Abstract
OBJECTIVE
This study aims to describe the population pharmacokinetics and pharmacodynamic target attainment of meropenem in critically ill children.
METHODS
The study involved a retrospective medical record review from a 189-bed, freestanding children's tertiary care teaching hospital of patients ages 1 to 9 years who received meropenem with concurrent therapeutic drug monitoring.
RESULTS
There were 9 patients ages 1 to 9 years (mean age, 3.1 ± 2.9 years) with a mean weight of 17.1 ± 11.9 kg who met the inclusion/exclusion criteria and were included in the pharmacokinetic analysis. Meropenem concentrations were best described by a 2-compartment model with first-order elimination, with an R2 and bias of 0.91 and 13.2 mg/L, respectively, for the observed versus population predicted concentrations, and an R2, bias, and imprecision of 1, 0.0675, and 1 mg/L, respectively, for the observed versus individual predicted concentrations. The mean total body drug clearance for the population was 6.99 ± 2.5 mL/min/kg, and Vc was 0.57 ± 0.47 L/kg. The calculated population estimate for the total volume of distribution was 0.78 ± 0.73 L/kg. Standard 0.5-hour meropenem infusions did not provide for appropriate pharmacodynamic exposures of 40% free time > minimum inhibitory concentration (40% fT > MIC) for Gram-negative organisms with susceptible MICs. Dosage regimens employing prolonged and continuous infusion regimens did provide appropriate pharmacodynamic exposures of 40% fT > MIC for Gram-negative organisms up to the break point for Pseudomonas aeruginosa of 4 mg/L.
CONCLUSION
These data suggest the reference dosage regimens for meropenem (20–40 mg/kg per dose every 8 hours) do not meet an appropriate pharmacodynamic target attainment in critically ill children ages 1 to 9 years. Based on these data, only the 3- to 4-hour prolonged infusion and 24-hour continuous infusion regimens were able to achieve an optimal probability of target attainment against all susceptible Gram-negative bacteria in critically ill children for 40% fT > MIC. Dosage regimens of 120 and 160 mg/kg/day as continuous infusion regimens may be necessary to achieve an optimal probability of target attainment against all susceptible Gram-negative bacteria in critically ill children for 80% fT > MIC. Based on these findings, confirmation with a larger, prospective investigation in critically ill children is warranted.
Keywords: antibiotic dosage, carbapenem, meropenem, pediatric, pharmacodynamics, pharmacokinetics, Pseudomonas aeruginosa
Introduction
Pediatric sepsis and septic shock affects approximately 30% of children admitted to the pediatric intensive care unit (PICU) and carries with it a 25% mortality rate, a median hospital cost per patient of $65,624 (interquartile range [IQR], $27,300–$169,624), and an increasing prevalence.1,2 From birth through childhood, many developmental changes naturally occur that can have a profound impact on drug exposure and the subsequent response.3 Further, pathophysiologic changes commonly occur during critical illness and can dramatically affect a drug's pharmacokinetics (PK) and pharmacodynamics (PD).4–13 Critically ill patients can have increases in their volume of distribution (Vd) due to fluid balance strategies and intravascular perfusion changes; such changes might reduce the peak concentrations achieved after an infusion. Furthermore, critically ill patients can often have sepsis-induced decreases or increases in cardiac output, which can result in hypoperfusion or hyperperfusion of the kidneys and alterations in drug clearance.12,14 Consequently, appropriate antibiotic treatment is a keystone in the management of critically ill children.
Meropenem is a carbapenem, commonly used in pediatric critical care because of its broad antimicrobial spectrum and favorable safety profile.15 The standard dosage regimen for meropenem ranges from 20 to 40 mg/kg per dose every 8 hours infused during 30 minutes, which has been suggested to achieve the PD target of 40% free time above the minimum inhibitory concentration (40% fT > MIC) in clinically stable children.16 However, this dosage regimen, in addition to standard doses of other β-lactams, has been shown to be suboptimal in critically ill children in certain scenarios, such as severe sepsis and shock, burns, and when extracorporeal therapies are employed.5–7,9,17 Therefore, the aim of this study was to assess the population PK and PD target attainment of meropenem in children admitted to the PICU who underwent routine therapeutic drug monitoring of meropenem for dosage optimization.
Materials and Methods
Patient Population and Study Design. St Christopher's Hospital for Children is a 189-bed, freestanding children's tertiary care teaching hospital in Philadelphia, Pennsylvania. The PICU consists of 33 critical care beds and provides care for children with burns, trauma, or congenital heart disease, and children on extracorporeal membrane oxygenation (ECMO) and continuous renal replacement therapy (CRRT). This study protocol was approved by the Drexel University College of Medicine Institutional Review Board, and the need for informed consent and assent was waived because meropenem therapeutic drug monitoring was part of our local standard of care. Patients admitted to the PICU who received meropenem for empiric or definitive therapy with an expected duration of ≥48 hours, were between 1 and 9 years of age, and met pediatric systemic inflammatory response syndrome (SIRS)/sepsis criteria were eligible for inclusion.18 Patients who had cystic fibrosis, who had acute or chronic renal failure with an estimated creatinine clearance <50 mL/min/1.73 m2, or who were receiving ECMO or CRRT were excluded from this PK analysis. Patients receiving other antibiotics, except aminoglycosides, were excluded because of assay interference.
A total of 1 to 2 blood samples per child were collected, and the samples were obtained, when possible, after a dose that permitted collection of each sample in succession as dictated by clinical care. For standard 30-minute infusion regimens, the first blood sample was typically obtained within 20 minutes from the end of the infusion but could be obtained up to 2 hours from the end of the infusion, and the subsequent blood sample(s) were obtained a minimum of 1 hour after the first sample collection. For prolonged (i.e., 3- to 4-hour infusions) or 24-hour continuous infusion regimens, blood samples were obtained at the end of an infusion and again 1 hour from the end of the infusion. Serum samples for meropenem were subsequently sent to the lab for immediate processing and concentration determination.
Meropenem Concentration Determination. Concentrations for the meropenem in serum were determined by bioassay and liquid chromatography–tandem mass spectrometry. For September 2009–September 2014, bioassay was the methodology used for meropenem concentration determination. The bioassays were performed at ARUP Laboratories (Salt Lake City, Utah). The reference organism that was used for the meropenem bioassay was Clostridium perfringens American Type Culture Collection 13,124. The standard curve for the meropenem bioassay ranged from 5 to 40 mg/L, with an interday assay variability that was less than 15% across all reference samples (linearity and correlation coefficients not provided by reference laboratory (personal communication with authors JC and AE). If samples were below the lower limit of determination on the standard curve, the reference laboratory reported a value of “undetectable,” so these values were not available for use during population modeling. For patients who were receiving concomitant aminoglycoside therapy, samples were mixed with a cellulose phosphate-binding agent to inactivate the aminoglycoside and prevent any interference with the reference organism (ARUP laboratories policy and procedure number ANTI545). From September 2014 through December 31, 2015, meropenem concentrations were determined by liquid chromatography–tandem mass spectrometry at Atlantic Diagnostic Laboratories (Bensalem, Pennsylvania). This method was accurate and precise at a linearity range of 0.5 to 500 mg/L with a correlation coeffcient of =0.99. The interday assay variability was less than 15% across all reference samples between 1 and 100 mg/L. In the event samples were outside the upper limit of determination on the standard curve, a 1:2 or 1:10 dilution was made until the sample was within the standard curve. If samples were below the lower limit of quantitation on the standard curve, the reference laboratory reported a value of “less than 0.5 mg/L.”
Population PK. Meropenem concentrations were modeled using Pmetrics, a non-parametric pharmacometric modeling and simulation package for R.19 One- and two-compartment models with zero order input and first-order elimination were evaluated as the base structural model. Models were differentiated using Akaike Information Criterion (AIC), the likelihood ratio test, and visual predictive checks of the observed versus predicted concentration plots.20 Weighting was conducted using the inverse of the assay SD squared. The final equation for weighting was SD = γ *[0.1182 + (0.0179*C)], where C is the meropenem concentration and γ is gamma, a multiplicative measure of all intraindividual variability other than the assay. The mean weighted error of predicted versus observed concentrations was used as the estimate of bias. The bias-adjusted mean weighted squared error was used for an estimate of precision.
After identifying a base structural model, covariate analysis was performed using linear regression (SPSS version 22, SPSS Inc, Chicago, Illinois) to determine a relationship between body weight, age, and estimated creatinine clearance and PK parameters. Significant covariates were then reentered into the model using AIC, the likelihood ratio test (change in −2 * log-likelihood of greater than 6.64 [(p < 0.01 with 1 degree of freedom]), and visual predictive checks to reassess for model improvement. Linear and allometric scaling techniques were also conducted again using AIC, the likelihood ratio test, and visual predictive check to reassess for model improvement.21,22 Secondary PK parameters were calculated from the primary parameters for comparison with other studies. Half-life (t½) was calculated as 0.693/beta, where beta is the root of the quadratic polynomial.23 Volume of the peripheral compartment (Vp) was calculated as (Vc*kcp)/kpc, where Vc is the volume of the central compartment and kcp and kpc are intercompartment transfer constants for that patient. Total Vd was calculated as the sum of Vc and Vp. Serum creatinine values were determined using an enzymatic colorimetric creatinine methodology that is standardized against IDMS using a Roche 800 Integra instrument. This method of serum creatinine determination was the same during the entire study period. Creatinine clearance (as defined by the estimated glomerular filtration rate) was calculated using the updated Schwartz equation.24
Monte Carlo Simulation. Monte Carlo simulation was conducted to determine the probability of target attainment (PTA) in 5000 simulated children aged 1 to 9 years with estimated creatinine clearances of 100 to 220 mL/min/1.73 m2, and with a weight range of 7 to 40 kg as was seen in this population. Pharmacokinetic parameter estimates were simulated from the mean and SD estimates of the population model and employing the resulting covariance matrix. All PK parameter estimates were simulated as log-Gaussian distributions. An unbound fraction of 0.98 was applied during simulation to determine the fT > MIC exposure for meropenem.15 Simulated meropenem dosage regimens included 20 mg/kg every 8 hours as a 0.5-hour and 4-hour infusion, 30 mg/kg every 8 hours as a 0.5- and 4-hour infusion, 40 mg/kg every 8 hours as a 0.5- and 4-hour infusion, 20 mg/kg every 6 hours as a 0.5- and 3-hour infusion, 30 mg/kg every 6 hours as a 0.5- and 3-hour infusion, and 40 mg/kg every 6 hours as a 0.5- and 3-hour infusion, in addition to 60, 90, 120, and 160 mg/kg every 24 hours as a continuous infusion. The % fT > MIC for meropenem was calculated for the range of MICs from 0.03 to 32 mg/L. A PD target of ≥40% fT > MIC was defined as the primary exposure threshold.16 Additionally, a fT > MIC target of 80% was included as a secondary PD threshold, given the impairment and/or absence of neutrophils in a PICU population and the risk for infection with organisms in which meropenem has little postantibiotic effect.25,26 An a priori PTA ≥90% was defined as optimal.
Results
Patients. From January 1, 2009, to December 31, 2015, there were 9 patients who met the inclusion/exclusion criteria and were included in this analysis. Baseline demographics and dosage regimen details are presented in Table 1. There was a total of 16 meropenem concentrations (median of 2 samples per patient; range, 1–2) obtained. Of the 16 meropenem concentrations, none were below the lower limit of detection; therefore, all were included in the analysis. Therefore, 16 concentrations contributed toward development of the PK model. The 16 concentrations that were included in the analysis were spread across the dosage interval as follows: 0 to 1 hour from the end of infusion (n = 3), 1 to 2 hours (n = 2), 2 to 3 hours (n = 4), 3 to 4 hours (n = 3), and >4 hours (n = 4).
Table 1.
Demographics and Baseline Characteristics of the 9 Pediatric Intensive Care Unit Patients
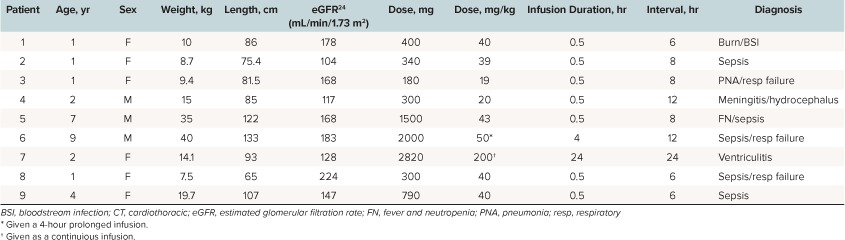
Population PK. Meropenem concentrations were best described by a 2-compartment model with first-order elimination as demonstrated by an AIC of −54.2 and log likelihood ratio of −69.68 versus an AIC of 71.6 and log likelihood ratio of 63.75 for the comparison between a 1-compartment and 2-compartment model, respectively, p-value < 0.01. A statistically significant relationship was not identified between body weight, age, or calculated creatinine clearance with the Vc or CL during the linear regression analyses, and the simple 2-compartment base model was demonstrated to be the superior model. The individual patient and population PK parameter estimates are presented in Tables 2 and 3, respectively. The mean total body CL (mL/min/kg) for the population was 6.99 ± 2.5 mL/min/kg and Vc (L/kg) was 0.57 ± 0.47 L/kg. The observed versus population predicted concentrations are provided in Figure 1. The R2 and bias were 0.91 and 13.1 mg/L, respectively, which are reasonable for population predictions. The observed versus individual predicted concentrations are provided in Figure 2. The R2, bias, and imprecision were 1, 0.0675, and 1 mg/L, respectively. The calculated population estimate for the mean total Vd was 0.78 ± 0.73 L/kg.
Table 2.
Individual Meropenem Population Pharmacokinetic Parameter Estimates for the 9 Pediatric Intensive Care Unit Patients
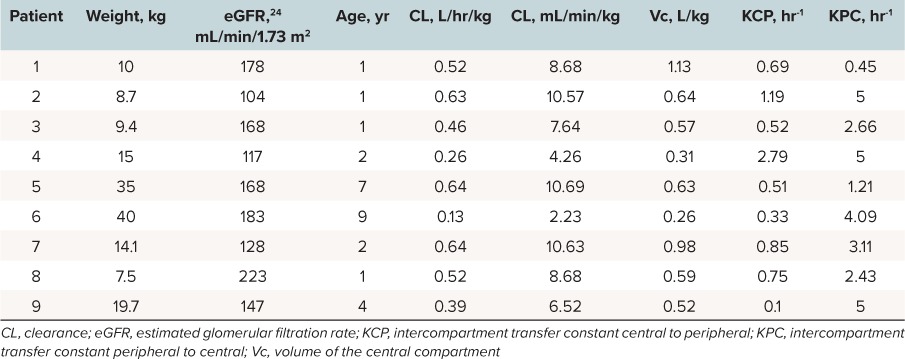
Table 3.
Mean Meropenem Population Pharmacokinetic Parameter Estimates for the 9 Pediatric Intensive Care Unit Patients

Figure 1.
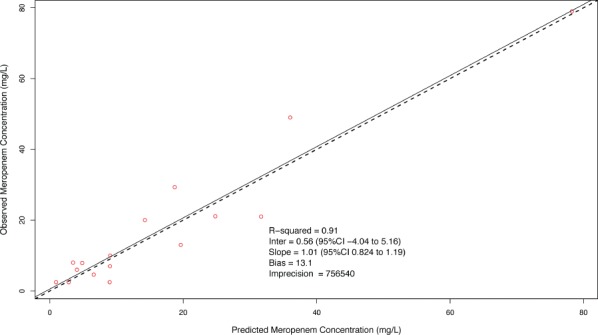
Observed versus population predicted meropenem concentration among 9 pediatric intensive care unit patients. Mean population parameter estimates were employed for calculation of concentrations.
Figure 2.
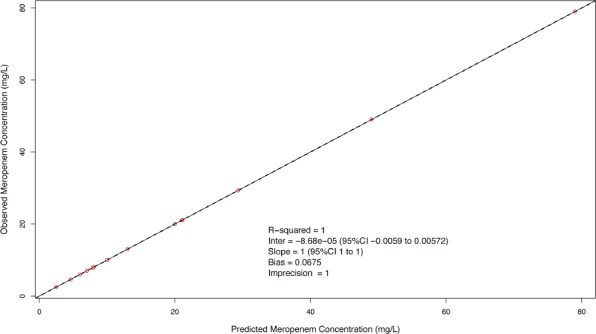
Observed versus individual predicted meropenem concentrations. Mean population parameters were used as the a priori Bayesian estimates.
Monte Carlo Simulations. Tables 4 and 5 display the PTAs for all simulated dosage regimens using 40% fT > MIC and 80% fT > MIC PD thresholds, respectively.
Table 4.
Probability of Target Attainment Using 40% Free Time > Minimum Inhibitory Concentration (40% fT > MIC) as the Pharmacodynamic Threshold for 5000 Simulated Pediatric Intensive Care Unit Children Receiving Meropenem as Conventional, Extended, and Continuous Infusion
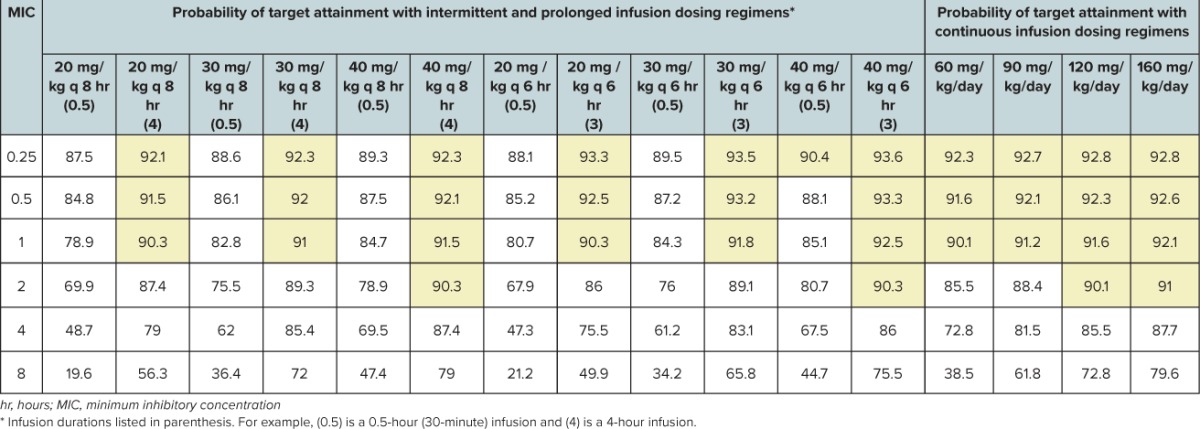
Table 5.
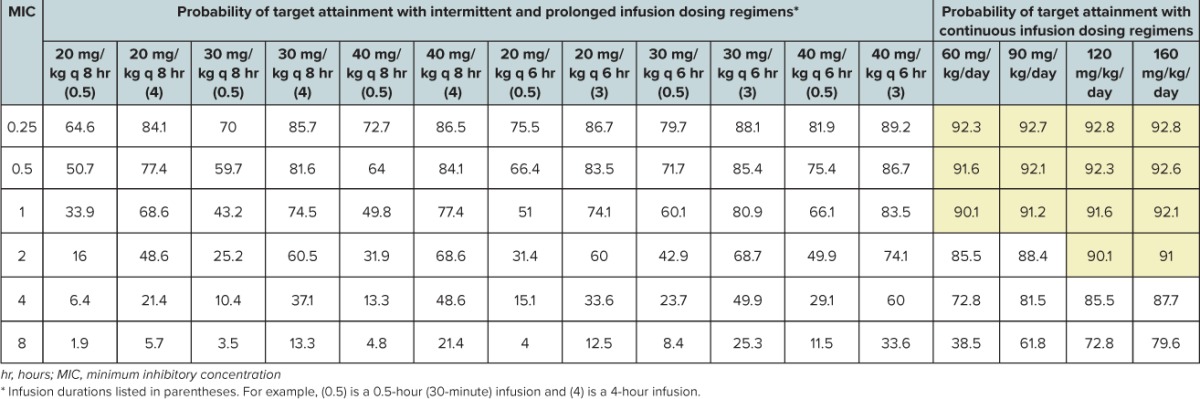
Probability Of Target Attainment Using 80% Free Time > Minimum Inhibitory Concentration (80% fT > MIC) as the Pharmacodynamic Threshold for 5000 Simulated Pediatric Intensive Care Unit Children Receiving Meropenem as Conventional, Extended, and Continuous Infusion
Discussion
Selection of an optimal dosage regimen in critically ill children is complicated by the lack of PK studies in this population and the different dosage recommendations available for meropenem, which appear to be weight based and dependent on age and type/severity of infection.27–29 Herein, we present PK for meropenem in critically ill young children and suggest dosage regimens based on contemporary PD concepts.
The PK of meropenem described in these 9 critically ill children differs from the PK data previously published.30,31 The population from the Blumer et al30 study was in generally good condition, the participants were clinically stable, and the participants either had completed a minimum of 2 days of conventional therapy for a specific bacterial infection or were receiving intravenous prophylactic antibiotics at ages 2 months to 12 years, whereas the Du et al31 population comprised clinically stable patients with suspected or proven bacterial meningitis who were ages 1 month to 17.3 years. The data from the Blumer et al30 and Du et al31 studies suggest the Vd is ~0.4 L/kg, with a clearance estimate range of 3 to 6 mL/min/kg compared with a Vd estimate of 0.78 ± 0.73 L/kg and a clearance estimate of 6.99 ± 2.5 mL/min/kg in the present investigation. A previous population PK analysis was conducted by Parker et al32 in children ages 2 months to 12 years using the previously published Blumer et al30 data. Parker et al32 concluded the Vd, clearance, and distributional clearance were markedly different in the younger (age <2 years) and lighter (<10 kg) patients. Interestingly, the clearance (~3 mL/min/kg) and Vd (~0.2 L/kg) estimates were even more significantly different than found in this investigation when stratified according to age and weight. Again, these patients were in generally good condition and were clinically stable; therefore, the stark contrast in the PK parameters between the 2 populations is not that surprising. More recently, Kongthavonsakul et al33 published a PK analysis of 14 children with severe infection, predominantly consisting of an oncology population with fever and neutropenia. The mean age was 7.1 ± 2.4 years, and the authors observed a mean clearance estimate of 5.4 mL/min/kg, which is faster than that reported in the package insert with a similar Vd estimate.15 We previously described the PK changes of piperacillin in a pediatric population with fever and neutropenia compared with a PICU population and similarly found the ICU population had more dramatic PK alterations.9,34 Our data suggest a larger meropenem Vd and faster CL than previously reported. As has been observed with other β-lactams, the larger Vd estimate for meropenem in our critically ill population was most likely primarily due to the underlying SIRS/sepsis pathophysiology and disease state, with good creatinine clearance estimates.9 β-Lactam antimicrobials display time-dependent killing. As such, the PD parameter associated with success for β-lactams is the time that free drug concentrations remain above the MIC (% fT > MIC).25 Therefore, drug CL is usually the most critical PK parameter to affect exposure, even though in this population the PK and PD changes were also significant as related to Vd. It is well established in adults that sepsis can cause enhanced blood flow to the kidneys and increase glomerular filtration, resulting in a state referred to as “augmented renal clearance,” and there is emerging pediatric data suggesting similar findings.17,35,36 This state of enhanced CL results in subtherapeutic concentrations of β-lactams and lower than anticipated fT > MIC.9,17,37 Further, although our clearance estimates appear to be larger than those with the previous populations, our data support the finding of augmented renal CL in this population, as demonstrated by CLs >7 mL/min/kg.
Our Monte Carlo simulation analysis was conducted to determine whether appropriate PD exposures can be achieved with current published meropenem dosage regimens.27–29 Our Monte Carlo analysis demonstrates that currently recommended doses of meropenem can be insufficient, particularly against Gram-negative bacteria with MICs in the susceptible range. This analysis suggests that the highest US Food and Drug Administration–approved meropenem dosage regimen of 40 mg/kg per dose every 8 hours given as a 0.5-hour infusion does not provide for an appropriate PD exposure at any susceptible MIC, let alone provide an optimal bactericidal PTA up to the meropenem break point of 4 mg/L for Pseudomonas aeruginosa. Our Monte Carlo simulation for a meropenem dosage regimen of 40 mg/kg per dose every 6 hours as a 0.5-hour infusion also does not even result in an optimal PTA up to the current Center for Laboratory Standards Institute susceptibility break point. The only regimens capable of providing an appropriate PTA up to the break point were the prolonged and continuous infusion regimens.
An understanding of PK changes in critically ill children and their impact on antimicrobial PD is crucial because suboptimal antimicrobial therapy is associated with worse outcomes, including longer durations of mechanical ventilation, ICU lengths of stay, and hospital lengths of stay, and increased mortality.37,38 Further, use of extracorporeal therapies, such as ECMO and CRRT, can also dramatically alter antimicrobial concentrations.39,40 Prolonged infusions of β-lactams have been found to increase the probability of attaining bactericidal activity by prolonging the time to reach maximum concentrations, thereby increasing fT > MIC.41–43 In clinical practice, prolonged infusions have also been associated with improved outcomes and demonstrated decreased mortality rates in critically ill patients.5–7,41,42 Too commonly, typical antimicrobial dosage regimens are used for critically ill, neonatal, and pediatric patients, as well as patients with complications. Considering that the infecting pathogen and MIC are not known when empiric therapy is initiated, empiric dosage regimens should be designed to provide for appropriate exposures in case the infecting pathogen is a multidrug-resistant organism. Considering the ICU setting, in addition to any potential extracorporeal therapies that may be used, the ability to achieve the PD target % fT > MIC can be reduced, demonstrating the “one dose fits all” theory is not appropriate and standard meropenem dosage regimens may not be appropriate.
Although the optimal PD target has not been identified in PICU patients, it has been suggested that the % f T > MIC should be maximized (i.e., 100%) in critically ill patients because in the absence of neutrophils, there is no post-antibiotic effect.25 A study by Ariano and colleagues26 in adult patients with fever and neutropenia identified 80% fT > MIC as the PD target predictive of efficacy for meropenem. Monte Carlo simulations demonstrate that the only regimens capable of providing 80% fT > MIC up to the Center for Laboratory Standards Institute break point are the 120 and 160 mg/kg/day continuous infusion regimens.
When new dosage regimens are suggested, an attempt to evaluate the safety of the regimen should also be conducted. For drugs that are renally eliminated, dosage regimens are usually created and subsequently modified based on the individual patients' renal function. With the increasing prevalence of drug-resistant organisms, higher than “standard” β-lactam doses have been used for less susceptible organisms. In an ICU setting in the presence of sepsis and shock, alterations in renal blood flow can affect a drug's PK.11 A reduction in glomerular filtration can cause drug accumulation and, potentially, adverse events. In general, β-lactam antimicrobials are generally well tolerated.44 In an adult ICU population, Beumier et al45 found a correlation between elevated β-lactam trough concentrations and neurologic deterioration in patients receiving meropenem and piperacillin, but not in patients receiving cefepime. Not surprisingly, the patients with diminished renal function were most likely to have elevated β-lactam trough concentrations. Monitoring of renal function is essential in any ICU population, and dosage adjustments should be made based on renal function and drug concentrations, when available. Using a simplified PK equation, dose = concentration * Vd * CL, it is evident that if the Vd and CL increase as a result of sepsis and augmented renal clearance, to achieve the desired concentration, the dose would need to be increased and/or the dosage interval shortened. As a result, the “higher” dosage recommendations are sometimes needed to achieve “standard” serum concentrations. Furthermore, we are not advocating for concentrations above those listed in the package insert which have been associated with US Food and Drug Administration approval and presumably successful outcomes; therefore, we would not expect a higher incidence of adverse events with the dosage regimens suggested in our analysis.
There are several limitations to this investigation. First, the study was conducted at a single center and included a mix of underlying disease states, so it is not possible to determine whether PK differ among different pediatric ICUs. Although by no means large, a sample size of 9 is still similar to other PK studies of meropenem and other anti-infectives in this age group. Second, patients with diminished renal function, which is seen with severe sepsis and septic shock, were not included in this analysis, so these results are not generalizable to that population. Third, 6 of the 9 patients included in this PK analysis were younger than 2 years, and this age group is known to renally eliminate drugs more rapidly than other pediatric age groups and typically has larger volumes of distribution for carbapenems. As such, the Monte Carlo simulation analysis may not be generalizable to other pediatric-age populations. Finally, we employed an opportunistic sampling strategy to inflict minimal pain on the child contributing blood samples. Although this was convenient for the child, sampling times as well as the sparse number of samples collected for each child may not have been ideal to fully characterize the distribution phase of meropenem in each individual child. We believe, however, that the use of the population PK approach and estimation of individual parameter estimates using the Bayesian priors is capable of providing a reasonable population PK estimate.
Conclusions
These data suggest the reference dosage regimens for meropenem (20–40 mg/kg per dose every 8 hours) do not meet an appropriate PD target attainment in critically ill children ages 1 to 9 years. Based on these data, only the 3- to 4-hour prolonged infusion and 24-hour continuous infusion regimens were able to achieve an optimal PTA against all susceptible Gram-negative bacteria in critically ill children for 40% f T > MIC. Dosage regimens of 120 and 160 mg/kg/day as continuous infusion regimens may be necessary to achieve an optimal PTA against all susceptible Gram-negative bacteria in critically ill children for 80% fT > MIC. Based on these findings, confirmation with a larger, prospective investigation in critically ill children is warranted.
Acknowledgments
This work was presented in part as an abstract presentation at the 44th Society of Critical Care Medicine Annual Congress, Phoenix, Arizona, abstract no. 1155, January 2015. The authors would like to thank Joseph L. Kuti, PharmD, from the Center for Anti-Infective Research and Development at Hartford Hospital in Hartford, Connecticut, for his teaching, insight, and guidance on the PK/PD modeling and simulation process.
Abbreviations
- AIC
Akaike Information Criterion
- CL
clearance
- CRRT
continuous renal replacement therapy
- ECMO
extracorporeal membrane oxygenation
- fT
free time
- MIC
minimum inhibitory concentration
- PD
pharmacodynamics
- PICU
pediatric intensive care unit
- PK
pharmacokinetics
- PTA
probability of target attainment
- SIRS
systemic inflammatory response syndrome
- Vd
volume of distribution
Footnotes
Disclosure Dr Cies is a consultant for Atlantic Diagnostic Laboratories and has received grants or honoraria from Allergan, Merck, and Thermo Fisher Scientific. Drs Moore and Chopra declare no conflicts or financial interests, and neither does Ms Enache. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Copyright Published by the Pediatric Pharmacy Advocacy Group. All rights reserved. For permissions, email: matthew.helms@ppag.org.
REFERENCES
- 1. Balamuth F, Weiss SL, Neuman MI, . et al. Pediatric severe sepsis in U.S. children's hospitals. Pediatr Crit Care Med. 2014; 15 9: 798– 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weiss SL, Fitzgerald JC, Pappachan J, . et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015; 191 10: 1147– 1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kearns GL, Abdel-Rahman SM, Alander SW, . et al. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003; 349 12: 1157– 1167. [DOI] [PubMed] [Google Scholar]
- 4. Carcillo JA, Doughty L, Kofos D, . et al. Cytochrome P450 mediated-drug metabolism is reduced in children with sepsis-induced multiple organ failure. Intensive Care Med. 2003; 29 6: 980– 984. [DOI] [PubMed] [Google Scholar]
- 5. Cies JJ, Moore WS Jr, Calaman S, . et al. Pharmacokinetics of continuous-infusion meropenem for the treatment of Serratia marcescens ventriculitis in a pediatric patient. Pharmacotherapy. 2015; 35 4: e32– e36. [DOI] [PubMed] [Google Scholar]
- 6. Cies JJ, Moore WS Jr, Conley SB, . et al. Pharmacokinetics of continuous infusion meropenem with concurrent extracorporeal life support and continuous renal replacement therapy: a case report. J Pediatr Pharmacol Ther. 2016; 21 1: 92– 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cies JJ, Moore WS Jr, Dickerman MJ, . et al. Pharmacokinetics of continuous-infusion meropenem in a pediatric patient receiving extracorporeal life support. Pharmacotherapy. 2014; 34 10: e175– e179. [DOI] [PubMed] [Google Scholar]
- 8. Cies JJ, Moore WS Jr, Miller K, . et al. Therapeutic drug monitoring of continuous-infusion acylovir for disseminated herpes simplex virus infection in a neonate receiving concurrent extracorporeal life support and continuous renal replacement therapy. Pharmacotherapy. 2015; 35 2: 229– 233. [DOI] [PubMed] [Google Scholar]
- 9. Cies JJ, Shankar V, Schlichting C, . et al. Population pharmacokinetics of piperacillin/tazobactam in critically ill young children. Pediatr Infect Dis J. 2014; 33 2: 168– 173. [DOI] [PubMed] [Google Scholar]
- 10. Ince I, de Wildt SN, Peeters MY, . et al. Critical illness is a major determinant of midazolam clearance in children aged 1 month to 17 years. Ther Drug Monit. 2012; 34 4: 381– 389. [DOI] [PubMed] [Google Scholar]
- 11. Roberts JA, Lipman J.. Antibacterial dosing in intensive care: pharmacokinetics, degree of disease and pharmacodynamics of sepsis. Clin Pharmacokinet. 2006; 45 8: 755– 773. [DOI] [PubMed] [Google Scholar]
- 12. Roberts JA, Roberts MS, Robertson TA, . et al. Piperacillin penetration into tissue of critically ill patients with sepsis--bolus versus continuous administration? Crit Care Med. 2009; 37 3: 926– 933. [DOI] [PubMed] [Google Scholar]
- 13. Smith BS, Yogaratnam D, Levasseur-Franklin KE, . et al. Introduction to drug pharmacokinetics in the critically ill patient. Chest. 2012; 141 5: 1327– 1336. [DOI] [PubMed] [Google Scholar]
- 14. Joukhadar C, Frossard M, Mayer BX, . et al. Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit Care Med. 2001; 29 2: 385– 391. [DOI] [PubMed] [Google Scholar]
- 15. Meropenem [package insert]. Wilmington, DE: Aztra Zeneca Pharmaceuticals LP. [Google Scholar]
- 16. Ellis JM, Kuti JL, Nicolau DP.. Use of Monte Carlo simulation to assess the pharmacodynamics of beta-lactams against Pseudomonas aeruginosa infections in children: a report from the OPTAMA program. Clin Ther. 2005; 27 11: 1820– 1830. [DOI] [PubMed] [Google Scholar]
- 17. De Cock PA, Standing JF, Barker CI, . et al. Augmented renal clearance implies a need for increased amoxicillin-clavulanic acid dosing in critically ill children. Antimicrob Agents Chemother. 2015; 59 11: 7027– 7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dellinger RP, Levy MM, Rhodes A, . et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013; 41 2: 580– 637. [DOI] [PubMed] [Google Scholar]
- 19. Neely MN, van Guilder MG, Yamada WM, . et al. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit. 2012; 34 4: 467– 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamaoka K, Nakagawa T, Uno T.. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978; 6 2: 165– 175. [DOI] [PubMed] [Google Scholar]
- 21. Holford N, Heo YA, Anderson B.. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013; 102 9: 2941– 2952. [DOI] [PubMed] [Google Scholar]
- 22. Meibohm B, Laer S, Panetta JC, . et al. Population pharmacokinetic studies in pediatrics: issues in design and analysis. AAPS J. 2005; 7 2: E475– E487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DeRyke CA, Kuti JL, Nicolau DP.. Pharmacodynamic target attainment of six beta-lactams and two fluoroquinolones against Pseudomonas aeruginosa, Acinetobacter baumannii, Escherichia coli, and Klebsiella species collected from United States intensive care units in 2004. Pharmacotherapy. 2007; 27 3: 333– 342. [DOI] [PubMed] [Google Scholar]
- 24. Schwartz GJ, Work DF.. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009; 4 11: 1832– 1843. [DOI] [PubMed] [Google Scholar]
- 25. Turnidge JD. The pharmacodynamics of beta-lactams. Clin Infect Dis. 1998; 27 1: 10– 22. [DOI] [PubMed] [Google Scholar]
- 26. Ariano RE, Nyhlen A, Donnelly JP, . et al. Pharmacokinetics and pharmacodynamics of meropenem in febrile neutropenic patients with bacteremia. Ann Pharmacother. 2005; 39 1: 32– 38. [DOI] [PubMed] [Google Scholar]
- 27. Lexi-Comp Online. Lexi-Comp Inc. Accessed March 19, 2017.
- 28. Pediatric & neonatal lexi-drugs online. Lexi-Comp, Inc. Accessed March 19, 2017.
- 29. Thomson Micromedex Web site. . http://uspdi.micromedex.com/. Accessed March 19, 2017.
- 30. Blumer JL, Reed MD, Kearns GL, . et al. Sequential, single-dose pharmacokinetic evaluation of meropenem in hospitalized infants and children. Antimicrob Agents Chemother. 1995; 39 8: 1721– 1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Du X, Li C, Kuti JL, . et al. Population pharmacokinetics and pharmacodynamics of meropenem in pediatric patients. J Clin Pharmacol. 2006; 46 1: 69– 75. [DOI] [PubMed] [Google Scholar]
- 32. Parker EM, Hutchison M, Blumer JL.. The pharmacokinetics of meropenem in infants and children: a population analysis. J Antimicrob Chemother. 1995; 36 suppl A: 63– 71. [DOI] [PubMed] [Google Scholar]
- 33. Kongthavonsakul K, Lucksiri A, Eakanunkul S, . et al. Pharmacokinetics and pharmacodynamics of meropenem in children with severe infection. Int J Antimicrob Agents. 2016; 48 2: 151– 157. [DOI] [PubMed] [Google Scholar]
- 34. Cies JJ, Jain J, Kuti JL.. Population pharmacokinetics of the piperacillin component of piperacillin/tazobactam in pediatric oncology patients with fever and neutropenia. Pediatr Blood Cancer. 2015; 62 3: 477– 482. [DOI] [PubMed] [Google Scholar]
- 35. Udy AA, Roberts JA, Boots RJ, . et al. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet. 2010; 49 1: 1– 16. [DOI] [PubMed] [Google Scholar]
- 36. Udy AA, Varghese JM, Altukroni M, . et al. Subtherapeutic initial beta-lactam concentrations in select critically ill patients: association between augmented renal clearance and low trough drug concentrations. Chest. 2012; 142 1: 30– 39. [DOI] [PubMed] [Google Scholar]
- 37. Weiss SL, Fitzgerald JC, Balamuth F, . et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med. 2014; 42 11: 2409– 2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muszynski JA, Knatz NL, Sargel CL, . et al. Timing of correct parenteral antibiotic initiation and outcomes from severe bacterial community-acquired pneumonia in children. Pediatr Infect Dis J. 2011; 30 4: 295– 301. [DOI] [PubMed] [Google Scholar]
- 39. Amaker RD, DiPiro JT, Bhatia J.. Pharmacokinetics of vancomycin in critically ill infants undergoing extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 1996; 40 5: 1139– 1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bressolle F, Kinowski JM, de la Coussaye JE, . et al. Clinical pharmacokinetics during continuous haemofiltration. Clin Pharmacokinet. 1994; 26 6: 457– 471. [DOI] [PubMed] [Google Scholar]
- 41. Courter JD, Kuti JL, Girotto JE, . et al. Optimizing bactericidal exposure for beta-lactams using prolonged and continuous infusions in the pediatric population. Pediatr Blood Cancer. 2009; 53 3: 379– 385. [DOI] [PubMed] [Google Scholar]
- 42. Lodise TP Jr, Lomaestro B, Drusano GL.. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis. 2007; 44 3: 357– 363. [DOI] [PubMed] [Google Scholar]
- 43. Tam VH, Louie A, Lomaestro BM, . et al. Integration of population pharmacokinetics, a pharmacodynamic target, and microbiologic surveillance data to generate a rational empiric dosing strategy for cefepime against Pseudomonas aeruginosa. Pharmacotherapy. 2003; 23 3: 291– 295. [DOI] [PubMed] [Google Scholar]
- 44. Goncalves-Pereira J, Povoa P.. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of β-lactams. Crit Care. 2011; 15 5: R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beumier M, Casu GS, Hites M, . et al. Elevated β-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol. 2015; 81 5: 497– 506. [PubMed] [Google Scholar]


