Abstract
There has been significant attention to the growing recognition that oocytes have a critical capacity to organize and govern surrounding somatic cells. Bone morphogenetic protein 15 (BMP-15) is an oocyte-secreted factor that has raised particular interest due to its established role in determining ovulation quota and female fertility in mammals. As a first step in determining whether there are species-specific differences in the BMP-15 system that may play causal roles in the differences in ovulation quota observed in different mammalian species, we here compare the molecular characteristics of BMP-15 of polyovulatory mice with that of monoovulatory humans. We found that, although human BMP-15 mature protein is readily produced, there are defects in the production of mouse BMP-15 mature protein in an in vitro system of transfected cells. The generation of chimeric constructs consisting of different combinations of mouse and human BMP-15 proregions, cleavage sites, and mature regions indicates that the defects in the production of mouse BMP-15 mature protein depend on the presence of the mouse BMP-15 proregion. The mouse proregion also caused a significant reduction in the production of human BMP-15 mature protein. The coexpression with a convertase cleavage enzyme, furin, results in complete processing of all these chimeras; however, no mouse mature protein is detected in either secreted or cell-confined forms except when associated with the human proregion. Based on the biological role of BMP-15, defects in the production of mouse BMP-15 mature protein could correlate with the high ovulation quota and litter size observed in mice.
Keywords: ovary, oocyte, folliculogenesis, TGF-β superfamily, pregnancy
Arapidly growing body of evidence points to the oocyte as a predominant driving force in the development, organization, and function of the somatic cells of ovarian follicles (1–3). Thus, a comprehensive understanding of the factors secreted by oocytes that have the capacity to influence the functions of granulosa cells is likely to provide tremendous insight into some of the regulatory aspects of female reproduction that have remained elusive, such as dominant follicle selection and the regulation of ovulation quota.
Of the factors secreted from oocytes, bone morphogenetic protein 15 (BMP-15) and growth and differentiation factor 9 (GDF-9) are a focus of research in reproductive biology because of their central role in the control of female fertility (4–10). In particular, there is compelling evidence that BMP-15 is a critical determinant of ovulation quota (11). Sheep that are heterozygous carriers of various point mutations in the bmp15 gene exhibit increased litter sizes due to precocious follicle development, which leads to an increase in ovulation quota (9, 10). By comparison, sheep that are homozygous carriers of bmp15 mutations are infertile because of follicle arrests at the primary stage.
Previous studies in our laboratory intending to elucidate the molecular mechanisms underlying the effects of the BMP-15 mutations revealed that the introduction of the sheep bmp15 mutations to human recombinant BMP-15 did not affect the biological activity of the mature BMP-15 proteins but, rather, acted to impair the posttranslational processing of the proproteins and the secretion of the mature proteins (12, 13). Interestingly, the effects of the BMP-15 mutations were realized only when the mutant BMP-15 proteins were coexpressed with GDF-9. These data introduced the concept that aberrant processing of BMP-15 proproteins may have critical ramifications for female fertility and provided direct molecular evidence that the intracellular interaction between BMP-15 and GDF-9 is also a critical aspect of female reproductive physiology.
Crucial roles of BMP-15 in female fertility have also been demonstrated in humans. Specifically, Di Pasquale et al. (14) identified a BMP-15 mutation in humans that is associated with hypergonadotropic ovarian failure due to ovarian dysgenesis. The mutation is an A–G transition at position 704 of the bmp15 gene and results in a nonconservative replacement of a tyrosine with a cysteine at amino acid residue 235 of the proregion of the BMP-15 proprotein. The patients with the BMP-15 mutation were sisters who inherited the mutation from their father. Interestingly, both patients had streak ovaries, a characteristic phenotype observed in the homozygous, but not heterozygous, BMP-15 mutant ewes.
In contrast to the pronounced effects on folliculogenesis that the BMP-15 mutations cause in sheep and humans, mice that are homozygous-deficient in BMP-15 because of targeted deletions of the second exon of the bmp15 gene (BMP-15-KO mice) exhibit minimal defects in follicle growth and development, and there are no observed aberrancies in the phenotype of heterozygous BMP-15-KO mice (6).
Among female mammals, there are two major reproductive strategies: one is to ovulate relatively large numbers of oocytes and have large litters, and the other is to restrict the number of follicles that become competent to ovulate, resulting in singleton or twin gestations (15). Currently, very little is known about the species-specific physiological regulatory systems that determine the differences in ovulation quota. Elucidation of the molecular, cellular, and endocrine mechanisms underlying the species-specific determination of ovulation quota is an area of fundamental importance in mammalian reproduction and would provide a basis for understanding some of the still-elusive aspects of human fertility.
The ability of BMP-15 to regulate determinative developmental events in folliculogenesis, together with the species-specific discrepancies in the defects caused by mutations in the bmp15 gene, has led to the hypothesis that BMP-15 could be a key factor in determining the specific ovulation quota observed in different species (4, 6, 8, 11, 16–18). As a first step in addressing this question, we investigate here the specific differences in the proteins produced by the bmp15 gene from a polyovulatory species, the mouse, and a monoovulatory species, the human. BMP-15, like other members of the TGF type β (TGF-β) superfamily, is produced as a preproprotein that must undergo specific processing and proteolytic cleavage before the secretion of the bioactive mature form of the molecule (19–21). Our recent data have shown that the point mutations in BMP-15 that cause aberrancies in the fertility of ewes are manifested through defects in the processing and secretion of the mature form of the protein, rather than through affecting the biological activity of the proteins (12, 13). Accordingly, in the present study, we have focused on elucidating molecular and biochemical differences between mouse and human BMP-15 proteins.
Materials and Methods
Reagents and Supplies. FBS, penicillin, streptomycin, and l-glutamine were purchased from Invitrogen. The anti-FLAG M2 monoclonal antibody was purchased from Sigma–Aldrich. All restriction enzymes were purchased from New England Biolabs. The transfection reagent FuGENE 6 was from Roche Molecular Biochemicals.
Construction of Expression Plasmids. The described plasmid phBMP-15F (21) was used in this study. To generate mouse BMP-15, full-length mouse BMP-15 cDNA was amplified by reverse transcription–PCR from mouse ovarian RNA and cloned in-frame at the upstream site of the FLAG epitope tag present in pCMV-Tag4A (Stratagene). The resultant plasmid was designated pmBMP-15F.
In addition, using site-directed mutagenesis and subcloning procedures as described (12, 13), we generated plasmids designed to express chimeric constructs consisting of various combinations of human and mouse BMP-15 proregions, cleavage sites, and mature regions. Briefly, a chimeric construct of mouse BMP-15 with the human cleavage site was generated by mutating the mouse cleavage site (RSVR) of pmBMP-15F to that of human BMP-15 (RRTR). This plasmid was designated pmhmBMP-15F. To produce a chimera consisting of the mouse BMP-15 mature region with the human proregion and cleavage site, we replaced the proregion of the pmhmBMP-15F plasmid with the human proregion. This plasmid was designated phhmBMP-15F. Likewise, the plasmid pmhhBMP-15F was constructed by replacing the propregion of phBMP-15F with the mouse proregion from the pmBMP-15F plasmid.
Production and Analysis of Recombinant Proteins. 293T cells (a human embryonic kidney cell line) or CHO cells (a Chinese hamster ovary cell line) were transiently transfected by using FuGENE 6. Twenty-four hours after transfection, the culture media were replaced with serum-free media, and the cells were cultured for 4 days before harvesting the conditioned media for protein analysis. When indicated, conditioned media were harvested after a 1-day culture in serum-free media. 293T cells were also cotransfected with each of the various constructs of the BMP-15 expression plasmid and a furin expression plasmid where indicated. Western immunoblotting analysis was performed as described (13, 21) under reducing conditions by using the anti-FLAG antibody.
For chemical crosslinking, a noncleavable crosslinker, bis-sulfosuccinimidyl suberate (BS3) (Pierce), was added to conditioned media containing BMP-15 at a final concentration of 1–1.5 mg/ml as described (12, 13). To evaluate N-linked glycosylation of proteins, conditioned media (15 μl) from cells transfected with the indicated plasmids were precipitated with 120 μl of acetone at –20°C and treated with PNGase F (New England Biolabs) according to the manufacturer's instructions. All data presented are representative examples of three runs of the whole experiment from the point of the transient transfection step.
Results
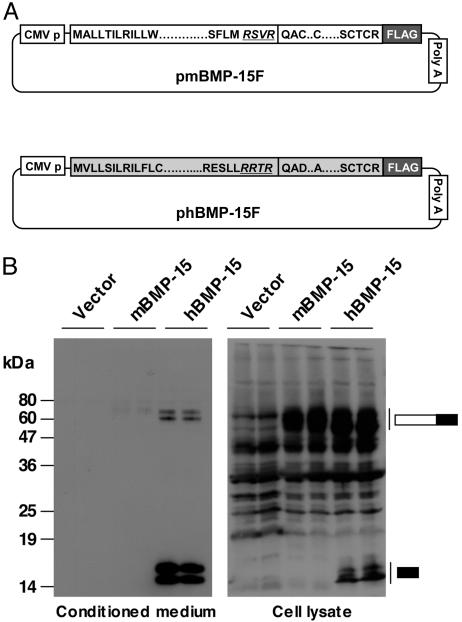
We began with a comparison of the production, processing, and secretion of mouse and human BMP-15 proteins, using transiently transfected 293T cells with expression plasmids encoding the cDNA of these genes tagged on the carboxyl terminus with the FLAG epitope (Fig. 1A). As expected (12, 13, 21), human BMP-15 was readily detectable by Western immunoblotting in the conditioned media of transfected cells (Fig. 1B). In contrast, despite numerous efforts using various transfection conditions, we repeatedly failed to detect mouse BMP-15 in the conditioned media from cells transfected with the mouse BMP-15 expression vector. To ensure that the mouse BMP-15 plasmid was successfully transfected and that the BMP-15 protein was transcribed and translated, we tested the lysates of transfected cells for FLAG immunoreactivity. Indeed, the mouse BMP-15 proprotein was detectable in cells transfected with the pmBMP-15F plasmid at expression levels that were similar to the levels of human BMP-15 proprotein inside cells transfected with the phBMP-15F plasmid (Fig. 1B). This finding suggests that the posttranslational processing of the mouse BMP-15 proprotein may be defective.
Fig. 1.
Expression of mouse and human BMP-15 by 293T cells. (A) Schematic drawing of mouse and human BMP-15 expression plasmids. (B) Conditioned media (Left) and cell lysates (Right) were from 293T cells transiently transfected with mouse or human BMP-15 expression plasmids. The samples were subjected to SDS/PAGE immunoblotting analysis by using anti-FLAG antibody. The migration of BMP-15 proteins is indicated by the bars to the right. The filled portions of the bars indicate the BMP-15 mature region, and the open portions of the bars indicate the BMP-15 proregion. Vector, pCMV-tag 4A.
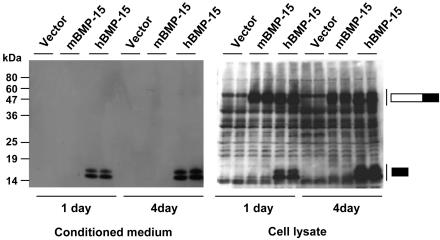
The defect in the processing of the mouse BMP-15 proprotein was not the result of culture time. In the cell lysates, the human BMP-15 mature protein and proprotein were present 1 day after transfection, and the levels of both human BMP-15 mature protein and proprotein were increased by 156% and 20%, respectively, after 4 days of culture (Fig. 2 Right). Under similar conditions, the BMP-15 mature protein levels were also increased by 83% in the conditioned medium (Fig. 2 Left). Therefore, the time course of human BMP-15 expression in 293T cells reflects continuous synthesis, processing, and secretion of proteins. In contrast, there was no observable change in the levels of either mouse BMP-15 mature protein or proprotein. Thus, it is likely that the mouse BMP-15 proprotein is targeted for degradation, rather than activation by proteolytic processing.
Fig. 2.
Time course of mouse and human BMP-15 expression by 293T cells. 293T cells were transiently transfected with the indicated expression plasmids. The conditioned media (Left) and cell lysates (Right) were analyzed after a 1- or 4-day culture by SDS/PAGE immunoblotting by using anti-FLAG antibody. The migration of BMP-15 proteins is indicated by the bars to the right. The filled portions of the bars indicate the BMP-15 mature region, and the open portions of the bars indicate the BMP-15 proregion. Vector, pCMV-tag 4A.
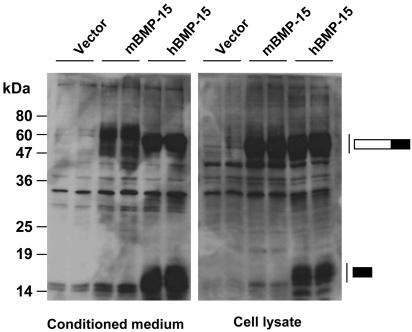
To assess whether the cell line contributes to the failure of mouse BMP-15 proprotein processing, we expressed mouse and human BMP-15 in CHO cells. As with human 293T cells, human BMP-15 mature protein and proprotein were present in conditioned media and cell lysates (Fig. 3). Regarding mouse BMP-15, expression of the proprotein, but not the mature protein, occurred in the CHO cells. Thus, the failure of mouse BMP-15 processing seemed unaffected by the expression system.
Fig. 3.
Expression of mouse and human BMP-15 by CHO cells. Conditioned media (Left) and cell lysates (Right) from CHO cells transiently transfected with mouse or human BMP-15 were subjected to SDS/PAGE immunoblotting analysis by using an anti-FLAG antibody. The migration of BMP-15 proteins is indicated by the bars to the right. The filled portions of the bars indicate the BMP-15 mature region, and the open portions of the bars indicate the BMP-15 proregion. Vector, pCMV-tag 4A.
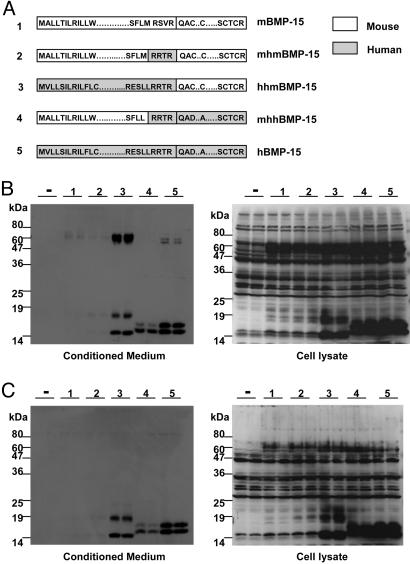
To identify the structural domain of the mouse or human BMP-15 responsible for the failure or success of BMP-15 proprotein processing, we generated a series of expression plasmids encoding chimeric proteins containing various combinations of mouse and human regions of the BMP-15 molecule (Fig. 4A). Because the proteolytic cleavage of proproteins depends on specific dibasic RXXR consensus cleavage sequences, we first investigated whether we could rescue the defective processing of mouse BMP-15 by replacing the cleavage sequence with that of human BMP-15 (Fig. 4A, chimera 2, mhmBMP-15). As shown in Fig. 4B, the human cleavage site was not sufficient for the successful cleavage of the mouse BMP-15 proprotein, and no mouse BMP-15 mature protein was detectable in either the conditioned media or the cell lysate of 293T cells expressing this chimera. Thus, the cleavage sequence is not responsible for the failure of mouse BMP-15 processing. Equal levels of the proprotein in the cells expressing mBMP-15 (Fig. 4A, chimera 1) and mhmBMP-15 indicate that the expression constructs were successfully transfected, transcribed, and translated (Fig. 4B Right, lanes 1 and 2).
Fig. 4.
Production of mouse and human chimeric BMP-15. (A) Schematic representation of the chimeric BMP-15 constructs containing combinations of the human and mouse preproregions, cleavage sites, and mature regions that were used in this study. (B) Conditioned media (Left) and cell lysates (Right) from 293T cells transiently transfected with chimeric BMP-15 constructs (indicated by numbers corresponding to the numbers of the chimeras in A) were subjected to SDS/PAGE immunoblotting analysis by using an anti-FLAG antibody. (C) 293T cells were transiently cotransfected with a furin expression plasmid together with chimeric BMP-15 constructs (indicated by numbers corresponding to the numbers of the chimeras in A). The conditioned media (Left) and cell lysates (Right) were then subjected to SDS/PAGE immunoblotting by using an anti-FLAG antibody. –, pCMV-tag 4A vector.
Interestingly, when the human BMP-15 proregion was fused to the mature region of mouse BMP-15 (Fig. 4A, chimera 3, hhmBMP-15), mouse BMP-15 mature protein accumulated in both the conditioned media and the cell lysate (Fig. 4B Left and Right, lane 3). Thus, the mouse BMP-15 proregion is a critical determinant in preventing the production of mouse BMP-15 mature protein. To determine whether the mouse proregion also prohibits the processing and secretion of human BMP-15 mature protein, we produced a chimera consisting of the mouse proregion fused to the mature region of human BMP-15 (Fig. 4A, chimera 4, mhhBMP-15). Cells expressing this construct were able to produce human BMP-15 mature protein, indicating that the mouse BMP-15 proregion does not preclude the production of human BMP-15 mature protein; however, when compared with hBMP-15 (Fig. 4A, chimera 5), it seems that the mouse BMP-15 proregion may cause a reduction in the processing of the proprotein (Fig. 4B Left, lanes 4 and 5).
To test whether overexpression of a proteolytic cleavage enzyme would enhance the processing of the mouse BMP-15 proprotein, we coexpressed furin, a prototype member of the subtilisin-like protein convertase family of cleavage enzymes (22), with wild-type and chimeric BMP-15 (Fig. 4C). Coexpression of human BMP-15 with furin resulted in a dramatic increase in the ratio of the mature protein to that of the proprotein, in particular of that in the cell lysate, demonstrating the ability of furin to cleave the human BMP-15 proprotein (compare lane 5 in Fig. 4 B and C Right). In contrast, coexpression of furin with mouse BMP-15 caused the BMP-15 proprotein to be reduced to nearly undetectable levels; however, there was still no detectable mature BMP-15 in the conditioned media or in cell lysates (lane 1 in Fig. 4 B and C Right).
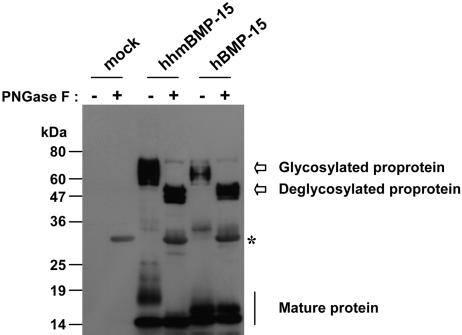
Because there have been, to our knowledge, no previous reports in which recombinant mouse mature BMP-15 was successfully produced, use of the hhmBMP-15 chimera provides the unique opportunity to characterize the mature form of mouse BMP-15 and to compare it with human BMP-15. A major discrepancy observed between mouse and human BMP-15 mature proteins is their migration pattern on SDS/PAGE gels. Both migrate as a doublet, but the human BMP-15 mature protein migrates at 16 and 17 kDa, whereas the mouse BMP-15 mature protein migrates at 15 and 18 kDa (Fig. 5). The 16-kDa band of human BMP-15 and the 15-kDa band of mouse BMP-15 are most likely unmodified monomeric forms of the mature protein. Although our previous studies (21) have demonstrated that the 17-kDa band of human BMP-15 does not represent an N- or O-linked glycosylated form of the molecule, the broad nature of the 18-kDa band of mouse BMP-15 is typical of N-linked glycosylated proteins. Consistent with this finding, there is an extra predicted N-linked glycosylation in the mature region of mouse BMP-15 that is not present in the human counterpart (20). To determine whether the 18-kDa band of mouse BMP-15 is indeed the N-glycosylated form of the protein, we treated mouse and human BMP-15 conditioned media with PNGase F. The PNGase F treatment had no effect on the bands of the human BMP-15 mature protein (Fig. 5), confirming our previously reported observation (21) that human BMP-15 mature protein is not N-glycosylated. In contrast, treatment of mouse BMP-15 mature protein with PNGase F resulted in the abolishment of the 18-kDa band and a concomitant increase in the intensity of the 15-kDa band. These results indicate that, unlike human BMP-15 mature protein, mouse BMP-15 mature protein is secreted as an N-glycosylated protein (18 kDa) as well as in the nonglycosylated form (15 kDa). It is noteworthy that PNGase F caused the BMP-15 proproteins to migrate at smaller sizes, indicating that the proregion of human BMP-15 is N-glycosylated (Fig. 5).
Fig. 5.
N-linked glycosylation of mouse and human BMP-15 mature protein. Conditioned media of 293T cells expressing either hhmBMP-15F or hBMP-15F were treated with PNGase F as indicated. The disappearance of the 18-kDa band of mouse BMP-15 mature protein during PNGase F treatment indicates that this band represents the N-glycosylated form of the molecule. The asterisk indicates the cross-reactivity of PNGase F under these immunoblotting conditions. mock, pCMV-tag 4A vector.
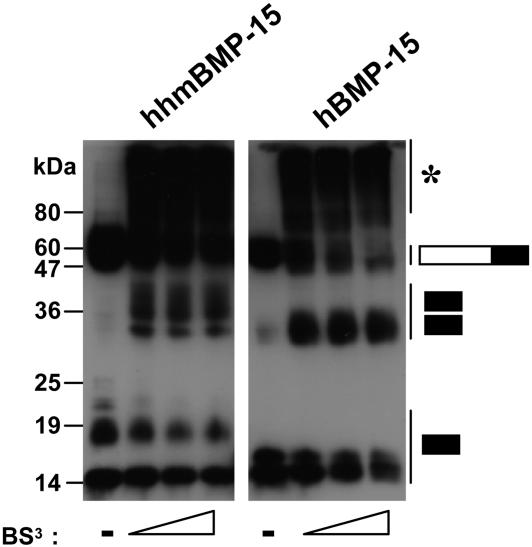
Our previous data have shown that human BMP-15 forms homodimers without forming disulfide bonds (12, 13). To test whether the mouse BMP-15 mature protein also dimerizes, we subjected conditioned media from cells expressing hhmBMP-15 or hBMP-15 to a water-soluble chemical crosslinker, bis-sulfosuccinimidyl suberate (BS3). In the presence, but not the absence, of the crosslinking chemical, the dimeric BMP-15 mature proteins were seen in both samples (Fig. 6). The dimer of mouse BMP-15 mature protein migrated as a broad band (30–40 kDa), consistent with both the glycosylated and nonglycosylated forms of the dimeric proteins.
Fig. 6.
Dimerization of mouse and human BMP-15 mature protein. Conditioned media of 293T expressing hhmBMP-15F (Left) or hBMP-15F (Right) were subjected to chemical cross-linking by using bis-sulfosuccinimidyl suberate (BS3) at final concentrations ranging from 0 to 1.5 mg/ml. The asterisk indicates high-molecular-mass molecules (65–180 kDa) that are formed upon cross-linking, most likely representing complexes of the mature domains bound to the prodomains. The migration of BMP-15 proteins is indicated by the bars to the right. The filled portions of the bars indicate the BMP-15 mature region, and the open portions of the bars indicate the BMP-15 proregion.
Discussion
A number of features of the bmp15 gene made it of immediate interest to researchers upon its discovery in 1998 (19, 20). First, BMP-15 is the closest homologue of GDF-9, and it exhibits a similar oocyte-specific expression pattern in the ovary. With respect to molecular characteristics, BMP-15 and GDF-9 are unique among members of the TGF-β superfamily of growth factors in that they lack the fourth of seven characteristic conserved cysteine residues (19, 20). This cysteine is of particular importance because it is responsible for forming the disulfide bond between the subunits of the mature dimer of most TGF-β superfamily members, which is also a characteristic feature of the TGF-β superfamily. Our recent data have demonstrated that, although they do not form covalently linked dimers as do most of the other TGF-β superfamily members, both mature BMP-15 and mature GDF-9 do form noncovalent dimers (12, 13). Additionally, when BMP-15 and GDF-9 are coexpressed in the same cells, mimicking their in vivo expression pattern in the oocyte, they also form heterodimers (13). As of yet, the functional physiological significance of the heterodimer remains unresolved.
Among members of the TGF-β superfamily, the nucleotide and amino acid sequences tend to be highly conserved between different species. For example, the amino acid sequences of the mature domain of mouse and human BMP-2 are absolutely conserved, as are the sequences of mouse and human BMP-4. GDF-9 is consistent with this trend of the TGF-β superfamily, with the amino acid sequence of the mature region of mouse and human GDF-9 being 96% identical (20). In contrast, BMP-15 does not exhibit a similar degree of cross-species homology. At the amino acid level, mouse and human BMP-15 mature domains share only 70% identity (20). The significance or consequence of the relatively low cross-species conservation of the BMP-15 amino acid sequence has not been directly resolved. However, it can be expected that significant differences in the structure of the mouse and human BMP-15 mature proteins may have important consequences for the role of BMP-15 in these species. Because of the established capacity of BMP-15 to regulate critical aspects of female reproductive physiology (21, 23–25), an important question can be raised: Could the relatively low cross-species differences in the mature protein of BMP-15 be causally associated with species-specific differences in ovulation quota?
The results of the present study show that, unlike human BMP-15, mouse BMP-15 is produced as a proprotein that is not cleaved into the mature form. Consequently, transfected cells expressing the mouse proprotein are unable to secrete mature BMP-15. Taken together, these results raise the intriguing possibility that oocyte BMP-15 mature protein may not be secreted in the mouse.
Based on our data, the human cleavage site is not sufficient for the proteolytic activation of the mouse BMP-15 proprotein. It follows, therefore, that the defects in processing are not simply due to an inefficient cleavage site. Interestingly, we show that mouse BMP-15 mature protein is efficiently produced in the presence of the human BMP-15 proregion. However, when the mouse proregion was fused to the mature region of human BMP-15, the mouse proregion did not prevent the cleavage of the chimeric proprotein or the secretion of the human BMP-15 mature protein. Therefore, the mouse BMP-15 proregion does not independently confer resistance to cleavage but, rather, must interact with the mouse mature region for the impairment of proprotein processing to occur.
In a previous study investigating the processing and cleavage of other BMP-related molecules, Constam and Robertson (26) reported results similar to our findings on the processing of BMP-15. Consistent with our present data, they reported that COS cells failed to process the nodal proprotein into mature nodal protein. Also similar to our findings, cotransfection with furin resulted in a dramatic reduction in the levels of the nodal proprotein, without a corresponding increase in the levels of the mature form of nodal. In Constam and Robertson's study (26), fusing the prodomain of dorsalin, another member of the TGF-β superfamily, to the mature domain of nodal resulted in the successful secretion of mature nodal. By contrast, the nodal prodomain prevented the secretion of mature dorsalin, as well as mature BMP-4, into the media of cells transfected with the respective chimeric constructs. Generally, the data from this study resemble our present results on the processing of BMP-15, and it is possible that similar mechanisms are involved in the defective processing of the respective proproteins. However, a significant difference in our data is that the mouse BMP-15 proregion was not able to prevent the secretion of human BMP-15 mature protein, whereas the nodal proregion completely abolished secretion of mature dorsalin and mature BMP-4. The slight reduction in the levels of the human BMP-15 mature protein secreted by cells expressing mhhBMP-15, as compared with that of cells expressing hBMP-15 (Fig. 4B), does, however, suggest that mouse BMP-15 may exhibit some destabilizing properties similar to the nodal proregion.
Based on our previous findings (12, 13) regarding the molecular manifestation of sheep BMP-15 mutations, we developed the hypothesis that the BMP-15 proprotein is susceptible to aberrancies in posttranslational processing and that such aberrancies may have direct consequences in female reproductive physiology. Intriguingly, the present data also point to the significance of the processing of the BMP-15 proprotein and extend the concept to suggest that species-specific differences in BMP-15 processing may be associated with, or causal to, species-specific differences in ovulation quota. A significant difference between the present study and our prior studies (12, 13) introducing the sheep point mutations into human BMP-15 is that the deleterious effects of the sheep mutations are only realized when the mutant BMP-15 is coexpressed with GDF-9, whereas, here, proper processing of the BMP-15 proproteins with a mouse proregion failed even when GDF-9 was not coexpressed. This discrepancy could suggest that there are species-specific differences in how the molecular interactions between GDF-9 and BMP-15 are manifested.
Another particularly important finding of the present study is the ability of the mouse proregion to impair the secretion of the human and mouse mature proteins. These data suggest that there are specific properties of the human BMP-15 proregion that are critical factors for processing of the human BMP-15 proprotein. In this respect, it is notable that the bmp15 mutation recently identified in women with hypergonadotropic ovarian failure is found in the proregion of the human BMP-15 proprotein (Y235C), also supporting the importance of the human BMP-15 proregion (14). Interestingly, generation of the mutant recombinant protein (BMP-15Y235C) revealed that mutant BMP-15 failed to exhibit stimulatory action on granulosa cell mitosis. In addition, the mutant protein antagonized the mitotic properties of wild-type human BMP-15. Because the BMP-15 mature protein is the biologically active form of the growth factor, the mutation in the BMP-15 proregion must result in the antagonism of mature BMP-15 through a still unidentified mechanism. In this regard, it is important to note that women who are heterozygous carriers of the Y235C mutation are infertile with streak ovaries, a phenotype that resembles that of the homozygous, but not heterozygous, mutant sheep. Thus, it is possible that, in those women, the mutated BMP-15 protein is capable of antagonizing the wild-type BMP-15 protein and/or other critical ovarian factors, such as GDF-9. Although a mechanism for this phenomenon remains unresolved, it is likely that the mutation in the proregion of human BMP-15 may affect the posttranslational processing of the factor, perhaps affecting binding interactions between the mature region and proregion of the BMP-15 proprotein. Collectively, when this study is considered with the data from the sheep mutations and the present data regarding the introduction of the mouse proregion, it becomes clear that the proper posttranslational processing of the BMP-15 proprotein is a critical aspect of female fertility in sheep as well as in humans.
In summary, the present data demonstrate that there are dramatic differences between human and mouse BMP-15 with respect to the processing of the proprotein and the subsequent secretion of the mature form of BMP-15. It will be interesting to determine whether reduced levels of secreted bioactive BMP-15 in vivo are causally correlated with the high ovulation quota and large litter size in animals such as mice. If that is the case, it could explain why the deletion of the bmp15 gene has only minimal effects on the ovarian phenotype in mice (6).
Acknowledgments
We thank Dr. G. F. Erickson for helpful discussion and critical reading of the manuscript, M. Wang for excellent technical assistance, and Andi Hartgrove for editorial assistance. This work was supported in part by National Institutes of Health (NIH) Grant R01 HD41494 (to S.S.) and by the National Institute of Child Health and Human Development/NIH through Cooperative Agreement U54HD12303 as part of the Specialized Cooperative Centers Program in Reproduction Research (to S.S.). R.K.M. was supported by NIH Fellowship Grant F32 HD41320 and a fellowship from the Giannini Family Foundation.
Author contributions: S.S. designed research; O.H. and R.K.M. performed research; and R.K.M. and S.S. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BMP-15, bone morphogenetic protein 15; GDF-9, growth and differentiation factor 9.
References
- 1.Matzuk, M. M., Burns, K. H., Viveiros, M. M. & Eppig, J. J. (2002) Science 296, 2178–2180. [DOI] [PubMed] [Google Scholar]
- 2.Eppig, J. J., Wigglesworth, K. & Pendola, F. (2002) Proc. Natl. Acad. Sci. USA 99, 2890–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hubner, K., Fuhrmann, G., Christenson, L. K., Kehler, J., Reinbold, R., De La Fuente, R., Wood, J., Strauss, J. F., III, Boiani, M. & Scholer, H. R. (2003) Science 300, 1251–1256. [DOI] [PubMed] [Google Scholar]
- 4.Shimasaki, S., Moore, R. K., Otsuka, F. & Erickson, G. F. (2004) Endocr. Rev. 25, 72–101. [DOI] [PubMed] [Google Scholar]
- 5.Dong, J., Albertini, D. F., Nishimori, K., Kumar, T. R., Lu, N. & Matzuk, M. (1996) Nature 383, 531–535. [DOI] [PubMed] [Google Scholar]
- 6.Yan, C., Wang, P., DeMayo, J., DeMayo, F. J., Elvin, J. A., Carino, C., Prasad, S. V., Skinner, S. S., Dunbar, B. S., Dube, J. L., et al. (2001) Mol. Endocrinol. 15, 854–866. [DOI] [PubMed] [Google Scholar]
- 7.Juengel, J. L., Hudson, N. L., Whiting, L. & McNatty, K. P. (2004) Biol. Reprod. 70, 557–561. [DOI] [PubMed] [Google Scholar]
- 8.Juengel, J. L., Hudson, N. L., Heath, D. A., Smith, P., Reader, K. L., Lawrence, S. B., O'Connell, A. R., Laitinen, M. P. E., Cranfield, M., Groome, N. P., et al. (2002) Biol. Reprod. 67, 1777–1789. [DOI] [PubMed] [Google Scholar]
- 9.Galloway, S. M., McNatty, K. P., Cambridge, L. M., Laitinen, M. P. E., Juengel, J. L., Jokiranta, T. S., McLaren, R. J., Luiro, K., Dodds, K. G., Montgomery, G. W., et al. (2000) Nat. Genet. 25, 279–283. [DOI] [PubMed] [Google Scholar]
- 10.Hanrahan, J. P., Gregan, S. M., Mulsant, P., Mullen, M., Davis, G. H., Powell, R. & Galloway, S. M. (2004) Biol. Reprod. 70, 900–909. [DOI] [PubMed] [Google Scholar]
- 11.Moore, R. K., Erickson, G. F. & Shimasaki, S. (2004) Trends Endocrinol. Metab. 15, 356–361. [DOI] [PubMed] [Google Scholar]
- 12.Liao, W. X., Moore, R. K. & Shimasaki, S. (2004) J. Biol. Chem. 279, 17391–17396. [DOI] [PubMed] [Google Scholar]
- 13.Liao, W. X., Moore, R. K., Otsuka, F. & Shimasaki, S. (2003) J. Biol. Chem. 278, 3713–3719. [DOI] [PubMed] [Google Scholar]
- 14.Di Pasquale, E., Beck-Peccoz, P. & Persani, L. (2004) Am. J. Hum. Genet. 75, 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gougeon, A. (1996) Endocr. Rev. 17, 121–155. [DOI] [PubMed] [Google Scholar]
- 16.McNatty, K. P., Juengel, J. L., Wilson, T., Galloway, S. M., Davis, G. H., Hudson, N. L., Moeller, C. L., Cranfield, M., Reader, K. L., Laitinen, M. P., et al. (2003) Reprod. Suppl. 61, 339–351. [PubMed] [Google Scholar]
- 17.Wu, X. & Matzuk, M. M. (2002) Rev. Endocr. Metab. Disord. 3, 27–32. [DOI] [PubMed] [Google Scholar]
- 18.Galloway, S. M., Gregan, S. M., Wilson, T., McNatty, K. P., Juengel, J. L., Ritvos, O. & Davis, G. H. (2002) Mol. Cell. Endocrinol. 191, 15–18. [DOI] [PubMed] [Google Scholar]
- 19.Laitinen, M., Vuojolainen, K., Jaatinen, R., Ketola, I., Aaltonen, J., Lehtonen, E., Heikinheimo, M. & Ritvos, O. (1998) Mech. Dev. 78, 135–140. [DOI] [PubMed] [Google Scholar]
- 20.Dube, J. L., Wang, P., Elvin, J., Lyons, K. M., Celeste, A. J. & Matzuk, M. M. (1998) Mol. Endocrinol. 12, 1809–1817. [DOI] [PubMed] [Google Scholar]
- 21.Otsuka, F., Yao, Z., Lee, T. H., Yamamoto, S., Erickson, G. F. & Shimasaki, S. (2000) J. Biol. Chem. 275, 39523–39528. [DOI] [PubMed] [Google Scholar]
- 22.Seidah, N. G. & Chretien, M. (1999) Brain Res. 848, 45–62. [DOI] [PubMed] [Google Scholar]
- 23.Otsuka, F., Yamamoto, S., Erickson, G. F. & Shimasaki, S. (2001) J. Biol. Chem. 276, 11387–11392. [DOI] [PubMed] [Google Scholar]
- 24.Otsuka, F. & Shimasaki, S. (2002) Proc. Natl. Acad. Sci. USA 99, 8060–8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otsuka, F. & Shimasaki, S. (2002) Endocrinology 143, 4938–4941. [DOI] [PubMed] [Google Scholar]
- 26.Constam, D. B. & Robertson, E. J. (1999) J. Cell Biol. 144, 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]