Abstract
DiGeorge syndrome (DGS) is one of the most frequently seen chromosomal abnormalities. The major genetic cause of DGS is a microdeletion on chromosome 22q11.2. Majority of the cases are diagnosed during their childhood. DGS is rarely considered and diagnosed in adulthood. Herein, we report the first case of a patient with DGS and azoospermia in the literature. Our patient was a 35-year-old male with mild dysmorphic features, hypernasal voice, mental retardation, and azoospermia. His laboratory tests and echocardiographic assessments were normal. Clinical clues to DGS were hypernasal voice and dysmorphic features with mild mental retardation. The diagnosis of DGS was confirmed by fluorescence in situ hybridization (FISH). Negative effects of cognitive disorders on reproductivity are already known; however, we haven’t find any studies in the literature that evaluated infertile patients with DGS using semen analysis, apart from these potential unfavourable effectc of cognitive disorders. Coexistence of DGS and azoospermia may be completely coincidental, but azoospermia can be also one of the unknown clinical features of this syndrome. Many patients with a mild phenotype of DGS may be underdiagnosed. DGS should be considered in adults who have mental, behavioral, or psychiatric disorders with mild dysmorphic features, even in the absence of classical features.
Keywords: Azoospermia, DiGeorge syndrome, phenotypic variability
Introduction
DiGeorge syndrome (DGS) is the most common microdeletion syndrome with an incidence of 1/4,000–1/6,000 live births[1,2], but the actual incidence is probably higher because of phenotypic variabilities and underdiagnosis of the patients with mild phenotype. Even if all patients with DGS have the same genetic etiology, they show a wide spectrum of phenotypic variabilities which leads to usage of many different terminologies for DGS. DGS is also known as velocardiofacial, Cayler cardiofacial, Shprintzen, conotruncal anomaly face, Takao, and Sedlackova syndrome. Both male and female sexes are affected equally. Most of the patients have a de novo deletion on chromosome 22q11.2, although approximately 10%–20% of them inherit the deletion from a parent, and in particular, maternal transmission is observed.[3,4] Because of the microdeletion, there is defective development of the third and fourth pharyngeal arches. DGS can affect multiple organs and systems. Characteristic features of DGS are congenital heart defects, velopharyngeal insufficiency with a hypernasal voice, thymic hypoplasia, immunodeficiency, cognitive impairments, hypocalcemia due to hypoparathyroidism, and facial dysmorphisms. Facial dysmorphic features are subtle and include hypertelorism, micrognathia, smooth philtrum, thin upper lip, deep-set eyes, a small chin, and a telecanthus with short palpebral fissures.[5] Most of the patients have a 3-megabase (Mb) deletion, but some have a 1.5-Mb distal deletion at 22q11.2, and a small group of patients have chromosomal rearrangements or point mutations.[6] Although deletions of different sizes have been identified, there is no correlation between the type or size of deletion and phenotype.[7]
The diagnosis of DGS is frequently made during childhood, athough it can be made antenatally during ultrasonographic investigation of pregnancy by identifying characteristic features of DGS (e.g., congenital heart disease). However, many mild cases without characteristic features may not be diagnosed until adulthood. DGS is routinely diagnosed by fluorescence in situ hybridization (FISH), multiplex ligation-dependent probe amplification, and array technologies.
Case presentation
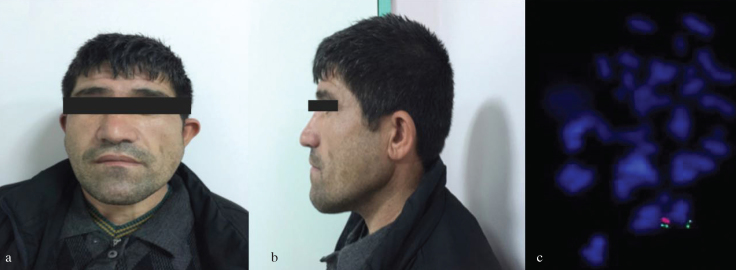
A non-consanguineous couple married for 10 years, namely, a 26-year-old female and her 35-year-old husband, was referred to our clinic for evaluation of primary infertility. Her physical examination was normal. She had regular menstrual cycles. Her husband was mildly retarded. He had a long face, cup-shaped ears, and facial asymmetry (Figures 1a, and b). His speech was hypernasal. His testicular and scrotal examinations were normal. Recurrent semen analyses of the case showed azoospermia. His routine blood tests, testosterone, luteinizing hormone and follicle stimulating hormone levels were within normal range. Cardiac echocardiographic assessment did not demonstrate any abnormalities. Chromosomal analyses of peripheral blood lymphocytes were performed using a G-banding procedure, and their karyotypes were normal. FISH analysis was performed using a Vysis DiGeorge region probe [Vysis, LSI TUPLE 1 (HIRA) spectrum orange/LSI ARSA spectrum green; Abbott Laboratories, Chicago, IL, USA] and showed a deletion at locus 22q11.2 (Figure 1c). We could not perform FISH analysis on his parents.
Figure 1. a–c.
Facial dysmorphism and FISH analysis of the patient. Facial asymmetry of the patient with a long face (a), Lateral view of the cup-shaped ear (b). FISH analysis of the patient using a LSI TUPLE 1 (DGS critical region) spectrum orange/LSI ARSA (control probe) spectrum green probe, the orange signal indicates deletion of the TUPLE 1 locus at 22q11.2 (c).
As the patient was mildly retarded, written informed consent was obtained from the patient’s wife for publication of this case report and accompanying images.
Discussion
In 1965, Dr. Angelo M. DiGeorge reported a case with congenital absence of a thymus and parathyroid glands. Later, congenital cardiac anomalies were discovered, and the syndrome was termed as DGS. DGS is the most common microdeletion syndrome, although its actual incidence is probably higher because of phenotypic variability and patients undiagnosed because of a mild phenotype.[8]
DiGeorge syndrome’s phenotypic presentations vary widely, and many patients have mild phenotypes. Unfortunately, the diagnosis of this syndrome can be extremely difficult. Facial dysmorphic features of patients with DGS can be easily overlooked; therefore, many mild cases without typical features may not be diagnosed until adulthood, such as our patient. DGS is not a rare microdeletion syndrome, and diagnosis should be considered in adults who have mental, behavioral, or psychiatric disorders with mild dysmorphic features, even in the absence of classical features of DGS, so that patients can benefit from genetic counseling.[9]
Most common genetic diseases and abnormalities that result in azoospermia are chromosome abnormalities, cystic fibrosis and Y chromosome microdeletions. But irrespective of genetic etiologies, intelligence may affect reproductivity.[10] It has been known that cognitive impairments have a negative impact on reproductive fitness of patients with DGS. The cause is unknown, although regardless of cognitive impairments, the male gender is a significant independent negative predictor of reproductive fitness in DGS.[11] DGS may affect sperm viability and count. We searched the literature for other infertile DGS cases with azoospermia but did not find a similar case. To the best of our knowledge, in English literature, this is the first case that presented a male infertile patient with DGS and azoospermia. Coexistence of DGS and azoospermia may be coincidental, but azoospermia can also be one of the unknown clinical features of this syndrome and the cause of low reproductive fitness in males. Evaluation of large groups of patients with DGS by semen analysis will determine a possible association of DGS, with male infertility and azoospermia.
Footnotes
Informed Consent: Written informed consent was obtained from patient’s wife who participated in this case.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – Y.Ş.; Design – A.Ö., Y.Ş.; Supervision – A.Ö., Y.Ş.; Resources – A.Ö., Y.Ş.; Materials – Y.Ş.; Data Collection and/or Processing – Y.Ş.; Analysis and/or Interpretation –A.Ö., Y.Ş.; Literature Search – A.Ö.; Writing Manuscript – A.Ö.;Critical Review – A.Ö., Y.Ş.; Other – A.Ö., Y.Ş.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Devriendt K, Fryns J, Mortier G, Van Tienen MN, Keymolen K. The annual incidence of DiGeorge/velocardiofacial syndrome. JMed Genet. 1998;35:789–90. doi: 10.1136/jmg.35.9.789-a. https://doi.org/10.1136/jmg.35.9.789-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA, et al. A Population-Based Study of the 22q11.2 Deletion:Phenotype, Incidence, and Contribution to Major Birth Defects inthe Population. Pediatrics. 2003;112:101–7. doi: 10.1542/peds.112.1.101. https://doi.org/10.1542/peds.112.1.101. [DOI] [PubMed] [Google Scholar]
- 3.McDonald-McGinn DM, Kirschner R, Goldmuntz E, Sullivan K, Eicher P, Gerdes M, et al. The Philadelphia story: The 22q11.2 deletion: Report on 250 patients. Genetic Counseling. 1999;10:11–24. [PubMed] [Google Scholar]
- 4.Cohen E, Chow EW, Weksberg R, Bassett AS. Phenotype of adults with the 22q11 deletion syndrome: A review. Am J Med Genet. 1999;86:359–65. doi: 10.1002/(sici)1096-8628(19991008)86:4<359::aid-ajmg10>3.0.co;2-v. https://doi.org/10.1002/(SICI)1096-8628(19991008)86:4<359::AID-AJMG10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swillen A, McDonald-Mcginn D. Developmental trajectories in22q11.2 deletion syndrome. Am J Med Genet Part C Semin MedGenet. 2015;169:172–81. doi: 10.1002/ajmg.c.31435. https://doi.org/10.1002/ajmg.c.31435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yagi H, Furutani Y, Hamada H, Sasaki T, Asakawa S, Minoshima S, et al. Role of TBX1 in human del22q11.2 syndrome. Lancet. 2003;362:1366–73. doi: 10.1016/s0140-6736(03)14632-6. https://doi.org/10.1016/S0140-6736(03)14632-6. [DOI] [PubMed] [Google Scholar]
- 7.Emanuel BS. Molecular mechanisms and diagnosis of chromosome 22q11.2 rearrangements. Dev Disabil Res Rev. 2008;14:11–8. doi: 10.1002/ddrr.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oskaarsdottir S, Vujic M, Fasth A. Incidence and prevalence ofthe 22q11 deletion syndrome: a population-based study in WesternSweden. Arch Dis Child. 2004;89:148–51. doi: 10.1136/adc.2003.026880. https://doi.org/10.1136/adc.2003.026880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Philip N, Bassett A. Cognitive, Behavioural and Psychiatric Phenotype in 22q11.2 Deletion Syndrome. Behav Genet. 2011;41:403–12. doi: 10.1007/s10519-011-9468-z. https://doi.org/10.1007/s10519-011-9468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadeghi-Nejad H, Farrokhi F. Genetics of Azoospermia: CurrentKnowledge, Clinical Implications, and Future Directions Part I. Urol J. 2007;4:192–206. [PubMed] [Google Scholar]
- 11.Costain G, Chow EWC, Silversides CK, Bassett AS. Sex differences in reproductive fitness contribute to preferential maternaltransmission of 22q11.2 deletions. J Med Genet. 2011;48:819–24. doi: 10.1136/jmedgenet-2011-100440. https://doi.org/10.1136/jmedgenet-2011-100440. [DOI] [PubMed] [Google Scholar]