Abstract
IMPORTANCE
Tobacco use disorder is associated with dysregulated neurocognitive function in the right inferior frontal gyrus (IFG)—one node in a corticothalamic inhibitory control (IC) network.
OBJECTIVE
To examine associations between IC neural circuitry structure and function and lapse/relapse vulnerability in 2 independent studies of adult smokers.
DESIGN, SETTING, AND PARTICIPANTS
In study 1, treatment-seeking smokers (n = 81) completed an IC task during functional magnetic resonance imaging (fMRI) before making a quit attempt and then were followed up for 10 weeks after their quit date. In study 2, a separate group of smokers (n = 26) performed the same IC task during fMRI, followed by completing a laboratory-based smoking relapse analog task. Study 1 was performed at Duke University Medical Center between 2008 and 2012; study 2 was conducted at the Medical University of South Carolina between 2013 and 2016.
MAIN OUTCOMES AND MEASURES
Associations between corticothalamic-mediated IC, gray-matter volume, and smoking lapse/relapse.
RESULTS
Of the 81 study participants in study 1 (cessation study), 45 were women (56%), with mean (SD) age, 38.4 (10.2) years. In study 1, smoking relapse was associated with less gray-matter volume (F1,74 = 28.32; familywise error P threshold = 0.03), greater IC task-related blood oxygenation level–dependent (BOLD) response in the right IFG (F1,78 = 14.87) and thalamus (F1,78 = 14.97) (P < .05), and weaker corticothalamic task-based functional connectivity (tbFC) (F1,77 = 5.87; P = .02). Of the 26 participants in study 2 (laboratory study), 15 were women (58%), with mean (SD) age, 34.9 (10.3). Similar to study 1, in study 2, greater IC-BOLD response in the right IFG (t23 = −2.49; β = −0.47; P = .02), and weaker corticothalamic tbFC (t22 = 5.62; β = 0.79; P < .001) were associated with smoking sooner during the smoking relapse-analog task. In both studies, corticothalamic tbFC mediated the association between IC performance and smoking outcomes.
CONCLUSIONS AND RELEVANCE
In these 2 studies, baseline differences in corticothalamic circuitry function were associated with mediated IC and smoking relapse vulnerability. These findings warrant further examination of interventions for augmenting corticothalamic neurotransmission and enhancing IC during the course of tobacco use disorder treatment.
Executive function and behavioral inhibition deficits play significant roles in substance use disorders.1 Converging evidence from animal and human models of addiction demonstrates that chronic exposure to psychoactive drugs produces neuroplasticity in prefrontal circuitry underlying executive function2,3 and inhibitory control (IC).1 Inhibitory control is subserved, in part, by a corticothalamic circuit including the right inferior frontal gyrus (IFG),4–6 presupplementary motor area,5,6 and the subthalamic nucleus,5 with the right IFG modulating the strength of the presupplementary motor area’s excitatory action on the subthalamic nucleus, in turn inhibiting motor output during IC.5
Compared with nonsmokers, smokers exhibit less gray-matter volume(GMV) in the corticothalamic pathway, including the IFG7–9 and thalamus,10,11 and greater functional magnetic resonance imaging (fMRI) blood oxygenation level-dependent (BOLD) response in the right IFG during neurocognitive tasks.12,13 Acute smoking abstinence further increases BOLD response in right IFG during IC14 and other neurocognitive tasks.13,15,16 Abnormalities in IFG structure and function implicate a compensatory mechanism by which smokers “overrecruit” the right IFG in an attempt to exert IC. These contrasting patterns of neural structure and function between smokers and nonsmokers may underlie differences in cognitive functioning12,17,18 and impulsivity,19 as well as withdrawal-induced cognitive disturbances in smokers; however, the effect of these neurophysiological disparities on inhibiting smoking behavior remains unknown.
Across 2 studies, we measured baseline GMV and fMRI BOLD response during a well-validated measure of IC20 to examine structural and functional correlates of the ability to resist smoking in adult daily smokers (Table 1 and Table 2). In study 1 (cessation study), 81 participants underwent a baseline fMRI session 1 month before a quit attempt, and smoking behavior was assessed during a 10-week postquit period. In a separate sample of smokers (study 2, laboratory study), 26 participants first completed an fMRI visit and then performed a smoking relapse analog task (SRT). We hypothesized that less GMV and greater IC task-related BOLD response in corticothalamic circuitry would be associated with smoking relapse (study 1), as well as ad lib smoking during the SRT (study 2).
Table 1.
Study 1 Demographics and Baseline Self-Report Measures
| Characteristic | Overall Sample (N = 81) | Abstinent (n = 41) | Relapsed (n = 40) | Statistical Value | P Value |
|---|---|---|---|---|---|
| Women, No. (%) | 45 (56) | 24 (58.5) | 21 (52.5) | 0.3, χ2 | .59 |
| Age, mean (SD), y | 38.4 (10.2) | 39.2 (11.4) | 37.6 (9.0) | 0.71, t79 | .48 |
| Educational level, No. (%) | |||||
| Some high school | 1 (1) | 0 | 1 (3) | 1.35, t79 | .72 |
| High school graduate | 11 (14) | 5 (12) | 6 (15) | ||
| Some college | 36 (44) | 18 (44) | 18 (45) | ||
| College graduate | 33 (41) | 18 (44) | 15 (38) | ||
| Treatment group, No. (%) | |||||
| Extinction | 43 (53) | 19 (46) | 24 (60) | 1.52, t79 | .22 |
| Usual brand | 38 (47) | 22 (54) | 16 (40) | ||
| Baseline clinical measures, mean (SD) | |||||
| Nicotine dependence, FTND score | 5.0 (1.8) | 4.6 (1.9) | 5.5 (1.6) | −2.27, t79 | .03 |
| Years smoking | 18.4 (9.6) | 19.5 (10.4) | 17.2 (8.6) | 1.1, t79 | .28 |
| Daily cigarettes | 19.2 (6.4) | 16.3 (5.1) | 22.1 (6.2) | −4.59, t79 | <.001 |
| Depressive symptoms, CESD score | 6.3 (4.8) | 7.1 (5.4) | 5.5 (4.1) | 1.52, t79 | .13 |
Abbreviations: CESD, Center for Epidemiological Studies–Depression; FTND, Fagerstrom Test of Nicotine Dependence.
Table 2.
Study 2 Demographics and Baseline Self-Report Measures
| Characteristic | Overall Sample (n = 26) |
|---|---|
| Women, No. (%) | 15 (58) |
| Age, mean (SD), y | 34.9 (10.3) |
| Years of education, mean (SD) | 14.1 (2.0) |
| Baseline clinical measures, mean (SD) | |
| Nicotine dependence, FTND score | 4.5 (1.7) |
| Years smoking | 15.4 (8.3) |
| Daily cigarettes | 14.8 (4.4) |
| Depressive symptoms, CESD score | 11.8 (7.6) |
Abbreviations: CESD, Center for Epidemiological Studies–Depression; FTND, Fagerstrom Test of Nicotine Dependence.
Methods
Participants and Procedures
Across Studies
Inclusion criteria were being in good health, right-handed, aged 18 to 55 years, and smoking 10 cigarettes or more per day. Exclusion criteria were significant health problems, contraindications for MRI, use of psychoactive medications, use of smokeless tobacco or nicotine replacement therapy, current drug or alcohol abuse, afternoon expired carbon monoxide level less than 10 ppm (Vitalograph Inc), and positive urine illicit drug screen, breath alcohol level (Alert breathalyzer; Columbia Laboratory Supplies), or urine pregnancy test.
The study was approved by the institutional review boards of Duke University School of Medicine for study 1 (cessation study) and the Medical University of South Carolina for study 2 (laboratory study). Participants gave written informed consent and received financial compensation.
Study 1
Smokers (n = 95) in the smoking cessation study (conducted at Duke University from 2008 to 2012) who were interested in quitting smoking were recruited via newspaper and internet advertisements. Participants underwent fMRI scanning 30 minutes after smoking a cigarette, were randomized to an experimental cessation treatment for 30 days before a quit attempt, and then returned to the laboratory to report their smoking behavior at 1, 3, 6, and 10 weeks after quitting (eMethods in the Supplement). Daily diaries of cigarette use, as well as levels of expired carbon monoxide, were collected at each visit to confirm abstinence (carbon monoxide level <8 ppm) or relapse. The primary smoking-cessation outcome variable was relapse defined as 7 consecutive days of smoking at least 1 cigarette per day.21,22 Following the baseline prequit fMRI scan, data from 14 participants were excluded from analyses: 8 withdrew before initiating a quit attempt, 3 for image artifacts, and 3 for incomplete imaging data, resulting in a final sample of 81 smokers (Table 1). Forty participants either met criteria for relapse or were lost to contact and were grouped as relapsed. Forty-one participants who did not meet the relapse criterion were grouped as abstinent. To promote truthful reports, participation and payment were not contingent upon abstinence.
Study 2
Smokers (n = 30) in the laboratory study (conducted at the Medical University of South Carolina from 2013 to 2016) were recruited via newspaper and internet advertisements and expressed no interest in quitting smoking. Participants underwent fMRI scanning twice: once 30 minutes after smoking a cigarette (sated) and once following 24 hours of abstinence (order randomized). To replicate study 1 methods, all study 2 data reported herein are from the sated condition. Immediately following scanning, participants performed a smoking relapse analog task (SRT). During the SRT, participants were presented with positive, negative, and neutral emotional images on a computer screen over 6-minute time blocks while also being provided with an open pack of their preferred brand of cigarettes, ashtray, and lighter. Participants received $1 for each 6-minute block that they did not smoke, up to 10 blocks over a 1-hour period. Following the first block, participants could stop the task and forfeit earning money to smoke 1 cigarette. Latency to initiate ad lib smoking was recorded and smoking topography (puff count and volume) was measured using the Clinical Research Support System. eTable 1 in the Supplement provides the SRT performance results. Four participants were excluded due to poor IC task accuracy (<75% Go trials correct, as described below). The final sample was 26 participants (Table 2).
Across studies, all participants completed a training session during which they practiced the IC task and completed a smoking-history questionnaire and the Fagerstrom Test of Nicotine Dependence (FTND); FTND scores ranged between 1 (low) and 9 (high).23 In addition, the participants were familiarized with the scanning environment using a mock MRI scanner.
Image Acquisition
Study 1
Neuroimaging data were collected on two 3T scanners (Signa Excite HD and MR750; GE Healthcare). A 3-dimensional, spoiled gradient recalled acquisition anatomical sequence was collected (field of view [FOV], 25.6 cm2; flip angle, 12°; 166 sections; 1-mm isotropic voxel), followed by an fMRI SENSE sequence (repetition time [TR], 1500 milliseconds; echo time [TE], 30 milliseconds; flip angle, 60°; 32 sections; voxel size, 4 × 4 × 3.8 mm).
Study 2
In the laboratory study, MRI scanning was conducted on a 3T scanner (Magnetom TrioTim; Siemens). A 3-dimensional MPRAGE anatomical sequence was collected (FOV, 25.6 cm2; flip angle, 9°; 192 sections; and 1-mm isotropic voxel), followed by an fMRI EP2D-BOLD sequence (TR, 2000 milliseconds; TE, 30 milliseconds; flipangle, 90°; 36 sections; and voxel size, 3.3 × 3.3 × 3.0 mm).
IC Task
The validated, fixed-jittered, event-related IC task20 included randomly presented colored circles of 3 types of trial: frequent gray (Go, 75.4%;n = 388), rare yellow(RareGo, 12.3%; n = 65), and rare blue (NoGo, 12.3%; n = 65) (eFigure 1 in the Supplement). Participants were instructed to press a button with their right index finger as quickly as possible following each Go and RareGo trial; and to refrain from pressing in response to a NoGo trial.
Image Processing
Voxel-Based Morphometry Data Processing
Structural images (study 1: n = 41 abstinent, n = 40 relapsed; study 2:n = 26)were preprocessed using the voxel-based morphometry 8 (VBM8) toolbox (http://dbm.neuro.uni-jena.de/vbm8) and statistical parametric mapping 12 (SPM12) (http://www.fil.ion.ucl.ac.uk/spm) according to a standard pipeline. Forward-deformation fields were calculated from each participant’s skull-stripped and rigid-body registered T1 image to warp functional data into Montreal Neurological Institute space.24
fMRI Data Processing
Preprocessing of functional images included slice-time correction and realignment25; motion outlier detection (framewise displacement >4 mm (approximately 1 acquisition voxel) http://www.nitrc.org/projects/artifact_detect) and correction (via nearest-neighbor interpolation); despiking at 4%of global mean (http://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html); coregistration of functional images to structural image; warping to Montreal Neurological Institute space using forward deformations, resampling to1.5-mm3 voxel size (ie, 3.375 μL) and smoothing with a 10-mm3 full width at half maximum (FWHM) Gaussian filter. Exclusion threshold for rapid motion was 20% of run length, but no participants exceeded this threshold (eTable 2 in the Supplement).
Task-Based Functional Connectivity Data Processing
Preprocessed fMRI data were uploaded into the conn14 toolbox (http://www.nitrc.org/projects/conn) for denoising and connectivity analyses. Using unsmoothed segmented tissue images, along with functionally defined regions of interest (ROIs), significant clusters were exported using MarsBaR (http://marsbar.sourceforge.net) from the NoGocorrect–RareGocorrect analysis of covariance fMRI model (study 1; eTable 3 in the Supplement). Mean time courses from the unsmoothed BOLD signal from each ROI were characterized with no additional principal components. Confounds (mean white matter, cerebrospinal fluid signal, and motion parameters) were regressed out of the mean signal for each ROI. A high-pass filter of 0.008 Hz was performed after confound regression (with no detrending).
Inhibitory Control Network Mask
An IC network mask was created in WFU PickAtlas (http://fmri.wfubmc.edu/software/pickatlas), including the right IFG, bilateral thalamus, subthalamic nucleus, presupplementary motor area, and left primary motor cortex (eFigure 2 in the Supplement). The ROI mask was used as an explicit mask in all analyses.
Statistical Analysis
General Statistical Considerations
For both studies, significance was defined at α = .05,with a cluster-determining threshold of P < .001, as determined by Monte Carlo simulations individually for each statistical parametric mapping SPM model (3dClustSim; https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html, May 2016). Specifically, 3dcalc was used to take the square root of the SPM model’s error variance image (ResMS) and 3dFWHMx was used to empirically determine the spatial smoothness of residual error in the model using the newly developed non-Gaussian autocorrelation function. Data from the National Institutes of Health, replicating the Beijing data sets,26 show that these settings, combined with a cluster-determining threshold of P < .001 and a 10-mm3 smoothing kernel, maintain a true false-positive rate of 5%for regular event-related designs.27 The required cluster extent (cubic millimeters) for each model is noted in the respective table footnotes. Due to nonstationarity in VBM data, we thresholded at P < .05 familywise error at the voxel level. When significant clusters were observed at this cluster-determining threshold, cluster-level familywise error P values and cluster extent threshold values are reported.
Covariates
In study 1, differences in demographic characteristics and baseline self-report measures between the two groups (abstinent, relapsed) were analyzed with χ2 and independent-sample, 2-tailed t tests (Table 1). Significant group differences were observed for nicotine dependence (FTND) and cigarettes per day. Cigarettes per day and FTND were positively correlated (r = 0.58; P < .001). The FTND results were associated with BOLD response in a priori ROIs, including presupplementary motor area (P = .004), right IFG (P = .03), and thalamus (P = .02), and was therefore included as a nuisance covariate in all models. Before hypothesis testing in study 1, we assessed whether the scanner used was a confound in the GMV or BOLD signal. A main effect of the scanner was observed in GMV (eTable 4 in the Supplement) and therefore was included as a covariate. No effect of the scanner was observed in the fMRI data or in the task-based functional connectivity (tbFC) ROI-ROI analysis (all P > .01). Visit order was included as a covariate in all study 2 models. Age, sex, and educational level are known confounds for morphometric analyses and thus were included as nuisance covariates in both studies. Functional connectivity is known to be particularly sensitive to rapid motion; therefore, the total number of interpolated volumes from motion-outlier detection was covaried at the second level in both studies.
Voxel-Based Morphometry
The VBM data were modeled at the second level in SPM12 using analysis of covariance for study 1 and time to smoke on the SRT in a regression analysis for study 2. For VBM analytic strategy, see the eMethods in the Supplement.
Experimental IC Task
Session data were entered into a first-level analysis using the general linear model25 to examine the BOLD response to each of 5 trial types: NoGocorrect, NoGoincorrect, RareGocorrect, RareGoincorrect, and Goincorrect. Each event was modeled as a delta regressor at the onset of the event and convolved with a canonical hemodynamic response function. Intrarun motion was removed through rigid body rotation and translation and parameters were included as covariates. A high-pass filter (0.008 Hz) was applied to remove slow signal drift. Finally, to examine successful IC-BOLD response, controlling for novelty detection, a NoGocorrect–RareGocorrect contrast image was generated (henceforth, IC-BOLD) and used for hypothesis testing. Within- and between-participants (study 2 only) main effects of trial type on accuracy, within-participants main effects of rare trial type on BOLD response, and between-participants main effects of group (study 1 only) on IC-BOLD response were each assessed via analysis of covariance. Next, the functional ROIs obtained from study 1 (right IFG and right thalamus) were used to obtain mean percent signal change from first-level models during IC (via MarsBaR). The main effect of task on BOLD response is shown in eFigure 3 and eTable 5 in the Supplement.
Task-Based Functional Connectivity
Corticothalamic tbFC was assessed at the second level based on first-level, voxelwise, Fisher-transformed correlation-coefficient maps.28 In study 1, tbFC between right IFG and right thalamus functional ROIs from the IC-BOLD analysis was assessed using the conn14 ROI-ROI explorer (via between participant analysis of covariance). In study 2, a second-level seed-voxel strategy was implemented, seeding the right IFG functional ROI from study 1 to examine tbFC within a right thalamus automated anatomical labeling mask, and regression was performed to examine corticothalamic tbFC as a function of time to smoke on the SRT.
Mediation Path and Post hoc Analysis
Mediation analyses were performed to test the a priori hypothesis that corticothalamic tbFC during IC mediates the association between IC accuracy and smoking outcomes, using bootstrapping (10000) with bias-corrected and accelerated (BCa) 95% CI.29 Associations between GMV, BOLD, and tbFC findings, as well as with behavioral measures, were assessed (eMethods in the Supplement).
Results
Inhibitory Control Task
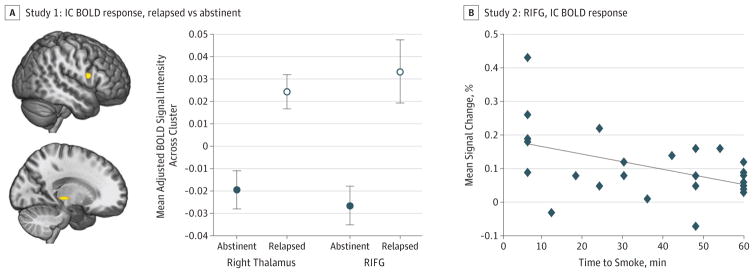
In study 1, inhibitory control task performance did not differ between the abstinent and relapsed groups (eFigure 4A and eTable 6A in the Supplement). A significant main effect of group demonstrated that, compared with those who remained abstinent, smokers who relapsed exhibited greater IC-BOLD responses in the right IFG (F1,78 = 14.87) and right thalamus (F1,78 = 14.97) (P <. 05) (Figure 1A and eTable 3 in the Supplement). Furthermore, greater IC-BOLD response in the right IFG was associated with worse IC accuracy (β78 = −0.234; R2 = 0.037; P = .04), but not in the right thalamus (P = .47).
Figure 1. Associations Between Inhibitory Control (IC) Blood Oxygenation Level–Dependent (BOLD) Response and Smoking Outcomes.
A, In study 1 (cessation study), abstinent smokers exhibited less baseline (ie, prequit attempt) IC task-related BOLD response in the right inferior frontal gyrus (IFG) (yellow cluster in top figure) and right thalamus (yellow cluster in bottom figure) than did smokers who relapsed (P<.001 cluster-determining threshold, cluster extent >186 mm3; 3dClustSim autocorrelation function) (eTable 3 in the Supplement). Error bars represent 1 SE. B, In study 2 (laboratory study), greater BOLD response in the right IFG during IC predicted a shorter time to smoke during the smoking relapse analog task (SRT) (β = −0.468; P = .02; adjusted R2 = 0.151) (eTable 3 in the Supplement). All imaging data were analyzed within an IC network mask (eFigure 2 in the Supplement).
In study 2, successful IC accuracy predicted time to smoke during the SRT (eFigure 4B in the Supplement). Analogous to study 1, greater IC-BOLD response in the right IFG predicted a shorter time to smoke during the SRT (β = −0.468; P = .02; adjusted R2 = 0.151) (t23 = −2.49; β = −0.47; P = 0.02) (Figure 1B and eTable 3 in the Supplement).
Corticothalamic tbFC
In study 1, stronger IC tbFC between the right IFG and thalamus (henceforth, corticothalamic) predicted successfully maintaining abstinence (F1,77 = 5.87; P = .02) (Figure 2A and eTable 7 in the Supplement) and IC accuracy (β77 = 0.229; R2 = 0.03; P = .04). In study 2, stronger ICtbFC in the corticothalamic circuit predicted a longer time to initiate smoking on the SRT (t22 = 5.62; β = 0.79; P < .001, adjusted R2 = 0.538) (Figure 2B and eTable 7 in the Supplement) and greater IC accuracy (β22 = 0.530; R2 = 0.249; P = .009).
Figure 2. Associations Between Corticothalamic Task-Based Functional Connectivity (tbFC) During Inhibitory Control (IC) and Smoking Outcomes.
A, In study 1 (cessation study), smokers who remained abstinent exhibited stronger baseline corticothalamic tbFC during IC than smokers who relapsed at baseline (ie, prequit attempt) (P = .02) (eTable 7 in the Supplement). Error bars represent 1 SE. B, In study 2 (laboratory study), stronger corticothalamic tbFC during inhibitory control predicted a longer time to initiate smoking on the smoking relapse analog task (SRT) (β = 0.791; P < .001, adjusted R2 = 0.538) (eTable 7 in the Supplement).
Mediation Path Analysis
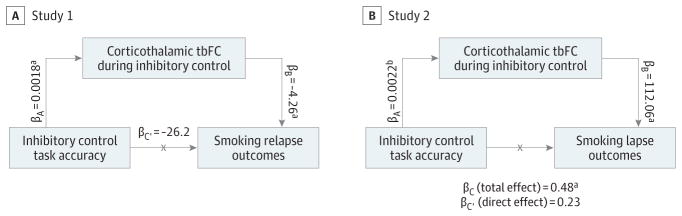
Corticothalamic tbFC mediated the association between IC accuracy and smoking relapse outcomes in study 1 (βi = −0.0078; BCa 95% CI, −0.022 to −0.0002) (Figure 3 and eTable 8A in the Supplement). In study 2, corticothalamic tbFC mediated the association between IC accuracy and time to smoke on the SRT, accounting for 51.7% of the total effect (βi = 0.25; BCa 95% CI, 0.02 to 0.66) (Figure 3 and eTable 8B in the Supplement).
Figure 3. Corticothalamic Task-Based Functional Connectivity (tbFC) During Inhibitory Control (IC) Mediates the Association Between IC Task Accuracy and Smoking Outcomes.
A, In study 1 (cessation study), there was a significant indirect effect of IC task accuracy on smoking relapse outcomes via corticothalamic task-based functional connectivity (tbFC) such that increasing IC task accuracy and corticothalamic tbFC predicted maintaining abstinence (βi = −0.0078; bias-corrected and accelerated [BCa] 95%CI, −0.022 to −0.0002 [binary coding: abstinent, 0; relapsed,1]). B, In study 2 (laboratory study), the indirect effect of IC task accuracy via tbFC accounted for 51.7%of the total effect on time to smoke during the smoking relapse analog task (βi = 0.25; BCa 95%CI, 0.02 to 0.66). eTable 8 in the Supplement provides additional details.
aSignificant at P < .05.
bP = .07.
Gray-Matter Volume
In study 1, compared with those who remained abstinent, smokers who relapsed exhibited significantly lower baseline GMV in the right IFG (F1,74 = 28.32; familywise error P threshold = 0.03; a 1.7% difference) (eFigure 5 and eTable 9 in the Supplement). Graymatter volume was not associated with IC accuracy. No significant associations between SRT and GMV were found in study 2.
Post hoc Analyses
Post hoc analysis findings evaluating associations between VBM, BOLD response, and behavior are presented in eResults in the Supplement. In study 1, the right IFGBOLD response during IC was found to be negatively associated with right IFGGMV (r74 = −0.353, P = .002). In study 2, semipartial correlation revealed a moderate negative association between IC task-related corticothalamic BOLD response and tbFC (r22 = −0.434, P = .03).
Discussion
These studies reveal that IC task-related BOLD response in the right IFG and corticothalamic tbFC are associated with the ability to resist smoking; also, right IFGGMV was associated with maintaining abstinence. Moreover, corticothalamic tbFC mediated the association between IC task accuracy and both smoking relapse and time to lapse. To our knowledge, these are the first studies to directly link corticothalamic-mediated IC to the ability to resist smoking.
The right IFG plays an important role in attentional control processes, including novelty detection,30 sustained attention,31 emotion-cognition interactions,32 and resolving emotional distraction during goal-directed processes.33–35 Smoking is an over-learned, prepotent response and smoking abstinence requires suppressing this response. Prior work has demonstrated that smoking abstinence disrupts the right IFGBOLD response during a broad array of neurocognitive tasks.13–16 However, previous studies did not report on whether the effects of acute abstinence on frontally mediated neurocognition predicted inhibiting subsequent smoking behavior.
In addition to the effect in the right IFG, we found that increased activity in the right thalamus, along with weaker tbFC between the IFG and thalamus, is associated with smoking lapse and relapse. The thalamus serves as an information relay36 between distinct neuroanatomical systems that subserve arousal and attention.37 Thalamic nuclei share reciprocal connections with38 and are functionally connected to39 the lateral prefrontal cortex. Corticothalamic loops mediate IC and executive function,40 whereas dysregulated corticothalamic circuitry is posited to represent a transdiagnostic factor in neuropsychiatric disorders.41 Weaker FC in corticothalamic circuitry has been reported across a spectrum of psychiatric disorders.42,43 With regard to smoking, chronic exposure to nicotine during development produces neuroplasticity in corticothalamic circuitry posited to mediate deficits in executive function.44
Finally, we found in the cessation study that greater right IFG GMV was associated with maintaining smoking abstinence. Morphometric data from smokers has shown that less GMV in bilateral IFG,9 and reduced GMV in the right dorsolateral prefrontal cortex more broadly, is associated with greater cue-induced craving.45 Together, these findings suggest that reduced prefrontal GMV may reduce IC over behavioral response to conditioned drugcues. This finding is consistent with the extant literature implicating drug addiction–related neuroplasticity in frontostriatal circuitry, which mediates cue-induced relapse,2,46 as being associated with frontally mediated behavioral inhibition.3
In addition to replicating the extant literature on dysregulated prefrontally mediated IC in substance use disorders,1 the present studies extend the literature by providing convergent findings that corticothalamic circuitry function mediates associations between IC and resisting smoking. Moreover, findings herein suggest that individual differences in corticothalamic circuitry function have important implications for smoking cessation and relapse vulnerability. Further research is needed to evaluate the therapeutic benefit of delivering precessation interventions (eg, brain stimulation,47 cognitive training,48,49 and combined medications50) to treat the neuropathophysiology of corticothalamic circuitry involved in smoking behavior.
Limitations
It remains unclear whether structural and functional differences among smokers reflect the effects of chronic exposure to toxic compounds found in combustible tobacco on neurovasculature,51 are a product of epigenetic changes52 or neuroplasticity53 following chronic nicotine use, or reflect a smoking endophenotype with impaired inhibitory control of behavior. Therefore, despite controlling for a number of variables known to affect GMV and neurocognition, longitudinal studies are needed that examine the effects of smoking/nicotine across development and following smoking cessation.
Conclusions
Findings from the present studies provide new insight into associations between corticothalamic-mediated IC and tobacco use disorder and suggest value in assessing the efficacy of novel treatments for IC deficits that undergird the maintenance of cigarette smoking.
Supplementary Material
eFigure 1. Inhibitory Control Task Overview
eFigure 2. Inhibitory Control Network Mask
eFigure 3. Main Effects of Correct Rare Trial Type on BOLD Response
eFigure 4. Inhibitory Control and Smoking Lapse/Relapse Behavior
eFigure 5. Gray-Matter Volume (GMV) Associated With Smoking Cessation Outcomes
eTable 1. Smoking Relapse Analog Task Performance and Smoking Topography (Study 2: Laboratory Study)
eTable 2. Motion Outliers During Inhibitory Control Task fMRI
eTable 3. BOLD fMRI IC Contrast and BOLD fMRI Percent Signal Change
eTable 4. Main Effects of MRI Scanner on Gray Matter Volume Measurement (Study 1: Cessation Study)
eTable 5. Main Effects of Inhibitory Control Task on BOLD Activation
eTable 6. Inhibitory Control Task Accuracies: Within-Subjects Main Effect of Trial Type on Accuracy
eTable 7. Corticothalamic Functional Connectivity
eTable 8. Inhibitory Control Task Accuracy, Corticothalamic Task-Based Functional Connectivity (tbFC), and Smoking Relapse: Mediation Path Analysis
eTable 9. Voxel-Based Morphometry—Gray Matter Volume (GMV) Analysis (Study 1: Cessation Study)
Key Points.
Question
What is the association between corticothalamic-mediated inhibitory control and smoking relapse vulnerability?
Findings
In 2 functional magnetic res onance imaging studies, a smoking cessation study (n = 81) and a laboratory-based smoking assessment (n = 26), corticothalamic circuitry function during inhibitory control was associated with smoking relapse vulnerability.
Meaning
Findings that baseline differences in corticothalamic circuitry function mediate inhibitory control and smoking relapse vulnerability warrant further examination of interventions for augmenting corticothalamic neurotransmission during the course of tobacco use disorder treatment.
Acknowledgments
Funding/Support: This research was supported by National Institute of Drug Abuse grants R01DA025876 for clinicaltrials.gov NCT00831155 (Dr McClernon) and R01DA033459 (Dr Froeliger); data analysis and manuscript preparation by grants R01DA033459 and R01DA038700 (Dr Froeliger), and support from T32DA07288 (Mr Bell) and K23 DA039294 (Dr Sweitzer).
Footnotes
Conflict of Interest Disclosures: None reported.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: Drs Froeliger and McClernon had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Froeliger, McConnell, McClernon.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Froeliger, McConnell, Bell, Sweitzer, Kaiser.
Critical revision of the manuscript for important intellectual content: Froeliger, McConnell, Sweitzer, Kozink, Hallyburton, Gray, McClernon.
Statistical analysis: Froeliger, McConnell, Bell, Sweitzer, McClernon.
Obtained funding: Froeliger, McClernon.
Administrative, technical, or material support: Froeliger, Eichberg, Hallyburton, McClernon.
Study supervision: Froeliger, Eichberg, McClernon.
Additional Contributions: James Purl, BS, and Jayce Doose, MEng (Center for Biomedical Imaging, Medical University of South Carolina), assisted with image acquisition and provided technical support. Susan Music, RT(R)(MR), Luke Pool, RT(R)(MR), and Natalie Goutkin, RT(R)(MR) (Duke University Medical Center), assisted with image acquisition.
References
- 1.Moeller SJ, Bederson L, Alia-Klein N, Goldstein RZ. Neuroscience of inhibition for addiction medicine: from prediction of initiation to prediction of relapse. Prog Brain Res. 2016;223:165–188. doi: 10.1016/bs.pbr.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Rae CL, Hughes LE, Anderson MC, Rowe JB. The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. J Neurosci. 2015;35(2):786–794. doi: 10.1523/JNEUROSCI.3093-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swann NC, Cai W, Conner CR, et al. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: electrophysiological responses and functional and structural connectivity. Neuroimage. 2012;59(3):2860–2870. doi: 10.1016/j.neuroimage.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brody AL, Mandelkern MA, Jarvik ME, et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55(1):77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- 8.Fritz HC, Wittfeld K, Schmidt CO, et al. Current smoking and reduced gray matter volume—a voxel-based morphometry study. Neuropsychopharmacology. 2014;39(11):2594–2600. doi: 10.1038/npp.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallinat J, Meisenzahl E, Jacobsen LK, et al. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006;24(6):1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- 10.Franklin TR, Wetherill RR, Jagannathan K, et al. The effects of chronic cigarette smoking on gray matter volume: influence of sex. PLoS One. 2014;9(8):e104102. doi: 10.1371/journal.pone.0104102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao Y, Tang J, Liu T, Chen X, Hao W. Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study. Addict Biol. 2012;17(6):977–980. doi: 10.1111/j.1369-1600.2010.00250.x. [DOI] [PubMed] [Google Scholar]
- 12.Froeliger B, Modlin LA, Kozink RV, et al. Frontoparietal attentional network activation differs between smokers and nonsmokers during affective cognition. Psychiatry Res. 2013;211(1):57–63. doi: 10.1016/j.pscychresns.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Froeliger B, Modlin LA, Kozink RV, Wang L, McClernon FJ. Smoking abstinence and depressive symptoms modulate the executive control system during emotional information processing. Addict Biol. 2012;17(3):668–679. doi: 10.1111/j.1369-1600.2011.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozink RV, Kollins SH, McClernon FJ. Smoking withdrawal modulates right inferior frontal cortex but not presupplementary motor area activation during inhibitory control. Neuropsychopharmacology. 2010;35(13):2600–2606. doi: 10.1038/npp.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Froeliger B, Modlin L, Wang L, Kozink RV, McClernon FJ. Nicotine withdrawal modulates frontal brain function during an affective Stroop task. Psychopharmacology (Berl) 2012;220(4):707–718. doi: 10.1007/s00213-011-2522-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozink RV, Lutz AM, Rose JE, Froeliger B, McClernon FJ. Smoking withdrawal shifts the spatiotemporal dynamics of neurocognition. Addict Biol. 2010;15(4):480–490. doi: 10.1111/j.1369-1600.2010.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernst M, Matochik JA, Heishman SJ, et al. Effect of nicotine on brain activation during performance of a working memory task. Proc Natl Acad Sci U S A. 2001;98(8):4728–4733. doi: 10.1073/pnas.061369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57(1):56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Yakir A, Rigbi A, Kanyas K, et al. Why do young women smoke? III: attention and impulsivity as neurocognitive predisposing factors. Eur Neuropsychopharmacol. 2007;17(5):339–351. doi: 10.1016/j.euroneuro.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Chikazoe J, Jimura K, Asari T, et al. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb Cortex. 2009;19(1):146–152. doi: 10.1093/cercor/bhn065. [DOI] [PubMed] [Google Scholar]
- 21.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 22.Shadel WG, Martino SC, Setodji C, et al. Lapse-induced surges in craving influence relapse in adult smokers: an experimental investigation. Health Psychol. 2011;30(5):588–596. doi: 10.1037/a0023445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 24.Froeliger B, McConnell PA, Stankeviciute N, McClure EA, Kalivas PW, Gray KM. The effects of N-acetylcysteine on frontostriatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: a double-blind, placebo-controlled fMRI pilot study. Drug Alcohol Depend. 2015;156:234–242. doi: 10.1016/j.drugalcdep.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friston KJ, Jezzard P, Turner R. Analysis of functional MRI time-series. Hum Brain Mapp. 1994;1(2):153–171. [Google Scholar]
- 26.Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox RW, Reynolds RC, Taylor PA. AFNI and clustering: false positive rates redux. doi: 10.1191/065862. [published online July 26, 2016]. bioRxiv. [DOI] [Google Scholar]
- 28.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 29.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- 30.Strange BA, Henson RN, Friston KJ, Dolan RJ. Brain mechanisms for detecting perceptual, semantic, and emotional deviance. Neuroimage. 2000;12(4):425–433. doi: 10.1006/nimg.2000.0637. [DOI] [PubMed] [Google Scholar]
- 31.Shallice T, Stuss DT, Alexander MP, Picton TW, Derkzen D. The multiple dimensions of sustained attention. Cortex. 2008;44(7):794–805. doi: 10.1016/j.cortex.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9(2):148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- 33.Dolcos F, Diaz-Granados P, Wang L, McCarthy G. Opposing influences of emotional and non-emotional distracters upon sustained prefrontal cortex activity during a delayed-response working memory task. Neuropsychologia. 2008;46(1):326–335. doi: 10.1016/j.neuropsychologia.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, LaBar KS, Smoski M, et al. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Res. 2008;163(2):143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26(7):2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci. 1998;18(21):8979–8989. doi: 10.1523/JNEUROSCI.18-21-08979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luria AR. The Working Brain: An Introduction to Neuropsychology. New York, NY: Basic Books; 1976. [Google Scholar]
- 38.Giguere M, Goldman-Rakic PS. Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol. 1988;277(2):195–213. doi: 10.1002/cne.902770204. [DOI] [PubMed] [Google Scholar]
- 39.Buchsbaum MS, Buchsbaum BR, Chokron S, Tang C, Wei TC, Byne W. Thalamocortical circuits: fMRI assessment of the pulvinar and medial dorsal nucleus in normal volunteers. Neurosci Lett. 2006;404(3):282–287. doi: 10.1016/j.neulet.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 40.Heyder K, Suchan B, Daum I. Cortico-subcortical contributions to executive control. Acta Psychol (Amst) 2004;115(2–3):271–289. doi: 10.1016/j.actpsy.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 41.Schulman JJ, Cancro R, Lowe S, Lu F, Walton KD, Llinas RR. Imaging of thalamocortical dysrhythmia in neuropsychiatry. Front Hum Neurosci. 2011;5:69. doi: 10.3389/fnhum.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nair A, Treiber JM, Shukla DK, Shih P, Muller RA. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain. 2013;136(pt 6):1942–1955. doi: 10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36(4):713–722. doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heath CJ, Picciotto MR. Nicotine-induced plasticity during development: modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56(suppl 1):254–262. doi: 10.1016/j.neuropharm.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, Salmeron BJ, Ross TJ, et al. Anatomical differences and network characteristics underlying smoking cue reactivity. Neuroimage. 2011;54(1):131–141. doi: 10.1016/j.neuroimage.2010.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45(5):647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Fattore L, Diana M. Drug addiction: an affective-cognitive disorder in need of a cure. Neurosci Biobehav Rev. 2016;65:341–361. doi: 10.1016/j.neubiorev.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Garland EL, Froeliger B, Howard MO. Mindfulness training targets neurocognitive mechanisms of addiction at the attention-appraisal-emotion interface. Front Psychiatry. 2014;4:173. doi: 10.3389/fpsyt.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McConnell PA, Froeliger B. Mindfulness, mechanisms and meaning: perspectives from the cognitive neuroscience of addiction. Psychol Inq. 2015;26(4):349–357. doi: 10.1080/1047840X.2015.1076701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McClure EA, Baker NL, Gipson CD, et al. An open-label pilot trial of N-acetylcysteine and varenicline in adult cigarette smokers. Am J Drug Alcohol Abuse. 2015;41(1):52–56. doi: 10.3109/00952990.2014.933839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanlon CA, Owens MM, Joseph JE, et al. Lower subcortical gray matter volume in both younger smokers and established smokers relative to non-smokers. Addict Biol. 2016;21(1):185–195. doi: 10.1111/adb.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mychasiuk R, Muhammad A, Ilnytskyy S, Kolb B. Persistent gene expression changes in NAc, mPFC, and OFC associated with previous nicotine or amphetamine exposure. Behav Brain Res. 2013;256:655–661. doi: 10.1016/j.bbr.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Viswanath H, Velasquez KM, Thompson-Lake DG, et al. Alterations in interhemispheric functional and anatomical connectivity are associated with tobacco smoking in humans. Front Hum Neurosci. 2015;9:116. doi: 10.3389/fnhum.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Inhibitory Control Task Overview
eFigure 2. Inhibitory Control Network Mask
eFigure 3. Main Effects of Correct Rare Trial Type on BOLD Response
eFigure 4. Inhibitory Control and Smoking Lapse/Relapse Behavior
eFigure 5. Gray-Matter Volume (GMV) Associated With Smoking Cessation Outcomes
eTable 1. Smoking Relapse Analog Task Performance and Smoking Topography (Study 2: Laboratory Study)
eTable 2. Motion Outliers During Inhibitory Control Task fMRI
eTable 3. BOLD fMRI IC Contrast and BOLD fMRI Percent Signal Change
eTable 4. Main Effects of MRI Scanner on Gray Matter Volume Measurement (Study 1: Cessation Study)
eTable 5. Main Effects of Inhibitory Control Task on BOLD Activation
eTable 6. Inhibitory Control Task Accuracies: Within-Subjects Main Effect of Trial Type on Accuracy
eTable 7. Corticothalamic Functional Connectivity
eTable 8. Inhibitory Control Task Accuracy, Corticothalamic Task-Based Functional Connectivity (tbFC), and Smoking Relapse: Mediation Path Analysis
eTable 9. Voxel-Based Morphometry—Gray Matter Volume (GMV) Analysis (Study 1: Cessation Study)