Abstract
Plakoglobin (PG) is a member of the Armadillo family of adhesion/signaling proteins and has been shown to play a critical role in the organization of desmosomes and tissue integrity. Because dissolution of intercellular junctions is frequently an initial step in the onset of epithelial cell migration, we examined whether loss of PG promotes cell motility by compromising adhesive strength. Keratinocyte cultures established from PG–/–mice exhibited weakened adhesion and increased motility in transwell migration assays; both were restored by reintroducing PG through adenoviral infection. Interestingly, single PG–/– cells also exhibited increased motility, which was suppressed by reintroducing PG, but not the closely related β-catenin. Whereas both N- and C-terminally truncated PG deletion mutants restored adhesion, only N-terminally deleted PG, but not C-terminally deleted PG, suppressed single-cell migration. Furthermore, both the chemical inhibitor PP2 and dominant-negative Src tyrosine kinase inhibited single-cell motility in PG–/– cells, whereas constitutively active Src overcame the inhibitory effect of PG. These data demonstrate that PG strengthens adhesion and suppresses motility in mouse keratinocytes, through both intercellular adhesion-dependent and -independent mechanisms, the latter of which may involve suppression of Src signaling through a mechanism requiring the PG C terminus.
Plakoglobin (PG) is a member of the Armadillo family of dual-functioning adhesion and signaling proteins. Similar to the closely related β-catenin, PG contains a central Armadillo repeat domain mediating the majority of its protein–protein interactions (1), flanked by N- and C-terminal domains, which are thought to regulate PG's function (2). PG can be found both in adherens junctions and desmosomes (3), where it links the cytoplasmic tail of classic or desmosomal cadherins to α-catenin or desmoplakin, which, in turn, associates with actin or intermediate filaments, respectively (1). PG's critical role in maintaining tissue integrity is underscored by skin and heart dysfunction in PG-null mice and patients with pathogenic homozygous mutations (4–7). In addition, keratinocyte cultures established from PG-null mice exhibit reduced adhesion in a mechanical dissociation assay (8).
The modulation of intercellular adhesion plays a central role in epithelial-to-mesenchymal transition, tumor progression and wound healing, and intercellular junction dissolution is thought to represent an important initial step in the onset of cell migration. The loss or inactivation of classic cadherins has been linked with tumor progression and increased cell migration (9). Likewise, a reduction in desmosomes or desmosomal proteins has been observed in certain tumors (10–13). Uncoupling desmosome attachment to intermediate filaments through expression of a dominant-negative (DN) desmoplakin (14) or loss of the desmosomal cadherin associated protein plakophilin 1 results in increased cell migration (15) correlated with a decrease in adhesive strength (16). These observations raise the question as to whether PG can also regulate cell motility through its regulation of intercellular adhesive strength.
Here, we directly examined PG's role in regulating cell motility by using keratinocyte cultures established from the skin of PG knockout mice. Our initial results show that PG suppresses keratinocyte motility in transwell migration assays in a calcium-dependent manner. However, further analysis of single-cell motility led to the unexpected finding that PG also suppresses motility through an intercellular adhesion-independent mechanism. Whereas both N- and C-terminally truncated PG deletion mutants restore adhesion, N-terminally deleted PG (ΔNPG), but not C-terminally deleted PG (ΔCPG), or the closely related β-catenin, is able to suppress single-cell motility. In addition, inhibition of the nonreceptor tyrosine kinase Src in PG–/– cells suppressed single-cell motility, whereas constitutively active (CA) Src antagonized the inhibitory effect of PG on cell motility in PG–/– cells. We conclude that PG strengthens adhesion and suppresses motility in keratinocytes, and that both cell–cell adhesion-dependent and -independent mechanisms contribute to PG's ability to suppress cell migration. Furthermore, our evidence is consistent with a mechanism by which PG suppresses single-cell motility through regulation of Src kinase-dependent signaling.
Materials and Methods
Cell Culture and Reagents. Keratinocyte cultures established from PG knockout (–/–) or heterozygous control (+/–) mouse skin (8) were cultured in CnT-02 medium (CellnTEC Advanced Cell Systems). In experiments where calcium concentrations were varied, cells were switched to calcium-free keratinocyte SFM (GIBCO/BRL and Invitrogen), supplemented with 10 ng/ml EGF and 10–10 M cholera toxin, and the calcium level was adjusted by using 1 M CaCl2. Kinase inhibitors PP2, SP600125, LY294002, U0126, and SB203580 were purchased from Calbiochem.
Generation of Adenoviruses and Adenoviral Transduction. The pAdeasy adenovirus-packaging system was kindly provided by W. G. Tourtellotte (Northwestern University Feinberg School of Medicine). Previously characterized myc-tagged, full-length (PGmyc), and N- (ΔNPGmyc), or C-terminal (ΔCPGmyc) deletion mutants of human PG (17) were used to generate recombinant viruses according to published protocols (18). Infection rates were monitored by using the GFP expressed in tandem with the construct of interest, and ≈90% infection was achieved for all constructs in all experiments. DN and CA Src adenoviruses were kindly provided by P. L. Stein (Northwestern University Feinberg School of Medicine).
Western Blot and Immunofluorescence. Western blot and immunofluorescence were performed as described (16). Primary antibodies used were: PG, 1407 (19); β-catenin, c-2206 (Sigma); rabbit anti-myc (20); GFP, JL-8 (Becton Dickinson); and rabbit anti-GAPDH (Novus Biologicals, Littleton, CO), mouse anti-v-Src (Oncogene Science, Cambridge, MA).
Dispase-Based Dissociation and Transwell Migration Assays. The dispase-based dissociation assay was performed as described (16). The transwell migration assay was modified from a published protocol (21). The undersides of Biocoat Cell Environment (Becton Dickinson) control inserts (24-well plates with a pore size of 8.0 μm) were coated with 0.1 mg/ml human placenta collagen (Sigma) at 37°C for 1 h. A total of 105 cells were plated into each insert and allowed to migrate at 37°C for 48 h. Migrating cells were fixed and stained with a DiffQuik kit (Dade Behring, Newark, DE). The total number of cells from 12 ×20 microscopic fields on each membrane was counted. Each experiment was performed in triplicate and the averages were compared by using Student's t test.
Colloidal Gold Migration Assay. The colloidal gold migration assay was modified from a published protocol (22). Keratinocytes in single-cell suspension were plated onto colloidal gold-coated coverslips and incubated at 37°C for 18 h. Images were taken with a Leica (Deerfield, IL) DMR microscope/Orca Hamamatsu (Ichinocho, Japan) digital camera system, and the area of individual tracks were measured by using openlab (Improvision, Lexington, MA). For colloidal gold migration assays using adenovirus-infected PG–/– cells, only the tracks of GFP-positive cells were counted. The average areas were compared by using Student's t test.
Results
PG Strengthens Keratinocyte Intercellular Adhesion in a Calcium-Dependent Manner. Previous studies (5) demonstrated that loss of PG expression caused severe skin fragility in PG knockout mice and decreased adhesion in keratinocytes established from the skin of these mice (8). Despite the lack of PG in PG–/– cells, the protein level and cellular localization of the closely related β-catenin were comparable in keratinocyte cultures established from the skin of PG knockout mice (PG–/–) and heterozygote control mice (PG+/–) (Fig. 7A, which is published as supporting information on the PNAS web site). To confirm the role of PG in maintaining keratinocyte intercellular adhesion, we first compared the adhesive strength of PG–/– and PG+/–cells by using a dispase-based mechanical dissociation assay. Keratinocyte monolayers were lifted from culture dishes by using dispase, a recombinant protease that cleaves cell–matrix, but not cell–cell, adhesion molecules. The resulting cell sheets were then subjected to mechanical stress and the number of fragments was used as an indicator of intercellular adhesive strength as described (16, 23). At 0.05 mM calcium, neither adherens junction nor desmosome components accumulated at cell–cell borders, based on immunofluorescence analysis (data not shown). Under these conditions, both PG+/– and PG–/–cell sheets dissociated into numerous fragments under sheer stress. At 0.2 mM calcium, a concentration at which adherens junction proteins were recruited to cell–cell borders in both cell lines, whereas desmosome formation was impaired in PG–/– cells (T.Y. and K.J.G., unpublished data), the integrity of epithelial cell sheets derived from both PG–/– and PG+/–cells increased dramatically and continued to increase with increasing calcium up to 1.2 mM (Fig. 1A). However, PG–/–sheets fragmented more than PG+/–sheets under all conditions that allowed intercellular junction formation (Fig. 1 A), supporting the idea that PG is required for strong intercellular adhesion. Moreover, we were able to manipulate intercellular adhesive strength by adjusting extracellular calcium concentration.
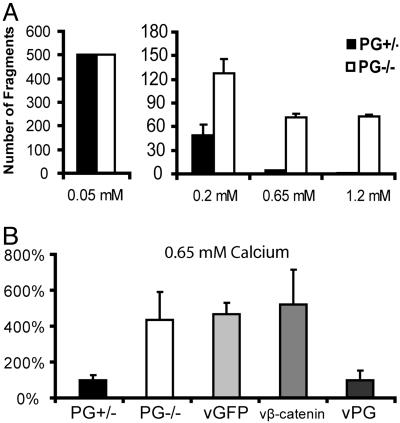
Fig. 1.
PG strengthens intercellular adhesion in mouse keratinocytes in a calcium-dependent manner. (A) Integrity of PG+/– and PG–/– cell monolayers at the indicated calcium concentration was assessed by using a dispasebased mechanical dissociation assay. Because the total fragment number at 0.05 mM calcium exceeded 500 for both PG+/– and PG–/– cells, precise values were not determined. (B) Adhesive strength of PG+/–, PG–/–, and PG–/– cells 24 h after infection with adenoviruses encoding GFP, β-catenin, or PG at 0.65 mM calcium concentration was examined by using the dispase-based dissociation assay. The y axis represents the total number of fragments in A and the percentage of fragments generated in response to mechanical stress compared with PG+/– cells in B.
The weakened adhesion in PG–/– cells was due to the loss of PG because reintroducing PG into PG–/– cells through adenoviral transduction (Fig. 7B) efficiently reduced the number of fragments to the level of PG+/–cells (Fig. 1B). Although β-catenin has been found in a complex with desmoglein in keratinocytes from PG–/–mice and was thought to compensate for PG's role in adhesion (24), overexpressing β-catenin in PG–/– cells did not restore adhesion (Fig. 1B). Therefore, PG strengthens intercellular adhesion in keratinocytes and its adhesion function cannot be replaced by β-catenin.
PG Suppresses Motility in a Transwell Migration Assay in a Calcium-Dependent Manner. Because cell dissociation is an initial step in cell migration, we tested whether weakened adhesion in PG–/– cells facilitates cell migration. A transwell migration assay was adopted as a model in which calcium-dependent intercellular contacts are present, thus allowing us to compare the impact of intercellular interactions on the overall migratory ability of two different cell populations. PG–/– and PG+/–cells were plated densely onto the porous membrane of tissue culture inserts and allowed to migrate for 48 h. Examination of the final cell numbers 48 h after plating in parallel cultures demonstrated that proliferation was comparable in the two cell lines during the assay period (data not shown). Because increasing extracellular calcium led to an increase in adhesive strength (Fig. 1A), we compared the ability of PG–/– and PG+/– to migrate in a range of calcium concentrations. In medium containing 0.2 mM calcium, 7-fold more PG–/– cells migrated through the filter than PG+/–cells. In medium containing 1.2 mM calcium, the motility of PG–/– cells was reduced to 50% of that in 0.2 mM calcium, but was still 4-fold greater than the PG+/– cells (Fig. 2A). The direct parallel relationship between the increase in intercellular adhesive strength (Fig. 1A) and decrease in motility exhibited by PG–/– cells at 1.2 mM calcium supports the idea that adhesion may negatively regulate motility. On the other hand, the motility of PG+/– cells was not greatly affected by the elevated calcium level (Fig. 2 A), although the adhesive strength in these cells increased >40-fold over this same range of calcium concentrations (Fig. 1A).
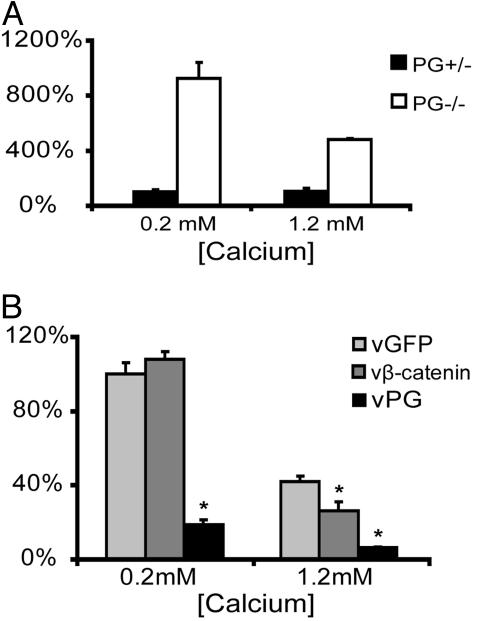
Fig. 2.
PG suppresses keratinocyte motility in a transwell migration assay in a calcium-dependent manner. (A) Motility of PG+/– and PG–/– cells was compared in a transwell migration assay at 0.2 and 1.2 mM calcium. The y axis represents the percentage of migrated cells compared with PG+/– cells at 0.2 mM calcium. (B) Motility of PG–/– cells transduced with adenoviruses encoding GFP, β-catenin, or PG was examined by using a transwell migration assay at 0.2 and 1.2 mM calcium. The y axis represents the percentage of migrated cells compared with vGFP-transduced PG–/– cells at 0.2 mM calcium. Asterisks represent statistically significant difference when compared with vGFP-transduced cells at the same calcium concentration by using Student's t test (t <0.05).
The increased motility of PG–/– cells was due to the loss of PG function as the motility of PG–/– cells infected with virus (v)PG was reduced by 80% compared with PG–/– cells infected with the GFP control viruses. β-catenin overexpression did not affect the motility of PG–/– cell in the transwell migration assay at 0.2 mM (Fig. 2B) or 0.65 mM calcium (data not shown), but did partially inhibit motility at 1.2 mM calcium (Fig. 2B).
PG Suppresses Single-Keratinocyte Motility Independent of Calcium Concentration. To determine whether the mechanism by which PG regulates motility wholly depends on intercellular adhesive strength, we examined the migration of single keratinocytes with a colloidal gold migration assay. Single-cell suspensions of PG–/– and PG+/– cells were plated sparsely onto gold particle-coated coverslips. The areas of the phagocytic tracks left by solitary migrating cells were measured after 18 h and used as an indicator of cell motility. Tracks left by single PG–/– cells covered six times more area than the PG+/– cells. In contrast to results obtained in the transwell migration assay, increasing calcium concentration did not affect single-cell motility in PG–/– or PG+/– cells (Fig. 3A).
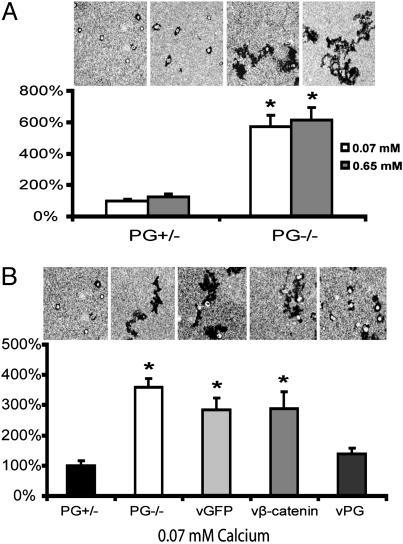
Fig. 3.
PG suppresses single-keratinocyte motility in a calcium-independent manner. (A) Single-cell motility of PG+/– and PG–/– cells at 0.07 and 0.65 mM calcium concentration, respectively. (B) Single-cell motility of PG+/–, PG–/–, and PG–/– cells transduced with adenoviruses encoding GFP, β-catenin, or PG at a 0.07-mM calcium concentration. In A and B, the y axis represents the percentage of the average area of individual tracks compared with PG+/– cells at 0.07 mM calcium. Asterisks represent a statistically significant difference by using Student's t test (t <0.05).
We then expressed PG in PG–/– cells through adenoviral transduction and repeated the colloidal gold assay, again comparing with cells transduced with GFP or β-catenin. Expression of PG, but not β-catenin or GFP, efficiently reduced single-cell motility to the level of PG+/– cells (Fig. 3B), demonstrating that PG also regulates single keratinocyte motility in a manner that is likely independent of β-catenin.
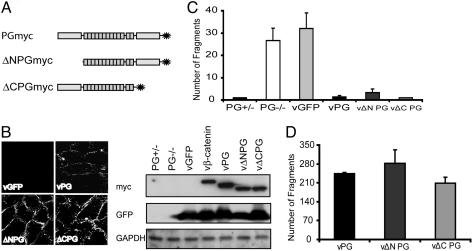
The N- and C-Terminal Domains Are Dispensable for PG-Dependent Strengthening of Intercellular Adhesion. To determine the functional domains of PG that regulate intercellular adhesion and cell motility, we generated adenoviruses encoding either an N- or a C-terminal deletion mutant PG (ΔNPGmyc and ΔCPGmyc) both of which retain the central Arm domain (Fig. 4A). Both mutant proteins were expressed at a level comparable to the wild-type protein and localized to cell–cell borders (Fig. 4B). In addition, both mutants restored adhesive strength to a level comparable with that of wild-type PG at 0.65 mM calcium (Fig. 4C). ΔNPGmyc was reproducibly less efficient than wild-type PG in restoring adhesive strength, but this difference was not always statistically significant (Fig. 4D).
Fig. 4.
Restoration of adhesive strength in cells expressing PG deletion mutants. (A) A diagram representing the N- or C-terminal deletion mutants of PG. PG+/–, PG–/–, and PG–/– cells transduced with vGFP, vβ-catenin, vPG, vΔNPG, or vΔCPG were subjected to Western blot and immunofluorescence analyses to examine the expression of exogenous protein in B, and adhesive strength at the 0.65-mM calcium level was examined by using dispase-based dissociation assay in C. (D) Adhesive strength at 0.65 mM calcium in PG–/– cells transduced with vPG, vΔNPG, or vΔCPG was examined by using a dispase-based dissociation assay. The number of rotations was increased to generate more sheer stress in this experiment.
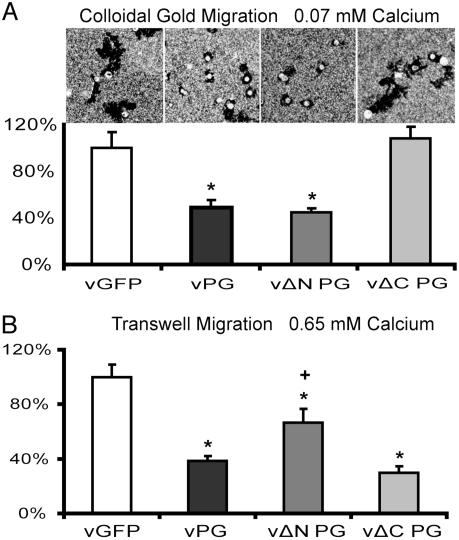
The C-Terminal Domain Is Required for PG-Dependent Suppression of Single-Keratinocyte Motility but Not Motility of Keratinocytes in Contact. To test whether the deletion mutants have similar effects on cell motility, PG–/– cells were infected with wild-type, ΔNPG, ΔCPG, or GFP control viruses, and then examined in the colloidal gold assay. Whereas PG lacking the N-terminal domain suppressed motility similar to wild-type PG, PG deletion mutants lacking the C-terminal domain failed to do so (Fig. 5A). However, ΔCPGmyc effectively inhibited migration in the transwell assay (Fig. 5B), possibly due to strengthened adhesion, which hampered the initiation of motility. Although ΔNPGmyc was sufficient to suppress single-cell motility, it did not inhibit motility in the transwell assay as efficiently as the wild-type PG. This finding may relate to ΔNPGmyc's somewhat reduced ability to restore adhesion (Fig. 4C), possibly due to compromised association with desmosomal cadherins.
Fig. 5.
The PG C terminus is required to suppress motility in the colloidal gold assay, but not the transwell assay. Motility of PG–/– cells transduced with vGFP, vPG, vΔNPG, or vΔCPG was examined by using the colloidal gold assay at 0.07 mM calcium in A and the transwell migration assay at 0.65 mM in B. The y axis in A represents the percentage of average area of individual tracks compared with vGFP-infected cells. The y axis in B represents the percentage of the average number of migrated cells compared with vGFP-infected cells. The asterisks represent the statistically significant difference from vGFP by using Student's t test (t <0.05). The number of migrating cells expressing vΔNPG and vΔCPG were also compared with vPG by using Student's t test. +, statistically significant difference (t <0.05).
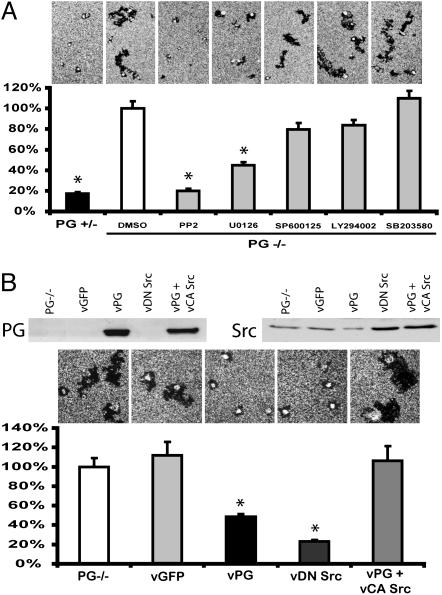
Role of Src Kinase in PG-Dependent Suppression of Single-Cell Motility. To test whether PG suppresses single-cell motility by interfering with signaling pathways known to regulate cell motility, we examined single PG–/– cell motility in the presence of various kinase inhibitors. Among the inhibitors we tested, the Src family inhibitor PP2 (25) efficiently suppressed motility of single PG–/– cells to the level of PG+/– cells. The mitogen-activated protein kinase kinase 1/2 inhibitor U0126 (26) also significantly decreased motility in PG–/– cells, although only by 60%. Meanwhile, inhibiting phosphoinositide 3 kinase, Jun N terminus kinase, and p38 by LY294002, SP600125, and SB203580 (27–29), respectively, did not significantly affect PG–/– motility in the colloidal gold migration assay (Fig. 6A).
Fig. 6.
Role for Src in PG-dependent regulation of single-keratinocyte motility. (A) Motility of PG–/– cells in the presence of 10 μM of the indicated kinase inhibitors at 0.07 mM calcium. Kinase inhibitors were added 2 h after plating cells onto coverslips. (B) Motility of PG–/– cells infected with indicated adenoviruses at 0.7 mM calcium. Expression level of PG and Src were examined by using Western blot. In both A and B, the y axis represents the percentage of average area of individual tracks compared with control PG–/– cells. The asterisks represent the statistically significant difference by using Student's t test (t <0.01).
Overexpressing a DN Src (30) by means of adenoviral infection efficiently suppressed single PG–/– cell motility (Fig. 6B), supporting the idea that elevated Src activity contributes to the increased motility observed in PG–/– cells. To test whether PG suppressed motility through the Src signaling pathway, PG–/– cells were coinfected with adenoviruses encoding PG and CA Src (30). Expression of CA Src overcame the inhibitory effect of PG on cell motility in PG–/– cells (Fig. 6B). These data suggest that PG may suppress single-cell motility by interfering with Src signaling in mouse keratinocytes.
Discussion
Intercellular adhesion molecules have long been touted as key regulators of cell motility, particularly during epithelial-to-mesenchymal transition, and loss of E-cadherin has been considered a hallmark of tumor metastasis (31). Although the importance of desmosomes in maintaining the noninvasive state is not as well understood, it has been shown that desmosomal molecules are down-regulated in certain cancers such as oral squamous cell carcinomas. Overexpression of desmosomal cadherins has been inversely correlated with invasive potential (32) and reduced motility (33). Here, we show that the desmosomal cadherin-associated protein PG suppresses motility in a transwell migration assay in a calcium-dependent manner that parallels its ability to restore adhesive strength in PG-null keratinocytes. More surprising, however, we also observed that PG regulates the intrinsic cell motility of single keratinocytes. Although PG expression has been positively correlated with cell transformation in certain cases (34), our findings are more consistent with the reported loss of PG in certain malignant tumors (4, 35) and invasive MCF-7 cells stably transfected with the human growth hormone gene (36).
In this study, variation of calcium levels was used as a tool to adjust intercellular adhesive strength, and its consequent effects on cell motility were assessed. Because calcium is a known regulator of integrin function (37), which plays a critical role in cell motility through the regulation of cell–matrix adhesion, it is possible that the inverse correlation between calcium level and migration in the transwell migration assay reflected the effects of calcium on cell–matrix adhesion. However, cell motility was not detectably affected by altering calcium concentrations in the single-cell colloidal gold migration assay, where cells were only in contact with the substrate. Thus, although we cannot rule out some effect of calcium on cell–substrate interactions in the transwell migration assay, our observations are consistent with the idea that increasing calcium concentration suppressed motility, at least in part, by strengthening intercellular adhesion.
The data presented here demonstrate that a desmosomal adhesion molecule can regulate single-cell motility in an adhesion-independent manner. Both p120 and β-catenin have been shown to promote motility, in the case of p120, in single-cell assays (38–40). However, unlike these Armadillo family members, PG suppresses, rather than promotes, motility. Also, because overexpressing β-catenin did not affect single-cell motility in PG–/– cells, it seems unlikely that PG is regulating keratinocyte motility through β-catenin signaling, as has been suggested to occur under certain circumstances (41, 42).
We identified the C-terminal domain of PG as being required to inhibit single-cell motility. The C terminus has been shown to regulate binding of PG to the lymphoid enhancer factor/T cell factor/DNA complex (41), and PG has been reported to either promote or interfere with β-catenin signaling, depending on cellular context (43). However, expression of a DN-TCF (44) had no detectable effect on motility of PG+/– or PG–/– cells in the single-cell assay, providing further support for the idea that PG does not suppress motility through β-catenin signaling (data not shown). Consistent with this hypothesis, β-catenin's role in migration did not require LEF/TCF signaling (38).
Among the kinase inhibitors we tested, the Src family kinase inhibitor PP2 was the most potent inhibitor of cell motility in PG–/– cells. U0126, the specific MEK1/2 inhibitor also inhibited single-cell motility, although not as efficiently as PP2. Taken together with previous findings that Src acts upstream of MEK pathway in promoting cell motility (45, 46), this observation suggests that Src may promote motility via the activation of multiple downstream pathways. Meanwhile, the fact that CA Src abrogated the inhibitory effects of PG introduced into null cells suggests that PG may suppress keratinocyte motility through inhibition of Src signaling. Given that Src specifically phosphorylates PG at Tyr-643 (47), it is plausible that PG may sequester Src from activating the motility machinery, and thus, inhibit migration in keratinocytes. Tyr-643 is located toward the end of the Armadillo domain, and the C terminus of PG has been shown to regulate the protein interactions in which the Armadillo domain takes part (2). Therefore, losing the C-terminal domain may hamper PG's interaction with Src, thus rendering ΔCPG incapable of suppressing single-cell motility.
In summary, we have shown that PG strengthens intercellular adhesion and inhibits motility of both cells in contact and single mouse keratinocytes. Recently, intravital imaging by using multiphoton microscopy revealed that during metastasis, carcinoma cells migrate away from the tumor mass as solitary amoeboid cells instead of a collection of cells in vivo (48). These data suggest that loss of PG in tumors may not only contribute to tumor progression by regulating cell growth (49, 50) but also cell invasion and metastasis through the regulation of adhesion and single cell motility.
Supplementary Material
Acknowledgments
We thank Drs. W. G. Tourtellotte and P. L. Stein for providing the reagents and technical assistance in the adenoviral work. We also thank Drs. L. Godsel and C. Gottardi (Northwestern University Feinberg School of Medicine) for helpful discussion and for critically reading the manuscript. This work was supported by National Institutes of Health Grants R01AR41836, R01AR43380, and Project 4 of P01 DE12328, and a Joseph L. Mayberry Endowment (to K.J.G.); Project 3 of National Institutes of Health Grant P01 DE12328 (to J.C.R.J.); National Institutes of Health Grant R01AR048266 (to A.P.K.); and Swiss National Science Foundation Grant 31-59456.99 (to E.J.M.).
Author contributions: T.Y., S.G., R.C., A.P.K., E.J.M., J.C.R.J., and K.J.G. designed research; T.Y. performed research; T.Y., R.C., A.P.K., and E.J.M. contributed new reagents/analytic tools; T.Y., S.G., R.C., A.P.K., E.J.M., J.C.R.J., and K.J.G. analyzed data; and T.Y. wrote the paper.
Abbreviations: PG, plakoglobin; ΔNPG, N-terminally deleted PG; ΔCPG, C-terminally deleted PG; DN, dominant-negative; v, virus; CA, constitutively active.
References
- 1.Zhurinsky, J., Shtutman, M. & Ben-Ze'ev, A. (2000) J. Cell Sci. 113, 3127–3139. [DOI] [PubMed] [Google Scholar]
- 2.Solanas, G., Miravet, S., Casagolda, D., Castano, J., Raurell, I., Corrionero, A., de Herreros, A. G. & Dunach, M. (2004) J. Biol. Chem. 279, 49849–49856. [DOI] [PubMed] [Google Scholar]
- 3.Cowin, P., Kapprell, H. P., Franke, W. W., Tamkun, J. & Hynes, R. O. (1986) Cell 46, 1063–1073. [DOI] [PubMed] [Google Scholar]
- 4.Aberle, H., Bierkamp, C., Torchard, D., Serova, O., Wagner, T., Natt, E., Wirsching, J., Heidkamper, C., Montagna, M., Lynch, H. T., et al. (1995) Proc. Natl. Acad. Sci. USA 92, 6384–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bierkamp, C., McLaughlin, K. J., Schwarz, H., Huber, O. & Kemler, R. (1996) Dev. Biol. 180, 780–785. [DOI] [PubMed] [Google Scholar]
- 6.McKoy, G., Protonotarios, N., Crosby, A., Tsatsopoulou, A., Anastasakis, A., Coonar, A., Norman, M., Baboonian, C., Jeffery, S. & McKenna, W. J. (2000) Lancet 355, 2119–2124. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz, P., Brinkmann, V., Ledermann, B., Behrend, M., Grund, C., Thalhammer, C., Vogel, F., Birchmeier, C., Gunthert, U., Franke, W. W. & Birchmeier, W. (1996) J. Cell Biol. 135, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caldelari, R., de Bruin, A., Baumann, D., Suter, M. M., Bierkamp, C., Balmer, V. & Muller, E. (2001) J. Cell Biol. 153, 823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wheelock, M. J. & Johnson, K. R. (2003) Annu. Rev. Cell Dev. Biol. 19, 207–235. [DOI] [PubMed] [Google Scholar]
- 10.Shinohara, M., Hiraki, A., Ikebe, T., Nakamura, S., Kurahara, S., Shirasuna, K. & Garrod, D. R. (1998) J. Pathol. 184, 369–381. [DOI] [PubMed] [Google Scholar]
- 11.Depondt, J., Shabana, A. H., Florescu-Zorila, S., Gehanno, P. & Forest, N. (1999) Eur. J. Oral Sci. 107, 183–193. [DOI] [PubMed] [Google Scholar]
- 12.Alroy, J., Pauli, B. U. & Weinstein, R. S. (1981) Cancer 47, 104–112. [DOI] [PubMed] [Google Scholar]
- 13.Alazawi, W. O., Morris, L. S., Stanley, M. A., Garrod, D. R. & Coleman, N. (2003) Virchows Arch. 443, 51–56. [DOI] [PubMed] [Google Scholar]
- 14.Setzer, S. V., Calkins, C. C., Garner, J., Summers, S., Green, K. J. & Kowalczyk, A. P. (2004) J. Invest. Dermatol. 123, 426–433. [DOI] [PubMed] [Google Scholar]
- 15.South, A. P., Wan, H., Stone, M. G., Dopping-Hepenstal, P. J., Purkis, P. E., Marshall, J. F., Leigh, I. M., Eady, R. A., Hart, I. R. & McGrath, J. A. (2003) J. Cell Sci. 116, 3303–3314. [DOI] [PubMed] [Google Scholar]
- 16.Huen, A. C., Park, J. K., Godsel, L. M., Chen, X., Bannon, L. J., Amargo, E. V., Hudson, T. Y., Mongiu, A. K., Leigh, I. M., Kelsell, D. P., et al. (2002) J. Cell Biol. 159, 1005–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palka, H. L. & Green, K. J. (1997) J. Cell Sci. 110, 2359–2371. [DOI] [PubMed] [Google Scholar]
- 18.He, T. C., Zhou, S., da Costa, L. T., Yu, J., Kinzler, K. W. & Vogelstein, B. (1998) Proc. Natl. Acad. Sci. USA 95, 2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaudry, C. A., Palka, H. L., Dusek, R. L., Huen, A. C., Khandekar, M. J., Hudson, L. G. & Green, K. J. (2001) J. Biol. Chem. 276, 24871–24880. [DOI] [PubMed] [Google Scholar]
- 20.Xiao, K., Allison, D. F., Kottke, M. D., Summers, S., Sorescu, G. P., Faundez, V. & Kowalczyk, A. P. (2003) J. Biol. Chem. 278, 19199–19208. [DOI] [PubMed] [Google Scholar]
- 21.Nieman, M. T., Prudoff, R. S., Johnson, K. R. & Wheelock, M. J. (1999) J. Cell Biol. 147, 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tjia, J. S. & Moghe, P. V. (2002) Tissue Eng. 8, 247–261. [DOI] [PubMed] [Google Scholar]
- 23.Calautti, E., Cabodi, S., Stein, P. L., Hatzfeld, M., Kedersha, N. & Paolo Dotto, G. (1998) J. Cell Biol. 141, 1449–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bierkamp, C., Schwarz, H., Huber, O. & Kemler, R. (1998) Development (Cambridge, U.K.) 126, 371–381. [DOI] [PubMed] [Google Scholar]
- 25.Hanke, J. H., Gardner, J. P., Dow, R. L., Changelian, P. S., Brissette, W. H., Weringer, E. J., Pollok, B. A. & Connelly, P. A. (1996) J. Biol. Chem. 271, 695–701. [DOI] [PubMed] [Google Scholar]
- 26.Favata, M. F., Horiuchi, K. Y., Manos, E. J., Daulerio, A. J., Stradley, D. A., Feeser, W. S., Van Dyk, D. E., Pitts, W. J., Earl, R. A., Hobbs, F., et al. (1998) J. Biol. Chem. 273, 18623–18632. [DOI] [PubMed] [Google Scholar]
- 27.Vlahos, C. J., Matter, W. F., Hui, K. Y. & Brown, R. F. (1994) J. Biol. Chem. 269, 5241–5248. [PubMed] [Google Scholar]
- 28.Cuenda, A., Rouse, J., Doza, Y. N., Meier, R., Cohen, P., Gallagher, T. F., Young, P. R. & Lee, J. C. (1995) FEBS Lett. 364, 229–233. [DOI] [PubMed] [Google Scholar]
- 29.Bennett, B. L., Sasaki, D. T., Murray, B. W., O'Leary, E. C., Sakata, S. T., Xu, W., Leisten, J. C., Motiwala, A., Pierce, S., Satoh, Y., et al.. (2001) Proc. Natl. Acad. Sci. USA 98, 13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabodi, S., Calautti, E., Talora, C., Kuroki, T., Stein, P. L. & Dotto, G. P. (2000) Mol. Cell 6, 1121–1129. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Ze'ev, A. (1997) Curr. Opin. Cell Biol. 9, 99–108. [DOI] [PubMed] [Google Scholar]
- 32.De Bruin, A., Muller, E., Wurm, S., Caldelari, R., Wyder, M., Wheelock, M. J. & Suter, M. M. (1999) Cell Adhes. Commun. 7, 13–28. [DOI] [PubMed] [Google Scholar]
- 33.Tselepis, C., Chidgey, M., North, A. & Garrod, D. (1998) Proc. Natl. Acad. Sci. USA 95, 8064–8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolligs, F. T., Kolligs, B., Hajra, K. M., Hu, G., Tani, M., Cho, K. R. & Fearon, E. R. (2000) Genes Dev. 14, 1319–1331. [PMC free article] [PubMed] [Google Scholar]
- 35.Amitay, R., Nass, D., Meitar, D., Goldberg, I., Davidson, B., Trakhtenbrot, L., Brok-Simoni, F., Ben-Ze'ev, A., Rechavi, G. & Kaufmann, Y. (2001) Am. J. Pathol. 159, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukhina, S., Mertani, H. C., Guo, K., Lee, K. O., Gluckman, P. D. & Lobie, P. E. (2004) Proc. Natl. Acad. Sci. USA 101, 15166–15171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leitinger, B., McDowall, A., Stanley, P. & Hogg, N. (2000) Biochim. Biophys. Acta 1498, 91–98. [DOI] [PubMed] [Google Scholar]
- 38.Wong, A. S. & Gumbiner, B. M. (2003) J. Cell Biol. 161, 1191–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller, T., Bain, G., Wang, X. & Papkoff, J. (2002) Exp. Cell Res. 280, 119–133. [DOI] [PubMed] [Google Scholar]
- 40.Grosheva, I., Shtutman, M., Elbaum, M. & Bershadsky, A. D. (2001) J. Cell Sci. 114, 695–707. [DOI] [PubMed] [Google Scholar]
- 41.Zhurinsky, J., Shtutman, M. & Ben-Ze'ev, A. (2000) Mol. Cell. Biol. 20, 4238–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simcha, I., Shtutman, M., Salomon, D., Zhurinsky, J., Sadot, E., Geiger, B. & Ben-Ze'ev, A. (1998) J. Cell Biol. 141, 1433–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin, T. & Green, K. J. (2004) Semin. Cell Dev. Biol. 15, 665–677. [DOI] [PubMed] [Google Scholar]
- 44.He, T. C., Sparks, A. B., Rago, C., Hermeking, H., Zawel, L., da Costa, L. T., Morin, P. J., Vogelstein, B. & Kinzler, K. W. (1998) Science 281, 1509–1512. [DOI] [PubMed] [Google Scholar]
- 45.Vindis, C., Cerretti, D. P., Daniel, T. O. & Huynh-Do, U. (2003) J. Cell Biol. 162, 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fincham, V. J., James, M., Frame, M. C. & Winder, S. J. (2000) EMBO J. 19, 2911–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miravet, S., Piedra, J., Castano, J., Raurell, I., Franci, C., Dunach, M. & Garcia De Herreros, A. (2003) Mol. Cell. Biol. 23, 7391–7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Condeelis, J. & Segall, J. E. (2003) Nat. Rev. Cancer 3, 921–930. [DOI] [PubMed] [Google Scholar]
- 49.Charpentier, E., Lavker, R. M., Acquista, E. & Cowin, P. (2000) J. Cell Biol. 149, 503–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simcha, I., Geiger, B., Yehuda-Levenberg, S., Salomon, D. & Ben-Ze'ev, A. (1996) J. Cell Biol. 133, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.