Abstract
Background
Osteosarcoma is a major bone malignancy in children and young adults, and it is highly heterogeneous. The clinical outcome of osteosarcoma is individual-dependent due to different genetic and pathological profiles. Although chemotherapy in combination with surgery has significantly improved the survival of localized disease, the prognostic improvement for metastatic patients is less marked. ECT2 (epithelial cell transforming sequence 2) is a transforming protein that can interact with Rho-like proteins of the Ras family and has been proven as an ontogenetic protein in cancer cell lines. We studied the clinical significance of ECT2 in osteosarcoma and explored its underlying oncogenic mechanisms.
Material/Methods
The protein expression pattern of ECT2 in osteosarcoma was investigated by immunohistochemical staining, and its association with clinicopathological characteristics was initially explored. The significance of ECT2 in predicting patient prognosis was verified by univariate and multivariate analyzes. Cellular experiments were conducted to explore underlying mechanisms of ECT2 in regulating osteosarcoma progression.
Results
High ECT2 expression was correlated with tumor metastasis and poor overall survival of osteosarcoma patients. ECT2 promotes cell invasion by modulating EMT process.
Conclusions
ECT2 is an independent prognostic factor for osteosarcoma and it can upregulate the metastatic capacity of osteosarcoma cells.
MeSH Keywords: Epithelial-Mesenchymal Transition, Neoplasm Metastasis, Osteosarcoma, Prognosis
Background
Osteosarcoma is a primary bone tumor that mostly affects children, adolescents, and young adults. It has been reported to be the third most common cancer in this age group, only behind lymphomas and brain tumors. The predilection site of osteosarcoma is the metaphysis of long bones such as the distal femur (43%), proximal tibia (23%), and proximal humerus (10%) [1–3]. Multimodal chemotherapy was introduced in the 1970s, and has since achieved dramatic prolonged survival for patients with localized disease, especially those responding well to the chemotherapy agents. However, osteosarcoma is generally aggressive and tends to occur as early distant metastasis [4]. Up to 20% of osteosarcoma patients present with imagological evidence of metastases at the time of diagnosis, with lung as the most prevalent metastatic location [5]. In addition, the risk of relapse after initial treatment of localized patients is approximately 30–40% within the first three years [6], and 90% of cases are lung metastases [7]. The five-year overall survival for those recurrent osteosarcoma patients is 23–29% [8]. Thus, efforts are needed to further improve clinical outcomes of osteosarcoma patients, including the need for studies on metastatic mechanisms, prognostic biomarkers, and novel chemotherapy or immunotherapy development.
Ect2 was firstly isolated from epithelial cells and identified as an oncogene due to its potency in inducing tumor formation in nude mice [9]. Later, it was revealed that ECT2 protein can interact with the Rho family proteins, including RhoA, RhoC, and Rac1 [10]. It functions as a guanine nucleotide exchange factor (GEF), which can be induced by growth factors and regulate cytokinesis [11]. The dysregulation and mislocalization of ECT2 can not only lead to malignant transformation [12], the phosphorylation status can also regulate oncogenetic pathways by modifying its structural conformation [13,14]. Moreover, in the past 10 year, the prognostic role of ECT2 in several cancer types has been reported, including glioma [15], lung cancer [16], oral cancer [17], hepatocellular carcinoma [18], ovarian cancer [19], as well as digestive cancers [20–22]. Even the involvement of ECT2 in the microRNA-233 signaling pathway has been reported in osteosarcoma cell lines [23,24], the ECT2 protein expression profiles and its significance in clinical practices of osteosarcoma still need further illustration.
In the current study, we initially investigated the protein expression and subcellular location of ECT2 in clinical osteosarcoma tissues. Then subsequently, the associations between ECT2 expression level and patient pathological features were analyzed. Kaplan-Meier univariate analysis and Cox multivariate analysis demonstrated that the high expression of ECT2 was correlated with unfavorable overall prognosis. Interestingly, cellular studies revealed that ECT2 overexpression has little effect on the proliferation of osteosarcoma cells, but can enhance the metastatic ability through upregulating epithelial-mesenchymal transition (EMT) process.
Material and Methods
Patients
A total of 49 patients diagnosed with osteoblastic osteosarcoma between June 2006 and June 2012 from Linyi People’s Hospital were enrolled in this study. All the patients underwent primary tumor resection and the final diagnosis was confirmed by routine pathology. We retrieved patient clinicopathological characteristics including age, sex, tumor location, histological grade, metastasis, and pathological response to chemotherapeutic agents. Written informed consent was obtained from all patients or their immediate family members. This study was approved by the Research Ethics Committee of Linyi People’s Hospital (Shandong, China).
Immunohistochemistry (IHC) and IHC evaluation
Biopsied osteosarcoma tissues before chemotherapy treatment were collected and embedded in paraffin. The detailed procedures for IHC staining have been described previously [25]. Briefly, tissue slides were treated as followed: de-waxed, rehydrated, antigen retrieval, goat serum blocking, primary antibody incubation (ECT2, Cat. #ab151503, Abcam, Cambridge, UK), secondary antibody incubation, and immunostaining using the DAB kit (Cat. #PW017, Sangon Biotech, Shanghai, China). Nonimmune serum was used as negative control.
The expression level of ECT2 protein was semi-quantified from the immunostaining results by two independent pathologists. The percentage of positive stained cells was calculated and scored as 0 (0–5% positive), 1 (5–25% positive), 2 (26–50% positive) and 3 (51–100% positive). The staining intensity was also scored as 0 (negative), 1 (weak, pale yellow), 2 (moderate, dark yellow), and 3 (strong, brown). The final immunoreactivity score (IRS) was calculated by multiplying the percentage and intensity scores (ranging from 0–9). To explore the role of ECT2 in osteosarcoma, patients were classified into two groups based on the IRS: low expression (IRS 0–4) and high expression (IRS 5–9).
Cell culture and transfection
Soas-2 cell line was selected due to its osteoblastic phenotype [26]. The cells were purchased from ATCC (American Type Culture Collection, Hongkong, China) and cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS, penicillin, and streptomycin at 37°C in 5% CO2.
Ect2 cDNA plasmid in pCMV3 vector was purchased from Abclonal Biotechnology (Cat. #HG18461-UT, MA, USA). The siRNAs were obtained from Santa Cruz Biotechnology (CA, USA) with the following sequences:
ECT2-siRNA: 5′-GAUAAAGGAUGAUCUUGAA-3′;
Scramble-siRNA: 5′-UUCUCCGAACGUGUCACGU-3′.
Both the overexpression and siRNA transfection were performed using Lipofectamine®2000 reagent (Cat. #11668019, ThermoFisher Scientific, PA, USA) as described previously [27].
Western blot
Cells were homogenized using NP-40 lysis buffer (Cat. #P0013F, Beyotime Biotechnology, Shanghai, China) after treatment. Total protein was extracted and quantified using Pierce BCA protein detection kit (ThermoFisher Scientific, PA, USA). Equal amounts of protein were loaded onto the SDS-PAGE gel, followed by transfer onto the PVDF membranes (Millipore Corp., Bedford, MA, USA). Blotting membranes were blocked for one hour using 0.5% BSA, and then incubated overnight with primary antibodies (ECT2, #ab151503, Abcam; N-cadherin, #sc-7939, Santa Cruz Biotechnology; E-cadherin, #sc-71009, Santa Cruz Biotechnology; Ki67, #sc-7846, Santa Cruz Biotechnology; p53, #10442-1-AP, ProteinTech; β-actin, #sc-47778, Santa Cruz Biotechnology. All 1: 1,000 dilutions) at 4 °C. The PVDF membranes were incubated for another 45 minutes at 25 °C with secondary antibodies and immunoreactivities were finally identified by quantifying the exposure intensity on films under ECL (Cat. #P0018, Beyotime Biotechnology, Shanghai, China) treatment.
MTT assay
Transfected cells were seeded into 96-well plates in triplicate at a density of 5,000 cells/well. After 24 hours, 48 hours, and 72 hours of cell culture, 20 μL of MTT (5 mg/mL) reagent was added to each well and incubated for another four hours at 37°C, then the medium was removed and 150 μL DMSO/well was added. The absorbance was measured at 490 nm on a microplate reader according to the manufacturer’s instructions.
Matrigel-Transwell assay
The invasion capacity of osteosarcoma cells was tested by Transwell assay. A total of 4×105 transfected cells were seeded into the upper side of a Matrigel-coated Transwell chamber (BD Biosciences, CA, USA). After cultured for another 12 hours, the membrane was fixed with 4% paraformaldehyde, stained with 0.05% crystal violet, and the invaded cells on the lower surface of the membrane were counted under a light microscope.
Statistics
The overall survival time was defined as the survival months from the date of tumor resection to June 2014 or the date of death. Survival curves were profiled with the Kaplan-Meier method using GraphPad Prism 6.01 software. The correlations between ECT2 expression and the clinicopathological parameters of osteosarcoma patients were evaluated by chi-square test. Univariate and multivariate analyzes were performed to figure out independent prognostic factors. All the data was tested by Student t-test, and p<0.05 was considered as statistically significant.
Results
Patient characteristics
For the 49 enrolled patients with osteoblastic osteosarcoma, 18 cases (36.7%) were female and 31 cases (63.3%) were male. The primary tumor location included tibia (15 cases, 30.6%), femur (20 cases, 40.8%), humeral (10 cases, 20.4%), and other locations (4 cases, 8.2%). Twenty-six patients (53.1%) were classified as high histological grade according to the Enneking staging system [28]. Twenty-eight patients (57.1%) presented with tumor metastasis at the time of diagnosis (25 cases with lung metastases and three cases with bone metastases). The patients’ responses to chemotherapy were evaluated according to the Huvos grading system, poor chemotherapy response showed <90% tumor necrosis while good chemotherapy response suggested ≥90% necrosis in the resected tumors. In our cohort, 30 cases (61.2%) were identified as good chemotherapy response, while the other 19 cases (38.8%) were poor response. Detailed patient information is shown in Table 1.
Table 1.
Association of ECT2 expression with clinicopathological characteristics of osteosarcoma patients.
| Variables | Cases (n=49) | ECT2 protein level | P value | ||
|---|---|---|---|---|---|
| Low (n=23) | High (n=26) | ||||
| Age (years) | ≤18 ys | 21 | 11 | 10 | 0.509 |
| >18 ys | 28 | 12 | 16 | ||
| Sex | Female | 18 | 8 | 10 | 0.790 |
| Male | 31 | 15 | 16 | ||
| Location | Tibia | 15 | 8 | 7 | 0.212 |
| Femur | 20 | 10 | 10 | ||
| Humeral | 10 | 2 | 8 | ||
| Others | 4 | 3 | 1 | ||
| Tumor grade | Low | 23 | 11 | 12 | 0.907 |
| High | 26 | 12 | 14 | ||
| Metastasis | Negative | 28 | 17 | 11 | 0.026* |
| Positive | 21 | 6 | 15 | ||
| Chemotherapy response | Good | 30 | 15 | 15 | 0.590 |
| Poor | 19 | 8 | 11 | ||
ECT2 – epithelial cell transforming sequence 2.
Expression of ECT2 in osteosarcoma tissues and its correlation with clinicopathological parameters
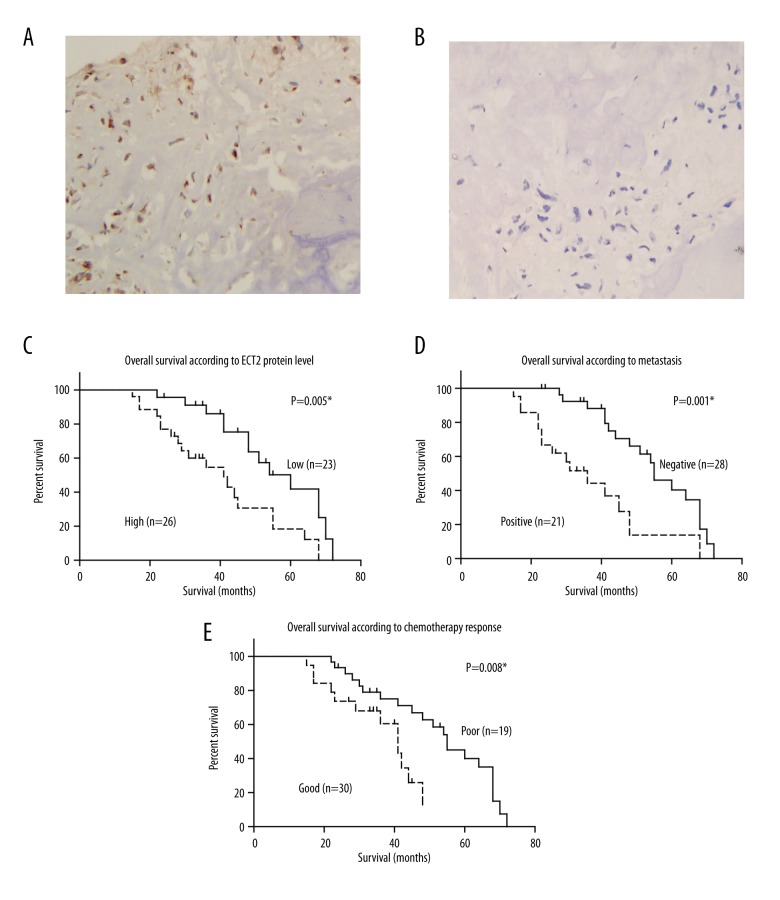
ECT2 protein was located in both the cytoplasm and nucleus of osteosarcoma cells, and patients showed distinct expression level of ECT2 (Figure 1A, 1B). According to the immunoreactivity of ECT2, 23 cases (46.9%) were classified as low expression and the other 26 cases (53.1%) as high expression. Chi-square test was performed to explore the associations between ECT2 protein level and patient characteristics, which demonstrated that high ECT2 expression was correlated with tumor metastasis (p=0.026, Table 1). This result indicates that ECT2 may function as an oncogenetic protein in the progression of osteosarcoma.
Figure 1.
Expression and prognostic role of ECT2 in osteosarcoma. (A) Representative high expression of ECT2 in osteosarcoma tissues by IHC. (B) Representative low expression of ECT2 in osteosarcoma tissues by IHC. (C) High protein expression of ECT2 indicates poor prognosis of osteosarcoma (p=0.005). (D) Positive tumor metastasis is correlated with unfavorable overall survival of osteosarcoma (p=0.001). (E) Patients with poor chemotherapy response show poorer clinical outcomes (p=0.008). The survival curves were profiled by Kaplan-Meier method and tested by Student t test. * p<0.05 indicates statistical significance. Magnification (400×).
High expression of ECT2 indicates poor overall survival of osteosarcoma patients
We further investigated whether ECT2 protein level was helpful in predicting prognosis of osteosarcoma. Kaplan-Meier analysis showed that the overall survival of patients with high ECT2 protein levels was poorer than those with low ECT2 levels (40.6±3.7 versus 55.4±3.5 months, p=0.005; Table 2, Figure 1C). Tumor metastasis (p=0.001, Figure 1D) and poor chemotherapy response (p=0.008, Figure 1E) also indicated unfavorable clinical outcomes.
Table 2.
Kaplan-Meier survival analysis of osteosarcoma patients.
| Variables | OS time (months) | 3-year OS (%) | P value | ||
|---|---|---|---|---|---|
| Mean ±SD | Median | ||||
| Age (years) | ≤18 ys | 46.5±4.4 | 41.0 | 59.6% | 0.850 |
| >18 ys | 48.6±3.5 | 48.0 | 77.6% | ||
| Sex | Female | 51.7±4.6 | 60.0 | 69.3% | 0.561 |
| Male | 45.5±3.5 | 44.0 | 69.5% | ||
| Location | Tibia | 48.4±5.4 | 48.0 | 65.0% | 0.577 |
| Femur | 51.8±3.2 | 51.0 | 88.2% | ||
| Humeral | 40.9±7.4 | 31.0 | 46.7% | ||
| Others | 41.3±11.9 | 23.0 | 50.0% | ||
| Tumor grade | Low | 47.8±3.6 | 48.0 | 72.0% | 0.551 |
| High | 47.7±4.2 | 45.0 | 67.7% | ||
| Metastasis | Negative | 55.1±2.9 | 55.0 | 88.1% | 0.001* |
| Positive | 36.9±4.2 | 36.0 | 44.2% | ||
| Chemotherapy response | Good | 52.5±3.3 | 55.0 | 75.0% | 0.008* |
| Poor | 36.3±2.8 | 41.0 | 60.5% | ||
| ECT2 protein level | Low | 55.4±3.5 | 60.0 | 86.0% | 0.005* |
| High | 40.6±3.7 | 41.0 | 54.6% | ||
OS – overall survival; ECT2 – epithelial cell transforming sequence 2; S.D – standard deviation.
To better elucidate the prognostic role of ECT2, we next enrolled the parameters that were statistical significant into Cox hazard regression study, including metastasis, chemotherapy response, and ECT2 protein level. The multivariate analysis demonstrated that ECT2 protein expression level can act as an independent prognostic factor for the overall survival (p=0.037, Table 3). Other independent risk factors included positive metastasis (p=0.018) and poor chemotherapy response (p=0.005).
Table 3.
Cox hazard regression analysis of osteosarcoma patients.
| Variables | HR | 95% CI | P value | |
|---|---|---|---|---|
| Metastasis | Negative | Reference | ||
| Positive | 2.50 | 1.17–5.35 | 0.018* | |
| Chemotherapy response | Good | Reference | ||
| Poor | 3.55 | 1.46–8.64 | 0.005* | |
| ECT2 protein level | Low | Reference | ||
| High | 2.26 | 1.05–4.88 | 0.037* |
ECT2 – epithelial cell transforming sequence 2; HR – hazard ration; CI – confidence interval.
ECT2 promotes cell invasion by modulating EMT process
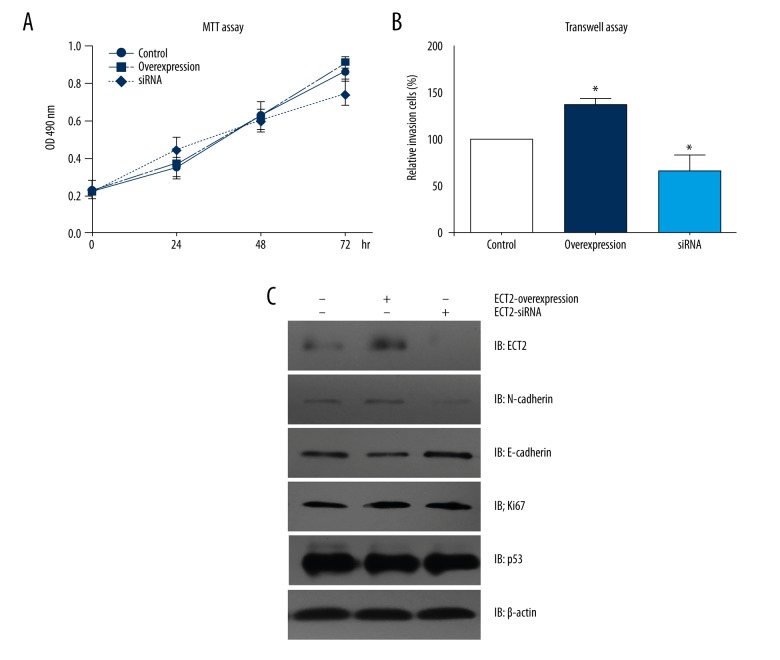
Since our clinical results implied a correlation between ECT2 expression and tumor metastasis, we next performed cellular studies to explore the underlying mechanisms. We did not find any significantly proliferative effect on ECT2 overexpression or siRNA knock-down (Figure 2A). Interestingly, the invasive capacity of Soas-2 cells was remarkably enhanced after ECT2 overexpression, whereas ECT2-siRNA inhibited the invasive process (Figure 2B).
Figure 2.
ECT2 promotes the invasion of osteosarcoma cells by upregulating EMT process. (A) ECT2 had little effect on the proliferation of osteosarcoma cells according to MTT assay. (B) Matrigel-Transwell assay revealed that ECT2 overexpression enhanced cell invasion, whereas ECT2-siRNA inhibited cell invasion. (C) Western blot results showed that ECT2 overexpression elevated the protein level of N-cadherin and downregulated the E-cadherin level. All the experiments were performed for at least three times, independently. The graphs’ lines showed the standard deviation. * indicated p<0.05 by Student t-test compared with control group.
Furthermore, Western blot results showed that ECT2 may not alter the proliferation and apoptosis of tumor cells, as reflected by Ki67 and p53 biomarkers, respectively. However, the protein level of N-cadherin was upregulated while E-cadherin was downregulated in ECT2-overexpression cells, whereas ECT2-siRNA showed opposite effects. Therefore, ECT2 protein may promote the EMT process in osteosarcoma cells, which subsequently enhances tumor metastasis.
Discussion
As one of the most common tumor types affecting adolescents and young adults, osteosarcoma has been postulated to be associated with rapid bone growth [29]. The five-year overall survival rate for localized osteosarcoma has been improved to about 65–70% after the combination of chemotherapy with surgical resection [30,31]. However, the high metastasis and relapse risk lead to an unsatisfactory clinical outcome. More and more studies are now focusing on studying the metastatic mechanisms and identifying novel prognostic biomarkers; simultaneously, preclinical and clinical trials are underway to test the efficiency of novel chemotherapy agents.
In this study, we explored the protein expression of ECT2 in clinical osteosarcoma tissues, which verified the correlations between ECT2 and tumor metastasis. Univariate and multivariate analyzes further identified ECT2 as an independent risk factor for the overall survival of osteosarcoma patients. Our clinical results not only expanded the current knowledge about the oncogenetic role of ECT2 in tumors, but also provided evidence for its potential application in predicting patient clinical outcomes.
Moreover, we performed in vitro studies to better investigate the role of ECT2 in osteosarcoma cells. The cellular results indicated that ECT2 enhanced the invasive capacity of tumor cells, whereas no significant effect on cell proliferation was found. Taking into consideration that EMT is one of the most important mechanisms in regulating tumor metastasis, we next tested the changes of EMT markers on ECT2 overexpression and knock-down. Immunoblots showed that ECT2 did indeed upregulate the expression of EMT proteins, although it showed little effect on cell proliferation markers.
Conclusions
Our study revealed that high protein expression of ECT2 in osteosarcoma tissues indicated poor clinical outcomes, and ECT2 promoted tumor metastasis through regulating the EMT process.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Gianferante DM, Mirabello L, Savage SA. Germline and somatic genetics of osteosarcoma – connecting aetiology, biology and therapy. Nat Rev Endocrinol. 2017;13(8):480–91. doi: 10.1038/nrendo.2017.16. [DOI] [PubMed] [Google Scholar]
- 2.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776–90. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 3.Jawad MU, Cheung MC, Clarke J, et al. Osteosarcoma: Improvement in survival limited to high-grade patients only. J Cancer Res Clin Oncol. 2011;137(4):597–607. doi: 10.1007/s00432-010-0923-7. [DOI] [PubMed] [Google Scholar]
- 4.Raymond AK, Jaffe N. Osteosarcoma multidisciplinary approach to the management from the pathologist’s perspective. Cancer Treat Res. 2009;152:63–84. doi: 10.1007/978-1-4419-0284-9_4. [DOI] [PubMed] [Google Scholar]
- 5.Bielack SS, Kempf-Bielack B, Delling GN, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776–90. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 6.Kempf-Bielack B, Bielack SS, Jürgens H, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23(3):559–68. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari S, Briccoli A, Mercuri M, et al. Postrelapse survival in osteosarcoma of the extremities: Prognostic factors for long-term survival. J Clin Oncol. 2003;21(4):710–15. doi: 10.1200/JCO.2003.03.141. [DOI] [PubMed] [Google Scholar]
- 8.Carrle D, Bielack S. Osteosarcoma lung metastases detection and principles of multimodal therapy. Cancer Treat Res. 2009;152:165–84. doi: 10.1007/978-1-4419-0284-9_8. [DOI] [PubMed] [Google Scholar]
- 9.Miki T, Fleming TP, Bottaro DP, et al. Expression cDNA cloning of the KGF receptor by creation of a transforming autocrine loop. Science. 1991;251(4989):72–76. doi: 10.1126/science.1846048. [DOI] [PubMed] [Google Scholar]
- 10.Miki T, Smith CL, Long JE, Eva A, Fleming TP. Oncogene ect2 is related to regulators of small GTP-binding proteins. Nature. 1993;362(6419):462–65. doi: 10.1038/362462a0. [DOI] [PubMed] [Google Scholar]
- 11.Tatsumoto T, Sakata H, Dasso M, Miki T. Potential roles of the nucleotide exchange factor ECT2 and Cdc42 GTPase in spindle assembly in Xenopus egg cell-free extracts. J Cell Biochem. 2003;90(5):892–900. doi: 10.1002/jcb.10750. [DOI] [PubMed] [Google Scholar]
- 12.Saito S, Liu XF, Kamijo K, et al. Deregulation and mislocalization of the cytokinesis regulator ECT2 activate the Rho signaling pathways leading to malignant transformation. J Biol Chem. 2004;279(8):7169–79. doi: 10.1074/jbc.M306725200. [DOI] [PubMed] [Google Scholar]
- 13.Hara T, Abe M, Inoue H, et al. Cytokinesis regulator ECT2 changes its conformation through phosphorylation at Thr-341 in G2/M phase. Oncogene. 2006;25(4):566–78. doi: 10.1038/sj.onc.1209078. [DOI] [PubMed] [Google Scholar]
- 14.Niiya F, Tatsumoto T, Lee KS, Miki T. Phosphorylation of the cytokinesis regulator ECT2 at G2/M phase stimulates association of the mitotic kinase Plk1 and accumulation of GTP-bound RhoA. Oncogene. 2006;25(6):827–37. doi: 10.1038/sj.onc.1209124. [DOI] [PubMed] [Google Scholar]
- 15.Sano M, Genkai N, Yajima N, et al. Expression level of ECT2 proto-oncogene correlates with prognosis in glioma patients. Oncol Rep. 2006;16(5):1093–98. [PubMed] [Google Scholar]
- 16.Hirata D, Yamabuki T, Miki D, et al. Involvement of epithelial cell transforming sequence-2 oncoantigen in lung and esophageal cancer progression. Clin Cancer Res. 2009;15(1):256–66. doi: 10.1158/1078-0432.CCR-08-1672. [DOI] [PubMed] [Google Scholar]
- 17.Iyoda M, Kasamatsu A, Ishigami T, et al. Epithelial cell transforming sequence 2 in human oral cancer. PloS One. 2010;5(11):e14082. doi: 10.1371/journal.pone.0014082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang SM, Ooi LL, Hui KM. Upregulation of Rac GTPase-activating protein 1 is significantly associated with the early recurrence of human hepatocellular carcinoma. Clin Cancer Res. 2011;17(18):6040–51. doi: 10.1158/1078-0432.CCR-11-0557. [DOI] [PubMed] [Google Scholar]
- 19.Huff LP, Decristo MJ, Trembath D, et al. The eole of Ect2 nuclear RhoGEF activity in ovarian cancer cell transformation. Genes Cancer. 2013;4(11–12):460–75. doi: 10.1177/1947601913514851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin Y, Yu Y, Shao Q, et al. Up-regulation of ECT2 is associated with poor prognosis in gastric cancer patients. Int J Clin Exp Pathol. 2014;7(12):8724–31. [PMC free article] [PubMed] [Google Scholar]
- 21.Luo Y, Qin SL, Mu YF, et al. Elevated expression of ECT2 predicts unfavorable prognosis in patients with colorectal cancer. Biomed Pharmacother. 2015;73:135–39. doi: 10.1016/j.biopha.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Wang HB, Yan HC, Liu Y. Clinical significance of ECT2 expression in tissue and serum of gastric cancer patients. Clin Transl Oncol. 2016;18(7):735–42. doi: 10.1007/s12094-015-1428-2. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Yin Z, Ning K, et al. Prognostic value of microRNA-223/epithelial cell transforming sequence 2 signaling in patients with osteosarcoma. Hum Pathol. 2014;45(7):1430–36. doi: 10.1016/j.humpath.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Xu J, Yao Q, Hou Y, et al. MiR-223/Ect2/p21 signaling regulates osteosarcoma cell cycle progression and proliferation. Biomed Pharmacother. 2013;67(5):381–86. doi: 10.1016/j.biopha.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Gao P, Li Z. Expression of G protein-coupled receptor 56 is an unfavorable prognostic factor in osteosarcoma patients. Tohoku J Exp Med. 2016;239(3):203–11. doi: 10.1620/tjem.239.203. [DOI] [PubMed] [Google Scholar]
- 26.Pautke C, Schieker M, Tischer T, et al. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res. 2004;24(6):3743–48. [PubMed] [Google Scholar]
- 27.Liu H, Xu Y, Zhang Q, et al. Correlations between TBL1XR1 and recurrence of colorectal cancer. Sci Rep. 2017;7:44275. doi: 10.1038/srep44275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–20. [PubMed] [Google Scholar]
- 29.Geller DS, Gorlick R. Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8(10):705–18. [PubMed] [Google Scholar]
- 30.Link MP, Goorin AM, Horowitz M, et al. Adjuvant chemotherapy of high-grade osteosarcoma of the extremity. Updated results of the Multi-Institutional Osteosarcoma Study. Clin Orthop Relat Res. 1991;(270):8–14. [PubMed] [Google Scholar]
- 31.Rosen G, Caparros B, Huvos AG, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49(6):1221–30. doi: 10.1002/1097-0142(19820315)49:6<1221::aid-cncr2820490625>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]