Abstract
Purpose
Regular use of aspirin is associated with improved survival for patients with colorectal cancer (CRC). However, the timing of and the subtype of CRC that would benefit the most from using aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) in relation to survival is unclear.
Patients and Methods
In all, 2,419 patients age 18 to 74 years with incident invasive CRC who were diagnosed from 1997 to 2008 were identified from population-based cancer registries in the United States, Canada, and Australia. Detailed epidemiologic questionnaires were administered at study enrollment and at 5-year follow-up. Survival outcomes were completed through linkage to national death registries. BRAF- and KRAS-mutation status, microsatellite instability, and CpG island methylator phenotype were also evaluated. Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% CIs for overall survival (OS) and CRC-specific survival.
Results
After a median of 10.8 years of follow-up since diagnosis, 381 deaths (100 as a result of CRC) were observed. Compared with nonusers, postdiagnostic aspirin-only users had more favorable OS (HR, 0.75; 95% CI, 0.59 to 0.95) and CRC-specific survival (HR, 0.44; 95% CI, 0.25 to 0.71), especially among those who initiated aspirin use (OS: HR, 0.64; 95% CI, 0.47 to 0.86; CRC-specific survival: HR, 0.40; 95% CI, 0.20 to 0.80). The association between any NSAID use after diagnosis and OS differed significantly by KRAS-mutation status (Pinteraction = .01). Use of any NSAID after diagnosis was associated with improved OS only among participants with KRAS wild-type tumors (HR, 0.60; 95% CI, 0.46 to 0.80) but not among those with KRAS-mutant tumors (HR, 1.24; 95% CI, 0.78 to 1.96).
Conclusion
Among long-term CRC survivors, regular use of NSAIDs after CRC diagnosis was significantly associated with improved survival in individuals with KRAS wild-type tumors.
INTRODUCTION
With improvements in early detection and treatment, the number of survivors of colorectal cancer (CRC) has grown to 1.2 million.1 Because of this large burden of CRC in the United States, identifying modifiable behaviors associated with better prognosis and long-term outcomes among CRC survivors has a major impact on public health. Several studies have demonstrated that long-term regular use of nonsteroidal anti-inflammatory drugs (NSAIDs) is associated with a lower risk of colorectal neoplasia.2-12 However, there is limited information on whether prediagnostic12-21 and postdiagnostic17-24 NSAID use differentially affect CRC survival. Some studies have found no association between prediagnostic NSAID use and survival,15-20 and others have observed more favorable survival among regular users of NSAIDs before diagnosis.12-14 Most of the current literature on postdiagnostic NSAID use has focused exclusively on aspirin.18-20,23,24 Information is limited on the changes in NSAID use from the pre- to postdiagnostic time periods and their associations with CRC survivorship.
We aim to investigate the association between survival and postdiagnostic use of aspirin and other NSAIDs and to evaluate whether this relationship differs according to time of use, patient characteristics, and tumor characteristics. To accomplish this, we analyzed data from population-based sites of the Colon Cancer Family Registry (CCFR).
PATIENTS AND METHODS
Study Population
The CCFR, an international consortium, has enrolled more than 32,000 participants with CRC, their relatives, and controls from six centers in Australia, Canada, and the United States.25,26 This analysis was restricted to patients with CRC who were identified from population-based cancer registries and were recruited through four CCFR study centers (Fred Hutchinson Cancer Research Center, Seattle, WA; Mayo Clinic, Rochester, MN; Cancer Care Ontario, Toronto, ON, Canada; and University of Melbourne, Melbourne, VIC, Australia). Eligible participants were diagnosed between 1997 and 2008 with incident cases of invasive adenocarcinoma of the colon and rectum and were age 18 to 74 years.
All participants completed a standard baseline questionnaire at enrollment (median, 9 months after diagnosis; interquartile range [IQR], 6 to 15 months after diagnosis), and those who were alive at approximately 5 years after baseline (median, 4.9 years; IQR, 4.6 to 5.5 years) were asked to complete a follow-up epidemiologic and medical history questionnaire. Additional descriptions of CCFR recruitment approaches have been published.25 All participants provided informed consent. The institutional review board at each of the CCFR sites approved the study protocol.
Pre- and Postdiagnostic NSAID use
Data on the use of aspirin and other NSAIDs were collected at the baseline and follow-up interviews. Non-aspirin NSAIDs included ibuprofen- and naproxen-based medications. We defined prediagnostic use as the reported use approximately 1 year before diagnosis of CRC assessed at the baseline interview and postdiagnostic use as being between baseline and the 5-year follow-up interview. At each time point, use of aspirin or other NSAIDs, the frequency of use, and the duration of use were assessed. Regular use of NSAIDs was defined as using the medications at least twice per week for more than 1 month. Ever users were persons who regularly used the medications, and nonusers were defined as those who used the medications other than regularly or never used such medications. Ever users were further divided into aspirin-only, other NSAID–only, and any-NSAID groups. The duration of postdiagnostic NSAID use was calculated as total years of using these mediations between baseline and 5-year follow-up. Change in use of aspirin or NSAIDs was categorized according to regular use of these medications during the pre- and postdiagnostic time periods: nonuse/nonuse as nonusers, any use/nonuse as discontinued users, nonuse/any use as initiated users, and any use/any use as continued users.
Tumor Characteristics
Tumor location was determined on the basis of International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes27 abstracted from cancer registry entries, pathology records, or medical records. Proximal colon cancer was defined as cancers in the cecum through transverse colon (C180, C182-184), distal colon cancers included cancers arising from the splenic flexure to sigmoid colon (C185-187), and rectal cancers consisted of cancers of the rectosigmoid junction or rectum (C199, C209). Patients with an unknown CRC primary site were classified as unknown. For tumor stage at diagnosis, the majority of patients had staging (I, II, III, or IV) according to the American Joint Committee on Cancer (AJCC, 7th edition) available. For a subset of individuals whose AJCC staging was missing, imputation from SEER summary staging and TNM staging was performed for each AJCC stage: I (SEER 1 and T1/T2), II (SEER 1 and T3; SEER 3/7 and N0, M0), III (SEER 3/4/7, and N1/N2, M0), and IV (SEER 7 and M1).
For tumor marker testing, DNA was extracted from paraffin-embedded formalin-fixed tumor tissues. Microsatellite instability (MSI) status was evaluated as previously described.25,28 For the majority of patients with available tumor samples (73%), MSI status was determined by using a 10-marker panel. Tumors were classified as MSI-high (MSI-H) if instability was observed in ≥ 30% of markers, and as microsatellite stable (MSS) or MSI-low (MSI-L) if otherwise. For the remaining patients, MSI status was determined on the basis of immunohistochemistry testing to evaluate the expression of the MLH1, MSH2, MSH6, and PMS2 proteins.29,30 Patients with positive staining for all markers were considered MSS/MSI-L; those who were negative on one or more markers were classified as MSI-H. Previous studies demonstrated a high concordance (97%) between these two methods.30,31 Our data showed a similar level of agreement (98.4%). Extracted tumor DNA was also tested for p.V600E BRAF mutation by using a fluorescent allele-specific polymerase chain reaction assay.32 For a subset of patients, coding sequence in KRAS exon 2 was amplified, and mutations were tested by using forward and reverse sequencing.33,34 Testing of the CpG island methylator phenotype (CIMP) was based on a validated five-marker DNA methylation assay (CACNA1G, IGF2, NEUROG1, RUNX3, and SOCS1).35-37 Tumors were classified as CIMP-positive if three or more of five markers had a percentage of methylated reference ratio ≥ 10 and as CIMP-negative otherwise.
Deaths
Follow-up for vital status, date of death, and cause of death was completed through linkage to population-based cancer registries, national death indices, and death certificates, with the most recent linkage occurring in January 2014. We used the ICD-9 or ICD-10 (depending on study site and year of linkage) to classify cause of death into CRC-specific deaths (ICD-9: 153.0-153.4, 153.6-153.9, or 154.0-154.1; ICD-10: C18.0-20.0 or C26.0), cardiovascular disease (CVD)–specific deaths (ICD-9: 390-459; ICD-10: I00-I99), or other causes.
Statistical Analysis
Of the 4,796 eligible population-based patients with CRC enrolled in CCFR, 3,430 were alive 5 years after diagnosis; 3,001 (88%) responded to the follow-up questionnaires. In addition, we excluded 295 individuals who had missing data on either pre- or postdiagnostic aspirin or non-aspirin NSAID use, 170 patients who were diagnosed more than 2 years before baseline, 114 who had follow-up interviews more than 6.5 years after baseline, and three participants who did not have a date of death recorded. A total of 2,419 patients with CRC were included in this analysis.
Delayed-entry Cox proportional hazards regression was used to estimate adjusted hazard ratios (HRs) and 95% CIs for the association of postdiagnostic NSAID use and duration and change in NSAID use with CRC-specific survival (CSS), CVD-specific survival, and overall survival (OS). Person time accrued from the date of follow-up interview to the date of death or the end of follow-up. Proportional hazards assumptions were examined by testing for a nonzero slope of the scaled Schoenfeld residuals as a function of survival time. Multivariable regression models were stratified on age at diagnosis and study center, in light of violation of the proportional hazards assumption, and adjusted for potential confounders selected a priori, including sex, body mass index, and smoking status at baseline, stage at diagnosis (as categorized in Table 1), and number of years between baseline and follow-up interviews (as a continuous variable). Analyses restricted to patients with stage I, II, or III CRC were also conducted.
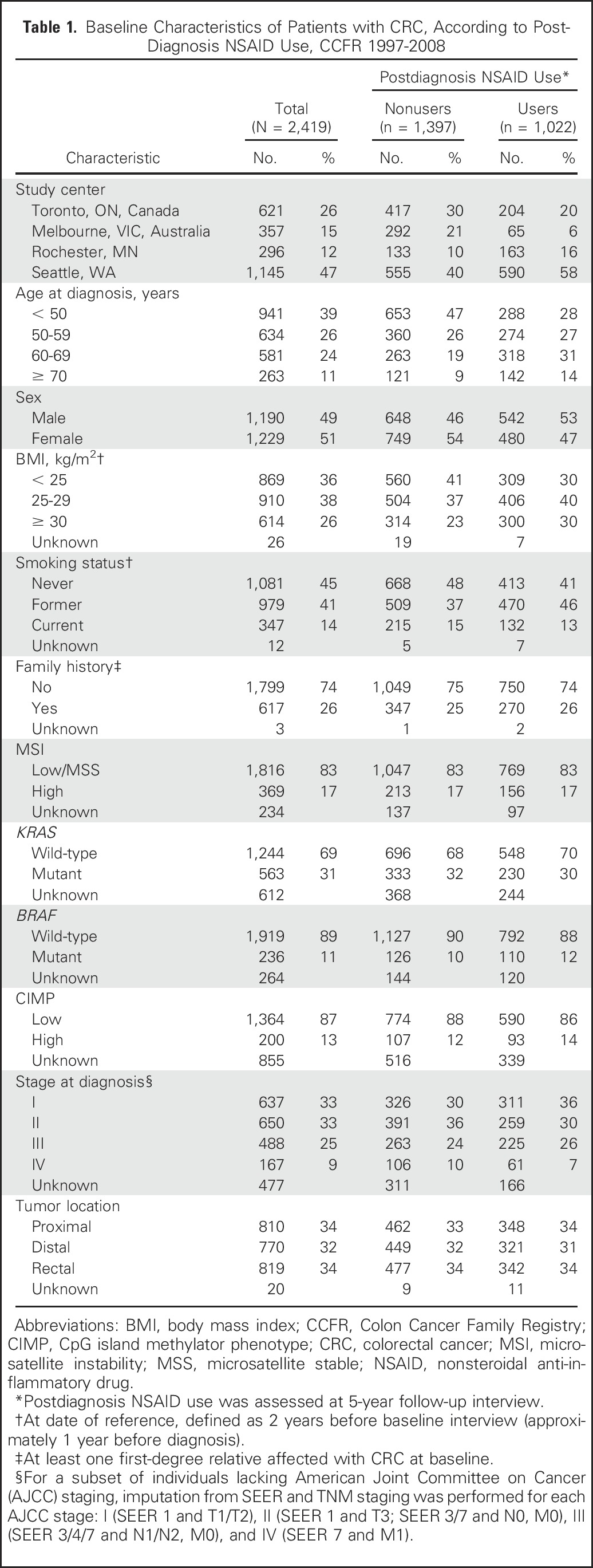
Table 1.
Baseline Characteristics of Patients with CRC, According to PostDiagnosis NSAID Use, CCFR 1997-2008
In addition, we evaluated the effect of aspirin-only and non-aspirin NSAID use separately, and conducted stratified analysis on any NSAID use after diagnosis in relation to OS and CSS by age at diagnosis, sex, family history (at least one first-degree relative affected with CRC, yes v no), tumor location, stage at diagnosis, CIMP, MSI status, KRAS-mutation status, and BRAF mutation status (as grouped in Table 3). Tests for interaction were performed by adding the cross-product term of postdiagnostic NSAID use and each of these factors in the regression models. P values were two-sided with significance level of .05. All statistical analyses were conducted by using SAS 9.3 (SAS Institute, Cary, NC).
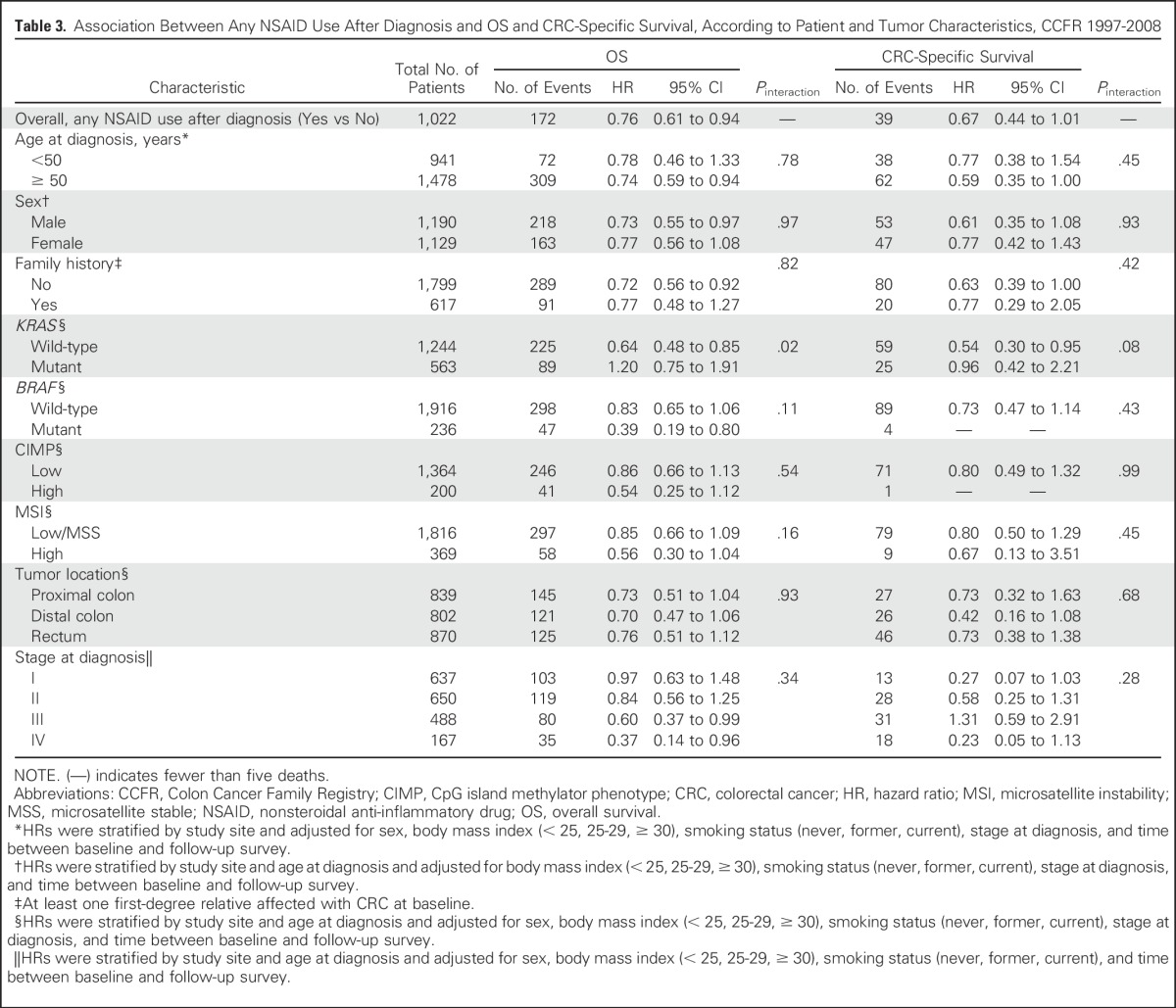
Table 3.
Association Between Any NSAID Use After Diagnosis and OS and CRC-Specific Survival, According to Patient and Tumor Characteristics, CCFR 1997-2008
RESULTS
Participants included in analyses of postdiagnostic NSAID use were mostly white (93%) with equal numbers of men and women who had an average age at diagnosis of 54 years (standard deviation, 11.3 years). Forty-two percent of participants regularly used either aspirin or other NSAIDs after a diagnosis of CRC. They were more likely to be older, male, overweight or obese, former smokers, and diagnosed with early-stage CRC compared with nonusers. The distributions of family history, tumor markers, and tumor location were nearly identical in these two groups (Table 1).
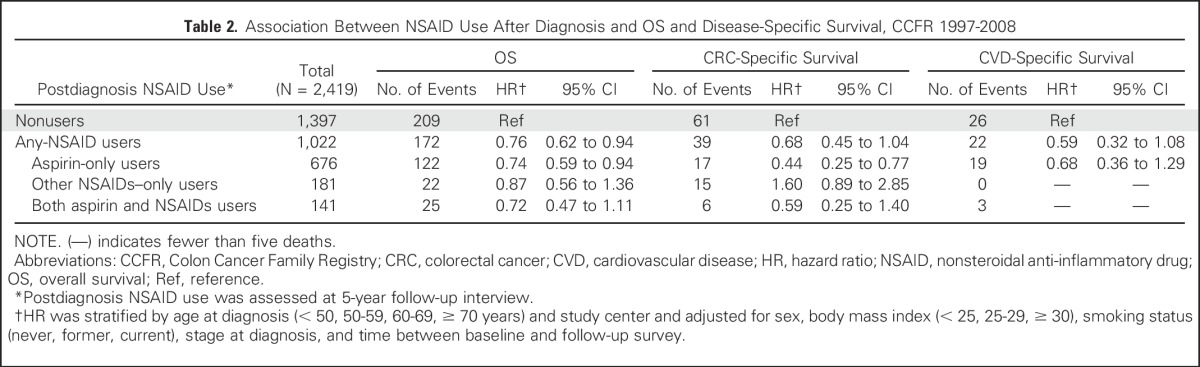
After the follow-up interview (median, 5.9 years since diagnosis; IQR, 5.5 to 6.5 years since diagnosis), we observed participants for a median of 4.9 years and recorded 381 deaths (16%; 100 died as a result of CRC). Regular users of any NSAIDs after diagnosis were associated with more favorable OS (HR, 0.76; 95% CI, 0.62 to 0.94). This association was similar among users of aspirin only, other NSAIDs only, or both aspirin and non-aspirin NSAIDs (Table 2). Statistical significance was lost, however, for users of other NSAIDs alone or both aspirin and other NSAIDs. For CSS, there was evidence of improved CSS among aspirin-only users compared with nonusers (HR, 0.44; 95% CI, 0.25 to 0.77). However, no statistically significant associations were observed among regular users of other NSAIDs only or both aspirin and other NSAIDs. Sensitivity analysis among patients with stage I to III CRC showed similar patterns of association.
Table 2.
Association Between NSAID Use After Diagnosis and OS and Disease-Specific Survival, CCFR 1997-2008
The observed association between postdiagnostic NSAID use and OS was limited to individuals with KRAS wild-type tumors (HR, 0.64; 95% CI, 0.48 to 0.85; Pinteraction = .02; Table 3) but not for those with KRAS-mutant tumors (HR, 1.20; 95% CI, 0.75 to 1.91). Patterns of association were similar with respect to CSS, although they did not reach statistical significance (Pinteraction = .08). The interaction between KRAS-mutation status and postdiagnostic NSAID use in relation to survival did not differ by family history (OS: P for three-term interaction = .08; CSS: P = .50; Appendix Table A1). In addition, there were some suggestive differences, although they were not statistically significant, in the association between NSAID use and OS by BRAF-mutant status (for BRAF-mutant tumors: HR, 0.39 [95% CI, 0.19 to 0.80]; for BRAF wild-type tumors: HR, 0.83 [95% CI, 0.65 to 1.06]; Pinteraction = .11). We found no evidence of interaction between postdiagnostic NSAID use and other patient or tumor characteristics in relation to survival.
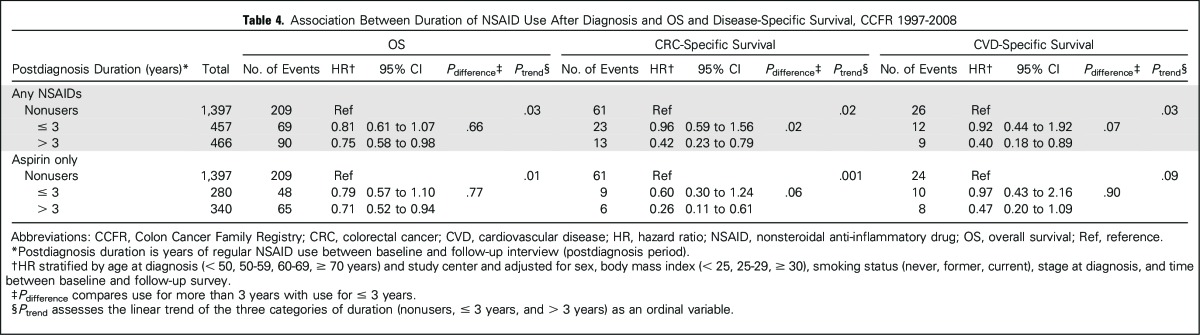
Duration-dependent relationships were observed for postdiagnostic NSAID use. In both any-NSAID and aspirin-only users, longer duration of use was associated with more favorable OS and CSS (Ptrend < .05; Table 4). Use of any NSAIDs for more than 3 years significantly improved CSS compared with shorter duration of use (P = .02; Table 4). In exploratory analysis, we found that the observed association between the duration of postdiagnostic NSAID use and OS remained only among participants with KRAS wild-type tumors (Pinteraction = .02; Appendix Table A2).
Table 4.
Association Between Duration of NSAID Use After Diagnosis and OS and Disease-Specific Survival, CCFR 1997-2008
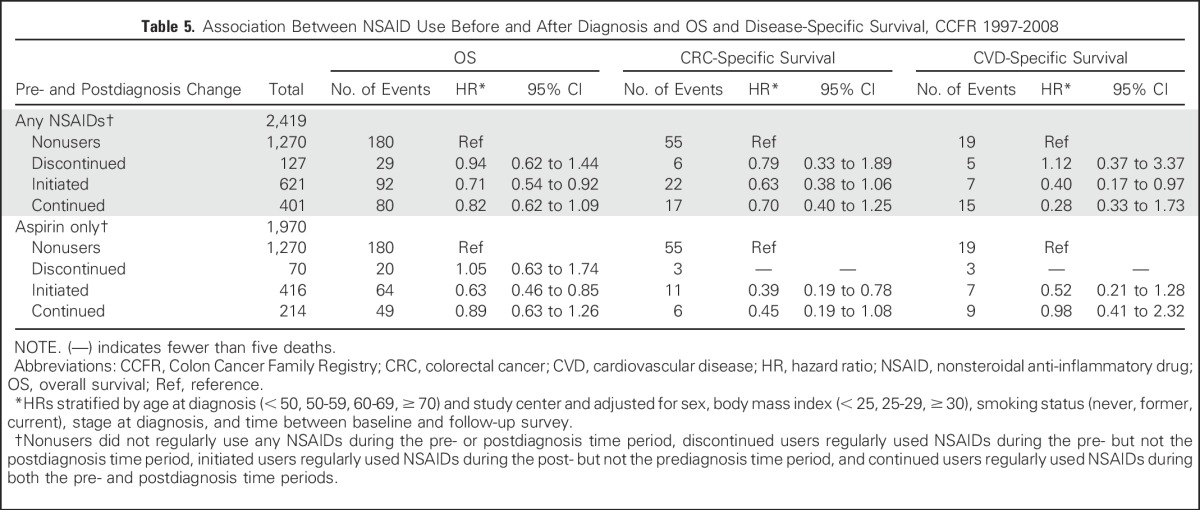
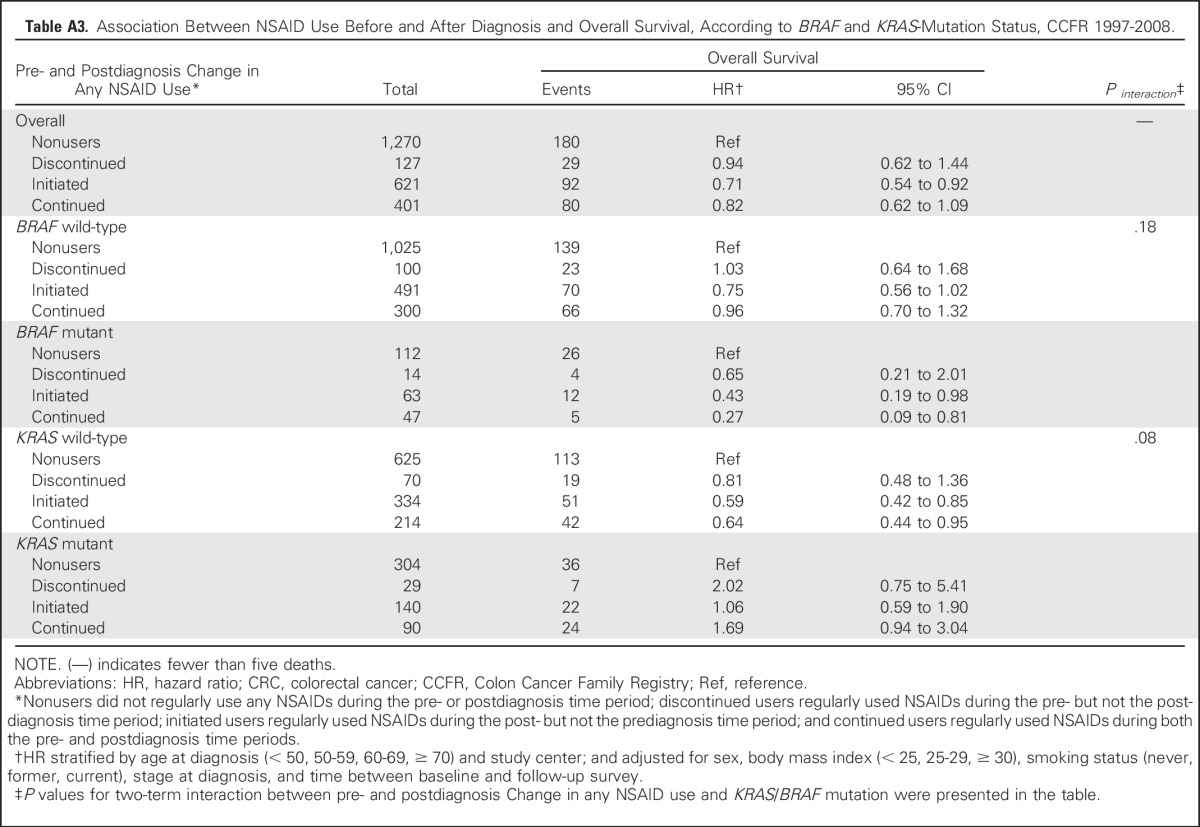
Among participants who reported any NSAID use before and after diagnosis, there were 1,315 never users (52%), 133 discontinued users (5%), 663 initiated users (26%), and 420 continued users (17%). The majority of participants who initiated or continued use of any NSAIDs were aspirin-only users (Table 5). In comparison with nonusers, initiated NSAID users experienced significantly better OS (HR, 0.71; 95% CI, 0.54 to 0.92), whereas no clear association was observed for continued and discontinued users. Similar patterns were observed for aspirin-only users. For CSS, initiated and continued aspirin-only users had improved CSS (HR, 0.39 [95% CI, 0.19 to 0.78] v HR, 0.45 [95% CI, 0.19 to 1.08]), although the latter did not reach statistical significance. When stratifying by KRAS-mutation status, we observed a similar pattern of association between pre- and postdiagnostic change in any NSAID use and OS among participants with KRAS wild-type tumors (Appendix Table A3).
Table 5.
Association Between NSAID Use Before and After Diagnosis and OS and Disease-Specific Survival, CCFR 1997-2008
DISCUSSION
In this population-based prospective study of long-term CRC survivors, we observed that the association between any NSAID use after diagnosis and OS seemed to be limited to individuals with KRAS wild-type tumors. The improved CSS may increase with longer duration of NSAID use after diagnosis. Our results also suggest that the association between aspirin use and OS was more pronounced among those who initiated aspirin use after diagnosis, whereas both initiated and continued users of aspirin after diagnosis seemed to be associated with more favorable CSS.
Our findings on the overall association between postdiagnostic use of aspirin and improved survival are consistent with existing literature.17-19,24,38 However, few studies had reported non-aspirin NSAID use after diagnosis in relation to survival. Our results suggest that postdiagnostic non-aspirin NSAID use may have a beneficial effect on OS, although it did not reach statistical significance. There was no evidence of a favorable association with CSS. Different patterns in use of these medications, instead of the type of NSAID, may partially explain their differential effects on CRC prognosis: non-aspirin NSAID users tended to be shorter-term and higher-dose users (on average 10.1 times per week; median duration of 1 year; IQR, 0.25 to 4 years), whereas regular users of aspirin were more likely to be medicated at a lower dose over a longer period of time (on average, 7.1 times per week; median duration of 4 years; IQR, 2 to 5 years). The importance of a cumulative pattern of NSAID use is further supported by our finding that longer duration of any NSAID use after diagnosis was associated with better CSS.
We also observed that the association between any NSAID use after diagnosis and improved survival is evident only among participants with KRAS wild-type tumors, but not for those with KRAS-mutant CRCs. An existing observational study39 reported that patients with PIK3CA-mutated tumors would benefit substantially from postdiagnostic aspirin use, but not those with wild-type PIK3CA tumors. Although it is unclear whether the pathway underlying the effect of NSAIDs on CRC prognosis by KRAS-mutation status differ from that by PIK3CA mutations, our finding supports the hypothesis that mutation of the KRAS proto-oncogene by activating the downstream Ras/mitogen-activated protein kinase (MAPK) pathway may lead to pro-proliferative and/or antiapoptotic effects that cannot be inhibited by NSAIDs. The suggestive interaction between BRAF and NSAID use after diagnosis, showing that only participants with BRAF-mutant CRC tumors might benefit from any NSAID use after diagnosis, indicates that the impact of NSAIDs may not uniformly affect prognosis between BRAF- and KRAS-mutant CRC tumors. Given the enriched family history sampling from the CCFR, we performed additional analysis by family history and found that the interaction between postdiagnostic NSAID use and KRAS mutations in relation to OS seemed to be limited to patients without family history, although three-term interaction did not reach statistical significance (P = .08). This is likely the result of limited sample size in the KRAS-mutant and family history–positive subgroup.
Several studies evaluated some aspects of the timing of aspirin use in relation to survival after CRC diagnosis; however, the findings were inconsistent.17,19,24Chan et al19 evaluated aspirin use after diagnosis according to prediagnosis use in two health professional cohorts (N = 1,279 patients with stage I to III CRC). They reported improved survival among individuals who initiated aspirin use after diagnosis compared with nonusers, similar to our findings. No association was observed in continued versus discontinued users. However, this study was not able to examine the association by additional tumor markers. In contrast, Walker et al17 only reported an association between continued use of aspirin and improved OS compared with discontinued users. Their study was not able to investigate disease-specific survival, and it had relatively short follow-up (3.1 years from diagnosis). More recently, a linkage study from Norway reported that continued, but not initiated, aspirin use after CRC diagnosis was associated with improved survival.24 Data linkage with the Norwegian Prescription Database limited their ability to adjust for potential confounders such as body mass index and smoking status and to include the use of over-the-counter aspirin, which was prevalent in Norway.40 Together, these studies suggest an association between postdiagnostic aspirin use and improved survival.17,19,24 However, it was inconclusive whether this association was influenced by prediagnostic aspirin use.
Limitations need to be considered when interpreting our results. Because we collected self-reported NSAID use retrospectively, misclassification of NSAID use may have occurred. Participants included in this study had to survive long enough after diagnosis to complete the follow-up questionnaire. Therefore our results may not apply to short-term survivors. In addition, without longitudinal information on all risk factors, our study design limited our ability to assess risk factors associated with mortality, specifically among long-term survivors for whom traditional risk factors might not impact survival in the same manner. Our study population was representative of the underlying source population, which was overwhelmingly white. Greater diversity is needed in future studies. Finally, the limited number of patients who used non-aspirin NSAIDs regularly led to reduced power for secondary analysis by tumor characteristics.
Our study also has many strengths. Patients were identified from population-based cancer registries, and therefore our results are generalizable to other white populations of long-term CRC survivors. Long follow-up time of the CCFR allowed us to explore not only OS, but also CSS and CVD-specific survival, and to investigate the effect of long-term NSAID use on survival. Standardized questionnaires with detailed information on types and duration of NSAID use before and after diagnosis, as well as potential confounders allowed us to explore different parameterization of NSAIDs and to estimate aspirin and non-aspirin NSAID use separately. Molecular characterization of CRC tumors enhanced our ability to conduct stratified analysis by tumor characteristics that were not addressed in previous studies.
In summary, postdiagnostic NSAID use was associated with improved survival among long-term CRC survivors diagnosed with KRAS wild-type tumors. Patients who initiated aspirin use after diagnosis had improved OS, whereas both initiated and continued use seemed to be associated with more favorable CSS. Although both observational and randomized studies provided convincing evidence of aspirin as an effective chemopreventive agent for CRC,8 aspirin is not generally recommended for primary prevention of CRC for people at average risk of CVD because of the potential risks. Aspirin for secondary prevention of CRC, particularly in subgroups such as those with KRAS wild-type tumors, is supported by our study. Future studies are needed to find the best timing, dose, and duration of aspirin use and to identify subgroups of patients with CRC for whom the benefits of aspirin outweigh its risk.
ACKNOWLEDGMENT
We thank all study participants of the Colon Cancer Family Registry and staff for their contributions to this project.
Appendix
Table A1.
Association Between Any NSAID Use After Diagnosis and Overall and CRC-Specific Survival, According to KRAS-Mutation Status and Family History, CCFR 1997-2008.
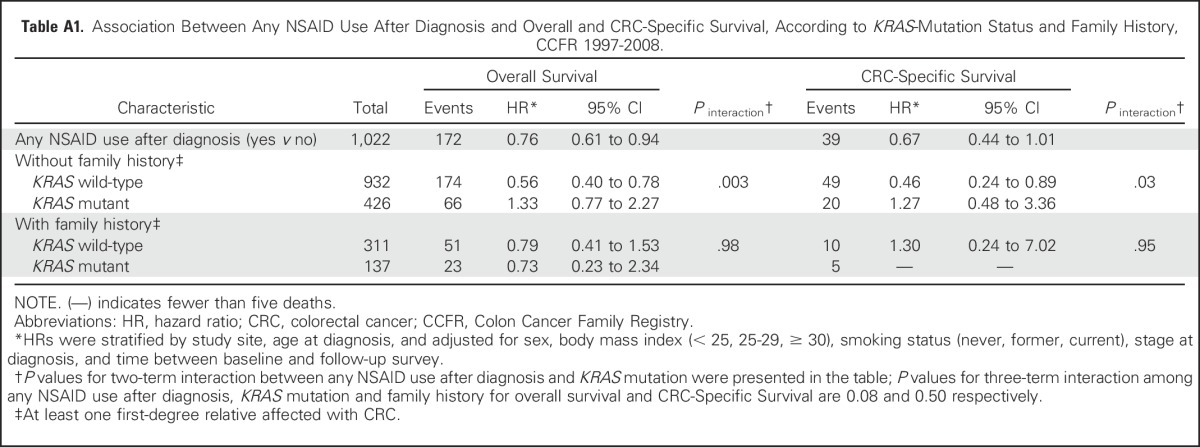
Table A2.
Association Between Duration of NSAID Use After Diagnosis and Overall and CRC-Specific Survival, According to BRAF and KRAS-Mutation Status, CCFR 1997-2008.
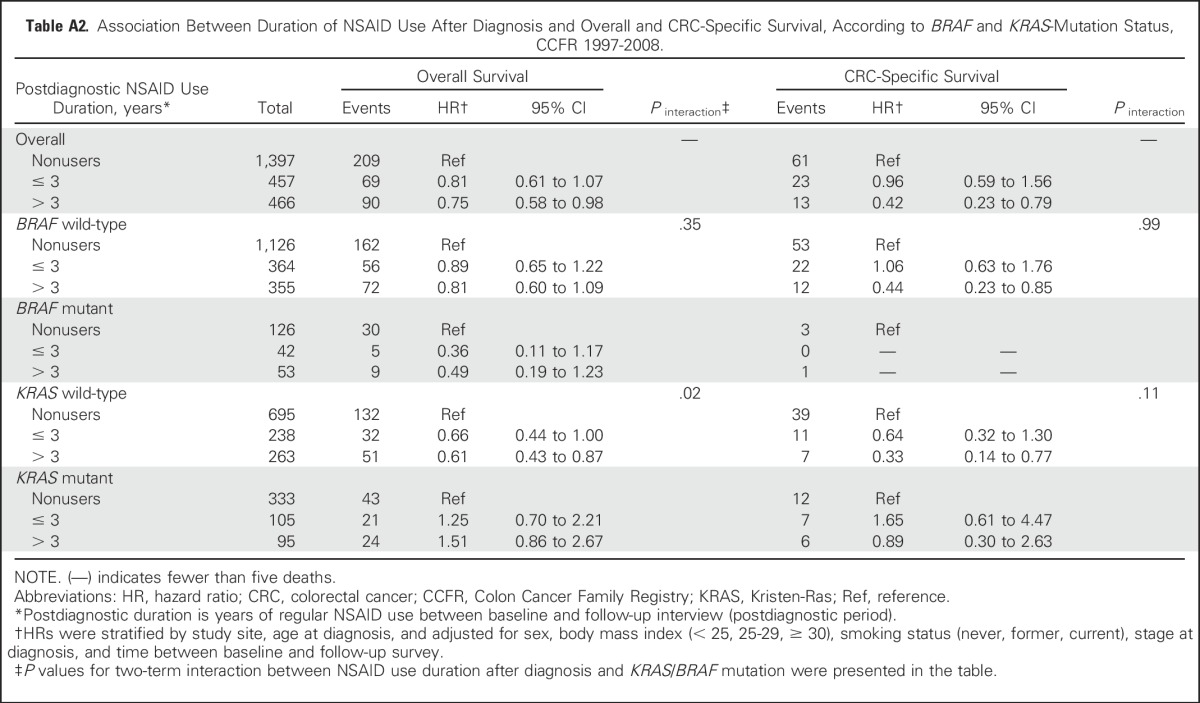
Table A3.
Association Between NSAID Use Before and After Diagnosis and Overall Survival, According to BRAF and KRAS-Mutation Status, CCFR 1997-2008.
Footnotes
Supported by Grant UM1 CA167551 from the National Cancer Institute (NCI) and through cooperative agreements with the following Colon Cancer Family Registry centers: Grants No. U01 CA074778 and U01/U24 CA097735 from the Australasian Colorectal Cancer Family Registry; U01/U24 CA074800 from the Mayo Clinic Cooperative Family Registry for Colon Cancer Studies; U01/U24 CA074783 from the Ontario Familial Colorectal Cancer Registry; and U01/U24 CA074794 from the Seattle Colorectal Cancer Family Registry; by Control No. N01 CN 67009 and N01 PC35142 and Contract No. HHSN2612013000121 from the SEER Program of the NCI (Fred Hutchinson Cancer Research Center), the Minnesota Cancer Surveillance System, the Victorian Cancer Registry (Australia), and the Ontario Cancer Registry (Canada); and Grant No. R01CA118699 from the NCI, National Institutes of Health (P. Laird, for Colon Cancer Family Register, CpG island methylator phenotype, and KRAS testing).
Presented at the 40th Annual Meeting of the American Society of Preventive Oncology, Columbus, OH, March 12-16, 2016 (preliminary results from the Seattle site of the Colon Cancer Family Registry only).
The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute, authors’ affiliated institutions, or any of the collaborating centers of the Colon Cancer Family Registry (CCFR), nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the CCFR.
AUTHOR CONTRIBUTIONS
Conception and design: Xinwei Hua, Scott V. Adams, Andrea N. Burnett-Hartman, Polly A. Newcomb
Provision of study materials or patients: Dennis J. Ahnen, Noralane M. Lindor, Polly A. Newcomb
Collection and assembly of data: Dennis J. Ahnen, Noralane M. Lindor, John A. Baron, Polly A. Newcomb
Data analysis and interpretation: Xinwei Hua, Amanda I. Phipps, Scott V. Adams, Sheetal Hardikar, Stacey A. Cohen, Jonathan M. Kocarnik, Dennis J. Ahnen, John A. Baron, Polly A. Newcomb
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Timing of Aspirin and Other Nonsteroidal Anti-Inflammatory Drug Use Among Patients With Colorectal Cancer in Relation to Tumor Markers and Survival
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Xinwei Hua
No relationship to disclose
Amanda I. Phipps
No relationship to disclose
Andrea N. Burnett-Hartman
No relationship to disclose
Scott V. Adams
Stock or Other Ownership: CVS Health, Gilead Sciences
Sheetal Hardikar
No relationship to disclose
Stacey A. Cohen
Research Funding: Boston Biomedical (Inst)
Travel, Accommodations, Expenses: Boston Biomedical
Jonathan M. Kocarnik
No relationship to disclose
Dennis J. Ahnen
Employment: Gastroenterology of the Rockies
Honoraria: Ambry Genetics, Cancer Prevention Pharmaceuticals
Consulting or Advisory Role: Cancer Prevention Pharmaceuticals
Speakers’ Bureau: Ambry Genetics
Noralane M. Lindor
Patents, Royalties, Other Intellectual Property: Royalties as editor for section in UpToDate.com
John A. Baron
Patents, Royalties, Other Intellectual Property: Together with Dartmouth College, I hold a use patent for chemopreventive use of calcium (currently not licensed) and a use patent for the colorectal chemopreventive use of aspirin (currently not licensed).
Polly A. Newcomb
No relationship to disclose
REFERENCES
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. : Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64:252-271, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Cole BF, Logan RF, Halabi S, et al. : Aspirin for the chemoprevention of colorectal adenomas: Meta-analysis of the randomized trials. J Natl Cancer Inst 101:256-266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron JA, Cole BF, Sandler RS, et al. : A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 348:891-899, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Sandler RS, Halabi S, Baron JA, et al. : A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med 348:883-890, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Friis S, Poulsen AH, Sørensen HT, et al. : Aspirin and other non-steroidal anti-inflammatory drugs and risk of colorectal cancer: A Danish cohort study. Cancer Causes Control 20:731-740, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Chan AT, Giovannucci EL, Meyerhardt JA, et al. : Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA 294:914-923, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flossmann E, Rothwell PM: Effect of aspirin on long-term risk of colorectal cancer: Consistent evidence from randomised and observational studies. Lancet 369:1603-1613, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Baron JA: Aspirin and NSAIDs for the prevention of colorectal cancer. Recent Results Cancer Res 181:223-229, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Giovannucci E, Egan KM, Hunter DJ, et al. : Aspirin and the risk of colorectal cancer in women. N Engl J Med 333:609-614, 1995 [DOI] [PubMed] [Google Scholar]
- 10. doi: 10.1093/jnci/djv170. Ait Ouakrim D, Dashti SG, Chau R, et al: Aspirin, ibuprofen, and the risk of colorectal cancer in Lynch syndrome. J Natl Cancer Inst 107(9), 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishihara R, Lochhead P, Kuchiba A, et al. : Aspirin use and risk of colorectal cancer according to BRAF mutation status. JAMA 309:2563-2571, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothwell PM, Wilson M, Elwin CE, et al. : Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 376:1741-1750, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Zell JA, Ziogas A, Bernstein L, et al. : Nonsteroidal anti-inflammatory drugs: Effects on mortality after colorectal cancer diagnosis. Cancer 115:5662-5671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coghill AE, Newcomb PA, Campbell PT, et al. : Prediagnostic non-steroidal anti-inflammatory drug use and survival after diagnosis of colorectal cancer. Gut 60:491-498, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coghill AE, Newcomb PA, Chia VM, et al. : Pre-diagnostic NSAID use but not hormone therapy is associated with improved colorectal cancer survival in women. Br J Cancer 104:763-768, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Din FV, Theodoratou E, Farrington SM, et al. : Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut 59:1670-1679, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Walker AJ, Grainge MJ, Card TR: Aspirin and other non-steroidal anti-inflammatory drug use and colorectal cancer survival: A cohort study. Br J Cancer 107:1602-1607, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCowan C, Munro AJ, Donnan PT, et al. : Use of aspirin post-diagnosis in a cohort of patients with colorectal cancer and its association with all-cause and colorectal cancer specific mortality. Eur J Cancer 49:1049-1057, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Chan AT, Ogino S, Fuchs CS: Aspirin use and survival after diagnosis of colorectal cancer. JAMA 302:649-658, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cardwell CR, Kunzmann AT, Cantwell MM, et al: Low-dose aspirin use after diagnosis of colorectal cancer does not increase survival: A case-control analysis of a population-based cohort. Gastroenterology 146:700-708.e2, 2014. [DOI] [PubMed]
- 21.Li P, Wu H, Zhang H, et al. : Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: A meta-analysis. Gut 64:1419-1425, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Domingo E, Church DN, Sieber O, et al. : Evaluation of PIK3CA mutation as a predictor of benefit from nonsteroidal anti-inflammatory drug therapy in colorectal cancer. J Clin Oncol 31:4297-4305, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Reimers MS, Bastiaannet E, Langley RE, et al. : Expression of HLA class I antigen, aspirin use, and survival after a diagnosis of colon cancer. JAMA Intern Med 174:732-739, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Bains SJ, Mahic M, Myklebust TA, et al. : Aspirin as secondary prevention in patients with colorectal cancer: An unselected population-based study. J Clin Oncol 34:2501-2508, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Newcomb PA, Baron J, Cotterchio M, et al. : Colon Cancer Family Registry: An international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev 16:2331-2343, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Colon Cancer Family Registry Informatics Center. http://coloncfr.org/
- 27. Fritz A, Percy C, Jack A, et al: International Classification of Diseases for Oncology (ed 3). Geneva, Switzerland, World Health Organization, 2013. [Google Scholar]
- 28.Boland CR, Thibodeau SN, Hamilton SR, et al. : A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58:5248-5257, 1998 [PubMed] [Google Scholar]
- 29.Shia J: Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: Part I. The utility of immunohistochemistry. J Mol Diagn 10:293-300, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindor NM, Burgart LJ, Leontovich O, et al. : Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol 20:1043-1048, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Cicek MS, Lindor NM, Gallinger S, et al. : Quality assessment and correlation of microsatellite instability and immunohistochemical markers among population- and clinic-based colorectal tumors results from the Colon Cancer Family Registry. J Mol Diagn 13:271-281, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchanan DD, Sweet K, Drini M, et al. : Risk factors for colorectal cancer in patients with multiple serrated polyps: A cross-sectional case series from genetics clinics. PLoS One 5:e11636, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliner K, Juan T, Suggs S, et al. : A comparability study of 5 commercial KRAS tests. Diagn Pathol 5:23, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alsop K, Mead L, Smith LD, et al. : Low somatic K-ras mutation frequency in colorectal cancer diagnosed under the age of 45 years. Eur J Cancer 42:1357-1361, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Ogino S, Cantor M, Kawasaki T, et al. : CpG island methylator phenotype (CIMP) of colorectal cancer is best characterised by quantitative DNA methylation analysis and prospective cohort studies. Gut 55:1000-1006, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weisenberger DJ, Siegmund KD, Campan M, et al. : CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet 38:787-793, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Hinoue T, Weisenberger DJ, Lange CP, et al. : Genome-scale analysis of aberrant DNA methylation in colorectal cancer. Genome Res 22:271-282, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reimers MS, Bastiaannet E, van Herk-Sukel MP, et al. : Aspirin use after diagnosis improves survival in older adults with colon cancer: A retrospective cohort study. J Am Geriatr Soc 60:2232-2236, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Liao X, Lochhead P, Nishihara R, et al. : Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med 367:1596-1606, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dale O, Borchgrevink PC, Fredheim OM, et al. : Prevalence of use of non-prescription analgesics in the Norwegian HUNT3 population: Impact of gender, age, exercise and prescription of opioids. BMC Public Health 15:461, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]