002/jmri.24363. [DOI] [PubMed] [Google Scholar]
- 5.Jeong HT, Kim MJ, Park MS, et al. Detection of liver metastases using gadoxetic-enhanced dynamic and 10- and 20-minute delayed phase MR imaging. J Magn Reson Imaging. 2012;35(3):635–643. doi: 10.1002/jmri.22880. [DOI] [PubMed] [Google Scholar]
- 6.Muhi A, Ichikawa T, Motosugi U, et al. Diagnosis of colorectal hepatic metastases: comparison of contrast-enhanced CT, contrast-enhanced US, superparamagnetic iron oxide-enhanced MRI, and gadoxetic acid-enhanced MRI. J Magn Reson Imaging. 2011;34(2):326–335. doi: 10.1002/jmri.22613. [DOI] [PubMed] [Google Scholar]
- 7.Park MJ, Kim YK, Lee MW, et al. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology. 2012;264(3):761–770. doi: 10.1148/radiol.12112517. [DOI] [PubMed] [Google Scholar]
- 8.Sano K, Ichikawa T, Motosugi U, et al. Imaging study of early hepatocellular carcinoma: usefulness of gadoxetic acid-enhanced MR imaging. Radiology. 2011;261(3):834–844. doi: 10.1148/radiol.11101840. [DOI] [PubMed] [Google Scholar]
- 9.Frydrychowicz A, Jedynak AR, Kelcz F, Nagle SK, Reeder SB. Gadoxetic acid-enhanced T1-weighted MR cholangiography in primary sclerosing cholangitis. J Magn Re-son Imaging. 2012;36(3):632–640. doi: 10.1002/jmri.23683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grazioli L, Bondioni MP, Haradome H, et al. Hepatocellular adenoma and focal nodular hyperplasia: value of gadoxetic acid-enhanced MR imaging in differential diagnosis. Radiology. 2012;262(2):520–529. doi: 10.1148/radiol.11101742. [DOI] [PubMed] [Google Scholar]
- 11.Motosugi U, Ichikawa T, Onohara K, et al. Distinguishing hepatic metastasis from hemangioma using gadoxetic acid-enhanced magnetic resonance imaging. Invest Radiol. 2011;46(6):359–365. doi: 10.1097/RLI.0b013e3182104b77. [DOI] [PubMed] [Google Scholar]
- 12.Zech CJ, Vos B, Nordell A, et al. Vascular enhancement in early dynamic liver MR imaging in an animal model: comparison of two injection regimen and two different doses Gd-EOB-DTPA (gadoxetic acid) with standard Gd-DTPA. Invest Radiol. 2009;44(6):305–310. doi: 10.1097/rli.0b013e3181a24512. [DOI] [PubMed] [Google Scholar]
- 13.Chung SH, Kim MJ, Choi JY, Hong HS. Comparison of two different injection rates of gadoxetic acid for arterial phase MRI of the liver. J Magn Reson Imaging. 2010;31(2):365–372. doi: 10.1002/jmri.22057. [DOI] [PubMed] [Google Scholar]
- 14.Motosugi U, Ichikawa T, Araki T. Rules, roles, and room for discussion in gadoxetic acid-enhanced magnetic resonance liver imaging: current knowledge and future challenges. Magn Reson Med Sci. 2013;12(3):161–175. doi: 10.2463/mrms.2012-0085. [DOI] [PubMed] [Google Scholar]
- 15.Davenport MS, Viglianti BL, Al-Hawary MM, et al. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology. 2013;266(2):452–461. doi: 10.1148/radiol.12120826. [DOI] [PubMed] [Google Scholar]
- 16.Pietryga JA, Burke LM, Marin D, Jaffe TA, Bashir MR. Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology. 2014;271(2):426–434. doi: 10.1148/radiol.13131988. [DOI] [PubMed] [Google Scholar]
- 17.Davenport MS, Caoili EM, Kaza RK, Hussain HK. Matched within-patient cohort study of transient arterial phase respiratory motion-related artifact in MR imaging of the liver: gadoxetate disodium versus gadobenate dimeglumine. Radiology. 2014;272(1):123–131. doi: 10.1148/radiol.14132269. [DOI] [PubMed] [Google Scholar]
- 18.Bashir MR, Castelli P, Davenport MS, et al. Respiratory motion artifact affecting hepatic arterial phase MR imaging with gadoxetate disodium is more common in patients with a prior episode of arterial phase motion associated with gadoxetate disodium. Radiology. 2015;274(1):141–148. doi: 10.1148/radiol.14140386. [DOI] [PubMed] [Google Scholar]
- 19.Motosugi U. Gadoxetic acid-induced acute transient dyspnea: the perspective of Japanese radiologists. Magn Reson Med Sci. 2015;14(2):163–164. doi: 10.2463/mrms.2014-0154. [DOI] [PubMed] [Google Scholar]
- 20.Motosugi U, Ichikawa T, Sano K, et al. Double-dose gadoxetic acid-enhanced magnetic resonance imaging in patients with chronic liver disease. Invest Radiol. 2011;46(2):141–145. doi: 10.1097/RLI.0b013e3181f9c487. [DOI] [PubMed] [Google Scholar]
- 21.Tanimoto A, Higuchi N, Ueno A. Reduction of ringing artifacts in the arterial phase of gadoxetic acid-enhanced dynamic MR imaging. Magn Reson Med Sci. 2012;11(2):91–97. doi: 10.2463/mrms.11.91. [DOI] [PubMed] [Google Scholar]
- 22.Motosugi U, Ichikawa T, Sou H, et al. Dilution method of gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI) J Magn Reson Imaging. 2009;30(4):849–854. doi: 10.1002/jmri.21913. [DOI] [PubMed] [Google Scholar]
- 23.Haradome H, Grazioli L, Tsunoo M, et al. Can MR fluoroscopic triggering technique and slow rate injection provide appropriate arterial phase images with reducing artifacts on gadoxetic acid-DTPA (Gd-EOB-DTPA)-enhanced hepatic MR imaging? J Magn Reson Imaging. 2010;32(2):334–340. doi: 10.1002/jmri.22241. [DOI] [PubMed] [Google Scholar]
- 24.American College of Radiology. Manual on contrast media. 9. Reston, Va: American College of Radiology; 2013. [Google Scholar]
- 25.Brismar TB, Dahlstrom N, Edsborg N, Persson A, Smedby O, Albiin N. Liver vessel enhancement by Gd-BOPTA and Gd-EOB-DTPA: a comparison in healthy volunteers. Acta Radiol. 2009;50(7):709–715. doi: 10.1080/02841850903055603. [DOI] [PubMed] [Google Scholar]
- 26.Frydrychowicz A, Nagle SK, D’Souza SL, Vigen KK, Reeder SB. Optimized high-resolution contrast-enhanced hepatobiliary imaging at 3 tesla: a cross-over comparison of gadobenate dimeglumine and gadoxetic acid. J Magn Reson Imaging. 2011;34(3):585–594. doi: 10.1002/jmri.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee MS, Lee JY, Kim SH, et al. Gadoxetic acid disodium-enhanced magnetic resonance imaging for biliary and vascular evaluations in preoperative living liver donors: comparison with gadobenate dimeglumine-enhanced MRI. J Magn Reson Imaging. 2011;33(1):149–159. doi: 10.1002/jmri.22429. [DOI] [PubMed] [Google Scholar]
- 28.Davenport MS, Bashir MR, Pietryga JA, Weber JT, Khalatbari S, Hussain HK. Dose-toxicity relationship of gadoxetate disodium and transient severe respiratory motion artifact. AJR Am J Roentgenol. 2014;203(4):796–802. doi: 10.2214/AJR.13.11587. [DOI] [PubMed] [Google Scholar]
- 29.Kondo H, Kanematsu M, Goshima S, et al. Body size indexes for optimizing iodine dose for aortic and hepatic enhancement at multidetector CT: comparison of total body weight, lean body weight, and blood volume. Radiology. 2010;254(1):163–169. doi: 10.1148/radiol.09090369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.