Abstract
GPR37 is an orphan G protein-coupled receptor that is predominantly expressed in the brain and found at particularly high levels in oligodendrocytes. GPR37 has been shown to exert effects on oligodendrocyte differentiation and myelination during development, but the molecular basis of these actions is incompletely understood and moreover nothing is known about the potential role(s) of this receptor under demyelinating conditions. To shed light on the fundamental biology of GPR37, we performed proteomic studies comparing protein expression levels in the brains of mice lacking GPR37 and its close relative GPR37-like 1 (GPR37L1). These studies revealed that one of the proteins most sharply decreased in the brains of Gpr37/Gpr37L1 double knockout mice is the myelin-associated glycoprotein MAG. Follow-up Western blot studies confirmed this finding and demonstrated that genetic deletion of Gpr37, but not Gpr37L1, results in strikingly decreased brain expression of MAG. Further in vitro studies demonstrated that GPR37 and MAG form a complex when expressed together in cells. As loss of MAG has previously been shown to result in increased susceptibility to brain insults, we additionally assessed Gpr37-knockout (Gpr37−/−) vs. wild-type mice in the cuprizone model of demyelination. These studies revealed that Gpr37−/− mice exhibit dramatically increased loss of myelin in response to cuprizone, yet do not show any increased loss of oligodendrocyte precursor cells or mature oligodendrocytes. These findings reveal that loss of GPR37 alters oligodendrocyte physiology and increases susceptibility to demyelination, indicating that GPR37 could be a potential drug target for the treatment of demyelinating diseases such as multiple sclerosis.
Keywords: GPCR, myelin, oligodendrocytes, cuprizone, demyelination, remyelination, multiple sclerosis
INTRODUCTION
GPR37 and GPR37L1 are closely related orphan G protein-coupled receptors (GPCRs) that are highly expressed in the CNS (Donohue et al., 1998; Leng et al., 1999; Marazziti et al., 1998; Marazziti et al., 1997; Valdenaire et al., 1998; Zeng et al., 1997). GPR37 is known to be a substrate of the E3 ubiquitin ligase parkin and related to the endothelin-B receptor, which gave rise to one of its alternative names, the parkin- associated endothelin-like receptor (Pael-R) (Imai et al., 2001). Because of its association with parkin, GPR37 has been most intensively studied in the context of parkinsonism and dopamine neurotransmission, and mice lacking Gpr37 have been shown to exhibit mild perturbations to the dopamine system (Imai et al., 2007; Marazziti et al., 2011; Marazziti et al., 2004; Marazziti et al., 2007). Conversely, a single study has examined the phenotype of mice lacking Gpr37L1 and revealed precocious development of the cerebellum (Marazziti et al., 2013). However, much remains to be learned about the function GPR37 and GPR37L1 in vivo (Smith, 2015).
Despite a predominant focus of previous in vivo work on the role of GPR37 in neurons, GPR37 is most highly expressed in oligodendroglia (Cahoy et al., 2008; Imai et al., 2001; Yang et al., 2016). The oligodendroglial lineage is represented by oligodendrocyte precursor cells (OPCs) and oligodendrocytes, the myelinating cells of the central nervous system into which OPCs terminally differentiate. GPCR signaling, including signaling by several orphan receptors, is emerging as a key factor in regulating the survival and differentiation of myelinating cells as well as maintenance of myelin (Chen et al., 2009; Deshmukh et al., 2013; Mogha et al., 2013; Simon et al., 2016). In Schwann cells, fingolimod, a drug used to treat multiple sclerosis (MS), downregulates Gpr37 mRNA expression and is accompanied by concomitant decreases in myelin expression (Heinen et al., 2015). Furthermore, mice lacking Gpr37 were recently shown to display precocious myelination during development and slightly increased thickness of myelin sheaths in adulthood, leading to the conclusion that GPR37 negatively regulates the progression of differentiation and myelination in oligodendrocytes (Yang et al., 2016). This study also found that loss of GPR37 in the developing brain resulted in increased phosphorylation of the extracellular signalregulated kinase (ERK) via a cAMP-dependent mechanism. However, little is known about the molecular basis by which GPR37 may regulate oligodendroglial physiology in the adult brain, and moreover nothing is known about the potential roles of GPR37 in demyelination or remyelination. We addressed these questions in the present study by performing proteomic analyses of brain tissue from mice lacking Gpr37, focusing on potential changes in the expression of oligodendroglial-expressed proteins, and also assessing Gpr37 knockout mice in the cuprizone model of demyelination.
MATERIALS & METHODS
Tissue preparation for mass spectrometry
Brain tissue was collected from three- month-old wild-type (WT) and Gpr37/Gpr37L1 double knockout (DKO) mice and then vortexed in urea lysis buffer (8M urea, 100 mM NaHPO4, pH 8.5), including HALT protease and phosphatase inhibitor cocktail (Pierce). All homogenization was performed using a Bullet Blender (Next Advance) per manufacturer protocols. Protein supernatants were transferred to 1.5 ml Eppendorf tubes, subjected to centrifugation at 14,000 rpm for 1 min and sonicated (Sonic Dismembrator, Fisher Scientific) 3 times for 5 s with 15 s intervals of rest at 30% amplitude to disrupt nucleic acids. Samples were subsequently vortexed. Protein concentrations were determined by the bicinchoninic acid (BCA) method, and samples frozen in aliquots at −80°C. Protein homogenates (100 µg) were then treated with 1 mM dithiothreitol (DTT) at 25°C for 30 minutes, followed by 5 mM iodoacetimide (IAA) at 25°C for 30 minutes in the dark. Protein was digested with 1:100 (w/w) lysyl endopeptidase (Wako) at 25°C overnight. Samples were then diluted with 50 mM NH4HCO3 to a final concentration of less than 2M urea and further digested overnight with 1:50 (w/w) trypsin (Promega) at 25°C. Resulting peptides were desalted with a Sep-Pak C18 column (Waters) and dried under vacuum.
LC-MS/MS analysis
Derived peptides were resuspended in loading buffer (0.1% formic acid, 0.03% trifluoroacetic acid, 1% acetonitrile). Peptide mixtures were separated on a self-packed C18 (1.9 um Dr. Maisch, Germany) fused silica column (25 cm × 75 uM internal diameter (ID); New Objective, Woburn, MA) by a Dionex Ultimate 3000 RSLCNano and monitored on a Fusion mass spectrometer (ThermoFisher Scientific, San Jose, CA). Elution was performed over a 120-minute gradient at a rate of 300 nl/min with buffer B ranging from 3% to 80% (buffer A: 0.1% formic acid in water, buffer B: 0.1 % formic in acetonitrile). The mass spectrometer cycle was programmed to collect at the top speed for 3 second cycles. The MS scans (400–1500 m/z range, 200,000 AGC, 50 ms maximum ion time) were collected at a resolution of 120,000 at m/z 200 in profile mode, and the HCD MS/MS spectra (1.6 m/z isolation width, 32% collision energy, 10,000 AGC target, 35 ms maximum ion time) were detected in the ion trap. Dynamic exclusion was set to exclude previous sequenced precursor ions for 20 seconds within a 10 ppm window. Precursor ions with +1 and +8 or higher charge states were excluded from sequencing.
Data Analysis for LC-MS/MS
RAW data for the samples was analyzed using MaxQuant v1.5.4.1 with Thermo Foundation 2.0 for RAW file reading capability. The search engine Andromeda, integrated into MaxQuant, was used to build and search a concatenated target-decoy Uniprot mouse reference protein database (retrieved April 20, 2015; 53,289 target sequences), plus 245 contaminant proteins from the common repository of adventitious proteins (cRAP) built into MaxQuant. Methionine oxidation (+15.9949 Da), asparagine and glutamine deamidation (+0.9840 Da), and protein N- terminal acetylation (+42.0106 Da) were variable modifications (up to 5 allowed per peptide); cysteine was assigned a fixed carbamidomethyl modification (+57.0215 Da). Only fully tryptic peptides were considered with up to 2 miscleavages in the database search. A precursor mass tolerance of ±20 ppm was applied prior to mass accuracy calibration and ±4.5 ppm after internal MaxQuant calibration. Other search settings included a maximum peptide mass of 6,000 Da, a minimum peptide length of 6 residues, 0.50 Da tolerance for low resolution MS/MS scans. Co-fragmented peptide search was enabled to deconvolute multiplex spectra. The false discovery rate (FDR) for peptide spectral matches, proteins, and site decoy fraction were all set to 1 percent. Quantification settings were as follows: requantify with a second peak finding attempt after protein identification has completed; match MS1 peaks between runs; a 0.7 min retention time match window was used after an alignment function was found with a 20 minute RT search space. Quantification of proteins was performed using summed peptide intensities given by MaxQuant. The quantification method only considered razor plus unique peptides for protein level quantification.
Western blotting
Protein samples were reduced and denatured in Laemmli buffer, loaded into 4–20% Tris-Glycine gels (Bio-Rad) for SDS-PAGE, and then transferred to nitrocellulose membranes (Bio-Rad). Blots were blocked with 2% milk (in 50mM NaCl, 10mM HEPES, pH 7.3 with 0.1% Tween-20 (Sigma)) and incubated with primary antibodies overnight at 4°C. Proteins were quantified using densitometry, performed with ImageJ software.
cDNA conversion and RT-PCR for Mag mRNA quantification
RNA was isolated from the brains of 3 WT and 3 Gpr37−/− adult mice (2–3 months of age) using TriZOL (Life technologies). cDNA was prepared using random hexamers and reverse transcription. Real-time RT-PCR (qPCR) was performed using DyNaMo Sybr Green qPCR kit (Thermo-Scientific). Changes in gene expression between WT and Gpr37−/− mice were assessed by ΔCt relative to Gapdh expression and fold change (2−ΔΔCt) of Mag mRNA expression in Gpr3T−/− mice compared to WT mice. Gapdh primers (forward: ACCACAGTCCATGCCATCAC; reverse: TCCACCACCCTGTTGCTGTA; ordered from IDT) were used as an internal control and Mag primers (forward: GTGCTGTGGTCGCCTTTGCC; reverse: CCCTCACCCCTACTACTCTC; ordered from IDT) were used to amplify MAG cDNA.
Cell culture
HEK-293T/17 cells were acquired from ATCC (Manassas, VA) and maintained in DMEM (Life Technologies) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin in a humid, 5% CO2, 37°C incubator. Cells were transfected using Mirus (Madison, WI) TransIT-LT1 according to the manufacturer’s protocol.
Coimmunoprecipitation
HEK-293T/17 cells were transfected in 10 cm dishes with 2µg of either empty vector plasmid, GFP-tagged human GPR37, or FLAG-tagged human M1 and 1µg of human MAG (Origene) DNA at 50% confluency. After a 72-hour incubation, cells were harvested in 500 µl of lysis buffer (1% Triton X-100, 25 mM HEPES, 150 mM NaCl, 10 mM MgCl2, 1 mM EDTA, HALT protease inhibitor mix, and 2% glycerol) and lysed by slowly rotating for 60 min at 4 °C. Cells were subjected to centrifugation to clear cellular debris, and soluble cell lysates were incubated with 60µl of protein A/G beads and 3uL of MAG antibody for 1 h at 4 °C. Following five washes with lysis buffer, beads were resuspended in 60 µl of 2X Laemmli buffer.
Generation of Knockout Mice and Maintenance of Mouse Colony
We obtained Gpr37 knockout (Gpr37−/−) mice from Jackson Laboratory (strain Gpr37tm1Dgen, stock number 005806) and Gpr37L1-knockout (Gpr37L1−/−) mice from the NIH Mutant Mouse Regional Resource Centers (strain Gpr37l1tm1Lex, stock number 011709-UCD). Following 10 backcrosses each with wild-type C57BL/6 mice (Jackson Laboratory), the Gpr37−/− and Gpr37L1−/− mice were crossed to develop the DKO line of mice. Genetic deletion of Gpr37 and/or Gpr37L1 was confirmed by DNA sequencing, and loss of GPR37 and/or GPR37L1 protein expression was confirmed by Western blotting of brain tissue samples with specific anti-GPR37 and anti-GPR37L1 antibodies (MAb Technologies).
All mice were maintained on a C57BL/6J background and housed on a 12-h light/dark cycle, with food and water available ad libitum. All experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Emory University. Age-matched WT controls were used in all experiments.
Cuprizone administration
A total of 90 WT mice and 91 Gpr37−/− mice between two and three months of age were used for the experiment, in which 80 WT mice and 81 Gpr37−/− mice were fed 0.2% cuprizone diet and 10 control animals from both groups received normal diet. A cohort of animals (10) from each group was sacrificed to evaluate demyelination at 0, 3, 4 and 6 weeks after cuprizone feeding. Remaining mice were removed from the cuprizone diet, returned to normal chow alone, and were sacrificed at week 9 (3 weeks into the remyelination phase) and week 12 (6 weeks into the remyelination phase) for luxol fast blue (LFB) staining and immunohistochemical (IHC) staining with NG2 or GSTπantibodies to visualize OPCs or oligodendrocytes, respectively.
Tissue collection
To harvest tissue, mice were transcardially perfused. Brains were removed, and coronal sections were post-fixed in 4% PFA with 2.5% glutaraldehyde. The corpus callosum was then removed from each brain and stored in 4% PFA with 2.5% glutaraldehyde for 3–5 days. The tissue was then dehydrated through graded alcohols and mounted into paraffin blocks. When ready for processing, slides were deparaffinized and rehydrated in 95% ethanol (EtOH). Tissue was sectioned into 5 µm serial sections, microtomed from the mounted tissue, and dried on glass slides prior to histology.
Luxol fast blue staining
Rehydrated tissue was incubated in LFB solution overnight at 60°C. After rinsing, tissue was incubated in 0.05% lithium carbonate and differentiated in 70% EtOH until grey and white matter could be distinguished. Samples were dehydrated in 95% EtOH, 100% EtOH, and xylene, then mounted on glass slides. Myelination of the corpus callosum was visualized using a Nikon Eclipse Ti microscope.
Immunohistochemistry
Rehydrated tissue was incubated in antigen retrieval buffer (10mM sodium citrate, 0.05% Tween 20), quenched in H2O2, and blocked at room temperature. The tissue was then incubated with primary antibodies (anti-NG2 or anti-GST-π) overnight at 4°C. Tissue was incubated with biotinylated secondary antibody followed by Vectastain ABC reagent. DAB (3,3’-diaminobenzidine) was used to develop the tissue, after which it was dehydrated through graded alcohols and xylene and then mounted on a glass coverslip. Microscopy was performed on a Nikon Eclipse Ti microscope.
RESULTS
GPR37 regulates MAG expression
To gain molecular insight into the roles of GPR37 and GPR37L1 in the CNS, brain tissue samples from double knockout (DKO) mice lacking both GPR37 and GPR37L1 were subjected to LC/MS, and the expression levels of brain proteins were evaluated. One of the most significantly decreased proteins identified in this screen was the myelin-associated glycoprotein MAG, which exhibited almost 70% lower expression in DKO vs. WT brain tissue (Table 1). Given this striking difference, in addition to the fact that GPR37 is found at its highest levels in oligodendrocytes (Cahoy et al., 2008; Imai et al., 2001; Yang et al., 2016), we focused further analyses of the proteomic data on other oligodendroglia-expressed proteins. However, none of the other oligodendroglia-expressed proteins assessed were found at significantly different levels between WT and DKO mice (Table 1).
Table 1.
Differentially expressed oligodendrocyte and myelin-associated proteins in brain of mice lacking GPR37 and GPR37L1
| Protein | P value | Percent Change DKO/WT |
Significant (p<0.01) |
|---|---|---|---|
| Myelin-associated glycoprotein (MAG) | 0.0004 | −69% | Yes |
| Myelin proteolipid protein (PLP) | 0.072 | 21% | No |
| 2,3-cyclic-nucleotide 3-phosphodiesterase (CNPase) | 0.53 | 6% | No |
| Glutathione S-transferase P1 (GSTπ) | 0.037 | 7% | No |
| Myelin basic protein (MBP) | 0.17 | 24% | No |
| Myelin-associated oligodendrocyte basic protein (MOBP) | 0.73 | 8% | No |
| Myelin-oligodendrocyte glycoprotein (MOG) | 0.99 | 0% | No |
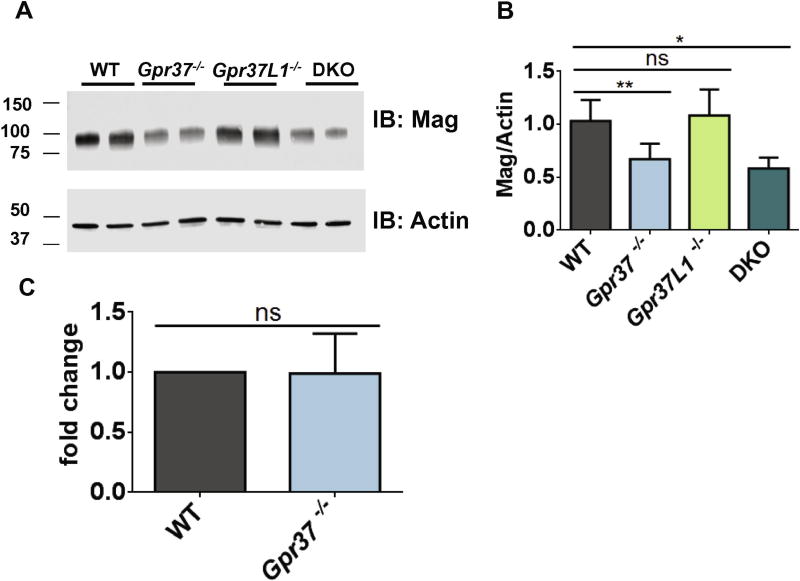
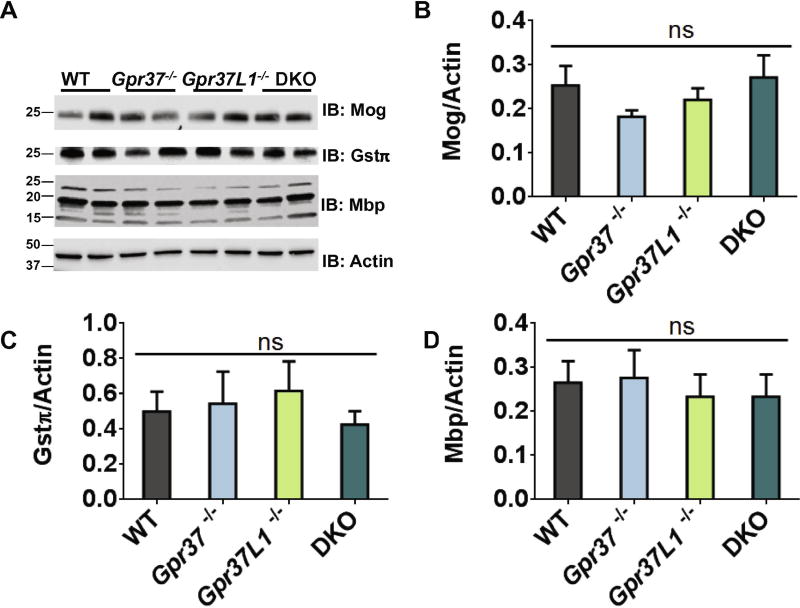
Western blot studies were performed next to provide a second independent measure of oligodendroglia-expressed proteins in DKO brain tissue. Moreover, brain tissue samples from Gpr37-knockout (Gpr37−/−) and Gpr37L1-knockout (Gpr37L1−/−) mice were also included in these studies to determine whether one receptor or the other might be more responsible for any observed alterations in the levels of oligodendroglia- expressed proteins. As in the proteomic analyses, MAG expression was found in the Western blot studies to be significantly decreased in DKO mice compared to WT (Figure 1A–B). Expression of MAG was decreased to a similar extent in brain tissue from Gpr37−/− mice. In contrast, MAG levels in brain tissue from Gpr37L1−/− mice were not significantly different from WT. Quantitative PCR of whole RNA extracted from WT and Gpr37−/− mouse brains revealed no difference in Mag mRNA expression (Figure 1C). These results indicate that GPR37 regulates expression of MAG at the protein level but not at the level of mRNA. Expression levels of a handful of other oligodendroglia-expressed proteins (GSTπ MOG, and MBP) were also assessed via Western blot and found to not be significantly different between WT, Gpr37−/−, Gpr37L1−/− or DKO brain tissue (Figure 2).
Figure 1. Expression of MAG protein, but not MAG mRNA, is decreased with loss of GPR37.
Representative Western blot images (A) and quantification (B) for total MAG protein in whole brain tissue samples from WT and Gpr37−/− mice showed significantly decreased MAG protein expression in Gpr37−/− and DKO mice, but not Gpr37L1−/− mice (n=8 per genotype, data analyzed using one-way ANOVA *p<0.01, **p<0.001). Molecular mass markers (in kDa) are shown on the left side of the blots. C) Relative Mag mRNA expression was not significantly different between whole brain samples from WT and Gpr37−/− mice, as assessed via qPCR
Figure 2. Expression of other oligodendrocyte and myelin proteins is unaffected by loss of GPR37.
Representative Western blot images (A) and quantification of expression of the myelin- associated proteins Mbp (B), Mog (C), and Gstπ(D) in whole brain tissue samples from WT and Gpr37−/− mice revealed no significant differences in protein expression. All protein levels were normalized to actin expression (n=8 per genotype). Molecular mass markers (in kDa) are shown on the left side of each immunoblot (IB).
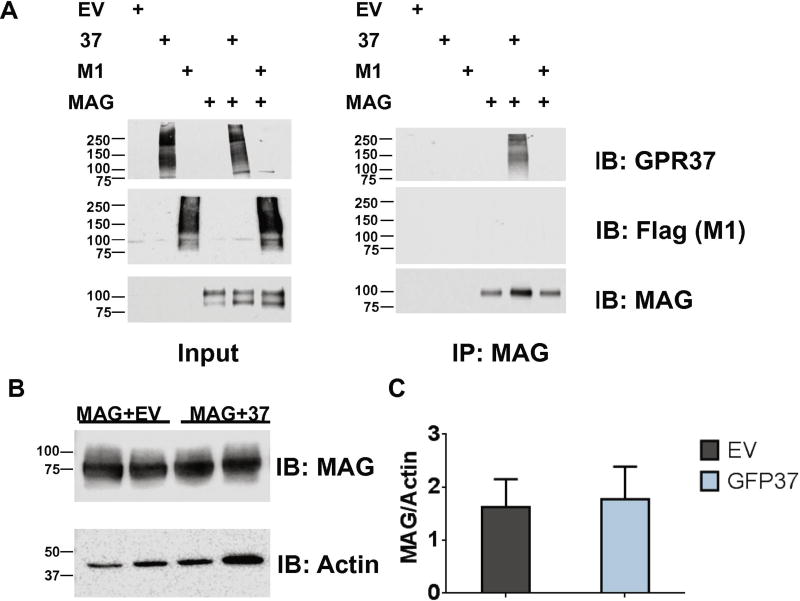
Given the specific regulation of MAG expression by GPR37 in brain tissue, we explored whether these two proteins might be found in complex together. Co-immunoprecipitation assays revealed that GPR37 robustly interacts with MAG in lysates derived from co-transfected HEK-293 cells (Figure 3A). The M1 muscarinic acetylcholine receptor (M1), another receptor known to be highly expressed in oligodendrocytes (Deshmukh et al., 2013), was also assessed for potential co- immunoprecipitation with MAG. However, no M1 co-immunoprecipitation with MAG was observed, suggesting a specific interaction between GPR37 and MAG rather than a general capacity of MAG to interact with GPCRs. We also assessed whether the effect of GPR37 on MAG expression in vivo could be recapitulated via co-expression of the two proteins in heterologous cells. However, transfection of MAG into HEK-293 cells in the absence and presence of GPR37 co-transfection resulted in no significant differences in MAG expression (Figure 3B–C). Thus, GPR37-mediated regulation of MAG expression in vivo may be dependent on cellular context or other factors not easily recapitulated in cultured cells.
Figure 3. GPR37 coimmunoprecipitates with MAG, but does not increase MAG expression upon co-transfection in vitro.
A) GFP-tagged GPR37, but not Flag-tagged M1 muscarinic acetylcholine receptor, coimmunoprecipitates with MAG. Molecular mass markers are shown (in kDa) on the left side of the blots. Both GFP-GPR37 and Flag-M1 exhibit higher-order oligomers in addition to bands corresponding to monomeric receptors. B-C) Cotransfection of GFP-tagged GPR37 with MAG did not increase MAG expression in HEK-293 cells. All protein levels were normalized to actin expression (n=7 per genotype). IP=immunoprecipitation, IB=immunoblot, GFP37=GFP-tagged GPR37, M1=Flag-tagged M1 muscarinic acetylcholine receptor, EV=Empty vector.
Loss of GPR37 in vivo increases susceptibility to demyelination
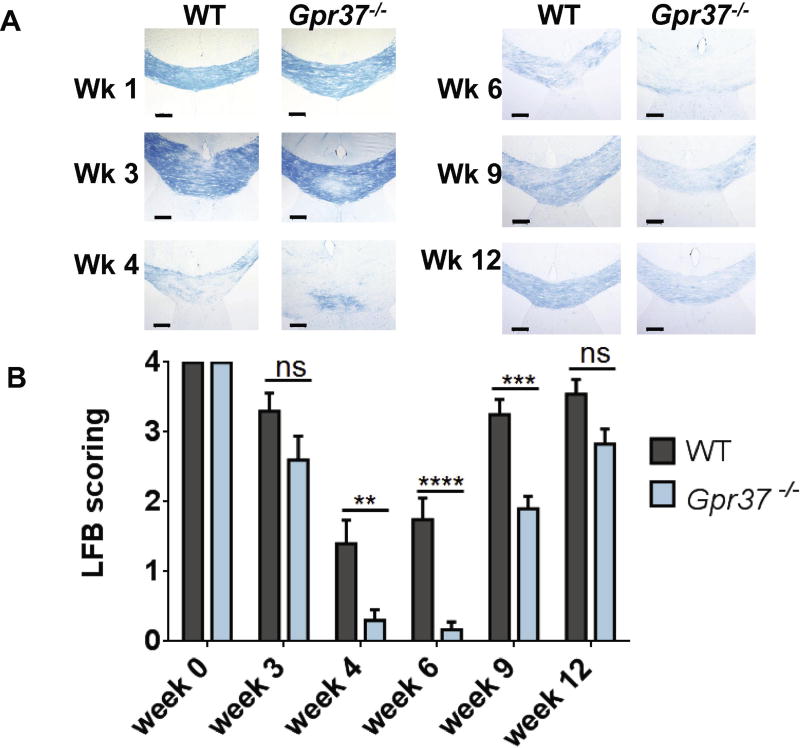
MAG is important in the development and maintenance of the myelin sheath (Li et al., 1998; Marcus et al., 2002). MAG-deficient mice exhibit a modest myelination phenotype (Li et al., 1994; Montag et al., 1994) and only exhibit more dramatic impairments following insults such as toxin treatment, aging or induced demyelination (Jones et al., 2013; Lassmann et al., 1997; Lopez et al., 2011; Weiss et al., 2000). Given the striking decrease in MAG expression that we observed in mice lacking GPR37, we assessed the susceptibility of these mice to demyelination induced by cuprizone. In these studies, Gpr37−/− vs. WT mice were fed chow with cuprizone for 6 weeks to induce demyelination, and then fed normal chow for 6 additional weeks to assess remyelination. Luxol fast blue (LFB) staining of myelin revealed a marked and progressive decrease in myelination for both WT and Gpr37−/− mice from week 0 to week 4 of cuprizone treatment (Figure 4). However, by week 6, the Gpr37−/− mice further progressed to nearly full demyelination, whereas demyelination in the WT mice was significantly less dramatic. Significant differences in myelination between WT and Gpr37−/− were exhibited at weeks 4, 6 and 9, with Gpr37−/− mice showing more extensive demyelination than WT animals in all cases. By week 12, remyelination had occurred in both WT and Gpr37−/− mice and no significant differences were observed between the two groups at the final time point examined (Figure 4).
Figure 4. Loss of GPR37 increases susceptibility to demyelination.
Representative images (A) and quantification (B) of luxol fast blue (LFB)-stained corpus callosum from cuprizone-treated mice. Gpr37−/− mice displayed significantly more demyelination than wild-type (WT) mice at weeks 4, 6, and 9 of cuprizone administration. LFB analysis of myelination was done by objective, blinded scoring on a scale of 0–4: 0=completely demyelinated, 1=25% myelinated, 2=50% myelinated, 3=75% myelinated, 4=100% myelinated. Data analyzed using two-way ANOVA with Sidak post-hoc analysis **p<0.01, ***p<0.001, ****p<0.0001
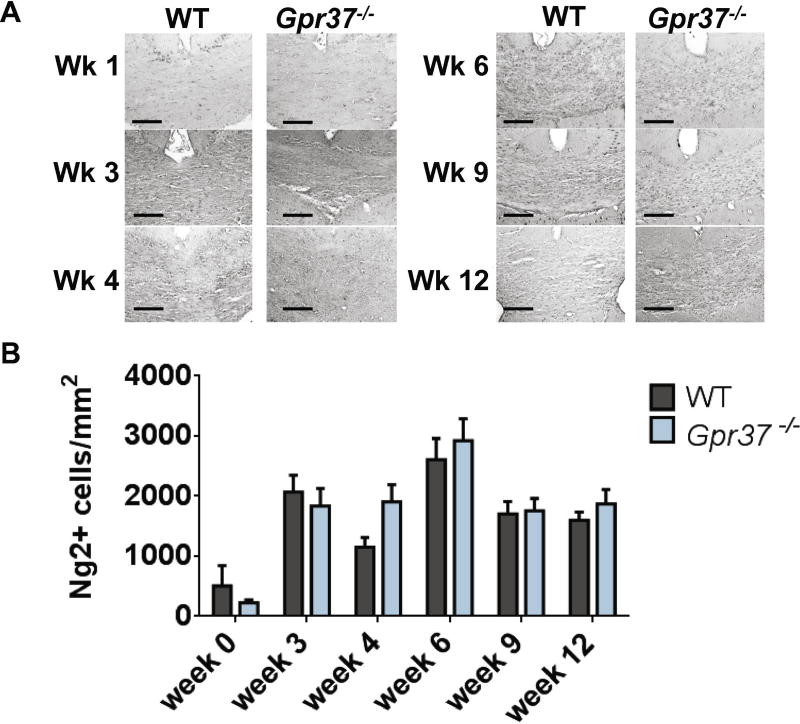
Differential responses to demyelinating insults can be accounted for by a variety of factors, including differences in oligodendrocyte survival, differences in OPC recruitment to damaged regions and/or differences in myelin stability (Franklin and Ffrench-Constant, 2008; Stangel and Trebst, 2006). To help distinguish between these possibilities, we evaluated whether Gpr37−/− mice displayed lesser recruitment of OPCs and/or greater loss of mature oligodendrocytes upon cuprizone-induced demyelination. During cuprizone administration, NG2-positive OPCs proliferate and migrate to sites of demyelination to replace lost oligodendrocytes (Matsushima and Morell, 2001; Stangel and Hartung, 2002). Indeed, analyses of NG2 immunostaining revealed substantial recruitment of OPCs in both WT and Gpr37−/− mice during cuprizone administration (Figure 5A). However, no significant differences in NG2 staining between WT and Gpr37−/− mice were observed at any time point, indicating that loss of GPR37 did not affect overall OPC number (Figure 5B).
Figure 5. Loss of GPR37 does not affect numbers of OPCs.
Representative images (A) and quantification (B) of NG2+ oligodendrocyte precursor cells (OPCs) in the corpus callosum of mice treated with cuprizone. No significant difference between wild-type (WT) and Gpr37−/− mice was observed at any time point.
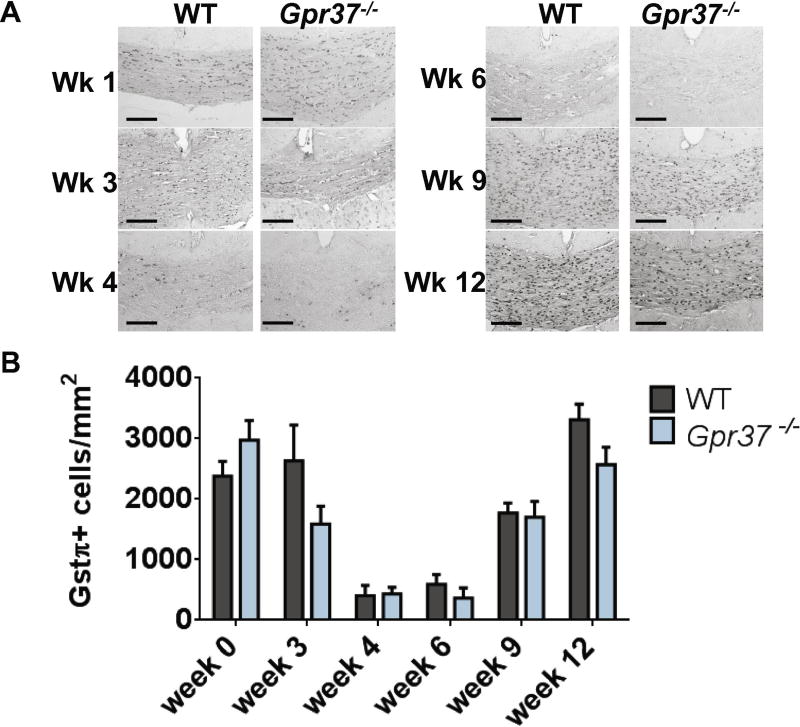
In parallel analyses, mature oligodendrocytes were assessed by immunohistochemical staining for GSTπ(Figure 6A). Staining for mature oligodendrocytes decreased from week 0 to week 6, as expected, as oligodendrocytes were lost to cuprizone-induced apoptosis. However, no significant differences in GSTπ staining were observed between WT and Gpr37−/− mice at any time point (Figure 6B). The lack of difference in either OPC or oligodendrocyte cell number between WT and Gpr37−/− mice during cuprizone-induced demyelination suggests that GPR37 specifically alters the process of myelination and/or the stability of myelin, rather than modulating OPC recruitment or exerting cytoprotective effects on mature oligodendrocytes.
Figure 6. Loss of GPR37 does not affect numbers of mature oligodendrocytes.
Representative images (A) and quantification (B) of Gstπ+ mature oligodendrocytes in the corpus callosum of mice treated with cuprizone. No significant difference between wild-type (WT) and Gpr37−/− mice was observed at any time point.
DISCUSSION
In this study, we describe regulation of MAG levels in vivo by GPR37 and enhanced susceptibility of Gpr37−/− mice to cuprizone-induced demyelination. GPR37 is an orphan GPCR that has not previously been associated with demyelinating disorders. This receptor, which is also known as the “parkin-associated endothelin receptor-like receptor” (Pael-R), is expressed predominantly in the brain (Donohue et al., 1998; Leng et al., 1999; Marazziti et al., 1998; Valdenaire et al., 1998; Zeng et al., 1997). Several peptides have been reported to bind to GPR37, including head activator peptide (Gandia et al., 2013; Rezgaoui et al., 2006) and prosaptide (Lundius et al., 2014; Meyer et al., 2013), but activation of GPR37 signaling by these peptides is modest at best and not observed at significant levels in all studies (Dunham et al., 2009; Coleman et al., 2016), and thus GPR37 is still considered an orphan receptor. Most of the focus of GPR37 research over the past twenty years has been on the potential role of GPR37 in Parkinson’s disease (Cahoy et al., 2008; Imai et al., 2002; Imai et al., 2001; Lundius et al., 2013; Murakami et al., 2004; Omura et al., 2006; Yang et al., 2003) and regulation of the dopamine system (Imai et al., 2007; Marazziti et al., 2011; Marazziti et al., 2004; Marazziti et al., 2007). In the absence of parkin, GPR37 is prone to aggregation and misfolding, resulting in cytotoxicity to dopaminergic neurons (Imai et al., 2002; Imai et al., 2001).
Although most work to date on GPR37 has focused on the receptor’s role in certain neuronal populations, such as dopaminergic neurons, the highest expression levels of GPR37 are observed in mature oligodendrocytes (Cahoy et al., 2008; Imai et al., 2001; Yang et al., 2016). Indeed, a recent report presented evidence that GPR37 is a negative regulator of myelination during development (Yang et al., 2016), but this report did not assess the effect of GPR37 on responses to demyelinating insults. In the present study, we observed that loss of GPR37 results in a striking increase in the extent of demyelination following cuprizone treatment. Additionally, we observed that overall numbers of OPCs and mature oligodendrocytes were unaffected by loss of GPR37 at all stages of cuprizone-induced demyelination. Furthermore, the fact that no statistically significant differences in number of OPCs or mature oligodendrocytes between WT and Gpr37−/− mice were observed during cuprizone-induced demyelination suggests that GPR37 may play a role in myelin repair distinct from the role it plays in primary myelination, similar to how distinct roles in primary myelination vs. remyelination have been identified for the p38αmitogen-activated protein kinase (MAPK) (Chung et al., 2015). These findings suggest that GPR37 regulates the stability of myelin or resistance of myelin itself to demyelination, rather than regulating OPC recruitment or behaving in a cytoprotective fashion in mature oligodendrocytes.
In addition to describing an effect of GPR37 in vivo on demyelination, we also report here that loss of GPR37 expression sharply reduces MAG expression in vivo. MAG is known to promote the stability of myelinated axons (Li et al., 1998; Marcus et al., 2002), and loss of MAG in mouse models results in a modest myelination phenotype (Li et al. 1994, Montag et al., 1994) unless the mice are challenged with insults such as toxin treatment, aging, or induced demyelination (Jones et al 2013, Lassman et al 1997, Lopez et al 2011, Weiss et al 2000). However, the mechanisms through which MAG mediates these effects are incompletely understood. Previous work has identified several trans-interacting partners of MAG localized to axons (Domeniconi et al., 2002; Liu et al., 2002; McKerracher and Rosen, 2015) and cis-interacting partners in oligodendrocytes (Umemori et al., 1994). The roles of the previously-identified interacting partners of MAG point to possible functions such as stabilizing MAG dimers to regulate the spacing between axons and the myelin sheath (Pronker et al., 2016) and localizing MAG to lipid rafts for reasons that remain unclear (Marta et al., 2004). Whether the interaction between GPR37 and MAG identified by the present study occurs in cis or in trans is currently unknown. Further work will be needed to resolve this issue and to address the question of whether the effect of loss of GPR37 on demyelination is connected to the decreased MAG levels observed upon loss of GPR37.
The mechanism by which GPR37 regulates MAG expression will also require further investigation. A previous report of a GPCR regulating MAG expression showed that co-transfection of MAG with the adhesion GPCR VLGR1 results in enhanced MAG levels, thereby recapitulating in cultured cells the MAG-stabilizing effect of VLGR1 observed in vivo (Shin et al., 2013). In contrast, no significant increases in MAG expression were observed in the present study upon co-transfection of MAG with GPR37 in HEK-293 cells in vitro. The regulation of MAG expression by GPR37 could possibly be dependent on cellular context and/or factors in vivo that are not present in vitro. We observed that MAG and GPR37 can associate in cells, but whether this interaction is related to the ability of GPR37 to regulate MAG stability remains uncertain at present. Future studies will be needed to dissect the structural determinants of the GPR37/MAG interaction and determine its functional significance.
CONCLUSIONS
Taken together, the data presented here reveal a novel role for GPR37 in influencing susceptibility to demyelinating insults. These findings also identify a decrease in expression of MAG, but not other oligodendrocyte-specific proteins, in mice lacking GPR37. GPCRs are common targets for therapeutic drugs (Santos et al., 2017), so the effects of GPR37 on demyelination and MAG levels in vivo reported here mark GPR37 as an intriguing potential drug target for the treatment of myelination disorders. Therefore, in addition to the future studies proposed above aimed at understanding the mechanism(s) by which GPR37 regulates MAG expression and demyelination, future work will also be needed to identify small molecule agonists and positive allosteric modulators capable of enhancing GPR37 activity. The results of the present study suggest that such compounds may have substantial therapeutic potential in the treatment of multiple sclerosis and other demyelination disorders.
Highlights.
Myelin-associated glycoprotein expression is lower in brains of mice lacking GPR37.
Loss of GPR37 results in increased susceptibility to demyelination.
GPR37 is identified as a target for understanding and treating demyelination.
Acknowledgments
We would like to thank Nick Seyfried and the staff of the Emory Integrated Proteomics Core, which is subsidized by the Emory University School of Medicine and is one of the Emory Integrated Core Facilities. We also thank Yue Feng (Emory), Yoland Smith (Emory), Ted Spack (Fast Forward), and Mike Webb (EMD Serono) for helpful advice and discussion during the course of these studies. This work was funded by the National Multiple Sclerosis Society Fast Forward Program and grant R01-NS088413 from the National Institutes of Health to RAH. Research reported in this publication was also supported in part by the Emory Neuroscience NINDS Core Facilities and NIH/NINDS under award number P30NS055077.
Abbreviations
- OPC
oligodendrocyte precursor cell
- GPR37L1
G protein-coupled receptor 37 like-1
- WT
wild-type
- Gpr37−/−
Gpr37 knockout
- Gpr37L1−/−
Gpr37L1 knockout
- DKO
Gpr37/Gpr37L1 double knockout
- M1
muscarinic acetylcholine receptor M1
- LFB
luxol fast blue
- MS
Mass Spectrometry
- MOG
myelin-oligodendrocyte glycoprotein
- MBP
myelin basic protein
- GSTπ
glutathione-s-transferase π
- VLGR1
very large G protein-coupled receptor
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen-activated protein kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:264–78. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wu H, Wang S, Koito H, Li J, Ye F, Hoang J, Escobar SS, Gow A, Arnett HA, Trapp BD, Karandikar NJ, Hsieh J, Lu QR. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat Neurosci. 2009;12:1398–406. doi: 10.1038/nn.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SH, Biswas S, Selvaraj V, Liu XB, Sohn J, Jiang P, Chen C, Chmilewsky F, Marzban H, Horiuchi M, Pleasure DE, Deng W. The p38alpha mitogen-activated protein kinase is a key regulator of myelination and remyelination in the CNS. Cell Death Dis. 2015;6:e1748. doi: 10.1038/cddis.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh VA, Tardif V, Lyssiotis CA, Green CC, Kerman B, Kim HJ, Padmanabhan K, Swoboda JG, Ahmad I, Kondo T, Gage FH, Theofilopoulos AN, Lawson BR, Schultz PG, Lairson LL. A regenerative approach to the treatment of multiple sclerosis. Nature. 2013;502:327–32. doi: 10.1038/nature12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, He Z, Filbin M. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–90. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- Donohue PJ, Shapira H, Mantey SA, Hampton LL, Jensen RT, Battey JF. A human gene encodes a putative G protein-coupled receptor highly expressed in the central nervous system. Brain Res Mol Brain Res. 1998;54:152–60. doi: 10.1016/s0169-328x(97)00336-7. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–55. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Gandia J, Fernandez-Duenas V, Morato X, Caltabiano G, Gonzalez-Muniz R, Pardo L, Stagljar I, Ciruela F. The Parkinson’s disease-associated GPR37 receptor-mediated cytotoxicity is controlled by its intracellular cysteine- rich domain. J Neurochem. 2013;125:362–72. doi: 10.1111/jnc.12196. [DOI] [PubMed] [Google Scholar]
- Heinen A, Beyer F, Tzekova N, Hartung HP, Kury P. Fingolimod induces the transition to a nerve regeneration promoting Schwann cell phenotype. Exp Neurol. 2015;271:25–35. doi: 10.1016/j.expneurol.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Imai Y, Inoue H, Kataoka A, Hua-Qin W, Masuda M, Ikeda T, Tsukita K, Soda M, Kodama T, Fuwa T, Honda Y, Kaneko S, Matsumoto S, Wakamatsu K, Ito S, Miura M, Aosaki T, Itohara S, Takahashi R. Pael receptor is involved in dopamine metabolism in the nigrostriatal system. Neurosci Res. 2007;59:413–25. doi: 10.1016/j.neures.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Imai Y, Soda M, Hatakeyama S, Akagi T, Hashikawa T, Nakayama KI, Takahashi R. CHIP is associated with Parkin, a gene responsible for familial Parkinson’s disease, and enhances its ubiquitin ligase activity. Mol Cell. 2002;10:55–67. doi: 10.1016/s1097-2765(02)00583-x. [DOI] [PubMed] [Google Scholar]
- Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell. 2001;105:891–902. doi: 10.1016/s0092-8674(01)00407-x. [DOI] [PubMed] [Google Scholar]
- Jones MV, Nguyen TT, Ewaleifoh O, Lebson L, Whartenby KA, Griffin JW, Calabresi PA. Accelerated axon loss in MOG35-55 experimental autoimmune encephalomyelitis (EAE) in myelin-associated glycoprotein-deficient (MAGKO) mice. J Neuroimmunol. 2013;262:53–61. doi: 10.1016/j.jneuroim.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Bartsch U, Montag D, Schachner M. Dying-back oligodendrogliopathy: a late sequel of myelin-associated glycoprotein deficiency. Glia. 1997;19:104–10. [PubMed] [Google Scholar]
- Leng N, Gu G, Simerly RB, Spindel ER. Molecular cloning and characterization of two putative G protein-coupled receptors which are highly expressed in the central nervous system. Brain Res Mol Brain Res. 1999;69:73–83. doi: 10.1016/s0169-328x(99)00092-3. [DOI] [PubMed] [Google Scholar]
- Li C, Trapp B, Ludwin S, Peterson A, Roder J. Myelin associated glycoprotein modulates glia-axon contact in vivo. J Neurosci Res. 1998;51:210–7. doi: 10.1002/(SICI)1097-4547(19980115)51:2<210::AID-JNR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Li C, Tropak MB, Gerlai R, Clapoff S, Abramow-Newerly W, Trapp B, Peterson A, Roder J. Myelination in the absence of myelin-associated glycoprotein. Nature. 1994;369:747–50. doi: 10.1038/369747a0. [DOI] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–3. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- Lopez PH, Ahmad AS, Mehta NR, Toner M, Rowland EA, Zhang J, Dore S, Schnaar RL. Myelin-associated glycoprotein protects neurons from excitotoxicity. J Neurochem. 2011;116:900–8. doi: 10.1111/j.1471-4159.2010.07069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundius EG, Stroth N, Vukojevic V, Terenius L, Svenningsson P. Functional GPR37 trafficking protects against toxicity induced by 6-OHDA, MPP+ or rotenone in a catecholaminergic cell line. J Neurochem. 2013;124:410–7. doi: 10.1111/jnc.12081. [DOI] [PubMed] [Google Scholar]
- Lundius EG, Vukojevic V, Hertz E, Stroth N, Cederlund A, Hiraiwa M, Terenius L, Svenningsson P. GPR37 protein trafficking to the plasma membrane regulated by prosaposin and GM1 gangliosides promotes cell viability. J Biol Chem. 2014;289:4660–73. doi: 10.1074/jbc.M113.510883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Di Pietro C, Golini E, Mandillo S, La Sala G, Matteoni R, Tocchini- Valentini GP. Precocious cerebellum development and improved motor functions in mice lacking the astrocyte cilium-, patched 1-associated Gpr37l1 receptor. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16486–91. doi: 10.1073/pnas.1314819110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Di Pietro C, Mandillo S, Golini E, Matteoni R, Tocchini-Valentini GP. Absence of the GPR37/PAEL receptor impairs striatal Akt and ERK2 phosphorylation, DeltaFosB expression, and conditioned place preference to amphetamine and cocaine. FASEB J. 2011;25:2071–81. doi: 10.1096/fj.10-175737. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Gallo A, Golini E, Matteoni R, Tocchini-Valentini GP. Molecular cloning and chromosomal localization of the mouse Gpr37 gene encoding an orphan G-protein-coupled peptide receptor expressed in brain and testis. Genomics. 1998;53:315–24. doi: 10.1006/geno.1998.5433. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Golini E, Gallo A, Lombardi MS, Matteoni R, Tocchini-Valentini GP. Cloning of GPR37, a gene located on chromosome 7 encoding a putative G-protein-coupled peptide receptor, from a human frontal brain EST library. Genomics. 1997;45:68–77. doi: 10.1006/geno.1997.4900. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Golini E, Mandillo S, Magrelli A, Witke W, Matteoni R, Tocchini- Valentini GP. Altered dopamine signaling and MPTP resistance in mice lacking the Parkinson’s disease-associated GPR37/parkin-associated endothelin- like receptor. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10189–94. doi: 10.1073/pnas.0403661101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Mandillo S, Di Pietro C, Golini E, Matteoni R, Tocchini-Valentini GP. GPR37 associates with the dopamine transporter to modulate dopamine uptake and behavioral responses to dopaminergic drugs. Proc Natl Acad Sci U S A. 2007;104:9846–51. doi: 10.1073/pnas.0703368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus J, Dupree JL, Popko B. Myelin-associated glycoprotein and myelin galactolipids stabilize developing axo-glial interactions. J Cell Biol. 2002;156:567–77. doi: 10.1083/jcb.200111047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marta CB, Taylor CM, Cheng S, Quarles RH, Bansal R, Pfeiffer SE. Myelin associated glycoprotein cross-linking triggers its partitioning into lipid rafts, specific signaling events and cytoskeletal rearrangements in oligodendrocytes. Neuron Glia Biol. 2004;1:35–46. doi: 10.1017/s1740925x04000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain pathology. 2001;11:107–16. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, Rosen KM. MAG, myelin and overcoming growth inhibition in the CNS. Front Mol Neurosci. 2015;8:51. doi: 10.3389/fnmol.2015.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RC, Giddens MM, Schaefer SA, Hall RA. GPR37 and GPR37L1 are receptors for the neuroprotective and glioprotective factors prosaptide and prosaposin. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9529–34. doi: 10.1073/pnas.1219004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogha A, Benesh AE, Patra C, Engel FB, Schoneberg T, Liebscher I, Monk KR. Gpr126 functions in Schwann cells to control differentiation and myelination via G-protein activation. J Neurosci. 2013;33:17976–85. doi: 10.1523/JNEUROSCI.1809-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag D, Giese KP, Bartsch U, Martini R, Lang Y, Bluthmann H, Karthigasan J, Kirschner DA, Wintergerst ES, Nave KA, et al. Mice deficient for the myelin-associated glycoprotein show subtle abnormalities in myelin. Neuron. 1994;13:229–46. doi: 10.1016/0896-6273(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Murakami T, Shoji M, Imai Y, Inoue H, Kawarabayashi T, Matsubara E, Harigaya Y, Sasaki A, Takahashi R, Abe K. Pael-R is accumulated in Lewy bodies of Parkinson’s disease. Ann Neurol. 2004;55:439–42. doi: 10.1002/ana.20064. [DOI] [PubMed] [Google Scholar]
- Omura T, Kaneko M, Okuma Y, Orba Y, Nagashima K, Takahashi R, Fujitani N, Matsumura S, Hata A, Kubota K, Murahashi K, Uehara T, Nomura Y. A ubiquitin ligase HRD1 promotes the degradation of Pael receptor, a substrate of Parkin. J Neurochem. 2006;99:1456–69. doi: 10.1111/j.1471-4159.2006.04155.x. [DOI] [PubMed] [Google Scholar]
- Pronker MF, Lemstra S, Snijder J, Heck AJ, Thies-Weesie DM, Pasterkamp RJ, Janssen BJ. Structural basis of myelin-associated glycoprotein adhesion and signalling. Nat Commun. 2016;7:13584. doi: 10.1038/ncomms13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezgaoui M, Susens U, Ignatov A, Gelderblom M, Glassmeier G, Franke I, Urny J, Imai Y, Takahashi R, Schaller HC. The neuropeptide head activator is a high-affinity ligand for the orphan G-protein-coupled receptor GPR37. J Cell Sci. 2006;119:542–9. doi: 10.1242/jcs.02766. [DOI] [PubMed] [Google Scholar]
- Shin D, Lin ST, Fu YH, Ptacek LJ. Very large G protein-coupled receptor 1 regulates myelin-associated glycoprotein via Galphas/Galphaq-mediated protein kinases A/C. Proc Natl Acad Sci U S A. 2013;110:19101–6. doi: 10.1073/pnas.1318501110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon K, Hennen S, Merten N, Blattermann S, Gillard M, Kostenis E, Gomeza J. The Orphan G Protein-coupled Receptor GPR17 Negatively Regulates Oligodendrocyte Differentiation via Galphai/o and Its Downstream Effector Molecules. J Biol Chem. 2016;291:705–18. doi: 10.1074/jbc.M115.683953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NJ. Drug Discovery Opportunities at the Endothelin B Receptor-Related Orphan G Protein-Coupled Receptors, GPR37 and GPR37L1. Front Pharmacol. 2015;6:275. doi: 10.3389/fphar.2015.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangel M, Hartung HP. Remyelinating strategies for the treatment of multiple sclerosis. Prog Neurobiol. 2002;68:361–76. doi: 10.1016/s0301-0082(02)00105-3. [DOI] [PubMed] [Google Scholar]
- Stangel M, Trebst C. Remyelination strategies: new advancements toward a regenerative treatment in multiple sclerosis. Curr Neurol Neurosci Rep. 2006;6:229–35. doi: 10.1007/s11910-006-0010-2. [DOI] [PubMed] [Google Scholar]
- Umemori H, Sato S, Yagi T, Aizawa S, Yamamoto T. Initial events of myelination involve Fyn tyrosine kinase signalling. Nature. 1994;367:572–6. doi: 10.1038/367572a0. [DOI] [PubMed] [Google Scholar]
- Valdenaire O, Giller T, Breu V, Ardati A, Schweizer A, Richards JG. A new family of orphan G protein-coupled receptors predominantly expressed in the brain. FEBS Lett. 1998;424:193–6. doi: 10.1016/s0014-5793(98)00170-7. [DOI] [PubMed] [Google Scholar]
- Weiss MD, Hammer J, Quarles RH. Oligodendrocytes in aging mice lacking myelin-associated glycoprotein are dystrophic but not apoptotic. J Neurosci Res. 2000;62:772–80. doi: 10.1002/1097-4547(20001215)62:6<772::AID-JNR3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Yang HJ, Vainshtein A, Maik-Rachline G, Peles E. G protein-coupled receptor 37 is a negative regulator of oligodendrocyte differentiation and myelination. Nat Commun. 2016;7:10884. doi: 10.1038/ncomms10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Nishimura I, Imai Y, Takahashi R, Lu B. Parkin suppresses dopaminergic neuron-selective neurotoxicity induced by Pael-R in Drosophila. Neuron. 2003;37:911–24. doi: 10.1016/s0896-6273(03)00143-0. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Su K, Kyaw H, Li Y. A novel endothelin receptor type-B-like gene enriched in the brain. Biochem Biophys Res Commun. 1997;233:559–67. doi: 10.1006/bbrc.1997.6408. [DOI] [PubMed] [Google Scholar]