Abstract
Background: Non-muscle invasive bladder cancer (NMIBC) is associated with high rates of recurrence, resulting in frequent follow-up cystoscopies. We evaluated the use of two point-of-care tests - the nuclear matrix protein 22 (NMP22) and urinary bladder cancer antigen (UBC) Rapid - compared to routine follow-up in patients with a previous history of NMIBC.
Methods: 31 patients with cystoscopy-verified active bladder cancer, and 44 follow-up patients without disease as confirmed by cystoscopy were prospectively enrolled. All urine samples were analyzed by voided urine and bladder washing cytology, NMP22 and UBC rapid test (qualitatively and quantitatively). The best cutoff (highest Youden index; ≥6.7 ng/ml) for the quantitative UBC was determined by receiver operating characteristic curves.
Results: Voided urine and barbotage cytology resulted in a sensitivity of 25.8% and 32.3%, and a specificity of 100% and 100%, while the NMP22 showed a sensitivity and specificity of 12.9% and 100%, respectively. The qualitative and quantitative UBC Rapid revealed a sensitivity of 61.3% and 64.5%, with a specificity of 77.3% and 81.8%. Barbotage cytology and qualitative UBC test proved to be the best dual combination with the highest overall sensitivity (77.4%). In contrast to barbotage cytology alone, sensitivity increased from 21.4% to 50% for detecting low-grade tumors, and from 43.8% to 100% for high-grade cancers, but reducing specificity from 100% to 77.3%.
Conclusion: Compared to urinary cytology, UBC tests alone as well as UBC tests in combination with bladder washing cytology revealed higher sensitivities in detecting low- and high-grade tumors, but at the expense of a lower specificity. Thus, currently cystoscopy cannot be replaced by any of the evaluated methods.
Keywords: Bladder Cancer, surveillance, recurrence, urine markers, cytology, NMP22, UBC, biomarkers
Introduction
Bladder cancer (BCa) is the eleventh most commonly diagnosed cancer in the world with an estimated 430 000 new cases reported in 2012. It also ranks fourteenth among the leading causes of cancer death 1-2. Given the steadily rising life expectancy and the rising costs of health care systems, BCa has become a global problem 1-2. For example, bladder cancer caused an expense of about 2.9 billion Euros for public health systems across the European Union in 2012, constituting 5% of the total health care expenses for cancer 3.
At the initial diagnosis, as many as 75% of patients with BCa present with non-muscle invasive disease confined to the mucosa or submucosa 4. The majority of patients in this stage of disease are successfully treated with endoscopic surgery, followed by adjuvant intravesical chemotherapy or Bacillus-Calmette Guérin (BCG) instillation treatment in the case of intermediate-risk and high-risk non-muscle invasive bladder cancer (NMIBC), 5. Non-muscle invasive cancer disease has a significant lower risk of cancer-specific mortality with a better long-term survival compared to muscle-invasive bladder cancer 4,6. Nevertheless, lifelong follow-up is needed in non-muscle invasive disease because of the high probability of recurrence (ranging from 15% to 61% at 1 year and from 31% to 78% at 5 years), especially in patients with carcinoma in situ (CIS) and T1 lesions with a high potential for malignancy 5,7. Thus, surveillance of NMIBC should be risk-adapted, with regular cystoscopies, urinary cytology and upper urinary tract imaging in the follow-up recommendation of current guidelines 8-9.
Cytopathology of the voided bladder or bladder washing urine specimens is a widely used and non-invasive test for the detection or in the surveillance of bladder cancer, with a high sensitivity of 84% in high-grade BCa 10-11. Although cytology is very specific (about 86%), its overall low sensitivity (48%) is a clear limitation. In fact, its sensitivity is a mere 16% for low-grade tumors 10-11. Moreover, the diagnostic accuracy of urinary cytology varies between study centers because of subjective cytology interpretation criteria, depending on the expertise and experience of cytopathologists requiring highly trained healthcare professionals, who may not be available in all areas 11-12. Over the years, various classification schemes for urinary cytology have been published, introducing the Paris System for Reporting Urine Cytology in 2013 13-14. Some limitations of prior classification schemes include a lack of rigorous definition of validated cytological criteria for individual categories and a lack of consensus for atypical categorization 14. Hence, significant efforts have been made to develop novel molecular and gene-based urinary tests that may reduce or, ideally replace, the frequency of endoscopy in patients under surveillance for BCa recurrence 15.
The aim of this prospective study was to evaluate and compare the diagnostic accuracy of urinary cytology (voided urine and bladder washing) with two urine-based point-of-care (POC) tests - the nuclear matrix protein 22 (NMP22)® BladderChek and the urinary bladder cancer antigen (UBC)® rapid (qualitative and quantitative) test - for the surveillance of patients with a previous history of non-muscle invasive bladder cancer (NMIBC).
Material and Methods
This prospective single-center study was approved by the local ethics committee (study number AN2016-0056; 360/4.7) of the Medical University of Innsbruck (Austria). Informed written consent for further urine analysis in addition to standard urine cytology was obtained from each patient prior to inclusion in the study. Patients with a previous history of NMIBC who had undergone routine oncological follow-up at our outpatient uro-oncology department between May 2016 and September 2016 were enrolled prospectively in the pilot study. To exclude a urinary tract infection, all patients underwent a routine urine dipstick analysis prior to cystoscopy. Symptomatic bacteriuria or a florid urinary tract infection was a contraindication for further investigation, and thus an exclusion criterion for the study.
The follow-up protocol included cystoscopy (flexible in men, rigid in women) in combination with voided urine and bladder washing cytology as the gold standard. In addition, all urinary samples were analyzed with two POC tests: the NMP22® BladderChek and the UBC® Rapid test (visually and quantitatively by the Omega 100 POC reader). The frequency of cystoscopy during follow-up depended on tumor histology and tumor grading. In accordance with the current European Association of Urology (EAU) guidelines and our institutional practice, patients with low-risk NMIBC underwent a cystoscopy every 3 months during the first year, and then once a year for 5 years. Intermediate-risk and high-risk NMIBC were followed by cystoscopy every 3 months during the first two years, at 6-month intervals for 5 years, and then once a year 8. Upper urinary tract imaging (CT-urography) was carried out once a year in high-risk NMIBC (according to the European Organization for Research and Treatment of Cancer scoring system and risk tables 5) or when a disease recurrence was detected. In case of cystoscopy-verified cancer recurrence or a positive cytology with no visible tumor, transurethral resection of the bladder (TURB) or PDD-guided, random-biopsies were performed. All cancer specimens obtained from TURB were investigated in regard of diagnosis, tumor grade (WHO 1973 and 2004) and stage (TNM 2009) by an experienced uropathologist.
Cytological evaluation of urinary specimens for exfoliated tumor cells was performed according to the Papanicolaou scheme. While classes 1 and 2 were considered negative, classes 4 and 5 were deemed suspicious and positive for bladder cancer 14. Patients with class 3 (atypical urothelial cells) cytological findings and negative cystoscopy underwent a cystoscopy and urine cytology control three months later.
The qualitative NMP22® BladderChek test (Alere, Waltham, Massachusetts, USA) and the UBC® Rapid test (Concile, Freiburg im Breisgau, Germany) were performed according to the manufacturers` protocols. Briefly, the NMP22 enzyme immunoassay is specific for the nuclear mitotic apparatus protein (NuMA), an abundant component of the nuclear matrix protein, which can be overexpressed in the nucleus of different cancer cells where aneuploidy is a common feature in driving tumor progression (such as epithelian ovarian cancer 16), reflecting mitotic activity of cells 17. The UBC® Rapid test measures the soluble fragments of cytokeratin 8 and 18, which play an active role in tumor invasion 18, by qualitative determination, as well as quantitatively using the Omega 100 POC reader (Concile, Freiburg im Breisgau, Germany).
Statistics
For the assessment of the diagnostic accuracy of voided urine and bladder wash cytology, NMP22 BladderChek® test, and qualitative UBC Rapid® test as compared to cystoscopy, data were tabulated in contingency tables. Sensitivity, specificity, positive and negative predictive values (PPV and NPV) were calculated. These analyses were also performed after stratification by tumor grade and tumor stage. For all tests, receiver operating characteristic (ROC) curves were plotted and the area under the curve (AUC) was calculated and tested for significance with the Mann-Whitney-U test. The optimal cutoff value for the quantitative UBC Rapid® test was determined as the value with the highest Youden index. To determine whether the combination of any two or more of the qualitative tests improves the diagnostic accuracy, we compared the sensitivity, specificity, positive and negative predictive value, and AUCs of various combined tests (deemed positive when any of the single tests yielded a positive result, otherwise negative) against the single test results. A significance level of α = 0.05 (two-tailed) was applied for all p-values. Statistical analyses were performed using the SPSS software, version 22 (IBM Corp., Armonk, NY).
Results
Baseline characteristics
In all 75 patients [21 (28.0%) women and 54 (72.0%) men] with a mean age of 70.6 years (median 70, range 38-91 years) were enrolled in this prospective study. All patients underwent a routine follow-up of a previous NMIBC. Tumor recurrence was noted in 31 (41.3%) of 75 patients, respectively. Histological investigation of TURB confirmed pTa, carcinoma in situ (CIS), pT1 and pT2 in 16 (51.6%), 6 (19.4%), 6 (19.4%) and 3 (9.6%) patients, respectively. Fifteen (48.4%) and 16 (51.6%) were classified as low-grade and high-grade tumors, respectively, according to the 2004 WHO classification system. Grades 1, 2, 3 were identified in 10 (32.3%), 9 (29%) and 12 (38.7%) tumors, respectively, based on the 1973 WHO classification.
Diagnostic accuracy of urinary cytology, the NMP22, the qualitative and the quantitative UBC Rapid test as single tests
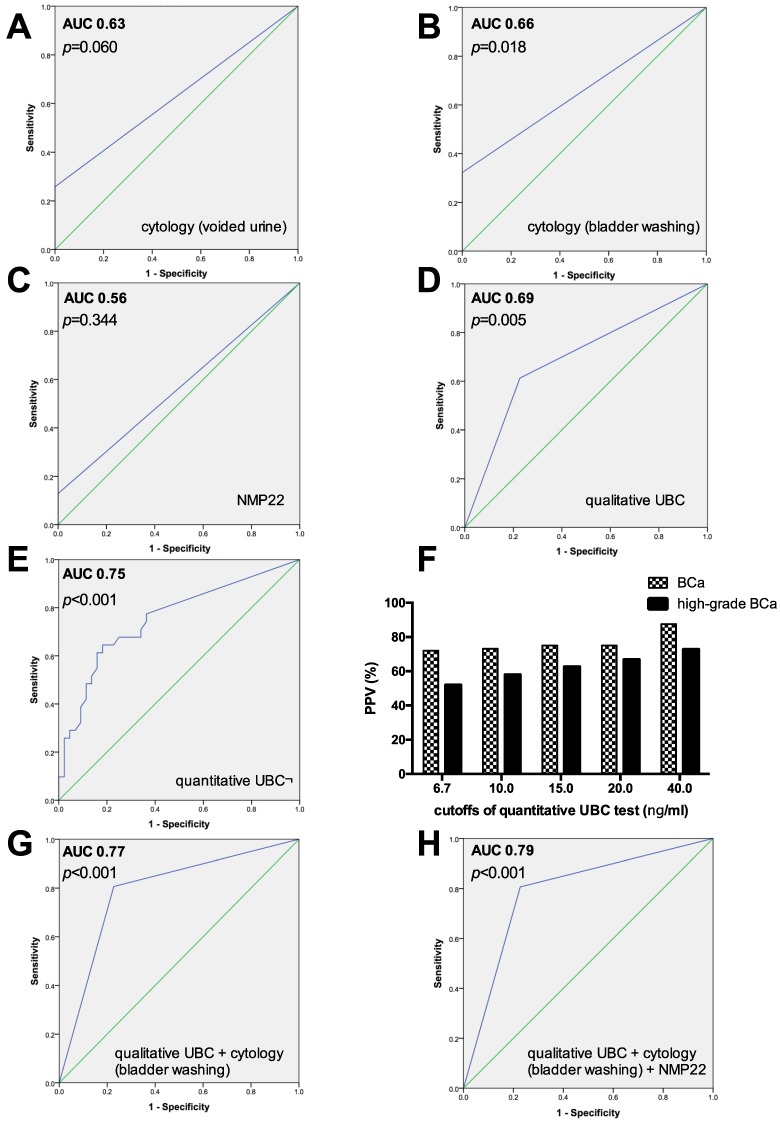
The sensitivities, specificities, PPVs and NPVs of the individual tests for predicting bladder cancer recurrence are shown in Table 1. ROC curves and AUCs for cytology (voided urine and bladder wash), NMP22, quantitative and qualitative UBC test are summarized in Figures 1A-1E, Figures 1G-1H.
Table 1.
Diagnostic accuracy including sensitivity, specificity, PPV and NPV for each single urinary marker such as NMP22® BladderChek, qualitative and quantitative (using the best cutoff of 6.7 ng/ml) UBC® Rapid test, and urine cytology (voided urine and bladder wash). Sensitivities were stratified by tumor grade and tumor stage.
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC (95% CI) |
P-value* | |
|---|---|---|---|---|---|---|
| NMP22 | 12.9 | 100.0 | 100.0 | 61.9 | 0.56 (0.43-0.70) |
0.344 |
| Low-grade | 7.1 | |||||
| High-grade | 18.8 | |||||
| pTa | 6.3 | |||||
| CIS | 16.7 | |||||
| pT1 | 40.0 | |||||
| pT2 | 0.0 | |||||
| UBC qualitative | 61.3 | 77.3 | 65.5 | 73.9 | 0.69 (0.57-0.82) |
0.005 |
| Low-grade | 28.6 | |||||
| High-grade | 87.5 | |||||
| pTa | 37.5 | |||||
| CIS | 83.3 | |||||
| pT1 | 100.0 | |||||
| pT2 | 66.7 | |||||
| UBC quantitative (best cutoff >6.7 ng/mL) | 64.5 | 81.8 | 71.4 | 76.6 | 0.75 (0.64-0.87) |
<0.001 |
| Low-grade | 35.7 | |||||
| High-grade | 87.5 | |||||
| pTa | 37.5 | |||||
| CIS | 100.0 | |||||
| pT1 | 100.0 | |||||
| pT2 | 66.7 | |||||
| Urinary cytology (voided urine) | 25.8 | 100.0 | 100.0 | 65.2 | 0.63 (0.49-0.76) |
0.060 |
| Low-grade | 7.1 | |||||
| High-grade | 43.8 | |||||
| pTa | 6.3 | |||||
| CIS | 66.7 | |||||
| pT1 | 20.0 | |||||
| pT2 | 66.7 | |||||
| Urinary cytology (bladder washing) | 32.3 | 100.0 | 100.0 | 67.7 | 0.66 (0.53-0.79) |
0.018 |
| Low-grade | 21.4 | |||||
| High-grade | 43.8 | |||||
| pTa | 18.8 | |||||
| CIS | 66.7 | |||||
| pT1 | 20.0 | |||||
| pT2 | 66.7 |
Figure 1.
A-E, G-H) ROC curves and AUC calculated for voided and barbotage cytology, NMP22 test, quantitative and qualitative UBC test as single investigations, and different combinations. F) Positive predictive value (PPV) of the quantitative UBC test in percentages (%), based on different cutoffs, concerning the probability of bladder cancer (BCa) and high-grade BCa.
Urinary cytology of voided urine and bladder washing yielded an overall sensitivity, specificity, PPV and NPV of 25.8% and 32.3%, 100% and 100%, 100% and 100%, and 65.2 and 67.7%, respectively. The AUC after ROC analysis was 0.63 for voided urine cytology (p=0.060), and 0.66 (p=0.018) for barbotage cytology. The calculated sensitivity of cytology (voided urine and bladder wash) was both 43.8% for high-grade tumors, but only 7.1% and 21.4% for low-grade tumors. The highest sensitivity was achieved for primary CIS (66.7% for voided and barbotage cytology) and pT2 tumors (66.7% for both, voided and bladder wash cytology).
The NMP22 BladderChek® test showed a low sensitivity of 12.9% and a high specificity of 100%; PPV and NPV were 100% and 61.9%, respectively. The AUC was 0.56, with no significant ability to predict tumor recurrence (p=0.344). The sensitivity of the NMP22 test for detecting low-grade and high-grade tumors was 7.1% and 18.8%, respectively.
The overall sensitivity, specificity, PPV and NPV of the visually (strong band intensity= positive) evaluated qualitative UBC® Rapid test was 61.3%, 77.3%, 65.5% and 73.9%, respectively. The sensitivity increased with tumor grade (low-grade vs. high-grade, 28.6% vs. 87.5%) and tumor stage (pTa vs. pT2, 37.5% vs. 66.7%).
For the quantitative UBC® Rapid test using the optimal threshold obtained by ROC analysis (cutoff ≥ 6.7 ng/ml), the sensitivity, specificity, PPV and NPV were 64.5%, 81.8%, 71.4% and 76.6%, respectively. This urinary POC test yielded the highest AUC value (0.75; p<0.001).
The positive predictive value of the quantitative UBC Rapid test for bladder cancer and high-grade bladder cancer was strongly correlated with increaseing cutoffs, Figure 1F. Specifically, the PPV (95% CI) of bladder cancer increased from 72.0% (58.1%-86.4%; cutoff ≥6.7 ng/ml), 73.1% (56.3%-89.5%; cutoff ≥10 ng/ml), 75% (53.9-93.8%; cutoff ≥15 ng/ml), 75% (50.0%-100%; cutoff ≥20 ng/ml) to 87.5% (50.0%-100%; cutoff ≥40 ng/ml). For high-grade BCa, the PPV was 51.9% (40.0%-66.7%) for a cutoff of ≥6.7 ng/ml, 57.9% (42.9%-76.5%) for ≥10 ng/ml, 62.5% (42.9%-83.3%) for ≥15 ng/ml, 66.7% (42.9%-90.9%) for ≥20 ng/ml and 72.7% (37.5%-100%) for a threshold of ≥40 ng/ml, Figure 1F.
Diagnostic accuracy for different combinations of urinary cytology with the NMP22, the quantitative and qualitative UBC Rapid test
Table 2 shows the results obtained by combining the individual diagnostic methods. Combining each urinary POC marker test with urinary cytology, barbotage cytology and the qualitative UBC Rapid test proved to be the best dual combination with the highest sensitivity (77.4%) and AUC value (0.77; 95% CI: 0.66-0.88). In contrast to bladder wash cytology alone, the sensitivity for the combination of barbotage cytology and the qualitative UBC for detecting low-grade and high-grade tumors increased from 21.4% to 50.0% and from 43.8% to 100%, but reducing specificity from 100% to 77.3% (Figure 1G and Table 2). Performing a triple combination, bladder wash cytology, the qualitative UBC Rapid and the NMP22 test resulted in a sensitivity, specificity, PPV and NPV of 80.6%, 77.3%, 71.4% and 85%, respectively (Figure 1H and Table 2).
Table 2.
Diagnostic accuracy including sensitivity, specificity, PPV and NPV for different combinations of NMP22® BladderChek, qualitative and quantitative (using the best cutoff of 6.7 ng/ml) UBC® Rapid test, and urine cytology (voided urine and bladder wash). Sensitivities were stratified by tumor grade and tumor stage.
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC (95% CI) | P-value* | |
|---|---|---|---|---|---|---|
| NMP22 + cytology (voided urine) | 32.3 | 100.0 | 100.0 | 67.2 | 0.66 (0.53-0.79) |
0.018 |
| Low-grade | 14.3 | |||||
| High-grade | 50.0 | |||||
| pTa | 12.5 | |||||
| CIS | 66.7 | |||||
| pT1 | 40.0 | |||||
| pT2 | 0.0 | |||||
| NMP22 + cytology (bladder washing) | 38.7 | 100.0 | 100.0 | 69.8 | 0.69 (0.56-0.82) |
0.005 |
| Low-grade | 28.6 | |||||
| High-grade | 50.0 | |||||
| pTa | 25.0 | |||||
| CIS | 66.7 | |||||
| pT1 | 40.0 | |||||
| pT2 | 66.7 | |||||
| UBC qualitative + cytology (voided urine) | 71.0 | 76.7 | 68.8 | 78.6 | 0.74 (0.62-0.85) |
<0.001 |
| Low-grade | 35.7 | |||||
| High-grade | 100.0 | |||||
| pTa | 43.8 | |||||
| CIS | 100.0 | |||||
| pT1 | 100.0 | |||||
| pT2 | 0.0 | |||||
| UBC qualitative + cytology (bladder washing) | 77.4 | 77.3 | 70.6 | 82.9 | 0.77 (0.66-0.88) |
<0.001 |
| Low-grade | 50.0 | |||||
| High-grade | 100.0 | |||||
| pTa | 56.3 | |||||
| CIS | 100.0 | |||||
| pT1 | 100.0 | |||||
| pT2 | 100.0 | |||||
| NMP22 + UBC qualitative + cytology (voided urine) | 74.2 | 76.7 | 69.7 | 80.5 | 0.75 (0.64-0.87) |
<0.001 |
| Low-grade | 42.9 | |||||
| High-grade | 100.0 | |||||
| pTa | 50.0 | |||||
| CIS | 100.0 | |||||
| pT1 | 100.0 | |||||
| pT2 | 0.0 | |||||
| NMP22 + UBC qualitative + cytology (bladder washing) | 80.6 | 77.3 | 71.4 | 85.0 | 0.79 (0.68-0.89) |
<0.001 |
| Low-grade | 57.1 | |||||
| High-grade | 100.0 | |||||
| pTa | 62.5 | |||||
| CIS | 100.0 | |||||
| pT1 | 100.0 | |||||
| pT2 | 0.0 | |||||
| UBC quantitative + cytology (voided urine) | 71.0 | 81.4 | 73.3 | 79.5 | 0.76 (0.64-0.88) |
<0.001 |
| Low-grade | 42.9 | |||||
| High-grade | 93.8 | |||||
| pTa | 43.8 | |||||
| CIS | 100.0 | |||||
| pT1 | 100.0 | |||||
| pT2 | 0.0 | |||||
| UBC quantitative + cytology (bladder washing) | 74.2 | 81.8 | 74.2 | 81.8 | 0.78 (0.67-0.89) |
<0.001 |
| Low-grade | 50.0 | |||||
| High-grade | 93.8 | |||||
| pTa | 50.0 | |||||
| CIS | 100.0 | |||||
| pT1 | 100.0 | |||||
| pT2 | 0.0 | |||||
| UBC quantitative + NMP22 + cytology (voided urine) | 74.2 | 81.4 | 74.2 | 81.4 | 0.78 (0.66-0.89) |
<0.001 |
| Low-grade | 50.0 | |||||
| High-grade | 93.8 | |||||
| pTa | 50.0 | |||||
| CIS | 100.0 | |||||
| pT1 | 100.0 | |||||
| pT2 | 0.0 | |||||
| UBC quantitative + NMP22 + cytology (bladder washing) | 77.4 | 81.8 | 75.0 | 83.7 | 0.79 (0.69-0.90) |
<0.001 |
| Low-grade | 57.1 | |||||
| High-grade pTa |
93.8 56.3 |
|||||
| CIS | 100.0 | |||||
| pT1 | 100.0 | |||||
| pT2 | 0.0 |
Discussion
In view of the fact that urinary cytology is of limited diagnostic value for detecting low-grade bladder tumors (in our series the sensitivity of bladder wash cytology was 21.4%) compared to high-grade tumors (up to 84% 10), novel molecular and gene-based urinary tests are currently being developed and tested, partially in clinical practice, with promising preliminary results 19-20. One example is a urinary assay combination of FGF3, TERT and OTX1, with a sensitivity of 57% for the detection of low-grade NMIBC, 83% for pT1 tumors and 72% for high-grade disease 21. Despite a negative cystoscopy, positive urinary test results were predictive of recurrence over time, in comparison to negative urine samples (58% vs. 36%), 21. Moreover, urinary miRNA analysis also seems to be a valuable method for the BCa surveillance 22. A urinary miRNA panel including six miRNAs detected cancer recurrence with a high sensitivity of 84.8% 22 to 88% 20, thus reducing cystoscopy rates by 30% in the validation cohort 20. However, the “ideal urine-based marker” for detecting BCa recurrence during surveillance would be rapid, noninvasive, easy to perform and interpret, and must possess not only a high specificity to reduce superfluous cystoscopies on oncological follow-up, but also a high sensitivity so that no patient with low-grade and high-grade bladder cancer will be missed 12. Nevertheless, there is a paucity of randomized trials concerning urinary molecular marker tests for evaluating the possibility of safely reducing cystoscopy rates during surveillance.
In this prospective study, we investigated the diagnostic value of two urinary-based POC tests (NMP22 BladderChek® and UBC Rapid®) versus urine cytology for the detection of BCa recurrence in the surveillance of patients with a previous history of NMIBC. A variety of urinary POC test systems are available on the market at the present time, permitting non-invasive and rapid determination of urinary markers, but their diagnostic accuracy is controversially discussed in a limited number of studies 23-24. The sensitivities are usually higher than those reported for urinary cytology alone, but at the cost of a lower specificity 15,24. Thus, the additional costs of further urinary markers in the surveillance protocol are not justified at the moment 25. The use of those markers for routine follow-up is not recommended in clinical practice by current guidelines, and remains a debated issue 8,24. Urinary tract infection, previous intravesical BCG or chemotherapy instillation therapy, mechanical stress due to instrumented urine sampling, benign conditions or hematuria (especially for the BTA stat test) were noted in connection with many urinary marker tests, resulting in frequent false positive results 15,24,26-28. Another limitation of the tests is the fact that they only permit a qualitative analysis, because they yield a simple positive or negative result. The overall sensitivity and specificity of the qualitative NMP22 BladderChek® test for detecting BCa recurrence has been reported to range from 16.4% to 55.7%, and 85.7% to 100%, respectively 29-31. These results are in line with our findings, which yielded a low overall sensitivity of 12.9% and a high specificity of 100% for the NMP22 test, and a similar low sensitivity when stratified by tumor grade (7.1% for low-grade and 18.8% high-grade tumors). Consequently, the NMP22 test did not significantly improve the diagnostic accuracy of urinary barbotage cytology alone (21.4% and 43.8% for low-grade and high-grade) compared to the combined assessment (NMP22 plus barbotage cytology: 28.6% and 50.0%).
For the urinary BC antigen (UBC) Rapid® test, the Concile Omega 100 reader was developed to obtain quantitative data in addition to the qualitative analysis 31. The sensitivity of the qualitative UBC Rapid test ranged from from 46.2% to 78.4%, and its specificity from 82.4% to 97.4% 31-36. These data concur with our own, which revealed an overall sensitivity and specificity of 61.3% and 77.3%, respectively. Whereas the sensitivity was low (28.6%) for detecting low-grade tumors, it increased to 87.5% for high-grade tumors and achieved the highest sensitivity of a single urinary marker test for detecting high-grade BCa.
Combined application of urinary cytology and molecular urine markers such as fluorescence in situ hybridization (FISH) or immunocytology (uCyt+) could improve the diagnostic accuracy of detecting BCa compared to cytology alone 37-38. We observed the highest overall sensitivity of 77.4% (AUC=0.77) for the dual combination of the qualitative UBC Rapid® test and bladder washing cytology, increasing the sensitivity for low-grade and high-grade tumors from 21.4% to 50%, and from 43.8% to 100%, compared to bladder washing cytology alone.
The diagnostic accuracy of the the quantitative UBC® Rapid POC system has been assessed in just four studies, which reported a sensitivity of 46.6% to 64.5% and a specificity ranging from 70.1% to 86.3%, respectively 31-32, 35-36. In our series, the quantitative UBC test using the best cutoff (highest Youden index) of ≥ 6.7 ng/ml showed the highest overall sensitivity and specificity of 64.5% and 81.8%, respectively, for a single urine marker test. Moreover, the probability of detecting BCa as well as high-risk BCa clearly increased with the cutoff value of the UBC rapid test 31. The PPV of the quantitative UBC was 42.7% and 21.9% for detecting BCa and high-risk tumors using a UBC concentration >10 ng/ml, and increased to 75% and 55% for a UBC concentration >60 ng/ml 31. This fact was confirmed in our study: we noted a PPV (95% CI) of 73.1% (56.3-89.5%) for detecting BCa using 10 ng/ml as a cutoff, and 87.5% (50-100%) for ≥40 ng/ml. For high-grade tumors, the PPV increased from 57.9% (42.9-76.5%) to 72.7% (37.5-100%), compared to the threshold of ≥10 ng/ml and ≥40 ng/ml. Nevertheless, neither quantitative UBC alone (sensitivity 35.7%) nor its combination with bladder washing cytology (sensitivity 50.0%) was sufficiently sensitive for detecting low-grade bladder cancers compared to high-grade tumors (87.5% and 93.8%), with a reduction of specificity from 100% to 81.4% using a dual combination (quantitative UBC and barbotage cytology).
The main limitation of this single-center pilot study is the small sample size of 75 patients, which limited the statistical interpretation with its quality and reproducibility as well. The merits of the investigation are worthy of mention: it was a prospective assessment of a homogeneous patient population with a previous history of NMIBC using a standardized oncological surveillance protocol including cystoscopy, voided urine and bladder washing cytology, with routine upper urinary tract imaging performed once a year in high-risk NMIBC patients and in all patients with cancer recurrence.
Conclusion
The overall sensitivity of both, the qualitative and the quantitative UBC Rapid test was higher, but its specificity was lower than that of urinary cytology alone, whereas the sensitivity of the qualitative NMP22 test remained consistently lower. The sensitivity for detecting low-grade tumors was similarly low in all analyzed single urinary molecular test methods (between 7.1% and 35.7%). The best dual combination of test methods with the highest overall sensitivity of 77.4% was achieved through the qualitative UBC Rapid test and bladder wash cytology. It revealed high-grade cancers in 100%, but low-grade tumors with a sensitivity of 50.0%, with a reduction of specificity from 100% to 77.3%. Based on these data, none of the assessed urinary marker tests alone or any combinations of these a) meets all of the criteria of an “ideal urine tumor marker” and thus, b) can currently replace cystoscopy for oncological BCa surveillance.
Acknowledgments
We thank Werner Uhl-Michelin of In Vitro Diagnostika for supporting this work, by providing the Omega 100 POC reader free of charge for this pilot study.
Abbreviations
- AUC
area under the curve
- BCa
bladder cancer
- CIS
carcinoma in situ
- NMIBC
non-muscle invasive bladder cancer
- NMP22
nuclear matrix protein 22
- NPV
negative predictive value
- NuMA
nuclear mitotic apparatus protein
- POC
point of care
- PPV
positive predictive value
- ROC
receiver operating curve
- TURB
transurethral resection of the bladder
- UBC
urinary bladder cancer antigen.
References
- 1.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol; 2016. Jun 28. pii: S0302-2838(16)30280-9. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010 Dec 15;127(12):2893–917. doi: 10.1002/ijc.25516. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Leal J, Luengo-Fernandez R, Sullivan R, Witjes JA. Economic Burden of Bladder Cancer Across the European Union. Eur Urol. 2016 Mar;69(3):438–47. doi: 10.1016/j.eururo.2015.10.024. doi: 10.1016/j.eururo.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Burger M, Catto JW, Dalbagni G. et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013 Feb;63(2):234–41. doi: 10.1016/j.eururo.2012.07.033. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Sylvester RJ, van der Meijden AP, Oosterlinck W. et al. Predicting recurrence and progression in individual patients with stage Ta, T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–5. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Steinmaus C, Ferreccio C, Acevedo J. et al. Increased lung and bladder cancer incidence in adults after in utero and early-life arsenic exposure. Cancer Epidemiol Biomarkers prev. 2014 Aug;23(8):1529–38. doi: 10.1158/1055-9965.EPI-14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soukup V, Babjuk M, Bellmunt J. et al. Follow-up after surgical treatment of bladder cancer: a critical analysis of the literature. Eur Urol. 2012 Aug;62(2):290–302. doi: 10.1016/j.eururo.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Babjuk M, Böhle A, Burger M, EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol; 2016. Jun 17. pii: S0302-2838(16)30249-4. doi: 10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 9.Kassouf W, Traboulsi SL, Schmitz-Dräger B. et al. Follow-up in non-muscle-invasive bladder cancer-International Bladder Cancer Network recommendations. Urol Oncol. 2016 Oct;34(10):460–8. doi: 10.1016/j.urolonc.2016.05.028. doi: 10.1016/j.urolonc.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 10.Yafi FA, Brimo F, Steinberg J, Aprikian AG, Tanguay S, Kassouf W. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol Oncol. 2015 Feb;33(2):66.e25–31. doi: 10.1016/j.urolonc.2014.06.008. doi: 10.1016/j.urolonc.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Têtu B. Diagnosis of urothelial carcinoma from urine. Mod Pathol. 2009 Jun;22(Suppl 2):S53–9. doi: 10.1038/modpathol.2008.193. doi: 10.1038/modpathol.2008.193. [DOI] [PubMed] [Google Scholar]
- 12.Shariat SF, Karam JA, Lotan Y, Karakiewizc PI. Critical evaluation of urinary markers for bladder cancer detection and monitoring. Rev Urol. 2008;10(2):120–35. [PMC free article] [PubMed] [Google Scholar]
- 13.VandenBussche CJ. A review of the Paris system for reporting urinary cytology. Cytopathology. 2016 Jun;27(3):153–6. doi: 10.1111/cyt.12345. doi: 10.1111/cyt.12345. [DOI] [PubMed] [Google Scholar]
- 14.Owens CL, Vandenbussche CJ, Burroughs FH, Rosenthal DL. A review of reporting systems and terminology for urine cytology. Cancer Cytopathol. 2013 Jan;121(1):9–14. doi: 10.1002/cncy.21253. doi: 10.1002/cncy.21253. [DOI] [PubMed] [Google Scholar]
- 15.Lokeshwar VB, Habuchi T, Grossman HB. et al. Bladder tumor markers beyond cytology: International Consensus Panel on bladder tumor markers. Urology. 2005 Dec;66(6 Suppl 1):35–63. doi: 10.1016/j.urology.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 16.Brüning-Richardson A, Bond J, Alsiary R. et al. NuMA overexpression in epithelial ovarian cancer. PLoS One. 2012;7(6):e38945.. doi: 10.1371/journal.pone.0038945. doi: 10.1371/journal.pone.0038945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng C. NuMA: a nuclear protein involved in mitotic centrosome function. Microsc Res Tech. 2000 Jun 1;49(5):467–77. doi: 10.1002/(SICI)1097-0029(20000601)49:5<467::AID-JEMT9>3.0.CO;2-V. Review. [DOI] [PubMed] [Google Scholar]
- 18.Southgate J, Harnden P, Trejdosiewicz LK. Cytokeratin expression patterns in normal and malignant urothelium: a review of the biological and diagnostic implications. Histol Histopathol. 1999 Apr;14(2):657–64. doi: 10.14670/HH-14.657. [DOI] [PubMed] [Google Scholar]
- 19.Sapre N, Anderson PD, Costello AJ, Hovens CM, Corcoran NM. Gene-based urinary biomarkers for bladder cancer: an unfulfilled promise? Urol Oncol. 2014 Jan;32(1):48.e9–17. doi: 10.1016/j.urolonc.2013.07.002. doi: 10.1016/j.urolonc.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Sapre N, Macintyre G, Clarkson M. et al. A urinary microRNA signature can predict the presence of bladder urothelial carcinoma in patients undergoing surveillance. Br J Cancer. 2016 Feb 16;114(4):454–62. doi: 10.1038/bjc.2015.472. doi: 10.1038/bjc.2015.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beukers W, van der Keur KA, Kandimalla R, FGFR3, TERT and OTX1 as urinary biomarker combination for surveillance of bladder cancer patients in a large prospective multicenter study. J Urol; 2016. Dec 31. pii: S0022-5347(16)32099-7. doi: 10.1016/j.juro.2016.12.096. [DOI] [PubMed] [Google Scholar]
- 22.Mengual L, Lozano JJ, Ingelmo-Torres M, Gazquez C, Ribal MJ, Alcaraz A. Using microRNA profiling in urine samples to develop a non-invasive test for bladder cancer. Int J Cancer. 2013 Dec 1;133(11):2631–41. doi: 10.1002/ijc.28274. doi: 10.1002/ijc.28274. [DOI] [PubMed] [Google Scholar]
- 23.Chou R, Gore JL, Buckley D. et al. Urinary Biomarkers for Diagnosis of Bladder Cancer: A Systematic Review and Meta-analysis. Ann Intern Med. 2015 Dec 15;163(12):922–31. doi: 10.7326/M15-0997. doi: 10.7326/M15-0997. [DOI] [PubMed] [Google Scholar]
- 24.van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol. 2005 Jun;47(6):736–48. doi: 10.1016/j.eururo.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Kamat AM, Karam JA, Grossman HB, Kader AK, Munsell M, Dinney CP. Prospective trial to identify optimal bladder cancer surveillance protocol: reducing costs while maximizing sensitivity. BJU Int. 2011 Oct;108(7):1119–23. doi: 10.1111/j.1464-410X.2010.10026.x. doi: 10.1111/j.1464-410X.2010.10026. [DOI] [PubMed] [Google Scholar]
- 26.Hennenlotter J, Huber S, Todenhöfer T. et al. Point-of-Care Tests for Bladder Cancer: The Influencing Role of Hematuria. Adv Urol. 2011;2011:937561.. doi: 10.1155/2011/937561. doi: 10.1155/2011/937561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raitanen MP, Kaasinen E, Lukkarinen O. et al. Finnbladder Group. Analysis of false-positive BTA STAT test results in patients followed up for bladder cancer. Urology. 2001 Apr;57(4):680–4. doi: 10.1016/s0090-4295(00)01055-4. [DOI] [PubMed] [Google Scholar]
- 28.Lüdecke G, Pilatz A, Hauptmann A, Bschleipfer T, Weidner W. Comparative analysis of sensitivity to blood in the urine for urine-based point-of-care assays (UBC rapid, NMP22 BladderChek and BTA-stat) in primary diagnosis of bladder carcinoma. Interference of blood on the results of urine-based POC tests. Anticancer Res. 2012 May;32(5):2015–8. [PubMed] [Google Scholar]
- 29.Grossman HB, Messing E, Soloway M. et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA. 2005 Feb 16;293(7):810–6. doi: 10.1001/jama.293.7.810. [DOI] [PubMed] [Google Scholar]
- 30.Smrkolj T, Mihelič M, Sedlar A, Sterle I, Osredkar J, Sedmak B. Performance of nuclear matrix protein 22 urine marker and voided urine cytology in the detection of urinary bladder tumors. Clin Chem Lab Med. 2011 Feb;49(2):311–6. doi: 10.1515/CCLM.2011.038. doi: 10.1515/CCLM.2011.038. [DOI] [PubMed] [Google Scholar]
- 31.Ritter R, Hennenlotter J, Kühs U. et al. Evaluation of a new quantitative point-of-care test platform for urine-based detection of bladder cancer. Urol Oncol. 2014 Apr;32(3):337–44. doi: 10.1016/j.urolonc.2013.09.024. doi: 10.1016/j.urolonc.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Hakenberg OW, Fuessel S, Richter K. et al. Qualitative and quantitative assessment of urinary cytokeratin 8 and 18 fragments compared with voided urine cytology in diagnosis of bladder carcinoma. Urology. 2004 Dec;64(6):1121–6. doi: 10.1016/j.urology.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Sánchez-Carbayo M, Herrero E, Megías J, Mira A, Soria F. Initial evaluation of the new urinary bladder cancer rapid test in the detection of transitional cell carcinoma of the bladder. Urology. 1999 Oct;54(4):656–61. doi: 10.1016/s0090-4295(99)00195-8. [DOI] [PubMed] [Google Scholar]
- 34.Mian C, Lodde M, Haitel A, Egarter Vigl E, Marberger M, Pycha A. Comparison of two qualitative assays, the UBC rapid test and the BTA stat test, in the diagnosis of urothelial cell carcinoma of the bladder. Urology. 2000 Aug 1;56(2):228–31. doi: 10.1016/s0090-4295(00)00664-6. [DOI] [PubMed] [Google Scholar]
- 35.Babjuk M, Kostírová M, Mudra K. et al. Qualitative and quantitative detection of urinary human complement factor H-related protein (BTA stat and BTA TRAK) and fragments of cytokeratins 8, 18 (UBC rapid and UBC IRMA) as markers for transitional cell carcinoma of the bladder. Eur Urol. 2002 Jan;41(1):34–9. doi: 10.1016/s0302-2838(01)00015-x. [DOI] [PubMed] [Google Scholar]
- 36.Ecke TH, Arndt C, Stephan C. et al. Preliminary Results of a Multicentre Study of the UBC Rapid Test for Detection of Urinary Bladder Cancer. Anticancer Res. 2015 May;35(5):2651–5. [PubMed] [Google Scholar]
- 37.Horstmann M, Patschan O, Hennenlotter J, Senger E, Feil G, Stenzl A. Combinations of urine-based tumour markers in bladder cancer surveillance. Scand J Urol Nephrol. 2009;43(6):461–6. doi: 10.3109/00365590903296837. doi: 10.3109/00365590903296837. [DOI] [PubMed] [Google Scholar]
- 38.Todenhöfer T, Hennenlotter J, Esser M. et al. Combined application of cytology and molecular urine markers to improve the detection of urothelial carcinoma. Cancer Cytopathol. 2013 May;121(5):252–60. doi: 10.1002/cncy.21247. doi: 10.1002/cncy.21247. [DOI] [PubMed] [Google Scholar]