Abstract
The mandibular condylar cartilage (MCC) is derived from periosteum and undergoes endochondral ossification during growth. Growth and remodeling of periosteal-derived bone is known to be modulated by estrogen signaling. Estrogen receptor alpha (ERα) and beta (ERβ) are the two main transmembrane receptors that mediate the majority of estrogen’s effects. In the appendicular skeleton, estrogen via ERα receptor signaling has been shown to mediate endochondral growth plate fusion in both males and females. However, the role of ERα in mediating growth of the mandibular condylar cartilage is unknown. To this end, we have characterized mandibular condylar cartilage growth in young (49 day-old) and adult (9 month-old) male ERαKO mice. In young mice, a significant increase in the number of mandibular condylar cartilage cells and a significant decrease in the expression of Col10, Runx2, and DMP1 was observed in the male ERαKO mice compared to WT. In 9 month old mice, we found a similar increase in the number of cells but no change in osteoarthritic histological scoring in ERαKO mice compared to WT mice. In summary, estrogen plays a role in mediating mandibular condylar maturation in young male mice. However, according to this study, it does not play a role in mediating long term growth or age-related mandibular condylar cartilage degeneration in males.
Keywords: estrogen, estrogen receptor alpha, mandibular condylar cartilage, temporomandibular joint
Introduction
It has been estimated that 10% of the US population has mandibular condylar cartilage growth abnormalities1, 2 and 70% of people over the age of 70 have radiographic evidence of TMJ degeneration.3 Thus, an understanding of parameters that regulate MCC growth is imperative for therapies to address growth abnormalities and age-related TMJ osteoarthritis.
Unlike most hyaline articular cartilages of the appendicular joints, the mandibular condylar cartilage (MCC) is derived from periosteal tissue and comprised of fibrocartilage.4 In the appendicular skeleton, estrogen signaling is known to mediate growth plate fusion. Estrogen signals through two receptor isoforms, estrogen receptor alpha (ERα) and beta (ERβ). Estrogen via ER alpha has been shown to irreversibly deplete growth plate resting zone cells and cause growth plate fusion in both sexes.4–7 In contrast, the role of estrogen receptor signaling in mediating TMJ growth is not fully deciphered. Ovariectomy has been shown to cause an increase in mandibular condylar cartilage thickness and an increase in proliferation that is reversed by estrogen replacement.8–10 We have previously found that ERβ deficiency results in an increase in mandibular condylar growth in young female but not in male mice.8, 11 However the role of ERα in mediating mandibular condylar growth and age-related TMJ degeneration is unknown.
Female ERαKO mice are associated with increased estrogen levels which may mask the mandibular condylar cartilage phenotype.12 Male ERαKO do not experience these increased levels. Thus, this study focuses on the characterization of the mandibular condylar phenotype in male mice. Specifically, changes to MCC thickness, cell number, cell density, cartilage-specific gene expression, and OARSI scores were investigated for 49-day and 9-month old male ERαKO and WT mice. Greater understanding of the role of estrogen receptor alpha in mediating mandibular condylar cartilage growth is critical in order to develop therapeutic intervention to promote mandibular condylar growth in craniofacial patients and to examine age-related TMJ degeneration
Materials and Methods
Mice
All experiments were performed based on approved animal welfare protocols from the Institutional Animal Care and Use Committee (IACUC, protocol #AAAH9166) from Columbia University. Breeding pairs of C57BL/6 ERαKO mice (heterozygous male, heterozygous female) were donated by Dr. Kenneth Korach from the National Institute of Environmental Health Sciences at the National Institutes of Health. Mice were genotyped and WT and homozygous ERαKO male mice were utilized for this study. Mice for histological analysis were injected intraperitoneally with 0.1 mg bromodeoxyuridine (BrdU) per gram body weight at 3 and 19 hours prior to euthanasia to track proliferating cells. Mice were sacrificed at 49 days (histological analysis: WT n = 5 and ERαKO n = 6; gene expression: WT n = 6 and ERαKO n = 7) or 9 months (histological analysis: WT n = 3 and ERαKO n = 6; gene expression: WT and ERαKO n = 7) for phenotypic analysis.
Histology and Histomorphometry
Histomorphometry techniques were employed to determine a global estrogen receptor alpha knockout phenotype in the mandibular condylar cartilage of male mice. Whole mouse heads were processed for histology as previously described.11 Sagittal serial sections of 5 μm thickness were made of the TMJ utilizing a Microm HM 355s microtome (Thermo Fisher Scientific, Waltham, MA, USA). Three to five sections from the sagittal plane representing the mid-coronal portion of the mandibular head were stained with hematoxylin and eosin (H&E) and Safranin-O (SafO) and used as the representative central sections for analysis.
Histomorphometry measurements were completed in a blinded, non-biased manner using the BioQuant computerized image analysis system (BioQuant, Nashville, TN, USA) and ImageJ (NIH). Cartilage thicknesses were determined within the cartilage region that was outlined from anterior to posterior to include all tissue that contained hypertrophic chondrocytes. Average thicknesses were taken for 3–5 sections per mouse and averaged within the group to determine overall average WT and ERαKO thicknesses. ImageJ was utilized to determine total cell numbers, cell density, hypertrophic cell numbers, and terminal hypertrophic cell diameter within the outlined cartilage region. Cell density was obtained by determining the total cells within the region and dividing by the total surface area within the region. Further histomorphometric analysis of the hypertrophic cells was conducted on 49-day samples. Hypertrophic cells were operationally defined as ≥ 10 μm as previously defined.13 Lastly, terminal hypertrophic cell diameter was determined following an adapted protocol from Weise et al.14 Specifically, the cartilage region was divided into 5 equally wide columns. Within each column, the terminal hypertrophic cell was defined as the last cell in the cartilage lacuna (light pink from eosin stain) adjacent to the subchondral bone (dark pink from eosin stain). The diameters of each of the 5 terminal hypertrophic chondrocytes were averaged for each section, averaged for each mouse, and averaged for each genotype.
BrdU Immunohistochemistry
BrdU immunohistochemical analysis to determine proliferating cells was completed using a BrdU staining kit following the manufacturer’s instructions (Invitrogen Corporation, Camarillo, CA, USA). To quantify BrdU, the labeling index (number of BrdU positive cells divided by the total number of cells) was calculated. Three to five sections, corresponding to the same anatomical region utilized to determine total cell number (mid-coronal), were counted for each group and the average index of these sections was used for the labeling index.
mRNA Extraction and Gene Expression
At both time points, mRNA from the mandibular condylar cartilage was isolated, purified and converted to cDNA as described 11. Real-time polymerase chain reaction (RT-PCR) was conducted to assess the relative levels of genes of interest using the ViiA™ 7 Real-Time PCR System (Applied Biosystems, Life Technologies) following the protocol detailed in Chen et al.11 Expression of each gene of interest was determined relative to the Gapdh housekeeping gene (MM99999915_g1) utilizing the ΔΔCT method. Gene expression was analyzed for the following markers: parathyroid hormone-related peptide (Mm00436057_m1), indian hedgehog (Mm00439613_m1), SRY-box containing gene 9 (MM00448840_m1), collagen type II (Mm00491889_m1), sclerostin (Mm00470479_m1), collagen type X ( Mm00487041_m1), vascular endothelial growth factor (Mm00437304_m1), runt-related transcription factor 2 (Mm00501578_m1), dentin matrix acidic phosphoprotein 1 (Mm00803833_g1), aggrecan ( Mm00545794_m1), matrix metalloproteinase 13 and 3 (Mm00439491_m1, Mm00440295_m1). All primers were purchased from Applied Biosystems.
OARSI Scoring
OARSI recommendations for histological scoring for osteoarthritic changes to cartilage in the mouse were employed.15 Three to five sections from the 9 month samples stained with safranin O were analyzed. Specifically, the depth of each cleft was determined and divided by the total cartilage thickness to determine the percentage of cleft penetration using ImageJ. These values were then averaged for each mouse and then averaged for each genotype.
Statistical Analysis
Values are presented as the mean ± standard deviation. Sample sizes are listed in the mice section of the methods. Statistical significance of differences among means was determined by a Student’s t-test. Statistical significance was defined by a 95% confidence interval (*p < 0.05) or a 99% confidence interval (^p < 0.01) as indicated in the figure legends.
Results
ERα deficiency inhibits mandibular cartilage maturation in young male mice
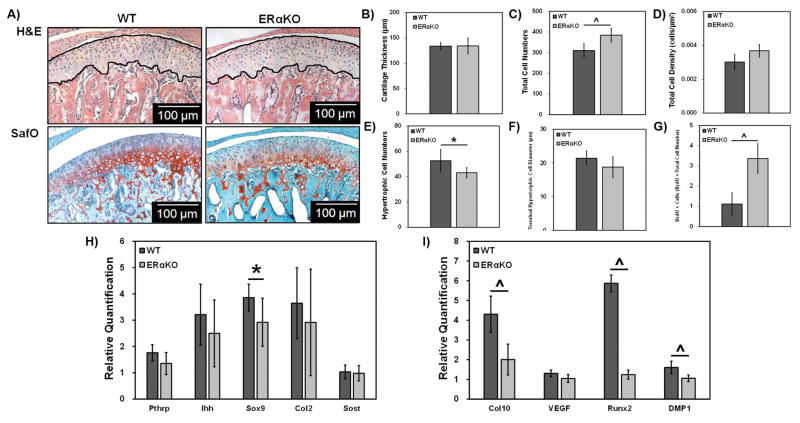
Representative H&E and safranin O images are shown in Figure 1A. Histomorphometric analysis revealed that 49 day-old ERαKO mice had a significant increase in the number of cells (Figure 1C), an increase proliferation (Figure 1G) and a decrease in the number of hypertrophic chondrocytes (Figure 1E) compared to male WT mice.
Figure 1. Histomorphometric and gene expression analysis of 49-day WT and ERαKO male mandibular condylar cartilage.
Representative H&E and SafO images for WT and ERαKO samples (A). The lines highlight the cartilage region measured. Histomorphometric analysis was conducted using ImageJ to determine cartilage thickness (B), total cell numbers (C), total cell density (D), hypertrophic cell numbers (E) and terminal hypertrophic cell diameter (F). Proliferating cells were marked with BrdU, counted, and normalized to total cell number (G). Real time PCR analysis of chondrocyte genes (H) and hypertrophy markers (I). For histomorphometric data, WT n=5 and ERαKO n = 6 and *p < 0.05, ^p < 0.01. For gene expression data, WT n=6 and ERαKO n = 7 and *p < 0.05, ^p < 0.01.
Results from real time PCR investigating mature (Figure 1H) and hypertrophic chondrocyte markers (Figure 1I) revealed a significant decrease in Sox9 and the hypertrophic maturation markers Col10, Runx2, and DMP1 with no significant change in the other markers of chondrocyte maturation in the male ERαKO mice compared to male WT mice.
9 month old male ERαKO mice have increased cell numbers but similar size and degeneration compared to male WT mice
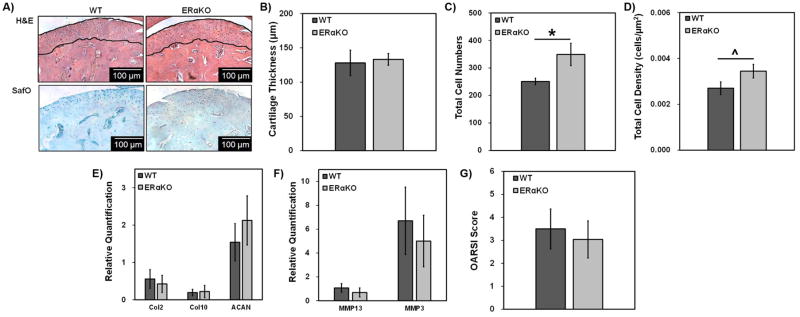
Representative H&E and safranin O images of 9-month ERαKO and WT MCC are shown in Figure 2A. At 9 months of age, male ERαKO mice exhibited a significant increase in cell numbers and cell density compared to the male WT mice (Figure 2C and 2D).
Figure 2. Histomorphometric, gene expression, and OARSI scoring analysis of 9-month WT and ERαKO male mandibular condylar cartilage.
Representative H&E and SafO images for WT and ERαKO samples (A). The lines highlight the cartilage region measured. Histomorphometric analysis was conducted using ImageJ to determine cartilage thickness (B), total cell numbers (C), and total cell density (D). Real time PCR analysis of chondrocyte-specific matrix genes (E) and MMPs (F). OARSI osteoarthritic scoring for WT and ERαKO 9-month mice (G). For histomorphometric and OARSI scoring data, WT n=5 and ERαKO n = 6 and *p < 0.05, ^p < 0.01. For gene expression data, WT n=6 and ERαKO n = 7 and *p < 0.05, ^p < 0.01.
Both WT and ERαKO male mice exhibited similar OARSI scores of 3.5 and 3, respectively, (Figure 2G) with no significant difference in chondrogenic or matrix enzyme gene expression (Figure 2E and 2F).
Discussion
In the appendicular skeleton, ERα deficiency has been shown to cause conflicting results in the growth plate of young male mice. For example, young male global ERαKO mice have a decrease, Col2 promoter-induced conditional ERαKO mice have no change, and osteocalcin promoter induced conditional ERαKO have increased femoral length.6, 12 In this study, our results are similar to what was found in the global ERαKO. However hormonal alterations of decreased IGF in the global ERαKO mice may be masking the local effect of ERα signaling in the mandibular condylar cartilage. Therefore, future studies using mandibular condylar cartilage-specific ERαKO mice that exhibit minimal hormonal alterations are needed in order to decipher the local role of ERα signaling in mandibular condylar cartilage growth.
Adult 9-month old ERαKO mice exhibited an increase in cell numbers and cell density in the mandibular condylar cartilage but no change in mandibular condylar cartilage thickness compared to age-matched male WT mice. The role of ERα in mediating axial skeletal growth in older male mice has not been described. In the female femur growth plate cartilage, it was shown that mice with cartilage specific ERα deletion had increased growth plate cartilage thickness at 1 year of age6 and global ERαKO female mice had increased femoral growth at 16–18 months of age.16 In the growth plate resting zone, there are a finite number of progenitor cells. Growth plate fusion involves depletion of these resting zone progenitor cells.17 In the long bone growth plate cartilages, estrogen via ERα promotes resting zone progenitor cell depletion and subsequent growth plate fusion.4, 6 In contrast, the mandibular condylar cartilage does not fuse and it appears that growth may continue after appendicular skeletal maturation.18 Therefore, it is probable that the number of progenitor cells in the MCC are not finite such that normal depletion does not occur until after 9 months in mice. Additional studies are needed to examine if ERα deficiency promotes growth in male mice past 9 months of age.
We have previously found that male and female WT mice develop signs of TMJ-OA at 6–9 months of age.19 In this study, we did not find any significant difference between TMJ OA scores between 9 month old male WT and male ERαKO mice. Previous work in female ERαKO mice illustrated no difference in cartilage degeneration scores but increased osteophyte formation in 6 month old ERαKO mice compared to WT mice.20 One of the hallmarks of TMJ-OA is acellularity of the cartilage.19 Therefore, it would be interesting to examine OA scores in older ERαKO mice to see if the increase in cell numbers we observed at 9 months has a protective role in age-related TMJ degeneration.
In summary, estrogen plays a role in mediating mandibular condylar maturation in young male mice. However, according to this study, it does not play a role in mediating long term TMJ growth or age-related mandibular condylar cartilage degeneration in males.
Clinical Relevance
The use of ERα antagonists to promote mandibular condylar growth or to prevent TMJ degeneration in males is not justified. However, the use of an estrogen receptor antagonist and/or agonist to promote or inhibit chondrocyte maturation may be an exciting option for MCC regenerative therapies.
Acknowledgments
The authors would like to acknowledge the funding sources for this work. Funding was supported by the National Institute for Health (R56 DE020097). Additionally, this material is based upon work supported by the National Institute for Health K12 TMJ Training Grant No. 5K12DE023583-02.
Footnotes
Disclosure: No competing conflict of interest exits for any of the authors.
References
- 1.Pirttiniemi P, Peltomaki T, Muller L, Luder HU. Abnormal mandibular growth and the condylar cartilage. European journal of orthodontics. 2009;31:1–11. doi: 10.1093/ejo/cjn117. [DOI] [PubMed] [Google Scholar]
- 2.Proffit WR, Fields HW, Jr, Moray LJ. Prevalence of malocclusion and orthodontic treatment need in the United States: estimates from the NHANES III survey. The International journal of adult orthodontics and orthognathic surgery. 1998;13:97–106. [PubMed] [Google Scholar]
- 3.Schmitter M, Essig M, Seneadza V, Balke Z, Schroder J, Rammelsberg P. Prevalence of clinical and radiographic signs of osteoarthrosis of the temporomandibular joint in an older persons community. Dento maxillo facial radiology. 2010;39:231–234. doi: 10.1259/dmfr/16270943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilsson O, Weise M, Landman EB, Meyers JL, Barnes KM, Baron J. Evidence that estrogen hastens epiphyseal fusion and cessation of longitudinal bone growth by irreversibly depleting the number of resting zone progenitor cells in female rabbits. Endocrinology. 2014;155:2892–2899. doi: 10.1210/en.2013-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. The New England journal of medicine. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 6.Börjesson AE, Lagerquist MK, Liu C, Shao R, Windahl SH, Karlsson C, et al. The role of estrogen receptor α in growth plate cartilage for longitudinal bone growth. J Bone Miner Res. 2010;25:2690–2700. doi: 10.1002/jbmr.156. [DOI] [PubMed] [Google Scholar]
- 7.Börjesson AE, Lagerquist MK, Windahl SH, Ohlsson C. The role of estrogen receptor α in the regulation of bone and growth plate cartilage. Cell Mol Life Sci. 2013;70:4023–4037. doi: 10.1007/s00018-013-1317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamiya Y, Chen J, Xu M, Utreja A, Choi T, Drissi H, et al. Increased mandibular condylar growth in mice with estrogen receptor beta deficiency. J Bone Miner Res. 2013;28:1127–1134. doi: 10.1002/jbmr.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okuda T, Yasuoka T, Nakashima M, Oka N. The effect of ovariectomy on the temporomandibular joints of growing rats. J Oral Maxillofac Surg. 1996;54:1201–1210. doi: 10.1016/s0278-2391(96)90352-3. [DOI] [PubMed] [Google Scholar]
- 10.Talwar RM, Wong BS, Svoboda K, Harper RP. Effects of estrogen on chondrocyte proliferation and collagen synthesis in skeletally mature articular cartilage. J Oral Maxillofac Surg. 2006;64:600–609. doi: 10.1016/j.joms.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Kamiya Y, Polur I, Xu M, Choi T, Kalajzic Z, et al. Estrogen via estrogen receptor beta partially inhibits mandibular condylar cartilage growth. Osteoarthr Cartilage. 2014;22:1861–1868. doi: 10.1016/j.joca.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vico L, Vanacker JM. Sex hormones and their receptors in bone homeostasis: insights from genetically modified mouse models. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2010;21:365–372. doi: 10.1007/s00198-009-0963-5. [DOI] [PubMed] [Google Scholar]
- 13.Mancilla EE, De Luca F, Uyeda JA, Czerwiec FS, Baron J. Effects of fibroblast growth factor-2 on longitudinal bone growth. Endocrinology. 1998;139:2900–2904. doi: 10.1210/endo.139.6.6032. [DOI] [PubMed] [Google Scholar]
- 14.Weise M, De-Levi S, Barnes KM, Gafni RI, Abad V, Baron J. Effects of estrogen on growth plate senescence and epiphyseal fusion. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6871–6876. doi: 10.1073/pnas.121180498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr Cartilage. 2010;18(Supplement 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Borjesson AE, Windahl SH, Karimian E, Eriksson EE, Lagerquist MK, Engdahl C, et al. The role of estrogen receptor-alpha and its activation function-1 for growth plate closure in female mice. American journal of physiology. Endocrinology and metabolism. 2012;302:E1381–1389. doi: 10.1152/ajpendo.00646.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schrier L, Ferns SP, Barnes KM, Emons JA, Newman EI, Nilsson O, et al. Depletion of resting zone chondrocytes during growth plate senescence. The Journal of endocrinology. 2006;189:27–36. doi: 10.1677/joe.1.06489. [DOI] [PubMed] [Google Scholar]
- 18.Pancherz H, Bjerklin K, Hashemi K. Late adult skeletofacial growth after adolescent Herbst therapy: a 32-year longitudinal follow-up study. American journal of orthodontics and dentofacial orthopedics: official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics. 2015;147:19–28. doi: 10.1016/j.ajodo.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Wadhwa S, Embree M, Ameye L, Young MF. Mice deficient in biglycan and fibromodulin as a model for temporomandibular joint osteoarthritis. Cells, tissues, organs. 2005;181:136–143. doi: 10.1159/000091375. [DOI] [PubMed] [Google Scholar]
- 20.Sniekers YH, van Osch GJ, Ederveen AG, Inzunza J, Gustafsson JA, van Leeuwen JP, et al. Development of osteoarthritic features in estrogen receptor knockout mice. Osteoarthritis and cartilage. 2009;17:1356–1361. doi: 10.1016/j.joca.2009.04.008. [DOI] [PubMed] [Google Scholar]