Abstract
The diaphragm muscle must be able to generate sufficient forces to accomplish a range of ventilatory and non-ventilatory behaviors throughout life. Measurements of transdiaphragmatic pressure (Pdi) can be conducted during eupnea, hypoxia (10% O2)-hypercapnia (5% CO2), chemical airway stimulation (i.e., sneezing), spontaneously occurring deep breaths (i.e., sighs), sustained airway or tracheal occlusion, and maximal efforts elicited via bilateral phrenic nerve stimulation, representing the full range of motor behaviors available by the diaphragm muscle. We provide detailed methods on the in vivo measurements of Pdi in mice.
Keywords: Diaphragm muscle, Mouse, Muscle force, Non-ventilatory behavior, Phrenic Nerve, Ventilatory behavior
1. Introduction
The diaphragm muscle sustains ventilation throughout the lifespan in mammals, as the primary muscle of inspiration. Fundamentally being able to measure transdiaphragmatic pressure (Pdi) and respiratory function is imperative. Failure of the respiratory system to sustain ventilation is an ultimate cause of death in many neuromuscular disorders including motor neuron diseases (i.e., Amyotrophic Lateral Sclerosis or Spinal Muscular Atrophy) and muscular dystrophies (i.e., Duchenne Muscular Dystrophy). The diaphragm muscle must be able to provide sufficient forces to accomplish a range of functions including ventilatory and high force non-ventilatory motor behaviors. Ventilatory behaviors include breathing room air at rest (i.e., eupnea) and breathing during various conditions where the chemical drive for ventilation is stimulated (e.g., hypoxia-hypercapnia, exercise). The diaphragm muscle also performs high force non-ventilatory behaviors such as sneezing, gagging and coughing, which are mostly related to maintenance and clearance of the airway. Each behavior requires varying amounts of force generation. Importantly, measurements of diaphragm muscle force generating capacity can be accomplished in vivo with the use of Pdi.
Force generation by the diaphragm muscle, similarly to other skeletal muscles, is accomplished by the recruitment of motor units (Figure 1), which exhibit diverse contractile and fatigue properties. Activation of the diaphragm motor units follows an orderly recruitment pattern (1), such that ventilatory behaviors recruit slow-twitch and fast-twitch fatigue-resistant motor units while maximum activation would recruit both slow- and fast-twitch motor units, including those that are more fatigable (2–6). Isometric force is correlated with peak root mean squared (RMS) electromyography (EMG) amplitude. In the diaphragm muscle, we have shown a significant positive correlation between RMS EMG and Pdi across motor behaviors (2, 3). Thus, RMS EMG can also be used to evaluate diaphragm muscle force (2, 3, 7–10). In skeletal muscles, weakness is best defined as a decrement in maximal force. Accordingly, for the diaphragm muscle, weakness is defined as a decrease in Pdi during maximal activation.
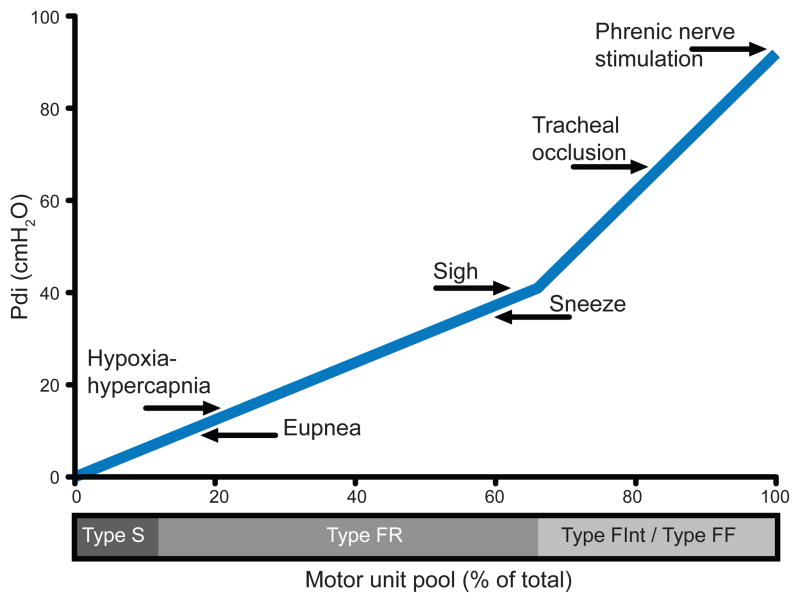
Figure 1.
The diaphragm muscle generates transdiaphragmatic pressures (Pdi; forces) to accomplish a range of motor behaviors from eupnea to high force non-ventilatory behaviors by the recruitment of different motor unit types. This model of motor unit recruitment for the mouse diaphragm muscle indicates that during ventilatory behaviors (eupnea and hypoxia-hypercapnia), type slow-twitch (S) and fast-twitch fatigue resistant (FR) motor units are recruited, while high force non-ventilatory behaviors (tracheal occlusion, and phrenic nerve stimulation) require the recruitment of type fast-twitch fatigue intermediate (FInt) and fast-twitch fatigable (FF) motor units. The Pdi during different motor behaviors are adapted from (15, 14, 24) and the motor unit pool is estimated from fiber type proportions of the mouse diaphragm muscle as determined by myosin heavy chain isoform expression (27, 29), both representing the adult male wild type mouse. This model of motor unit recruitment highlights the importance of measuring Pdi across motor behaviors, not just during eupnea.
To understand the function of the diaphragm muscle, it is necessary to examine force generation across the full range of motor behaviors (Figure 1). The percent of maximal Pdi generated during ventilatory behaviors varies across species, but in general, accomplishing eupneic breathing requires ~10–27% of maximal Pdi (2, 11–15). For this reason, even in conditions in which an entire hemi-diaphragm has been paralyzed by unilateral phrenic denervation, the intact hemi-diaphragm muscle is still able to generate forces required for ventilatory behaviors (3). Similarly, in old age when the diaphragm muscle is significantly weakened by sarcopenia, ventilatory behaviors are not compromised (15). In contrast, the ability of the diaphragm muscle to generate high force, non-ventilatory behaviors is compromised, highlighting the importance of examining the full range of behaviors that the diaphragm muscle can accomplish.
Clinically the measurement of Pdi has an important role in understanding the ability of the diaphragm muscle to generate force. Measurements of Pdi have been conducted in a range species including humans (16, 17), sheep (18), dogs (19), cats (11), piglets (20, 21), rabbits (22, 23), hamsters (12, 13), rats (2, 6, 3) and mice (14, 15, 24). The current position statement of the American Thoracic and the European Respiratory Societies outlines the accepted use of Pdi (25), with the most traditional methodology involving a dual balloon catheter system, with balloons spanning the diaphragm muscle in the thoracic and abdominal cavity. This methodology has been adapted for use with solid-state pressure transducers, which has proven especially useful in smaller animals such as mice (14, 15, 24).
2. Materials
2.1 Technical Equipment & Preparation
Connect a pressure transducer (e.g., Millar Instruments MPVS-300; Houston TX) to an analog to digital converter (e.g., ADInstruments PowerLab System 16/35; Colorado Springs, CO). (See Note 1)
Set up LabChart Pro for acquisition of data signal, alternative data acquisition software could be LabView or comparable software. Note there is a freely available reader for any post-hoc analysis (LabChart Reader) available through ADInstruments.
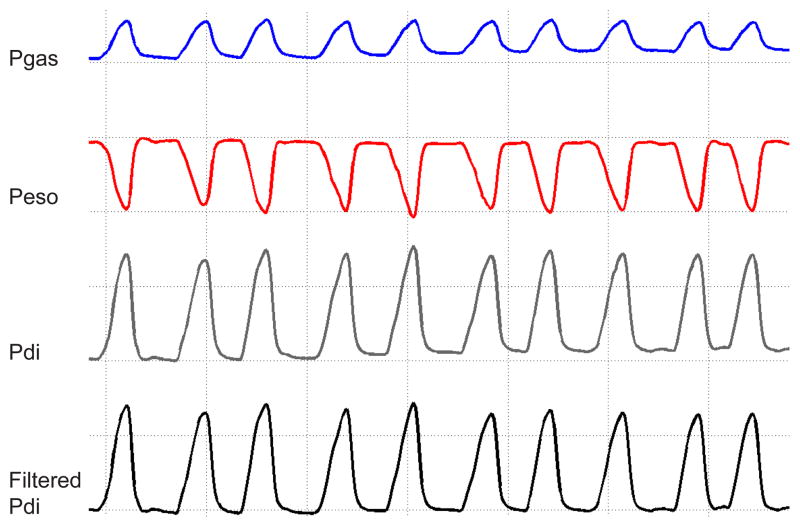
Signal should be acquired with at least 4 channels: two of which will be live data recordings of 1) thoracic (esophageal, Peso) pressure; 2) abdominal (gastric, Pgas) pressure; and two that will represent the data acquired: 3) pressure difference, i.e., Pdi; and 4) filtered Pdi signal using a 0.3–30 Hz band-pass to center the power of the signal and remove any noise related to cardiac activity (Figure 2).
Equilibrate two Mikro-Tip® solid-state pressure catheters (Millar Instrument; 3.5 F, #SPR-524) in individual syringes filled with saline for at least 30 minutes.
Calibration of the solid-state pressure catheters should follow the manufacture specification. Briefly, a two-point calibration between 0 and 30 cm H2O using a manometer is sufficient, since the solid-state pressure catheters are highly linear across the physiological range of Peso and Pgas comprising the Pdi measurement. This reliability and linear range is advantageous compared to the more commonly used balloon-tipped system. (See Note 2)
Figure 2.
Measurements of gastric (Pgas) and esophageal (Peso) pressures are used to calculate the transdiaphragmatic pressure (Pdi; Pgas – Peso). The raw pressure signals were band-pass filtered (0.3–30 Hz), to center the signal and remove any possible artifact noise due to cardiac activity. This tracing represents 10 inspiratory events during eupnea from an adult male wild type mouse. Note the gridlines are a guide to visualize the effects of filtering on the baseline.
3. Methods
3.1 Mouse Preparation
-
1
Weigh and anesthetize mice. Consider the use of fentanyl (10 mcg/kg), droperidol (0.2 mg/kg), and diazepam (5 mg/kg). This anesthetic regimen is consistent with limited instrumentation of the animal and has minimal impact on ventilation or parameters of skeletal muscle function (26).
-
2
Maintain mouse on a heating pad, consider using thermometer and pulse oximetry (e.g. MouseOX, Starr Life Science Corp., Oakmont PA) to monitor animal vital signs throughout the procedure. Note that stressing the animal to maintain thermoregulation may result in changes in ventilation so it is critical to maintain body temperature.
-
3
To constrain abdominal movements and maintain near isometric conditions, bind the abdomen of the mouse using a thick woven gauze or elastic bandage. Taking care to make sure the binding is below the base of the rib cage, and covering the body of the mouse.
-
4
Position and stabilize the mouse supine on a surgical board. The use of a magnetic board (e.g., Braintree Scientific, ACD 014) will allow for precise controlled retraction and positioning of the mouse. Note alternative positioning, such as prone, is possible but should be standardized across the experiment, since gravity and posture may affect Pdi.
-
5
Secure the upper airway of the mouse open at the mouth by the use of a rubber band or 4-0 suture attached to the incisors.
-
6
Tracheal cannulation (Figure 3): begin by making a vertical midline incision ~25 mm distal to the jaw, near the hyoid bone to just proximal of the sternum. Retract the skin on both the right and left side. Bluntly split and dissect away the submaxillary gland to expose the trachea and larynx. Using a dissecting microscope carefully split and dissect away the sternohyoid and sternothyroid muscle layers surrounding the trachea. Place two pieces of 5-0 silk suture, each ~10 cm long under the trachea at the level of the cricoid cartilage. Cut the trachea approximately two rings just below the cricoid cartilage. Carefully advance the cannula into the trachea, then using the two previously placed sutures, tie two basic square knots to secure the cannula in place. Take care to tie the knots between cartilage rings on the smooth muscle portion of the trachea. Trim excess suture. In general, a 19G blunt tipped metal cannula (e.g., Fishman #Z512119) is sufficient for mice, but very small (<15 g) or frail/old mice may require a smaller cannula such as a 21G (e.g., Fishman #Z512121). Note the use of thin wall cannulas is beneficial. (See Note 3)
-
7
Exposure of phrenic nerves (Figure 4): bilaterally expose the phrenic nerves for maximal stimulation during behavioral testing. Following placement of tracheal cannula, bluntly dissect the sternocleidomastoid muscle and retract carefully. Using the dissecting microscope, take care to avoid the jugular veins, carotid arteries and nerve plexus (ansa cervicalis) while dissecting deeply to reach the brachial plexus and phrenic nerve. Using the trachea as a reference, the phrenic nerve will be dorsal, lateral and parallel. Also running parallel will be the vagus nerve, which has a much larger diameter and is located more medial between the phrenic nerve and the trachea, next to the carotid and jugular vessels. The brachial plexus will run at roughly a 45° angle from the trachea. Note in some species there may be an accessory phrenic nerve (from lower cervical roots) that joins the main phrenic nerve lower in the neck and which may require additional dissection. (See Note 3)
Figure 3.
Anatomical schematic with the structures and landmarks necessary for the tracheal cannulation procedure in the mouse. Note this view is observed following the vertical midline incision, retraction of the skin and blunt dissection of the submaxillary gland to expose the trachea. The photographs highlight the same view; with a view of the sternothyroid muscle overlying the trachea, which needs to be retracted to expose the trachea, and tracheostomy followed by cannulation with a 19G blunt tipped cannula. Note in this view the sternothyroid muscle is retracted and sutures are placed securing the cannula between cartilage rings.
Figure 4.
Anatomical schematic showing the structures and landmarks related to phrenic nerve within the neck of the mouse. The phrenic nerve innervates the diaphragm muscle on the corresponding side of the muscle. Following dissection into the anterior aspect of neck, the brachial plexus and phrenic nerve’s run on both side of the trachea. Using the trachea as a reference, the phrenic nerve will be dorsal, lateral and parallel. The photographs highlight a zoomed in view; with the phrenic nerve and brachial plexus on the lateral side of the trachea.
3.2 Pdi Measurement of Motor Behaviors
-
8
Insert solid-state pressure catheters, one at a time, into the esophagus through the mouth of the mouse (Figure 5). Adjust placement of one of the catheters while monitoring pressure until it becomes positive (Pgas indicating gastric placement in abdominal cavity). Advance the second catheter to obtain the maximum negative pressure (Peso indicating esophageal placement in thoracic cavity). While inserting catheters, it is useful to use a blunt forceps to hold tongue, which will aid in advancing the catheters. Note there is no need to use lubrication for insertion of the catheters. Correct placement of the catheters can be confirmed post mortem.
-
9
Once catheters are placed spanning the diaphragm muscle, begin recording while animals breathe room air (eupnea) - recommended duration of at least 5 minutes.
-
10
To chemically stimulate breathing, expose the animals (in a chamber) to a hypoxia and/or hypercapnia gas mixture containing: 10% O2, 5–7% CO2, balance N2 - recommended duration of at least 5 minutes of acclimatization followed by 2 minutes for data analysis.
-
11
Spontaneously occurring sighs can be noted during ventilatory behaviors, in general the amplitude for a sigh will be at least 2 times that of eupnea in the mouse (see Figure 6). This criterion for sighs will allow for proper identification during analysis.
-
12
Inspiratory efforts against an occluded trachea provide a stronger neural drive for diaphragm activation. To accomplish tracheal occlusion the top of the tracheal cannula is completely obstructed for 15 seconds. Typically, this time will allow analysis ~10 escalating efforts, and the 5 maximal efforts are used for analysis. As an alternative, if tracheal cannulation is not conducted (e.g., with survival procedures), obstruction of the upper airway can be achieved by completely occluding the mouth and nose.
-
13
Maximal bilateral phrenic nerve stimulation provides a measure of maximal Pdi for referencing other efforts. When stimulating the phrenic nerves ensure that the electrodes make good contact with the nerve by freeing the area connective tissue, and by placing a few drops of mineral oil in the exposed area to aid in electrical isolation. Use straight bipolar electrodes on each nerve (e.g., FHC #PBSD08075; Bowdoin ME) and stimulate the nerve up to three times using a Grass stimulator together with a stimulation isolation unit (e.g., Grass Technologies, Warwick, RI) using the following settings determined in separate studies (27): 150 Hz (maximal tetanic force), 0.5 ms pulse duration, in 300 ms duration trains at supramaximal intensity (determined by increasing stimulus current until maximal Pdi response is elicited). Allow at least 1 minute between each stimulation train. Recommend analysis of only the maximal amplitude of the three evoked responses.
-
14
Sneezing induced with capsaicin (Sigma; M2028), using a 10 μl Hamilton syringe to inject 30 μM (30% alcohol) into both nostrils, ~5 μl per side. Allow 5 minutes of recording and analyze any sneezing that is seen. Note, while the sneeze response is robust and consistent in the rat, not all mice will sneeze and the response can be more variable. In general the amplitude for a sneeze will be at about 2 times that of eupnea. Alternative concentrations of capsaicin may be considered (28). Note that you must clean the syringe thoroughly with water and 70% alcohol after use.
-
15
Allow at least 5 minutes between each behavior to allow for Pdi amplitudes to return to eupneic values. Consider completing behaviors in the order presented; 1) eupnea; 2) hypoxia-hypercapnia; 3) sighs; 4) tracheal occlusion; 5) maximal phrenic nerve stimulation; and 6) sneezing.
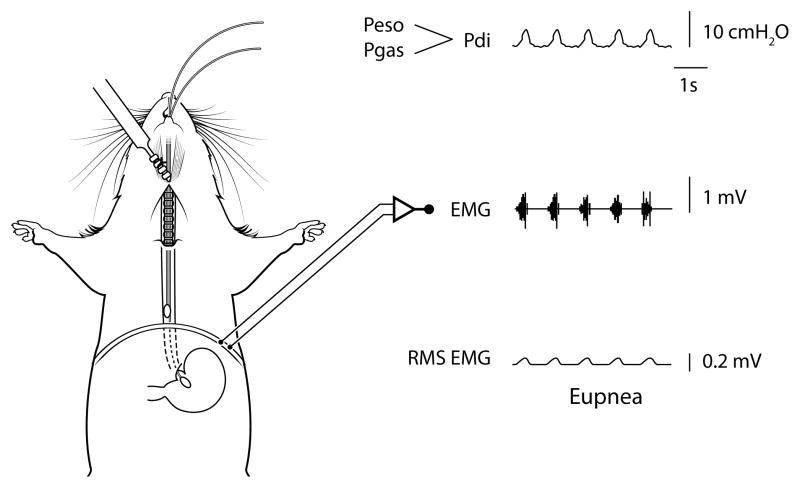
Figure 5.
Anatomical schematic showing placement of the solid-state pressure catheters for measurement of gastric (Pgas) and thoracic esophageal (Peso) pressures in mice - note the pair of catheters are advanced into the stomach (Pgas) and esophagus in the thoracic cavity (Peso) through the mouth. The difference between Pgas and Peso is used to determine transdiaphragmatic pressure (Pdi; see raw tracing). The Pdi amplitude is significantly correlated to peak root mean squared (RMS) of diaphragm muscle electromyography (EMG) activity (2, 3). Raw tracings are provided for Pdi, diaphragm EMG, and RMS EMG.
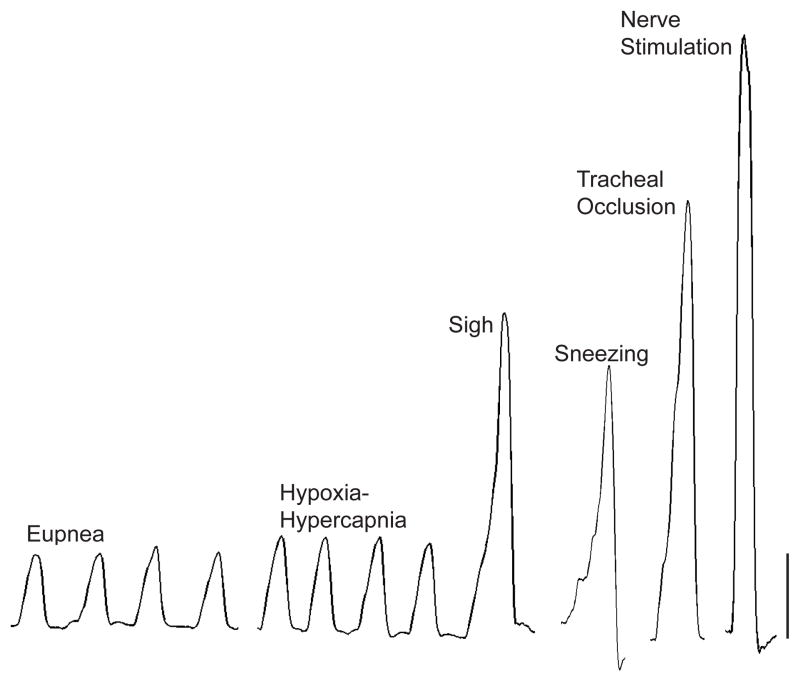
Figure 6.
Representative transdiaphragmatic pressure (Pdi) tracings during various motor behaviors in an adult mouse. Increasing Pdi (force) is found from eupnea, hypoxia-hypercapnia, sighs, sneezing, tracheal occlusion, and maximal phrenic nerve stimulation in mice. Bar represents 10 cmH2O.
3.3 Analytical Procedures
-
16
The primary outcome is the Pdi amplitude across motor behaviors (Figure 6). (See Note 4)
-
17
Analysis of Pdi amplitude across motor behaviors can be done through various analytical platforms such as LabChart, MATLAB, R, C, Java, or Python.
-
18
Additional parameters of analysis may include amplitude duration, inspiratory time, expiratory time, and estimates of drive (24). Additionally, respiratory frequency and frequency of naturally occurring sighs can be determined during ventilatory behaviors.
4. Notes
All specific equipment and product numbers listed is what our lab uses for Pdi; many aspects can be conducted with comparable equipment or platforms.
All cleaning and storage of the solid state pressure catheters should follow the manufacturer specification.
The Pdi procedure can be conducted during survival procedures and thus longitudinally in the same animal, with the omission of tracheal cannulation and maximal bilateral nerve stimulation.
Appropriate inclusion and exclusion criteria based on Pdi amplitude should be determined a priori and based on maximal values from phrenic nerve stimulation.
Acknowledgments
Any procedures conducted on animals in the development of these methods were conducted following institutional protocols and animal care guidelines, in compliance with National Institute of Health Guidelines.
This work was supported by grants from National Institute of Health R01-AG-044615 and R01-HL-096750 (CBM & GCS), T32-HL105355 (SMG), and the Mayo Clinic.
References
- 1.Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126(3287):1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- 2.Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol. 2010;173(1):101–106. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill LC, Mantilla CB, Sieck GC. Impact of unilateral denervation on transdiaphragmatic pressure. Respir Physiol Neurobiol. 2015;210:14–21. doi: 10.1016/j.resp.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sieck GC, Ferreira LF, Reid MB, Mantilla CB. Mechanical properties of respiratory muscles. Comprehensive Physiology. 2013;3(4):1553–1567. doi: 10.1002/cphy.c130003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantilla CB, Sieck GC. Impact of diaphragm muscle fiber atrophy on neuromotor control. Respir Physiol Neurobiol. 2013;189(2):411–418. doi: 10.1016/j.resp.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantilla CB, Sieck GC. Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors. Respir Physiol Neurobiol. 2011;179(1):57–63. doi: 10.1016/j.resp.2011.06.028. S1569-9048(11)00241-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantilla CB, Greising SM, Zhan WZ, Seven YB, Sieck GC. Prolonged C2 spinal hemisection-induced inactivity reduces diaphragm muscle specific force with modest, selective atrophy of type IIx and/or IIb fibers. J Appl Physiol. 2013;114(3):380–386. doi: 10.1152/japplphysiol.01122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Targeted Delivery of TrkB Receptor to Phrenic Motoneurons Enhances Functional Recovery of Rhythmic Phrenic Activity after Cervical Spinal Hemisection. PloS One. 2013;8(5):e64755. doi: 10.1371/journal.pone.0064755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Localized Delivery of Brain-Derived Neurotrophic Factor-Expressing Mesenchymal Stem Cells Enhances Functional Recovery following Cervical Spinal Cord Injury. J Neurotrauma. 2015;32(3):185–193. doi: 10.1089/neu.2014.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantilla CB, Gransee HM, Zhan WZ, Sieck GC. Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury. Exp Neurol. 2013;247C:101–109. doi: 10.1016/j.expneurol.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol. 1989;66(6):2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- 12.Sieck GC. Physiological effects of diaphragm muscle denervation and disuse. Clin Chest Med. 1994;15(4):641–659. [PubMed] [Google Scholar]
- 13.Sieck GC. Neural control of the inspiratory pump. NIPS. 1991;6:260–264. [Google Scholar]
- 14.Greising SM, Sieck DC, Sieck GC, Mantilla CB. Novel method for transdiaphragmatic pressure measurements in mice. Respir Physiol Neurobiol. 2013;188(1):56–59. doi: 10.1016/j.resp.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greising SM, Mantilla CB, Medina-Martinez JS, Stowe JM, Sieck GC. Functional Impact of Diaphragm Muscle Sarcopenia in both Male and Female Mice. Am J Physiol Lung Cell Mol Physiol. 2015;309(1):L46–52. doi: 10.1152/ajplung.00064.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polkey MI, Harris ML, Hughes PD, Hamnegard CH, Lyons D, Green M, Moxham J. The contractile properties of the elderly human diaphragm. Am J Respir Crit Care Med. 1997;155(5):1560–1564. doi: 10.1164/ajrccm.155.5.9154857. [DOI] [PubMed] [Google Scholar]
- 17.Tolep K, Higgins N, Muza S, Criner G, Kelsen SG. Comparison of diaphragm strength between healthy adult elderly and young men. Am J Respir Crit Care Med. 1995;152(2):677–682. doi: 10.1164/ajrccm.152.2.7633725. [DOI] [PubMed] [Google Scholar]
- 18.Bazzy AR, Haddad GG. Diaphragmatic fatigue in unanesthetized adult sheep. J Appl Physiol. 1984;57(1):182–190. doi: 10.1152/jappl.1984.57.1.182. [DOI] [PubMed] [Google Scholar]
- 19.Hubmayr RD, Sprung J, Nelson S. Determinants of transdiaphragmatic pressure in dogs. J Appl Physiol. 1990;69(6):2050–2056. doi: 10.1152/jappl.1990.69.6.2050. [DOI] [PubMed] [Google Scholar]
- 20.Watchko JF, Mayock DE, Standaert A, Woodrum DE. Postnatal changes in transdiaphragmatic pressure in piglets. Pediatr Res. 1986;20:658–661. doi: 10.1203/00006450-198607000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Howell S, Maarek JM, Fournier M, Sullivan K, Zhan WZ, Sieck GC. Congestive heart failure: differential adaptation of the diaphragm and latissimus dorsi. J Appl Physiol. 1995;79(2):389–397. doi: 10.1152/jappl.1995.79.2.389. [DOI] [PubMed] [Google Scholar]
- 22.Sassoon CS, Gruer SE, Sieck GC. Temporal relationships of ventilatory failure, pump failure, and diaphragm fatigue. J Appl Physiol. 1996;81(1):238–245. doi: 10.1152/jappl.1996.81.1.238. [DOI] [PubMed] [Google Scholar]
- 23.Sassoon CS, Caiozzo VJ, Manka A, Sieck GC. Altered diaphragm contractile properties with controlled mechanical ventilation. J Appl Physiol. 2002;92(6):2585–2595. doi: 10.1152/japplphysiol.01213.2001. [DOI] [PubMed] [Google Scholar]
- 24.Medina-Martinez JS, Greising SM, Sieck GC, Mantilla CB. Semi-Automated Assessment of Transdiaphragmatic Pressure Variability across Motor Behaviors. Respir Physiol Neurobiol. 2015 doi: 10.1016/j.resp.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 26.Ingalls CP, Warren GL, Lowe DA, Boorstein DB, Armstrong RB. Differential effects of anesthetics on in vivo skeletal muscle contractile function in the mouse. J Appl Physiol. 1996;80(1):332–340. doi: 10.1152/jappl.1996.80.1.332. [DOI] [PubMed] [Google Scholar]
- 27.Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC. Diaphragm muscle sarcopenia in aging mice. Expl Gerontolo. 2013;48(9):881–887. doi: 10.1016/j.exger.2013.06.001. doi: http://dx.doi.org/10.1016/j.exger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costanzo MT, Yost RA, Davenport PW. Standardized method for solubility and storage of capsaicin-based solutions for cough induction. Cough. 2014;10:6. doi: 10.1186/1745-9974-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greising SM, Medina-Martínez JS, Vasdev AK, Sieck GC, Mantilla CB. Analysis of muscle fiber clustering in the diaphragm muscle of sarcopenic mice. Muscle Nerve. 2015;52(1):76–82. doi: 10.1002/mus.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]