Abstract
Objective
The aim of the present study was to investigate the diagnostic value of strain elastography (SE) of testicular tissues in infertile population. We also evaluated the correlation between SE results with semen parameters and hormone profiles of the patients.
Material and methods
A total of 61 patients and 122 testes were evaluated. Patients who were evaluated in an andrology outpatient clinic with the diagnosis of infertility and referred to radiology department for investigation of reproductive organs between June 2015 and January 2016 were included. Patients were divided into two groups according to semen analyses results as normal (Group 1) and abnormal (Group 2). Hormone profiles, semen analyses, B-mode, coloured Doppler ultrasonography and sonoelastography examinations were performed for each patient. Measurements of testicular volumes, resistive indices (RI) in intraparenchymal arteries, strain, strain ratio (SR) and presence of varicocele were recorded.
Results
Mean age of participants was 33.7±6.3 years. Mean testicular volumes (Group 1, 19.41±4.8 mL, and Group 2, 17.64±3.62 mL) were significantly different between groups (p=0.023). Mean SRs were also different between Groups 1 and 2 (0.12±0.08 vs. 0.22±0.18, p<0.001). Testicular volumes were directly proportional with SRs in Group 1. Strain values had inverse relationship with sperm concentration and total motile sperm counts in Group 2 (p=0.01). SRs were found to be positively correlated with RI and sperm morphology in Group 2 (p<0.05). Although FSH values showed significant difference among groups, any correlation between FSH and elastographic parameters could not be displayed.
Conclusion
Strain elastography results were found to be significantly different in patients with abnormal sperm counts. This technique may provide promising results, however, further large scale studies may help to clarify the value of this imaging modality in the assessment of male infertility.
Keywords: Male infertility, semen analysis, sperm, strain elastography
Introduction
Infertility is defined as the inability of a non-contracepting couple to achieve spontaneous pregnancy within one year.[1] One in six couples suffer from problems when attempting to conceive child and seek treatment for infertility. A male factor is responsible in half of the cases, however, 15% of the cases are still classified as unexplained infertility.[2] In addition to physical examination and semen analysis, a scrotal ultrasonography (US) may help to demonstrate obstruction or testicular dysgenesis.[3] US is the first-line imaging modality used to evaluate male genital tract due to its noninvasiveness, safety and absence of exposure to radiation.[1]
It is well-known that conventional US is limited to the functional analysis of testicular tissue, but elastography is a promising technique in this field. Recent technical advances in US applications and post-processing developments have provided new insights for the structural and functional evaluation of testicular tissue.[4] Elastography which was first described by Ophir et al.[5] is a relatively new imaging technique that displays the images of tissue stiffness. Elastography creates images of tissue stiffness which can be thought as an extension to the ancient palpation techniques; however, it gives better spatial localisation information and is less subjective.[4] Two main types of elastography are currently in use. One is real-time elastography and the other is strain elastography (SE).[6] In this modality, tissue displacement in response to gentle pressure is used to compute and image tissue strain. SE measures the strain response of tissues in real time from sonography signals during externally applied compression–decompression cycles.[6] As a semiquantitative elastographic technique, SE compares the elasticity and stiffness of target tissues with nearby normal ones.[7] Basic assumption of SE is that soft tissues can be more deformable than hard ones which can be displayed on US as a colour map overlaid to the grey scale image.[8] The resultant strain ratio (SR) represents the ratio of stiffness of the target and normal tissues. Studies reported that objectively measured tissue stiffness by SE may be used as a diagnostic marker in clinical practice for the differentiation between benign and malignant tissues in various organs.[9–12]
The use of SE for the evaluation of testicular tissues is a new concept. Aigner et al.[13] reported that elastography can be used with high sensitivity rate to differentiate benign and malignant testicular lesions. In terms of infertility assessment, Schurich et al.[4] stated that SE can be used for structural analysis of testicular parenchyma and became an additional method for detecting pathological tissue alterations. They also noted that elasticity pattern of a testis seems to be related to the testicular volume and function. However, data about the results of SE on testicular tissues are still limited. In this study, it has been hypothesized that abnormal semen parameters might be related to testicular parenchymal abnormalities those not depicted by conventional US. Therefore, the aim of the present study was to evaluate the diagnostic value of SE of testicular tissues in infertile men with normal and abnormal semen parameters. It is also aimed to correlate SE results of testicular tissues with semen analysis parameters such as concentration, motility and morphology and hormone profiles of infertile patients.
Material and methods
Patients
Men who were between 23–45 years of age, clinically diagnosed with primary infertility and did not receive any previous fertility treatment constituted the study population. Men who were married for more than one year and who were referred from andrology outpatient clinic to radiology department for the investigation of testicles with scrotal US were enrolled in the study. This study was performed between June 2015 and January 2016 in a university hospital. Each patient was evaluated by a detailed history taking, physical examination, a semen analysis and endocrine profiles. A total of 61 men were divided into two groups according to the results of semen analyses. Group 1 included 31 men with normal semen parameters whereas 30 men in group 2 had abnormal sperm features. Participants who did not undergo semen analysis and had a history of undescended testis, orchiectomy or testicular biopsy were not included in the study. Additionally, patients with testicular atrophy, trauma, acute or chronic orchitis, testicular mass, testicular microlithiasis, hydrocele and infarct were excluded. Local ethics committee approved the study design and an informed consent was obtained from each patient.
Ultrasonographic evaluation
Participants underwent both B-mode sonography and free hand real-time SE examination in the supine position with a digital sonography scanner (Aplio 400, Toshiba Medical Systems Corporation, Otawara, Japan) supplied with SE software and using a linear 12 MHz multifrequency transducer. All imaging modalities were performed by an experienced radiologist. First, locations, contours, echo patterns and volumes of both testes were evaluated with grayscale sonograms. Then, intraparencymal flow rates and resistive indices were measured with spectral Doppler US (Figure 1). Afterwards, real time elastograhy was performed for both testes of the patients. During probe movement, grayscale sonograms of the testicular tissues were visualized adjacent to elastographic images on the screen. Images were obtained from middle portion of testis and scrotal skin layers. Due to the ovoid shape of the testis, upper and lower poles could not be included into images. After 10–12 compression–decompression cycles, acquisition of elastographic images was finalized, and images were produced automatically on the ultrasound machine by comparing two adjacent frames during tissue compression and decompression. Waveforms formed during compression and decompression appeared on the elastographic screen with sinusoidal shapes above and below the baseline of the waveform scale, respectively. The most relevant duration and strength of compression and decompression were determined according to compression-time scale which was appeared at the lower left part of the screen (Figure 2). Measurements were performed during the decompression phase since there was no pressure from the outside, therefore, only the internal dynamics were measured in this phase. SR values of tissues were measured by putting multiple equally sized regions of interest (ROIs) on the testicular tissue (A) and scrotal subcutaneous fatty tissue (B). SR value (B/A) reflecting the feature of stiffness was automatically calculated on the sonography machine by comparing normal testicular tissue (A) to the adjacentj scrotal subcutaneous fatty tissue (B) for each patient and mean values were obtained (Figure 3). SR value increases when the tissue is harder (stiffer) (B). To prevent the variability of the technique, three measurements were performed for each patient.
Figure 1.
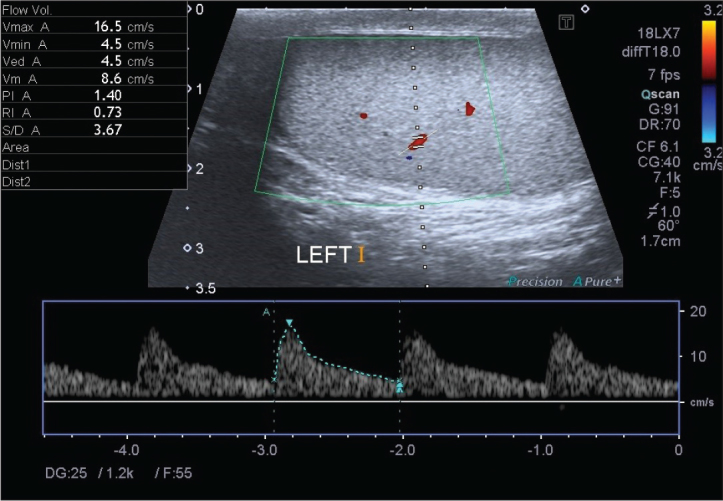
The spectral Doppler ultrasonography image of the left testis. Measurement of blood flows by spectral Doppler ultrasonography
Figure 2.
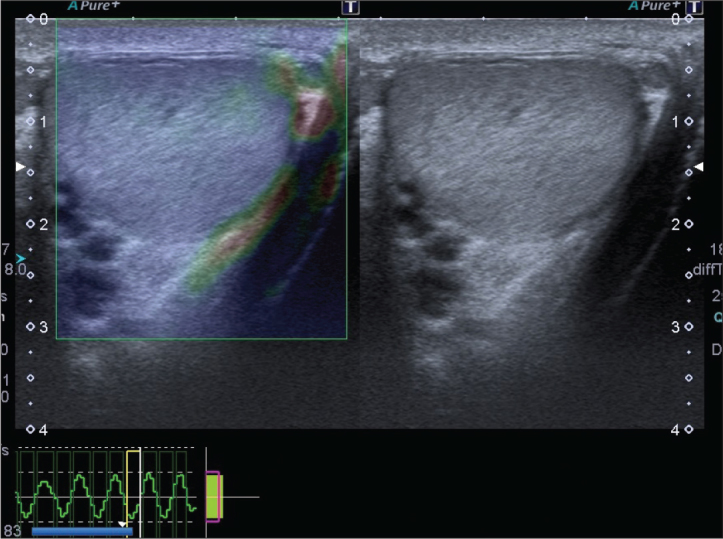
The elastography–ultrasonography image of the left testis. The velocity profile during compression and decompression
Figure 3.
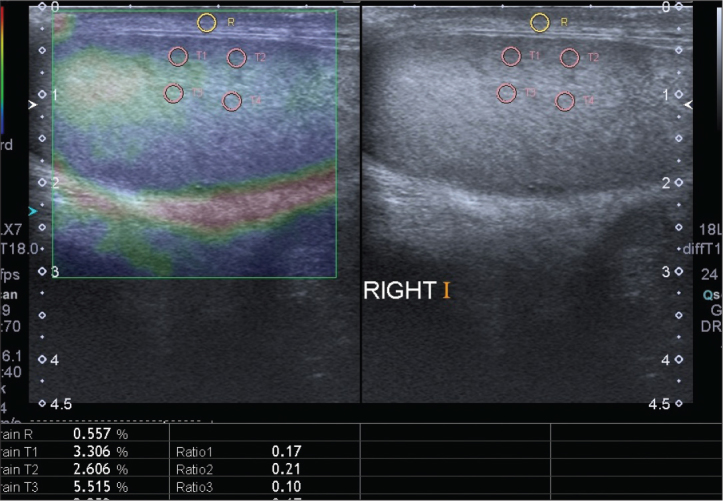
The elastography–ultrasonography image of the testis. The yellow region of interest (ROI) is on the skin. Lower four circles indicate the ROIs in subcapsular and intraparancyhmal area
Participants were also evaluated according to the presence of varicoceles. A colour Doppler scrotal US was performed on all patients and the largest diameter of veins of plexus pampiniformis was measured. A diameter of more than 2 mm was accepted as varicocele.[14]
Statistical analysis
A post-hoc power analysis with mean strain ratios and standard deviations showed that the study’s power was 78.06 % with an effect size of 0.71 (α=0.05) (GPower software, F. Faul, University of Kiel, Germany). Statistical analysis was performed by using Statistical Package for Social Sciences (IBM SPSS Statistics, Armonk, NY, USA) 21.0 software. Data were presented as mean±SD. Continuous variables were evaluated by mean and SD, and compared by Studen’s T test. Correlation of serum hormone measures (T, FSH and estradiol) and semen parameters with SE results were tested by Spearman’s correlation and Mann-Whitney U test. A P value of less than 0.05 was accepted as statistically significant.
Results
Demographic data of the patients were presented in Table 1. In this study, 122 testes of 61 patients (31 with normal and 30 with abnormal semen parameters) were investigated in terms of grayscale sonograms and SE. Of the 30 patients in Group 2, 16 had oligozoospermia, 9 had asthenozoospermia and 5 had teratozoospermia. None of the patients had Oligoasthenoteratozoospermia syndrome. According to grayscale sonograms, the lowest and the highest testicular volumes were 8.8 mL and 35.6 mL, respectively. Mean testicular volumes of the patients in Group 1 were significantly higher than the men of Group 2 (19.41±4.8 vs. 17.64±3.62, p=0.023). Testicular volume was positively correlated with sperm concentration in Group 1 (p=0.004). Mean strain values were statistically insignificant; however, mean SRs were significantly lower in Group 1 (0.12±0.08 vs. 0.22±0.18, p<0.001).
Table 1.
Demographic data and clinical characteristics of study population
| Group 1 | Group 2 | p | |
|---|---|---|---|
| Number of patients (n) | 31 | 30 | |
| Mean age (years) | 33.39±6.45 | 34.3±6.7 | 0.444 |
| Mean testicular volume (mL) | 19.41±4.8 | 17.64±3.62 | 0.023* |
| Mean resistive indices | 0.56±0.09 | 0.56±0.09 | 0.957 |
| Mean strain values | 5.27±2.37 | 5.46±2.54 | 0.657 |
| Mean strain ratios | 0.12±0.08 | 0.22±0.18 | <0.001* |
| Number of spermatozoa (nx106/mL) | 62.2±33.2 | 11.5±28.1 | <0.001* |
| Morphology (%) | 5.1±1.63 | 0.27±0.58 | <0.001* |
| Mean number of total motile sperm (%) | 37.58±3.66 | 9.2±10.68 | <0.001* |
| Mean FSH (mU/mL) | 4.71±1.59 | 12.5±17.98 | 0.001* |
| Mean estradiol (pg/mL) | 29.81±7.08 | 33.48±11.33 | 0.054 |
| Mean total testosterone (ng/dL) | 390.18±148.76 | 399.56±179.83 | 0.754 |
FSH: follicle stimulating hormone
Student t-test;
statistical significance
There was significant negative correlation between strain values and total sperm count in Group 2 (r=−0.33, p=0.01); however, there was no association between those measures in Group 1. Additionally, there was inverse relationship between strain values and total motile sperm count in Group 2 (r=−0.32, p=0.01). SRs were found to be significantly correlated with sperm morphology and RI in Group 2 (r=0.273 and 0.272; p=0.03 and 0.04 respectively). SRs were also found to be positively correlated with testicular volumes in Group 1, however, this association was insignificant for Group 2 (r=0.357, p<0.01).
Varicocele was present in 11 men in Group 1 and 12 men in Group 2 (p=0.526). In Groups 1, and 2, the patients had Grade 1 (n=6 vs. 5), 2 (n=3 vs. 4), and 3 (n=2 vs. 3) varicoceles Grade of varicocele was not significantly different between groups and statistical analyses did not reveal any effect of presence and degree of varicocele on elastography findings. Although FSH levels were significantly different between two groups, multivariate analysis between FSH and elastographic findings revealed no significant correlation in both groups.
Discussion
Infertility is a common public health issue since more than 15% of couples may be affected and subsequent psychosocial problems may impair partner relationships. Imaging modalities are becoming more commonly used to define the reasons of infertility. Among them, with recent technological advancements, US is the first option for male genital tract evaluation. In addition to B-mode and color Doppler US, elastography with strain and shear wave developments were reported to be used in the investigation of testicular tissues and spermatogenesis.[4,15,16]
Strain elastography is based on the principle that compression produces strain within the tissue and the amount of strain is lower in stiffer tissue than in softer.[17] Since carcinomas were found to be stiffer than adjacent normal tissues and studies reported that histologically different tumors may display different elasticity patterns according to tissue architecture,[7,10] this technique has been successfully applied to differentiate focal lesions of testis, breast, prostate, pancreas and liver.[14,18–20]
Recently, shear wave elastography (SWE) which combines B-mode image with color-coded US generating a quantitative image SWE (kPa) of the tissue stiffness started to be used to exhibit different hardness among different tissue regions in real-time conditions.[21] With the use of SWE, different elasticity values dependent on testicular volume and functional properties have been demonstrated.[22] SWE produces more reproducible results than other forms of sonoelastography. Transformation and change in tissue hardness should be therefore confidently documented by SWE. However, we do not have this modality in our radiology department and, for this reason, the study was performed with SE.
A few recent studies investigating the use of elastography in order to clarify the reason of infertility have been published in the literature.[4,23,24] Tissue elasticity generally correlates with pathological conditions of the testicular parenchyma In most of the cases, a normal testicular biopsy excludes the diagnosis of testicular pathologies.[25] Some focal testicular lesions such as testicular microlithiasis, azoospermia and lesions with a diameter of <10 mm, particularly if they are not palpable, were investigated with the use of sonoelastography technique.[23,26] The results of testicular biopsies of azoospermic male patients showed that along with the increasing grade of histological criteria, diameter of seminiferous tubules and height of spermatogenic epithelium are gradually reduced, while the thickness of the lamina propria is gradually increased.[23] Authors concluded that tissue stiffness was increased in azoospermic patients and SR may be useful for diagnosing azoospermia. Similarly, the present study demonstrated that the elasticity of testicular tissue had a negative correlation with sperm concentration and total motile sperm count.
Some studies reported that SR may provide more objective data with a higher diagnostic accuracy than elastography.[27–29] However, normal testicular tissues were compared with pathological lesions in those studies. In contrast, we defined two ROIs as middle of testis (A) and subcutaneous fatty tissue of corresponding scrotum (B) in this study. It can be criticised that lack of ROIs being at the same plane and different contraction patterns of scrotal skin during examination which was thought to be secondary to cremasteric reflex may affect SR levels for each patient.[30,31] However, those issues were speculated as the interest of other research studies. Further studies evaluating the relationship between strain elastography results and testicular sperm retrieval rates are needed to increase the diagnostic accuracy of this imaging modality. Our data revealed that SR values were significantly higher in patients with abnormal semen parameters. Positive correlations between SR, sperm morphology and RI were established in that patient group.
Many studies stated that testicular volume may be a predictor of spermatogenetic function. Tijani et al.[32] reported that testis volume was significantly different in fertile and infertile groups. Besides, they showed that testis volume measured by US was correlated with the severity of oligozoospermia. Other studies also revealed that testicular volume had a significant association with semen volume, sperm count and motility.[33,34] In their preliminary study Condorelli et al.[35], reported that testis volume was associated with some biofunctional sperm parameteres and stated that the biofunctional sperm parameters worsen with decreasing testicular volume. Schurich et al.[4] reported that elastography can be used for structural analysis of testicular tissues in order to find out any pathological tissue alterations. They also determined that the elasticity pattern of the testis seemed to be related to testicular function and volume. Consistent with that report, we found out that testicular volume was correlated with strain indices of patients in group 1.
Our study also determined a significant relationship between testis volume and sperm counts in Group 1. Since seminiferous tubules which constitute 80% of testicular volume were responsible from spermatogenesis, it can be hypothesized that testicular volume can be related to sperm count. It may also explain why testis volume was higher in Group 1 with normal sperm counts. However, we failed to demonstrate any association between testis volume and sperm count in Group 2. Besides, we could not show that sperm morphology and total motile sperm count were related to testicular volume in both groups. The reasons of failure to demonstrate any association between testis volume and semen parameters may be the small number of patient population and differences in the inclusion criteria.
Pinggera et al.[36] stated that measurement of RI of intratesticular arteries demonstrated an increased testicular vascular resistance in patients with pathological sperm features compared to patients with normal ones. It was also noted that testicular volumes of groups were not significantly different. Similar results were reported in other studies.[37–40] Present study showed that although RI values were not different in groups with normal and pathological semen parameters, SRs were found to have a positive relationship with RIs in Group 2.
Dede et al.[24] investigated the relationship between elastography scores, serum FSH levels and varicocele. They concluded that testicular elasticity was inversely correlated with serum FSH and varicocele grade. However, in our study, groups were homogenous for the presence of varicocele and no association between varicocele and tissue elasticity was found. Besides, we could not find out any correlation between FSH levels and elastography findings among groups.
One major limitation of our study was the low number of study population which prevented us from classifying patients into categories of oligozoospermia, azoospermia and teratozoospermia and to investigate their relationships with sonoelastographic findings. Besides, participants were not evaluated according to tobacco smoking, alcohol intake and any other endocrinological problems which may affect sperm characteristics.
In conclusion, elastographic techniques are becoming more popular in the evaluation of male infertility. With the aid of SE, we have demonstrated that testicular elasticity was inversely related to sperm parameters. In addition, SR may correlate with sperm morphology and testicular volume. Further studies with large patient populations which investigate the relationship between SE values and sperm features may provide new insights into the noninvasive investigation of testicular tissues in infertile men.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Kahramanmaraş Sütçü İmam University School of Medicine.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – M.A.S., Ö.E.; Design – M.A.S., Ö.E., B.K., M.B.; Supervision – B.K., M.B.; Resources – M.A.S., Ö.E.; Materials – M.A.S., Ö.E., F.K.; Data Collection and/or Processing – M.A.S., F.K.; Analysis and/or Interpretation – M.A.S., F.K., S.R.; Literature Search – M.A.S., F.K.; Writing Manuscript – F.K., M.A.S.; Critical Review – M.B., B.K., S.R.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, Dohle G, et al. European Association of Urology Working Group on Male Infertility. Eur Urol. 2012;62:324–32. doi: 10.1016/j.eururo.2012.04.048. https://doi.org/10.1016/j.eururo.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 2.WHO. WHO Manual for the Standardized Investigation and Diagnosis of the Infertile Couple. Cambridge University Press; Cambridge: 2000. [Google Scholar]
- 3.Ammar T, Sidhu PS, Wilkins CJ. Male infertility: the role of imaging in diagnosis and management. Br J Radiol. 2012;85:S59–68. doi: 10.1259/bjr/31818161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schurich M, Aigner F, Frauscher F, Pallwein L. The role ofultrasound in assessment of male fertility. Eur J Obstet GynecolReprod Biol. 2009;144(Suppl 1):S192–8. doi: 10.1016/j.ejogrb.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 5.Ophir J, Céspedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: aquantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111–34. doi: 10.1177/016173469101300201. https://doi.org/10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 6.Talreja SM, Tomar V, Yadav SS, Jaipal U, Priyadarshi S, Agarwal N, et al. Comparison of sonoelastography with sonourethrographyand retrograde urethrography in the evaluation of male anteriorurethral strictures. Turk J Urol. 2016;42:84–91. doi: 10.5152/tud.2016.99223. https://doi.org/10.5152/tud.2016.99223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onur MR, Poyraz AK, Bozgeyik Z, Onur AR, Orhan I. Utility ofsemiquantitative strain elastography for differentiation betweenbenign and malignant solid renal masses. J Ultrasound Med. 2015;34:639–47. doi: 10.7863/ultra.34.4.639. https://doi.org/10.7863/ultra.34.4.639. [DOI] [PubMed] [Google Scholar]
- 8.Inci MF, Kalayci TO, Tan S, Karasu S, Albayrak E, Cakir V, et al. Diagnostic value of strain elastography for differentiation betweenrenal cell carcinoma and transitional cell carcinoma of kidney. Abdom Radiol (NY) 2016;41:1152–9. doi: 10.1007/s00261-016-0658-2. https://doi.org/10.1007/s00261-016-0658-2. [DOI] [PubMed] [Google Scholar]
- 9.Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, et al. EFSUMB guidelines and recommendations onthe clinical use of ultrasound elastography. Part 1: Basic principlesand technology. Ultraschall Med. 2013;34:169–84. doi: 10.1055/s-0033-1335205. [DOI] [PubMed] [Google Scholar]
- 10.Tan S, Ozcan MF, Tezcan F, Balci S, Karaoglanoglu M, Huddam B, et al. Real-time elastography for distinguishing angiomyolipoma from renal cell carcinoma: preliminary observations. AJR. 2013;200:369–75. doi: 10.2214/AJR.12.9139. https://doi.org/10.2214/AJR.12.9139. [DOI] [PubMed] [Google Scholar]
- 11.Rago T, Santini F, Scutari M, Pinchera A, Vitti P. Elastography:new developments in ultrasound for predicting malignancy inthyroid nodules. J Clin Endocrinol. 2007;92:2917–22. doi: 10.1210/jc.2007-0641. https://doi.org/10.1210/jc.2007-0641. [DOI] [PubMed] [Google Scholar]
- 12.Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–50. doi: 10.1148/radiol.2391041676. https://doi.org/10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 13.Onur MR, Poyraz AK, Ucak EE, Bozgeyik Z, Ozercan IH, Ogur E. Semi-quantitative strain elastography of liver masses. JUltrasound Med. 2012;31:1061–7. doi: 10.7863/jum.2012.31.7.1061. https://doi.org/10.7863/jum.2012.31.7.1061. [DOI] [PubMed] [Google Scholar]
- 14.Aigner F, De Zordo T, Pallwein-Prettner L, Junker D, Schäfer G, Pichler R, et al. Real-time sonoelastography for the evaluation oftesticular lesions. Radiology. 2012;263:584–9. doi: 10.1148/radiol.12111732. https://doi.org/10.1148/radiol.12111732. [DOI] [PubMed] [Google Scholar]
- 15.Arslan H, Sakarya ME, Atilla MK. Clinical value of power Doppler sonography in the diagnosis of varicocele. J Clin Ultrasound. 1998;26:229. doi: 10.1002/(sici)1097-0096(199805)26:4<229::aid-jcu13>3.0.co;2-e. https://doi.org/10.1002/(SICI)1097-0096(199805)26:4<229::AID-JCU13>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Lv F, Tang J. Shear wave elastography (SWE) is reliablemethod for testicular spermatogenesis evaluation after torsion. IntJ Clin Exp Med. 2015;8:7089–97. [PMC free article] [PubMed] [Google Scholar]
- 17.Ophir J, Kallel F, Varghese T, Alam SK, Krouskop T, Garra BS, et al. Elastography. Optical and Acoustical Imaging of BiologicalMedia. 2001;4:1193–212. https://doi.org/10.1016/S1296-2147(01)01255-0. [Google Scholar]
- 18.Daniaux M, Auer T, De Zordo T, Junker D, Santner W, Hubalek M, Jaschke W, Aigner F. Strain Elastography ofBreast and Prostata Cancer: Similarities and Differences. Rofo. 2016;188:253–8. doi: 10.1055/s-0041-106540. [DOI] [PubMed] [Google Scholar]
- 19.Dyrla P, Gil J, Florek M, Saracyn M, Grala B, Jędrzejewski E, et al. Elastography in pancreatic solid tumours diagnoses. PrzGastroenterol. 2015;10:41–6. doi: 10.5114/pg.2015.48994. https://doi.org/10.5114/pg.2015.48994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Q, Ling W, Lu C, Li J, Ma L, Quan J, et al. Hepatocellular carcinoma: stiffness value and ratio to discriminate malignant frombenign focal liver lesions. Radiology. 2015;275:880–8. doi: 10.1148/radiol.14131164. https://doi.org/10.1148/radiol.14131164. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Lv F, Tang J. Shear wave elastography (SWE) is reliablemethod for testicular spermatogenesis evaluation aftertorsion. Int JClin Exp Med. 2015;8:7089–97. [PMC free article] [PubMed] [Google Scholar]
- 22.De Zordo T, Stronegger D, Pallwein-Prettner L, Harvey CJ, Pinggera G, Jaschke W, et al. Multiparametric ultrasonography ofthe testicles. Nat Rev Urol. 2013;10:135–48. doi: 10.1038/nrurol.2012.255. https://doi.org/10.1038/nrurol.2012.255. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Du J, Wang ZQ, Li FH. The value of sonoelastographyscores and the strain ratio in differential diagnosis of azoospermia. J Urol. 2012;188:1861–6. doi: 10.1016/j.juro.2012.07.031. https://doi.org/10.1016/j.juro.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 24.Dede O, Teke M, Daggulli M, Utangaç M, Baş O, Penbegül N. Elastography to assess the effect of varicoceles on testes: a prospective controlled study. Andrologia. 2016;48:257–61. doi: 10.1111/and.12440. https://doi.org/10.1111/and.12440. [DOI] [PubMed] [Google Scholar]
- 25.Cerilli LA, Kuang W, Rogers D. A practical approach to testicularbiopsy interpretation for male infertility. Arch Pathol Lab Med. 2010;134:1197–204. doi: 10.5858/2009-0379-RA.1. [DOI] [PubMed] [Google Scholar]
- 26.Pastore AL, Palleschi G, Maceroni P, Manfredonia G, Autieri D, Cacciotti J, et al. Correlation between semiquantitative sonoelastography and immunohistochemistry in theevaluation of testicularfocal lesions. Cancer Imaging. 2014;14:29. doi: 10.1186/s40644-014-0029-6. https://doi.org/10.1186/s40644-014-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhi H, Xiao XY, Yang HY, Wen YL, Ou B, Luo BM, et al. Semi-quantitating stiffness of breast solid lesions in ultrasonic elastography. Acad Radiol. 2008;15:1347–53. doi: 10.1016/j.acra.2008.08.003. https://doi.org/10.1016/j.acra.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Thomas A, Degenhardt F, Farrokh A, Wojcinski S, Slowinski T, Fischer T. Significant differentiation of focal breast lesions: calculation of strain ratio in breast sonoelastography. Acad Radiol. 2010;17:558–63. doi: 10.1016/j.acra.2009.12.006. https://doi.org/10.1016/j.acra.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Fischer T, Peisker U, Fiedor S, Slowinski T, Wedemeyer P, Diekmann F, et al. Significant differentiation of focal breastlesions: raw data-based calculation of strain ratio. Ultraschall Med. 2012;33:372–9. doi: 10.1055/s-0031-1273222. https://doi.org/10.1055/s-0031-1273222. [DOI] [PubMed] [Google Scholar]
- 30.Bingöl-Koloğlu M, Tanyel FC, Anlar B, Büyükpamukçu N. Cremasteric reflex and retraction of a testis. J Pediatr Surg. 2001;36:863–7. doi: 10.1053/jpsu.2001.23956. https://doi.org/10.1053/jpsu.2001.23956. [DOI] [PubMed] [Google Scholar]
- 31.Stein RJ, Santos S, Nagatomi J, Hayashi Y, Minnery BS, Xavier M, et al. Cool (TRPM8) and hot (TRPV1) receptors in the bladderand male genital tract. J Urol. 2004;172:1175–8. doi: 10.1097/01.ju.0000134880.55119.cf. https://doi.org/10.1097/01.ju.0000134880.55119.cf. [DOI] [PubMed] [Google Scholar]
- 32.Tijani KH, Oyende BO, Awosanya GO, Ojewola RW, Yusuf AO. Assessment of testicular volume: A comparison of fertile andsub-fertile West African men. African J Urol. 2014;20:136–40. https://doi.org/10.1016/j.afju.2014.05.001. [Google Scholar]
- 33.Kumar S, Mohsen N, Vineeth VS, Malini SS. Assessment ofTesticular Volume in Correlation with Spermiogram of InfertileMales in South India. Advanced Studies in Biology. 2013;5:327–35. https://doi.org/10.12988/asb.2013.3317. [Google Scholar]
- 34.Kristo A, Dani E. The Correlation between Ultrasound Testicular Volume and Conventional Semen Parameters in Albanian Subfertile Males. Macedonian Journal of Medical Sciences. 2014;7:464–6. https://doi.org/10.3889/oamjms.2014.081. [Google Scholar]
- 35.Condorelli R, Calogero AE, La Vignera S. Relationship betweentesticular volume and conventional or nonconventional spermparameters. Int J Endocrinol. 2013;2013:145792. doi: 10.1155/2013/145792. https://doi.org/10.1155/2013/145792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinggera GM, Mitterberger M, Bartsch G, Strasser H, Gradl J, Aigner F, et al. Assessment of the intratesticular resistive index bycolour Doppler ultrasonography measurements as a predictor ofspermatogenesis. BJU Int. 2008;101:722–6. doi: 10.1111/j.1464-410X.2007.07343.x. https://doi.org/10.1111/j.1464-410X.2007.07343.x. [DOI] [PubMed] [Google Scholar]
- 37.Biagiotti G, Cavallini G, Modenini F, Vitali G, Gianaroli L. Spermatogenesis and spectral echo-colour Doppler traces from the main testicular artery. BJU Int. 2002;90:903. doi: 10.1046/j.1464-410x.2002.03033.x. Y908. [DOI] [PubMed] [Google Scholar]
- 38.Akcar N, Turgut M, Adapınar B, Ozkan IR. Intratesticular arterial resistance and testicular volume in infertile men with subclinical varicocele. J Clin Ultrasound. 2004;32:389–93. doi: 10.1002/jcu.20059. https://doi.org/10.1002/jcu.20059. [DOI] [PubMed] [Google Scholar]
- 39.Semiz I, Tokgöz Ö, Tokgoz H, Voyvoda N, Serifoglu I, Erdem Z. The Investigation of Correlation Between Semen Analysis Parameters and Intraparenchymal Testicular Spectral Doppler Indices in Patients With Clinical Varicocele. Ultrasound Q. 2014;30:33–40. doi: 10.1097/RUQ.0000000000000055. https://doi.org/10.1097/RUQ.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 40.Polat H, Sarica MA, Bulut HT, Yucel MO, Gok A, Cift A, et al. The relationship between mean platelet volume and other platelet indices with testicular artery blood flow and fertility: a preliminary study. Int J Clin Exp Med. 2015;8:11554–8. [PMC free article] [PubMed] [Google Scholar]


