Abstract
Objective
For in vitro tissue engineering in urology, stem cells are commonly isolated from tissue specimens obtained during open or endoscopic surgery. Within the context of the present study our aim was to isolate cells from human urine by an alternative and safe technique rather than using the indicated method.
Material and methods
After human urine samples had been collected from young and healthy donors via urethral catheterization, cells were precipitated by centrifugation and cultured. Following this isolation procedure, cells were characterized by immunocytochemical method using specific antibodies.
Results
When these cells were characterized by immunocytochemical methods using specific antibodies some of them were positive for mesenchymal stem cell marker CD90 while the others were labelled with urothelial marker cytokeratin 7. When all these results were taken into consideration, urothelial cells together with stem cells were observed in the urine- derived cell population.
Conclusion
According to the results obtained from this study human urine may be preferred as an alternative stem cell and urothelial cell source in that this method is and easily reproducible non-invasive method.
Keywords: Cell-based therapy, human urine, stem cell, urine derived cell, urothelial cell
Introduction
In tissue engineering and cell-based therapeutic applications, cells are traditionally obtained from biopsies using an invasive approach but this method may lead to donor-site morbidity. Additionally, this cell isolation process which includes both enzymatic and mechanic digestions decreases clonal growth capabilities of cells. Therefore, noninvasive procedures are highly desirable to increase the viability of primary monolayer cell cultures, particularly of autologous cells. It is known that functional tissue regeneration and success of cell therapy are enhanced by these cells because they do not cause any immune response or rejection.[1] Nevertheless, there is a handicap in the use of autologous somatic cells because of their limited proliferation capacity. To overcome this limitation, studies have especially focused on autologous stem cells derived from a variety of adult tissues such as muscle, bone marrow and adipose tissue.[2] Moreover, it has recently been demonstrated that autologous stem cells can also be obtained from urine by a noninvasive and low- cost technique.[3,4] Therefore, urine can be an alternative autologous stem cell source for cell- based therapies.
Although there are some techniques in the literature for maintaining cell viability during handling of human urine, exact conditions need to be determined. For example, urine preservation conditions which are suitable for retaining proliferation and multipotent differentiation capabilities of stem cells in fresh urine samples have been explained in only one paper.[2] Additionally, little data are available about age, gender and health status of urine donors.[1,2,4–6] Therefore, development of a reliable method for preservation of cells in urine will increase the amount of high quality cells obtained, and also will alleviate cell damage caused by storing them in urine. Furthermore, if young and healthy people are chosen as urine donors, urine-derived stem cells will have higher capability for expansion in culture and also for differentiation toward different lineages.
Urothelium biopsy specimens are traditionally used to acquire urothelial cells for clinical trials and urological tissue engineering applications.[5] These specimens are usually obtained by surgery under general anesthesia with a lot of risk for donor such as infection, pain and bleeding. Thus, urine- derived urothelial cells may be an excellent alternative cell source especially because they are easily obtained from a donor using a noninvasive technique.
The aim of the present study was twofold: a) to reveal the importance of human urine as a mesenchymal stem cell and urothelial cell source and b) to optimize this new noninvasive method and to determine the most suitable conditions (health status and age of donors, duration of transportation, formulation of culture media) for the culture of human urine- derived cells (hUDCs).
Material and methods
Isolation and cultivation of human urine- derived cells
The present work was performed after an approval was obtained from Clinical Trials Ethics Committee of Ege University and human urine samples were collected from patients who gave their informed consent.
Basically two types of cells were investigated: i.e. human urine- derived stem cells (hUDSCs) and human urine- derived urothelial cells (hUCs). To isolate these cells, fresh urine samples were collected from lower urinary tract of six ASA class I patients (healthy patients) who were scheduled for elective surgery in Celal Bayar University Hospital.
With the aim of enhancing success in cell isolation and cultivation techniques, some inclusion and exclusion criteria were determined for the participation of donors to the research. For example, patients who had diabetes, infectious (hepatitis, AIDS, etc.) and oncological (bladder, kidney, etc.) diseases and patients who were using drugs continuously because of any chronic diseases were excluded from this experiment. Additionally, particularly young people (between the ages of eighteen and thirty) were also included in the present study.
To isolate hUDCs, urine collection was made via urethral catheterization performed for patients scheduled for elective surgery not for this study particularly. Urine samples collected from drainage tubes of catheter bags were used. After the valve of the tube was opened aseptically, the urine sample was withdrawn into a sterile injection syringe from tube opening. Following the completion of this process, the valve was closed again carefully to prevent patient from any infection. Additionally, we paid attention to collect urine samples within the first 4–5 hours after it had drained into catheter bag.[7]
In the process of harvesting the hUDCs, six fresh urine samples (average amount of 100 mL per sample) were immediately transferred to the laboratory under sterile conditions approximately at 4°C. Each sample was centrifuged (Eppendorf Centrifuge 5417R, North America) at 500g for 5 minutes and the cell pellets were gently resuspended in sterile phosphate buffer saline (PBS) solution. After the cell suspension was centrifuged at 500 g for 5 minutes for the second time, supernatant was discarded and the cells were collected with initiation medium which was a 1:1 mixture of keratinocyte serum free medium (KSFM, Gibco-Invitrogen, Scotland) and embryonic fibroblast medium (EFM). EFM contained DMEM (Biochrom AG, Germany) and Ham’s F12 (Biochrom AG, Germany) in the ratio of 3:1, respectively. The initiation medium also included 10% fetal bovine serum (FBS, Biochrom AG, Germany), 0.4 μg/mL hydrocortisone (Sigma, USA), 0.1 mM non-essential amino acids (PAA, USA), 0.01% insulin, human transferrin and selenious acid (ITS) premix (BD, USA), 2.5 μg/mL epidermal growth factor (EGF, Sigma, USA), 30 ng/mL cholera toxin (Calbiochem, Germany) and 50 μg/mL gentamycin (HyClone, USA). The suspended cells in this medium were transferred to a six well cell culture plates (Greiner Bio-one, Germany), and incubated at 37°C in a 5% CO2 and air humidified incubator (Thermo, Heraeus HeraCell 150, USA). Culture media was changed every other day and the cells were split when they reached to 70–80% confluency.[8,9]
Immunocytochemical analysis
In order to characterize the isolated hUDCs, they were cultured on coverslips and stained with specific antibodies such as cytokeratin 7, CD45 and CD90 (Abcam, Germany) by immunocytochemical techniques. In this experiment, cytokeratin 7 antibody was used as an urethelial cell marker. Additionally, CD45 and CD90 antibodies are the best known negative and positive selectable markers respectively for human mesenchymal stem cells.
At the initial step of the immunocytochemical analysis, the hUDCs which were cultured on glass coverslips were rinsed with PBS and then fixed in 4% paraformaldehyde solution (USB, United States) for 15 minutes at room temperature. Following the fixation period, cells were permeabilized in 0.2% Triton X-100 (AppliChem, Germany) solution for 10 minutes at room temperature and then rinsed with PBS. At the next step, in order to prevent nonspecific antibody binding, hUDCs were incubated in 3% bovine serum albumin (BSA, Sigma, USA) solution. After blocking, the cells were incubated with primary antibodies overnight in humidified chamber at 4°C. Following this period, the cells were incubated with fluorescein- conjugated secondary antibodies and nucleic acid stain 4’,6-diamidino-2-phenylindole (DAPI) (Sigma, USA) for 45 minutes in dark and humidified chamber at room temperature. Following this period, coverslips were permanently mounted onto microscope slides using immunofluorescence mounting media. Then, immunostained cells were examined under fluorescence microscope (Leica DMIL, Germany).
Results
When hUDCs were harvested from fresh urine samples (average amount of 100 mL per sample) and cultured in 1:1 mixture of KSFM and EFM, nucleated cells were observed within 2 days after initial seeding (Figures 1a and b). On the first day of the cultivation, all isolated cells demonstrated epithelial morphology (Figure 1a). But on the second day of the cultivation, after changing the growth medium, fibroblastic cells appeared on the surface of the culture media together with epithelial cell colonies (Figure 1b). Approximately within 12 days, hUDCs reached to 80–90% confluency (Figures 1c, d and e). It is necessary to explain the fact that similar findings were obtained from all six fresh urine samples collected during this study.
Figure 1. a–e.
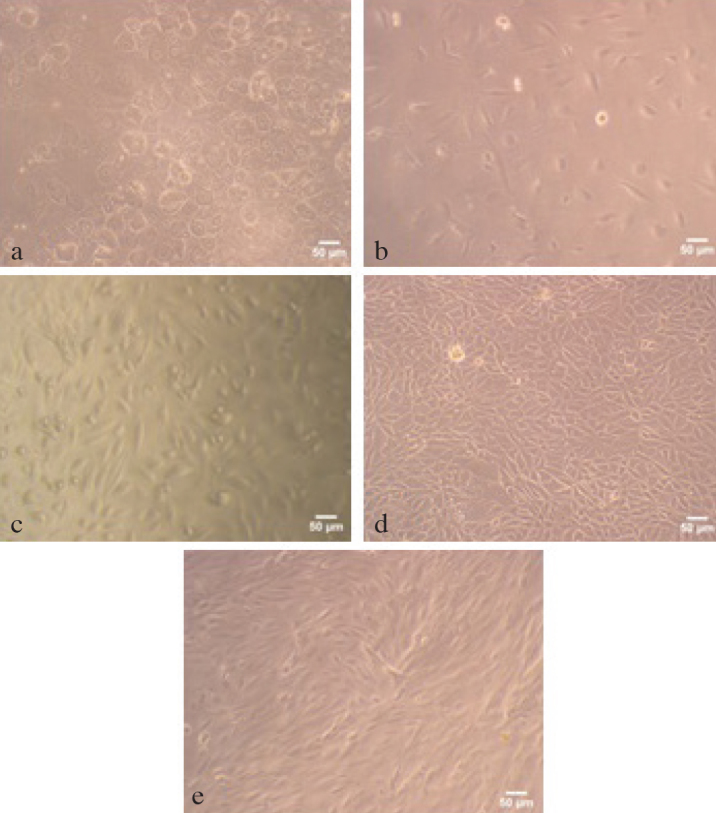
Inverted light microscope images of hUDCs. (a) The first day of primary culture of hUDCs. (b) Cell images from second day of culture. (c) Approximately half of the culture area was covered with hUDCs at the fifth day of primary culture. (d, e) This distinct colonization was shown in microscope images which were taken from different areas of culture dish on the twelfth day of culture. The scale bars indicated 50 μm
It was clearly observed that the cell population isolated from human urine wasn’t morphologically uniform, particularly within the first 10 days of culture. Although fibroblastic and urothelial cells were found in combination within the first 8–9 days of culture (Figure 1b and c), they were colonized by themselves and these colonies were spread on different areas of plate. This distinct colonization was shown in microscope images which were taken from different areas of culture dish on the twelfth day of culture (Figure 1d and e).
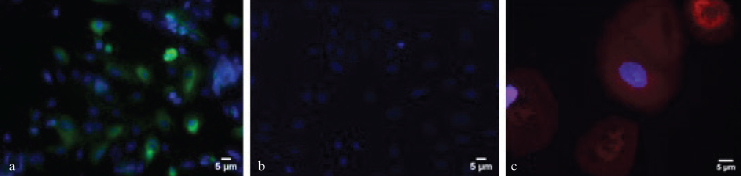
To determine immunocytochemical characteristics of hUDCs, they were immunostained with fluorescence labelled specific antibodies. When these cells were examined under fluorescence microscope, it was determined that some of hUDCs were positive for urothelial cell marker cytokeratin 7, while the others were negative for the same antibody (Figure 2a). Furthermore, some hUDCs were colonized separately from cobblestone area forming urothelial cells and labelled with mesenchymal stem cell marker CD90. Additionally, these CD90 positive cells were negative for hematopoietic stem cell marker CD45 (Figure 2b and c).
Figure 2. a–c.
Urine- derived cells were immunostained with specific monoclonal antibodies. (a) Some of the cells were positive for urothelial cell marker cytokeratin 7 (green fluorescence) while the others were negative for the same antibody. (b) hUDCs were not labelled with haematopoietic marker CD 45. (c) Some hUDCs in culture were stained with mesenchymal stem cell marker CD90 and emitted red fluorescence. All of the cells were counterstained with nuclear stain DAPI (blue fluorescence). Each scale bar indicated 5 μm
Discussion
In tissue engineering applications and cell therapies, use of autologous cells is always desirable because the risk of immune rejection can be eliminated by using them.[8] Especially autologous mesenchymal stem cells derived from patient’s own tissues such as bone marrow and adipose tissue are preferred due to their high proliferation capacity. Additionally, recent studies have indicated that the cells isolated from voided urine or urine catheterized from urinary tract have multi-lineage differentiation capability which are highly expandable and posses stem cell features.[1,2,10–13]
One of the most important results of the present study is that that human urine contains mesenchymal stem cells together with urothelial cells. This result is in compliance with the results other studies published in recent years.[4,6,8,9]
The previous studies have been supported by the current study with expanding knowledge about the importance of age and health status of urine donor, collection method of urine samples and also conditions of transportation to the laboratory. For instance, rates of success in isolation and cultivation of hUDCs can be enhanced by using freshly collected urine samples. Previous reports indicated that if exposure time to urine is more than five hours, then hUDCs lose their viability because of remarkable decrease in nutrients contained in urine and changes in pH.[1,2,7] Furthermore, the preliminary data of the present study showed the presence of a strong correlation between donor’s age and number of healthy cells in culture. For example, cells which were obtained from diabetic donors were never isolated and cultured successfully.[4,6,9] Contrary to other studies, higher cell viability was observed in the culture when the urine samples were transported to the laboratory at 4°C instead of room temperature.
In the present study, we didn’t come across with any microbial contamination in hUDCs which were isolated via urethral catheter from lower urinary tract. This result is in compliance with the results of the previous studies which suggested that isolation of hUDCs from urinary tract was more effective than isolation from voided urine. Additionally, it was found that the hUDCs obtained from lower urinary tract were similar to the cells isolated from voided urine in terms of morphology and marker expression profile.[1,3] In future studies, both urothelial cells and stem cell-like cells which can be isolated from fresh human urine should be characterized by different specific antibodies.
The findings of the present study have revealed that human urine can serve as an urothelial (hUCs) and mesenchymal stem cell (hUDSCs) source when it is processed with an appropriate technique.
Additionally, hUDSCs should be investigated for their differentiation capacity to the different cell types such as urothelial cells, osteoblasts, smooth muscle cells and also they induce pluripotent stem cells (IPS). In this way, relevant scientific information about human urine cells will have been supported and usage of these cells will be more widespread in various applications of regenerative medicine.
Acknowledgements
The authors would like to thank to Prof. Nejdet Kandemir for his helpful and constructive comments that greatly contributed to improving the final version of the paper.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Ege University School of Medicine.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – S.E.T., G.T.K., İ.T., E.M., S.İ.D.G.; Design – S.E.T., G.T.K., İ.T., E.M., S.İ.D.G.; Supervision – S.E.T., G.T.K., İ.T., E.M., S.İ.D.G.; Resources – S.E.T., G.T.K., İ.T., E.M., S.İ.D.G.; Materials – S.E.T., G.T.K., İ.T., E.M., S.İ.D.G.; Data Collection and/or Processing – S.E.T., G.T.K., İ.T., E.M., S.İ.D.G.; Analysis and/or Interpretation – S.E.T., G.T.K., İ.T., E.M., S.İ.D.G.; Literature Search – S.E.T., G.T.K., İ.T., E.M., S.İ.D.G.; Writing Manuscript – S.E.T., G.T.K., İ.T., E.M., S.İ.D.G.; Critical Review – S.E.T., G.T.K., İ.T., E.M., S.İ.D.G.; Other – S.E.T., G.T.K., İ.T., E.M., S.İ.D.G.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: Secil Erden Tayhan, one of the researchers of this study, was finantially supported by TUBITAK-BIDEB 2211 National Scholarship Programme for PhD Students.
References
- 1.Chun SY, Kim HT, Lee JS, Kim MJ, Kim BS, Kim BW, et al. Characterization of urine-derived cells from upper urinary tract in patients with bladder cancer. Urology. 2012;79:1186. doi: 10.1016/j.urology.2011.12.034. e1–e7. [DOI] [PubMed] [Google Scholar]
- 2.Lang R, Liu G, Shi Y, Bharadwaj S, Leng X, Zhou X, et al. Self-renewal and differentiation capacity of urine-derived stem cells after urine preservation for 24 hours. PLoS One. 2013;8:e53980. doi: 10.1371/journal.pone.0053980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodin A, Bharadwaj S, Wu S, Gatenholm P, Atala A, Zhang Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials. 2010;31:8889–901. doi: 10.1016/j.biomaterials.2010.07.108. https://doi.org/10.1016/j.biomaterials.2010.07.108. [DOI] [PubMed] [Google Scholar]
- 4.Wu S, Liu Y, Bharadwaj S, Atala A, Zhang Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials. 2011;32:1317–26. doi: 10.1016/j.biomaterials.2010.10.006. https://doi.org/10.1016/j.biomaterials.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Fossum M, Gustafson CJ, Nordenskjöld A, Kratz G. Isolation and in vitro cultivation of human urothelial cells from bladder washings of adult patients and children. Scand J Plast Recons. 2003;37:41–5. doi: 10.1080/alp.37.1.41.45. https://doi.org/10.1080/alp.37.1.41.45. [DOI] [PubMed] [Google Scholar]
- 6.Liu G, Pareta RA, Wu R, Shi Y, Zhou X, Liu H, et al. Skeletal myogenic differentiation of urine-derived stem cells and angiogenesis using microbeads loaded with growth factors. Biomaterials. 2013;34:1311–26. doi: 10.1016/j.biomaterials.2012.10.038. https://doi.org/10.1016/j.biomaterials.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adamowicz J, Kloskowski T, Tworkiewicz J, Pokrywczyńska M, Drewa T. Urine is a highly cytotoxic agent: Does it influence stem cell therapies in urology. Transplant. 2012;44:1439–41. doi: 10.1016/j.transproceed.2012.01.128. https://doi.org/10.1016/j.transproceed.2012.01.128. [DOI] [PubMed] [Google Scholar]
- 8.Mafi P, Hindocha S, Mafi R, Griffin M, Khan WS. Adult mesenchymal stem cells and cell surface characterization – a systematic review of the literature. The Open Orthop J. 2011;5:253–60. doi: 10.2174/1874325001105010253. https://doi.org/10.2174/1874325001105010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, McNeill E, Tian H, Soker S, Andersson KE, Yoo JJ, et al. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008;180:2226–33. doi: 10.1016/j.juro.2008.07.023. https://doi.org/10.1016/j.juro.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Dong X, Zhang T, Liu Q, Zhu J, Zhao J, Li J, et al. Beneficial Effects of Urine-Derived Stem Cells on Fibrosis and Apoptosis of Myocardial, Glomerular and Bladder Cells. Mol Cell Endocrinol. 2016;427:21–32. doi: 10.1016/j.mce.2016.03.001. https://doi.org/10.1016/j.mce.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Kang HS, Choi SH, Kim BS, Choi JY, Park GB, Kwon TG, et al. Advanced Properties of Urine Derived Stem Cells Compared to Adipose Tissue Derived Stem Cells in Terms of Cell Proliferation, Immune Modulation and Multi Differentiation. J Korean Med Sci. 2015;30:1764–76. doi: 10.3346/jkms.2015.30.12.1764. https://doi.org/10.3346/jkms.2015.30.12.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kloskowski T, Nowacki M, Pokrywczynska M, Drewa T. Urine-A Waste or the Future of Regenerative Medicine. Med Hypotheses. 2015;84:344–9. doi: 10.1016/j.mehy.2015.01.019. https://doi.org/10.1016/j.mehy.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Qin D, Long T, Deng J, Zhang Y. Urine-Derived Stem Cells for Potential Use in Bladder Repair. Stem Cell Res Ther. 2014;69:1–10. doi: 10.1186/scrt458. https://doi.org/10.1186/scrt458. [DOI] [PMC free article] [PubMed] [Google Scholar]