Abstract
OBJECTIVES
This study aims to determine the prevalence and factors associated with unrounded doses ordered via a computerized prescriber order entry (CPOE) system among children during a 1-week reference period.
METHODS
This retrospective, cross-sectional study included children younger than 18 years admitted during a 7-day period. An unrounded dose was defined as an unrounded actual dose (eg, dose calculated to the tenths place for non–neonatal intensive care (non-NICU) patients and dose calculated to the hundredth place for NICU patients) or unrounded volume per dose [eg, <0.1 mL for non-NICU patients and <0.01 mL for NICU patients]. A multilevel logistic regression model was used to determine the prevalence and factors associated with unrounded doses via a CPOE system with adjustment for clustering effects.
RESULTS
A total of 395 patients were admitted with 391 receiving medications. The overall prevalence of unrounded doses was 30% among the 2426 doses administered. Patients on the NICU team had the highest prevalence of unrounded doses. The odds of an unrounded dose were 4% (adjusted odds ratio, 0.96; 95% confidence interval, 0.94–0.98) lower with each additional kilogram increase in weight after controlling for age, route, scheduled versus as-needed administration, and cluster effects.
CONCLUSIONS
The prevalence of unrounded doses was higher than in previous studies. It was higher in smaller children after controlling for age, medication-related variables, and clustering. Future studies should focus on the role of CPOE in preventing unrounded and unmeasurable doses and if these strategies affect clinical outcomes (eg, adverse drug events).
Keywords: computerized prescriber order entry, dose rounding, medications, medication safety, pediatrics, rounding tolerance
Introduction
Medication errors in pediatric patients frequently result from errors in dosage, including dose miscalculations for weight-based medications.1,2 Implementation of computerized prescriber order entry (CPOE) systems has been shown to reduce these medication errors, because weight-based doses can be automatically calculated.3 However, computerized doses are often calculated to several decimal places, producing unreasonable and unmeasurable doses. When such doses are ordered, it is impossible to administer the exact dose intended, and this may result in increased risk of measurement and therapeutic errors.
Recent reports have highlighted the importance of ensuring that pediatric doses are reasonable and easily measured.4–6 However, few data exist concerning the prevalence and impact of unrounded doses ordered through CPOE systems. The purpose of this study was to determine the prevalence and factors associated with unrounded medication doses ordered for pediatric patients in the inpatient setting.
Methods
Study Design. This retrospective, cross-sectional study was conducted in a tertiary-care, academic hospital licensed for 314 pediatric inpatient beds and 40 labor/delivery inpatient beds. Data were included for all pediatric patients admitted during the 1-week period of August 11 to 17, 2013. Patients were identified through the institution's electronic medical record (EMR), Meditech (Medical Information Technology Inc, Westwood, MA). At the time of data collection, the CPOE ordering process in Meditech involved an automatic dose calculator for medications prescribed using a weight-based or body surface area–based dosage regimen. With this system, volume calculations are based on the weight per volume ratio in the drug dictionary strength field in Meditech. The dose calculator did not round the dose automatically, and it reported doses out to the ten-thousandths place. Parenteral nutrition and intravenous (IV) fluids were excluded from the analysis of the total medication count because IV infusion pumps at our institution are preset to round the infusion rate to the nearest tenths place, regardless of the ordered rate. Other medications that are traditionally not dispensed in unit doses were included in the total number of medications but were not included for further analysis ([eg, otic and ophthalmic drops, inhaled medications, topical medications, and continuous infusions (eg, patient-controlled analgesia, and sedative and analgesic infusions]). Insulin doses were also excluded from analysis because insulin syringes could be used to measure extremely small doses.
Study Objectives and Data Collection. Data collection included demographics and service provider (eg, pediatric surgery, neonatology). All medications received during the study period were collected and included dose (eg, mg and mg/kg), dosage formulation, and dosage frequency. Each medication order was counted as a distinct medication. Thus, patients could have had more than one order for a given medication. All medications were placed into 1 of 24 classes according to the American Hospital Formulary Service Pharmacologic-Therapeutic Classification system.7
The primary objective of this study was to determine the prevalence of unrounded doses with our CPOE system during the study period. For the purpose of this study, a dose was considered unrounded if the actual dose or volume per dose were deemed unmeasurable as defined by the study criteria. An unrounded dose was defined as a dose calculated to <0.1 unit (eg, mg, mcg) for non–-neonatal intensive care unit (non-NICU) patients and a dose calculated to <0.01 unit (eg, mg, mcg) for NICU patients. An unrounded volume per dose was defined as the corresponding volume of medication calculated to <0.1 mL for non-NICU patients and volume dose calculated to <0.01 mL for NICU patients. Secondary objectives included describing patient characteristics of children with unrounded doses (eg, total number of medications, service provider) and identification of medication classes and therapeutic agents most commonly ordered with unrounded doses. An additional objective was to determine the medication and patient factors associated with unrounded doses.
Statistical Analyses. Descriptive statistics were used to describe and summarize dose, patient, and service provider characteristics. Multilevel logistic regression was used to identify factors associated with unrounded doses adjusted for clustering effects due to medications being nested within patients and patients being nested within service providers. For simplicity, medications were treated as level 1 units, and patients as level 2 units. Service provider level characteristics or attributes were disaggregated to level 2 units by assigning service provider information to each patient served by the same provider. At the second level, the intercept parameters from each service provider were modeled as random effects. These intercepts allow for between–service provider heterogeneity in the prevalence proportions of unrounded doses, and they represent the overall prevalence levels for each service provider, adjusted for medication- and patient-level covariates. The medication-related factors (level 1) of interest included: medication class, drug dose (e.g, mg and mg/kg), dosage formulation, route of administration, and dosage frequency. Patient factors (level 2) included: age, weight, and service provider attributes. Potential interaction and confounding effects within and across levels were examined. Prevalence of unrounded doses in strata defined by medication class and dosage form was also examined and adjusted for potential confounding effects of patient and service provider factors and cluster effects. Maximum-likelihood parameter estimation procedures were used assuming any missing data were missing at random. All statistical tests were evaluated using SAS with an a set at 0.05.
Results
During the study period, 395 patients were admitted. Of these, 391 patients received at least 1 medication, whereas 4 patients received no medications. Four patients had substantial missing data and were excluded. Of the remaining 387 patients, the median age was 0.932 years (interquartile range [IQR], 1 month to 6.0 years), and the median weight was 8.6 kg (IQR, 3.22–20.80). There was a wide heterogeneity in the number of medications ordered in this cohort, with a median of 5 (IQR, 2–10). Figure 1 also provides a list of service providers.
Figure 1.
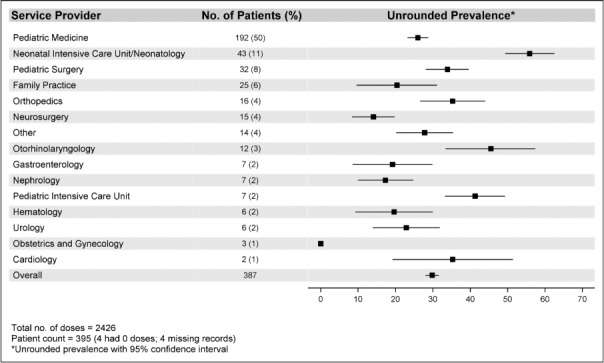
Prevalence of unrounded doses based on primary service provider.
A total of 3112 medications were administered in the 387 patients who received medications during the study. There were 686 medications that were not analyzed for appropriate dose rounding (eg, inhaled medications, otic drops, insulin, and continuous infusions). The total number of medications analyzed was 2426. Of the 2426 medications, 723 (29.8%) were unrounded. In the null model (the multilevel random intercept logistic regression model without covariates), there was substantial service provider–to–service provider variation in the prevalence of unrounded doses, intracluster correlation coefficient of 0.30 (95% confidence interval [CI], 0.25–0.37). The full model reduced the unexplained variance of service providers to 0.12 (95% CI, 0.07–0.18), indicating the prevalence of unrounded doses is best explained by patient and medication factors.
The prevalence of unrounded doses ranged from 0% (obstetrics-gynecology) to 55.9% (95% CI, 49.3%–62.5%: NICU; Figure 1). The overall mean of log odds of unrounded doses was −1.0415, with a corresponding estimated probability of unrounded doses on average of 0.260, which is slightly lower than the observed prevalence of 0.298. For doses that were unrounded based on the volume, the prevalence ranged from 0% (obstetrics-gynecology) to 31.2% (95% CI, 23.6%–38.9%: NICU), which was lower than the total overall prevalence of unrounded doses.
Figure 2 describes the frequency of rounded and unrounded medication doses per medication class. The most common medication classes of both rounded and unrounded doses included central nervous system, gastrointestinal, and anti-infective medications, which accounted for 71% of all doses and an average unrounded prevalence of 32% (95% CI, 29.7%–34.1%). The remaining 14 medication classes had a varying total number of doses (median, 17; IQR, 8–79), resulting in heterogeneous unrounded prevalence rates with wide confidence intervals: from 0% (local anesthetics) to 80% (95% CI, 44.94%–100.00%: serums, toxoids, and vaccines).
Figure 2.
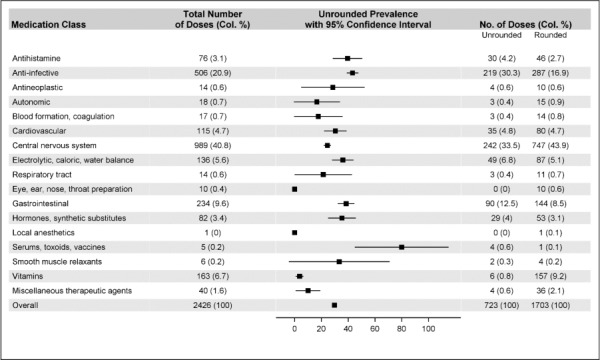
Prevalence of unrounded doses based on medication classes (n = 2426).
Figure 3 shows a comparison of medication characteristics, including dosage form, route of administration, and frequency of rounded medications compared with unrounded medications. The most common dosage forms for both rounded and unrounded medications were injections and liquid formulations. Intravenous and oral were the leading routes of administration, accounting for 88% of all doses. The odds of an unrounded dose was lower for those administered medications intramuscularly compared with intravenously (OR, 0.033; 95% CI, 0.015–0.075), but they did not differ significantly between other routes compared with the intravenous route.
Figure 3.
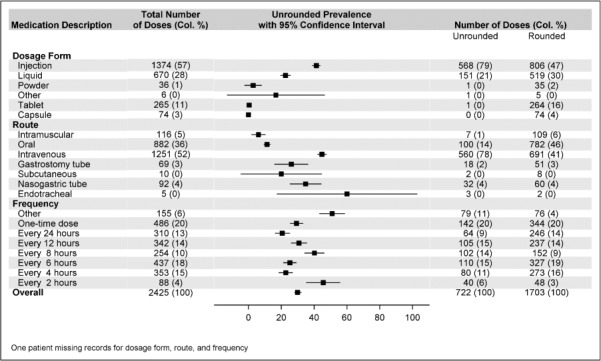
Prevalence of unrounded doses based on medication dosage form, route, and frequency (n = 2426).
There was a wide heterogeneity in the dosage frequency. A secondary analysis was performed for the variable dose frequency by excluding doses given at “other” frequencies and creating an ordinal set for those given once, daily, and every 12, 8, 6, 4, or 2 hours. The odds of an unrounded dose did not differ by dosage frequency. However, measured nominally, the only odds ratio (OR) differing was between doses administered every 8 hours versus daily (reference): (OR, 1.921; 95% CI, 1.184–3.117), after controlling for age, weight, route, form, and clustering. The odds of an unrounded dose was 4% (OR, 0.96; 95% CI, 0.94–0.98) lower with each additional kilogram increase in weight after controlling for age, route, scheduled versus as-needed administration, and cluster effects.
Discussion
This is the first study to describe the prevalence of unrounded doses and corresponding dosage volumes in pediatric patients in an inpatient setting. In this study, the definition of an unrounded dose was defined as either an actual dose or volume per dose calculated to <0.1 unit (eg, mg, mcg) or <0.1 mL for non-NICU patients, or an actual dose or volume per dose calculated to <0.01 unit (eg, mg, mcg) or <0.01 mL for NICU patients. We chose to allow for more significant digits in NICU patients, where weight-based dosage may result in a necessary small dose. In this study, we noted a prevalence in unrounded doses of 30%, which was higher than expected. Specifically, prevalence was higher in children with smaller weights and with children receiving every 8 hours versus daily dosage.
Two previous studies have investigated the occurrence of unmeasurable volume doses in pediatric patients.4–6 Morecroft and colleagues5,6 evaluated oral and IV liquid medications administered during a 5-week period to non-NICU and NICU patients at 2 hospitals in northern England. They defined unmeasurable doses as doses that could not be measured by their smallest syringe size. The investigators identified 196 of 1599 medications (12.3%) that were unmeasurable based on this definition. They concluded that anti-infective and analgesic agents accounted for most of the unmeasurable doses, which is similar to the findings in the present study. Another study by Caldwell and Rackham4 performed a point prevalence study of unmeasurable doses during 3 nonconsecutive days within a level 3 NICU. They defined an unmeasurable dose as one that was difficult to calculate by nursing staff. Based on this definition, they found 31 of 261 medications (11.8%) were unmeasurable. Similar to our study, these investigators collected the prevalence of unmeasurable doses during a short period of time. Both of these studies reported a lower prevalence of unmeasurable doses compared with our study. However, it is difficult to compare these study findings to our own because they used vague definitions. The definition of unmeasurable doses of these studies focused on whether it could be measured accurately based on a syringe or by nursing staff. It is feasible that if these investigators had used a definition similar to our own that they may have found different results.
In an effort to establish a more consistent definition of appropriateness of dose-rounding, Johnson and colleagues8 conducted an interview of 19 pediatric health care professional experts. The investigators also conducted a literature review and used a Delphi model to establish consensus recommendations for appropriateness of dose rounding in pediatric patients. These experts provided dose-rounding recommendations for 120 oral medications in children. The applicability of this panel's recommendations is limited to oral medications and has limited utility for the IV medications evaluated in this study.
There are significant concerns that if children receive unrounded doses, they may be at risk for potential complications in the inpatient and outpatient settings. In this study, we found that the odds of an unrounded dose were 4% lower with each additional kilogram increase in weight when controlling for age, route, scheduled versus as-needed administration, and cluster effects. It is likely that this finding may be due to the fact that weights for neonates and infants are often recorded in our CPOE system to the ten-thousandth place. Studies have suggested that dosage errors are the most common type of medication errors that may lead to potential adverse drug events (ADEs) in the pediatric population.2,9 Kaushal and colleagues10 have noted that e-prescribing may prevent up to 21% of ADEs. However, most e-prescribing systems do not generate easily administered doses. This may be due to the required use of multiple decimal places as a result of weight-based dosage, and the uncertainty of rounding numbers with multiple significant figures. In addition to these considerations, many clinicians may use these e-prescribing systems in the inpatient setting to generate outpatient medications. Thus, patients may be discharged home on these unrounded doses, potentially creating confusion for their caregivers. In one study, parents whose children were prescribed oral liquid medication in the emergency department were assessed to see if they could accurately measure the dose of liquid medications.11 The investigators found 41.1% of parents made an error in measuring the prescribed dose. It is very probable that these unrounded doses may lead to even greater complexity in administering medications and could lead to an increase in dose-dependent ADEs or subtherapeutic doses.
To address concerns about unrounded and unmeasurable doses, a number of institutions have implemented protocols in response to this issue. Three studies have described the implementation of dose standardization practices for oral and IV medications for the most common doses administered.9,12–14 These protocols have resulted in a variety of positive benefits, including a decrease in wastage and a potential cost savings. Despite these findings, it may be difficult to implement in all institutions. Other institutions have used pharmacist dose-rounding policies. These policies involve pharmacists adjusting doses by a certain percentage as approved by a pharmacy and therapeutics committee policy. These policies can be labor intensive. Based on the findings of this project, our institution implemented a dose-rounding rule within our EMR system. A description of the dose-rounding rule is provided in the Table. The rule is available for approximately 250 of the most commonly used IV and oral liquid medications. When an unrounded medication dose is entered, the EMR prompts the prescriber that the ordered dose could be rounded to a specified recommended dose. The provider must then accept or reject this proposed dose. The maximum difference between the ordered dose and the specified recommended dose will not exceed 5%. Although the rule addresses unrounded actual doses, it does not evaluate the corresponding dosage volume. Therefore, even if the dose looks appropriate, this could still result in an unmeasurable dose based on the volume with commercially available or extemporaneously prepared IV and oral liquids. It should be noted that this dose-rounding rule has not been validated. It is our belief that following implementation of this rule, that we can decrease our prevalence of unrounded doses, because the adjusted prevalence based on dosage volume was lower than the overall prevalence of unrounded doses. Future efforts will be focused on implementing protocols or policies to address issues with doses based on the corresponding volume of liquid medications. However, before this dose-rounding rule can be recommended, it must first be validated, and the investigator team has further plans to assess the impact of this intervention on unrounded doses.
Table.
Dose Rounding Rule
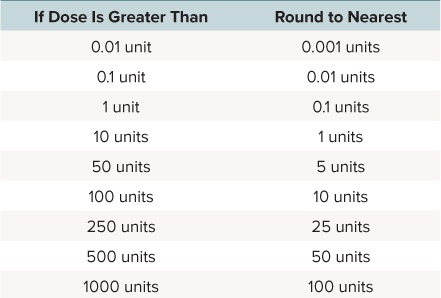
There are several limitations with this study. First, this was a retrospective study during a short study period. Prevalence was calculated during a 1-week period. There were 395 patients of the 13,690 total number of admissions in 2013, which accounted for only 2.9% of patients during 2013. Despite the limited sample size, this study had a study period similar to those of other previous studies, but it had a larger sample size.4–6 Next, this study was performed at a single center, and prescribing practices and EMR systems may differ at other institutions. In addition, we did not compare clinical outcomes (eg, ADEs, therapeutic response) between unrounded and rounded doses. Last, there is a lack of consistent definition of what an unmeasurable or unrounded dose is, as well as which specific agents are appropriate to round. The previous study by Johnson and colleagues8 provides some guidance, but their consensus recommendations have limited utility to many medications used in the inpatient setting. Without an appropriate understanding of the number of significant digits that account for an unmeasurable dose as well as what constitutes an appropriate rounding percentage per agent, it is difficult to provide dosage recommendations for all agents, specifically for medications with a narrow window of therapeutics. Future studies should focus on the extent to which rounding recommendations are accepted or overridden by prescribers, as well as any incidences of ADEs that may arise related to rounding.
Conclusions
In this study, the prevalence of unrounded doses based on the actual dose or dosage volume was 30%. Patients on the NICU team had the highest prevalence of unrounded doses. The prevalence was higher in smaller children after controlling for age, route, scheduled versus as-needed administration, and cluster effects. Future studies should focus on the role of CPOE and EMR systems in preventing unrounded and unmeasurable doses and if these strategies affect clinical outcomes (eg, ADEs).
Acknowledgments
This study was presented in poster form at the 24th Annual Meeting of the Pediatric Pharmacy Advocacy Group in May 2015, Minneapolis, Minnesota, and the 50th Annual American Society of Health-System Pharmacists Midyear Clinical Meeting in December 2015, New Orleans, Louisiana. We also acknowledge Arthur Owora, DrPH, MPH, formerly research biostatistician for the University of Oklahoma College of Pharmacy, for his contributions to the project.
Abbreviations
- ADE
adverse drug event
- CI
confidence interval
- CPOE
computerized prescriber order entry
- EMR
electronic medical record
- IV
intravenous
- NICU
neonatal intensive care unit
- OR
odds ratio
Footnotes
Disclosures Dr Jamie Miller is on the speaker's bureau for Chiesi, USA, Inc. The rest of the authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
Copyright Published by the Pediatric Pharmacy Advocacy Group. All rights reserved. For permissions, email: matthew.helms@ppag.org
REFERENCES
- 1. Stucky ER; American Academy of Pediatrics Committee on Drugs; American Academy of Pediatrics Committee on Hospital Care. . Prevention of medication errors in the pediatric inpatient setting. Pediatrics. 2003; 112 2: 431– 436. [DOI] [PubMed] [Google Scholar]
- 2. Kaushal R, Bates DW, Landrigan C, . et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001; 285 16: 2114– 2120. [DOI] [PubMed] [Google Scholar]
- 3. Potts AL, Barr FE, Gregory DF, . et al. Computerized physician order entry and medication errors in a pediatric critical care unit. Pediatrics. 2004; 113 1, pt 1: 59– 63. [DOI] [PubMed] [Google Scholar]
- 4. Caldwell NA, Rackham O.. Children's doses should be measurable. Arch Dis Child. 2010; 94 4: 313. [DOI] [PubMed] [Google Scholar]
- 5. Morecroft CW, Gill A, Caldwell NA, . et al. Are prescribed doses of medicine for children measurable? Arch Dis Child. 2012; 97: e18. [Google Scholar]
- 6. Morecroft CW, Caldwell NA, Gill A.. Prescribing liquid medication: can the dose be accurately given? Arch Dis Child. 2013; 98 10: 831– 832. [DOI] [PubMed] [Google Scholar]
- 7. McEvoy GK, Snow ED, . AHFS: Drug Information. Bethesda, MD: American Society of Health-System Pharmacists; 2012: 1125– 1126. [Google Scholar]
- 8. Johnson KB, Lee CK, Spooner SA, . et al. Automated dose-rounding recommendations for pediatric medications. Pediatrics. 2011; 128 2: e422– e428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robinson CA, Siu A, Meyers R, . et al. Standard dose development for medications commonly used in the neonatal intensive care unit. J Pediatr Pharmacol Ther. 2014; 19 2: 118– 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaushal R, Barker KN, Bates DW.. How can information technology improve patient safety and reduce medication errors in children's health care? Arch Pediatr Adolesc Med. 2001; 155 9: 1002– 1007. [DOI] [PubMed] [Google Scholar]
- 11. Yin HS, Dreyer BP, Ugboaja DC, . et al. Unit of measurement used and parent medication dosing errors. Pediatrics. 2014; 134 2: e354– e361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee B, Graner K.. Medication dose rounding and standardization of pediatric doses: a guideline for logical dosing. J Pediatr Pharmacol Ther. 2011; 6 2: 141. [Google Scholar]
- 13. MacKay MW, Holley M, Cash J, . et al. Dose standardization of oral liquid medications in a pediatric hospital. Hosp Pharm. 2005; 40 7: 582– 587. [Google Scholar]


