Abstract
Understanding the minimal dose of physical activity required to achieve improvement in physical functioning and reductions in disability risk is necessary to inform public health recommendations. To examine the effect of physical activity dose on changes in physical functioning and the onset of major mobility disability in The Lifestyle Interventions and Independence for Elders (LIFE) Study. We conducted a multicenter single masked randomized controlled trial that enrolled participants in 2010 and 2011 and followed them for an average of 2.6 years. 1,635 sedentary men and women aged 70–89 years who had functional limitations were randomized to a structured moderate intensity walking, resistance, and flexibility physical activity program or a health education program. Physical activity dose was assessed by 7-day accelerometry and self-report at baseline and 24 months. Outcomes included the 400 m walk gait speed, the Short Physical Performance Battery (SPPB), assessed at baseline, 6, 12, and 24 months, and onset of major mobility disability (objectively defined by loss of ability to walk 400 m in 15 min). When the physical activity arm or the entire sample were stratified by change in physical activity from baseline to 24 months, there was a dose-dependent increase in the change in gait speed and SPPB from baseline at 6, 12, and 24 months. In addition, the magnitude of change in physical activity over 24 months was related to the reduction in the onset of major mobility disability (overall P < 0.001) (highest versus the lowest quartile of physical activity change HR 0.23 ((95% CI:0.10–0.52) P = 0.001) in the physical activity arm. We observed a dose-dependent effect of objectively monitored physical activity on physical functioning and onset of major mobility disability. Relatively small increases (> 48 minutes per week) in regular physical activity participation had significant and clinically meaningful effects on these outcomes.
Trial registration: ClinicalsTrials.gov NCT00116194
Introduction
Physical inactivity is among the strongest predictors of physical disability in elders[1,2]. Furthermore, exercise prevents or improves conditions that underlie disability in older adults, including falls[3–6], hip fracture[7,8], cardiovascular disease[9,10], and diabetes[10–12]. Longitudinal studies suggest regular physical activity is associated with reduced mortality and risk of physical disability[13,14]. Several studies have demonstrated beneficial effects of physical activity programs on functional outcomes in older adults[15–19].
An important but understudied issue in physical activity trials in older adults is intervention adherence and the minimum dose necessary to achieve benefit. Previous physical activity intervention trials have reported that adherence among older adults is generally high (70–85%)[18,20,21]. However, many of these studies were of relatively short duration (6–18 months), and measured physical activity adherence by self-report or other indices of participation such as session attendance. To address gaps in knowledge regarding physical activity for disability prevention, the Lifestyle Interventions and Independence for Elders Study (the LIFE Study), a Phase 3 multicenter RCT compared a supervised moderate-intensity walking and resistance training physical activity program to a health education program on the incidence of major mobility disability was conducted[22]. The LIFE study demonstrated that a structured moderate intensity physical activity program reduced major mobility disability by 18% and persistent mobility disability by 28%, compared with a health education program[23].
In the present study, we examined whether differences in the dose of physical activity performed were associated with improvements in physical functioning and reductions in disability incidence. This analysis was an exploratory aim of the LIFE study. We also examined the stability/consistency of these results by examining the dose of physical activity using both objectively measured daily physical activity using accelerometry and self-reported minutes of walking plus weight training exercise.
Materials and methods
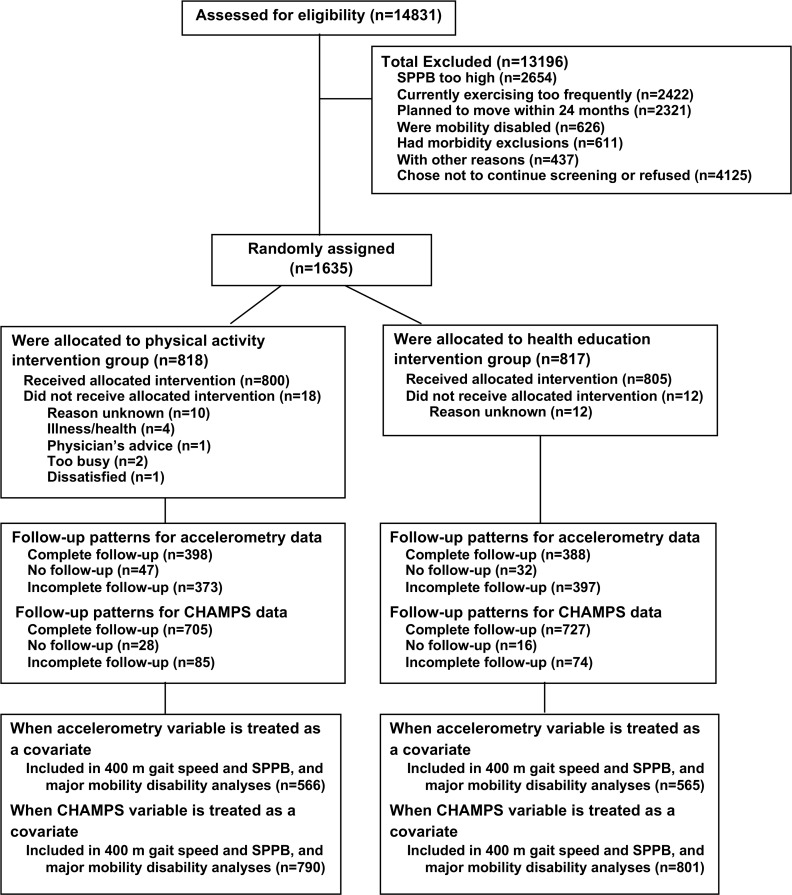
From February 2010 to December 2011, 14,831 participants were screened for the LIFE study at eight field centers; 1,635 of these participants were eligible and randomized (818 to physical activity and 817 to health education) (Fig 1).
Fig 1. Flow of participants through the trial.
Eligibility
Comprehensive details of the LIFE study have been published previously[22,24]. Briefly, the LIFE study eligibility criteria were designed to target older persons (age 70–89 years) who were: a) inactive (reporting <20 min/week in the past month performing regular physical activity and <125 min/week of moderate physical activity); b) at high risk for mobility disability determined by a Short Physical Performance Battery (SPPB) score of 9 or lower[25,26]; c) able to walk 400 meters in <15 minutes; d) had no major cognitive impairment (Modified Mini-Mental State Examination [27](3MSE)) and e) able to safely participate in the intervention. This study was approved by the Institutional Review Board (IRB) at University of Florida, University of Pittsburgh, Pennington Biomedical Research Institute, Wake Forest University, Yale University, Stanford University, Northwestern University, and Tufts University and written informed consent was obtained from all participants.
Study interventions
Physical activity intervention
The physical activity intervention included walking-based aerobic, resistance, flexibility, and balance training in a supervised class setting (2 x per week) with additional home-based physical activity goals[28]. The goal was to progressively move participants towards at least 150 minutes per week of moderate intensity physical activity, consistent with the 2008 Physical Activity Guidelines for Americans report[29].
Walking was the primary mode of physical activity, given its widespread popularity and ease of administration across a broad segment of the older adult population[30,31]. Participants also completed a 10-minute lower extremity resistance training program with ankle weights after walking exercise, a set of balance exercises, and a brief lower extremity stretching routine. Individualized home-based walking goals supplemented the supervised program.
Health education intervention
The health education intervention provided age-specific health information about “successful aging”. Health education consisted of workshops on topics relevant to older adults (e.g., negotiating the health care system, travel safety, nutrition, etc.). Each session included a brief program of seated upper extremity stretching/flexibility exercises. Health education classes met weekly for the first 26 and from week 27 on the program was offered two times per month and participants were prompted to attend at least once per month.
Study assessments
Participants were evaluated at baseline, 6, 12, and 24 month clinic visits by staff masked to intervention arm allocation. Home, telephone, and proxy assessments were attempted if the participant could not come to the clinic. Since objective measures of physical activity were assessed through month 24 on all participants (i.e. minimum follow-up was 24 months), we have confined our analysis to this time frame.
Short Physical Performance Battery (SPPB)
The SPPB consists of a standing balance test, a usual pace 4 meter walk, and five timed repeated chair stands[32]. Each performance measure is assigned a categorical score ranging from 0 (inability to complete the test) to 4 (best performance possible) and a summary score ranging from 0 (worst performance) to 12 (best performance) was calculated.
400 m walk test and assessment of major mobility disability
Participants were asked to walk 400 m at their usual pace, without overexertion, for 10 laps on a course defined by 2 cones placed 20 m apart. They could stop and rest for up to 1 minute for fatigue or related symptoms. Gait speed was calculated from the time to complete the walk and the distance covered.
Major mobility disability was defined as the inability to complete the 400 m walk test within 15 min without sitting and without the help of another person or walker[22]. Use of a cane was acceptable. When major mobility disability could not be objectively measured because the participant was not able to come to the clinic and lacked a suitable walking course at their home, institution or hospital, an alternative adjudication of the outcome was based on directly observed inability to walk 4 m in <10 sec, or self-, proxy-, or medical record-reported inability to walk across a room. If participants met these alternative criteria, it was considered that they would also not be able to complete the 400 m walk within 15 minutes.
Accelerometry
Participants were instructed to wear an accelerometer (Actigraph GT3X) on their hip for seven consecutive days except during sleep, showering/bathing, and swimming. Movement was captured along the vertical axis in 1-minute epochs, and non-wear time was defined as 90 minutes of consecutive zero counts[33]. An outlier minute was defined as having an activity count value that exceeded all of the participant’s non-outlier minutes and additionally the activity count value had to exceed the nearest value for the day by 1000 and exceed the median of the 2nd highest daily value by 3500. Data were considered valid if apparent wearing evidence was ≥ 600 minutes per day for at least five days. Data reduction focused on technical and procedural errors as well as evident outliers. Ultimately, data from a total of 1,131 participants (566 from the physical activity intervention, 565 from the health education intervention) were used in this analysis. To be included in the analysis, they must have had a baseline measure and at least one of the follow-up measure.
Total physical activity was calculated as the average number of daily activity counts per minute. This measure correlates with energy expenditure assessed using oxygen consumption in older adults[34]. In the absence of well-accepted evidence-based accelerometry cut-points for physical activity intensity in older adults, a cut-point was set that demarcated time during activity (> 760 activity counts/minute)[35,36]. Data were expressed in min/week, and adjusted for wear time.
Self-reported physical activity
Participants completed a shortened version of the Community Health Activities Model Program for Seniors (CHAMPS-5) physical activity questionnaire[37]. The CHAMPS-5 assessed the average weekly minutes spent in walking and weight training from 5 specific items in this instrument related to these specific activities. These items queried frequency and duration of engagement in moderate to heavy strength training, light strength training, walking uphill or hiking up hill, walking fast or briskly for exercise, and walking leisurely for exercise or pleasure.
Hospitalizations
Because physical activity participation may be influenced by inter-current illness, we used reported hospitalizations as a measure of inter-current illness. At each contact, participants (or proxies if the participant was not available) were questioned about hospitalizations since the last visit. All records for hospitalizations were obtained and adjudicated independently by two experts who were blinded to randomization arm.
Statistical analysis plan
The mean and standard deviation (SD) of change in physical activity from baseline by randomized arm at each visit were computed. The Wilcoxon rank sum test was used to compare the change in physical activity between randomization groups. Baseline characteristics, stratified by quartiles of change from baseline in accelerometer-determined physical activity at 24 months (interquartile ranges derived from physical activity arm) and by intervention arm, included means (± SD) for continuous variables or counts (%) for discrete variables. Analysis of variance was used to compare continuous characteristics across quartiles of change and a chi-square test was used to compare discrete characteristics across quartiles of change.
Physical activity measures, including minutes spent in activity associated with >760 activity counts/minute (by accelerometry) and CHAMPS-reported min/week in walking/weight training were the primary predictors of interest. They were categorized based on quartiles of changes at 24 months in the physical activity arm in the models. Baseline measures and changes to follow-up from baseline at 6, 12, and 24 months (treated as continuous measures or quartiles) were included in the analysis as an index of the dose of physical activity achieved. The outcomes included the SPPB, 400 m walk speed, and onset of major mobility disability. Analyses were initially restricted to the physical activity arm alone; however, we also examined these relationships using the same quartiles for both groups combined to examine changes in physical activity that occurred irrespective of intervention assignment. SPPB and 400 m walk speed were analyzed using mixed effects models with an unstructured parameterization for longitudinal covariance. For the analyses of the physical activity arm, models contained the following terms: field center and sex (both used to stratify randomization), baseline outcome measure, clinic visit, hospitalization, baseline physical activity measure, quartile of change in the physical activity measure, and quartile of change in the physical activity measure and clinic visit. For the entire sample analysis (both physical activity and health education), we additionally adjusted for randomization arm. Least squares means and their standard errors were presented from these models.
The effect of change in physical activity on time until major mobility disability was tested using a time-dependent Cox regression model, stratified by field center and sex. Failure time was measured from the time of randomization. Follow-up was censored at the first occurrence of loss-to-follow-up, death or the month 24 assessment. For participants who did not have any outcome assessments, a half hour of follow-up time was assigned, since we knew that they completed the 400 m walk at baseline. The models contained the following terms: baseline and change in physical activity measure, hospitalization, and randomization arm when the entire sample was used.
Results and discussion
The change in accelerometer-determined activity and the change in self-reported walking plus weight training were significantly greater in the physical activity arm than health education at all time points (P < 0.001) (Table 1.). Baseline characteristics by quartile of accelerometer-determined physical activity are presented in Tables 2 and 3. Baseline differences in number of chronic conditions were observed in the PA arm and in gait speed in both arms across quartiles.
Table 1. Change in physical activity from baseline by randomized arm (differences between physical activity and health education P < 0.001 (calculated using the Wilcoxon rank sum test) between PA and HE at all time points.
| Change in minutes of physical activity (>760 activity counts/min) | |||
| 6 months | 12 months | 24 months | |
| Physical activity | 31.3 ± 120.4a N = 518 |
19.0 ± 122.7 N = 519 |
-8.9 ± 118.2 N = 466 |
| Health education | -19.6 ± 139.3 N = 509 |
-25.7 ± 130.5 N = 511 |
-46.2 ± 132.2 N = 452 |
| Change in minutes of self-reported walking plus weight training minutes (from CHAMPS) | |||
| Physical activity | 151.6 ± 194.8 N = 774 |
141.9 ± 195.5 N = 761 |
133.7 ± 204.4 N = 724 |
| Health education | 29.1 ± 163.2 N = 796 |
36.8 ± 170.2 N = 777 |
29.4 ± 167.6 N = 737 |
amean change ± standard deviation
Table 2. Baseline characteristics of the physical activity program participants by quartiles of change in accelerometer-determined physical activity (minutes per week above 760 counts per minute change between baseline and 24 months).
| Q1a (n = 117) | Q2 (n = 116) | Q3 (n = 116) | Q4 (n = 117) | p-value | |
|---|---|---|---|---|---|
| Age (years) | 78.1 ± 5.1b | 79.0 ± 5.1 | 79.6±5.7 | 77.9±5.3 | 0.06 |
| Female No. (%) |
80 (68.4%)c | 76 (65.5%) | 74 (63.8%) | 75 (64.1%) | 0.88 |
| Race No. (%) |
0.56 | ||||
| White | 81 (69.2%) | 92 (79.3%) | 89 (76.7%) | 92 (78.6%) | |
| African American | 30 (25.6%) | 18 (15.5%) | 22 (19.0%) | 21 (18.0%) | |
| Other | 6 (5.1%) | 6 (5.2%) | 5 (4.3%) | 4 (3.4%) | |
| No. of chronic conditions | 1.8 ± 1.1 |
1.6 ± 1.1 |
2.0±1.3 |
1.6±1.1 |
0.05 |
| Body mass index (kg/m2) | 31.4 ± 6.4 | 29.7 ± 5.3 | 30.1±5.8 | 29.9±5.8 | 0.12 |
| Short Physical Performance Battery (SPPB) score |
7.6±1.5 | 7.5±1.5 | 7.3±1.7 | 7.5±1.7 | 0.49 |
| 400 m gait speed (m/sec) |
0.84 ± 0.16 | 0.84 ± 0.16 | 0.80±0.15 | 0.87±0.18 | 0.01 |
aQ1: < -66; Q2: -66 ─ -8; Q3: -8–43; Q4: ≥ 43 (minutes per week above 760 counts per minute change between baseline and 24 months)
bData are means ± standard deviations
cn (%).
Table 3. Baseline characteristics of the health education program participants by quartiles of change in physical activity by accelerometry (minutes per week above 760 counts/minute change between baseline and 24 months).
| Q1a (n = 152) | Q2 (n = 134) | Q3 (n = 105) |
Q4(n = 61) | ||
|---|---|---|---|---|---|
| Age (years) | 78.5 ± 5.3b | 79.5 ± 5.3 | 79.5 ± 5.2 | 77.8 ± 5.3 | 0.07 |
| Female | 109 (71.7%)c | 88 (65.7%) | 71 (67.6%) | 50 (82.0%) | 0.12 |
| Race | 0.64 | ||||
| White | 126 (82.9%) | 102 (76.1%) | 79 (75.2%) | 51 (83.6%) | |
| African American |
19 (12.5%) | 24 (17.9%) | 18 (17.1%) | 8 (13.1%) | |
| Other | 7 (4.6%) | 8 (6.0%) | 8 (7.6%) | 2 (3.3%) | |
| No. of chronic conditions | 1.7 ± 1.1 | 1.9 ± 1.1 |
1.8±1.1 |
1.7±1.1 |
0.18 |
| Body mass index (kg/m2) | 30.1 ± 5.8 | 30.7 ± 6.4 | 30.4 ± 6.0 | 30.2 ± 6.4 | 0.81 |
| Short Physical Performance Battery score |
7.6 ± 1.4 | 7.2 ± 1.6 | 7.4 ± 1.5 | 7.3 ± 1.8 | 0.26 |
| 400 m walking speed (m/sec) |
0.86 ± 0.15 | 0.81 ± 0.16 | 0.82 ±0.18 | 0.83 ± 0.15 | 0.02 |
aQ1: < -66; Q2: -66 ─ -8; Q3: -8–43; Q4: ≥ 43 (minutes per week above 760 counts per minute change between baseline and 24 months)
bData are means ± standard deviations
cn (%).
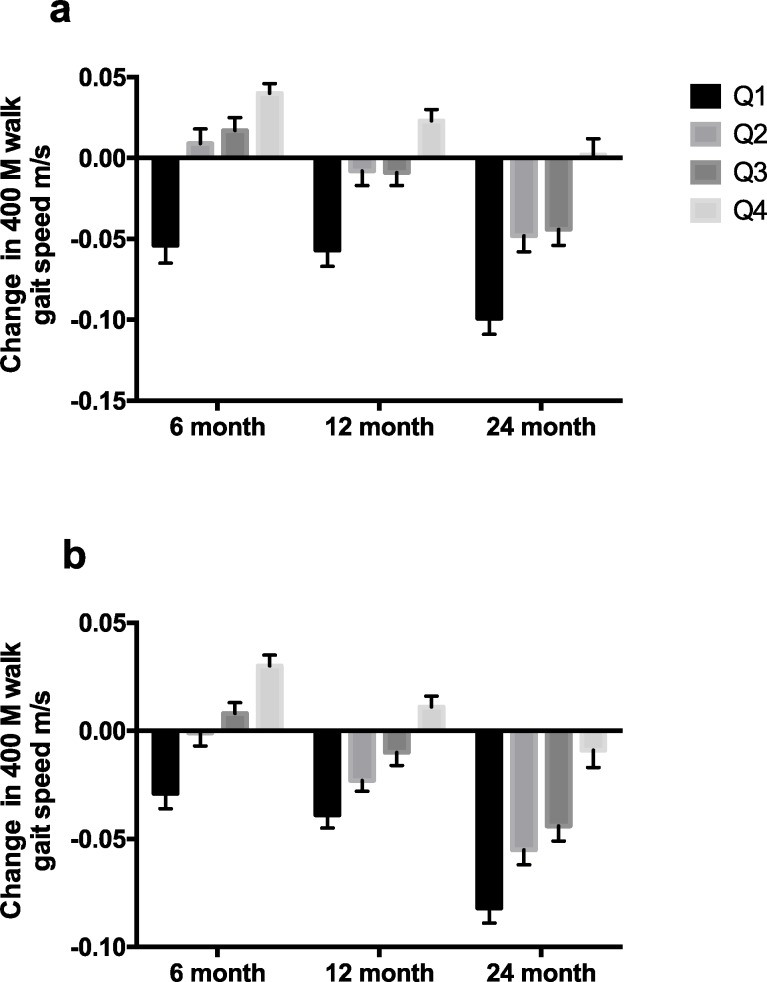
The mixed model analyses revealed statistically significant and clinically meaningful differences of the effect of quartile of change in accelerometry-based measures of change in activity from baseline to 24 month on the change in 400 m gait speed (Fig 2) for the physical activity arm over time (P < 0.001). The difference in gait speed change from lowest to the highest quartiles was between 0.05 and 0.10 m/s. This finding also held for the entire sample (Fig 2) (P < 0.001). These relationships remained when hospitalization was removed from the models (data not shown).
Fig 2.
Change in 400 m walk gait speed (m/s) compared to baseline gait speed at 6, 12, and 24 months according to quartiles of change physical activity by accelerometry from baseline to 24 months, (a.) physical activity arm alone and (b.) entire group combined (least square means ± SE). Overall effect P < 0.0001. Effects within each time point P < 0.0001.
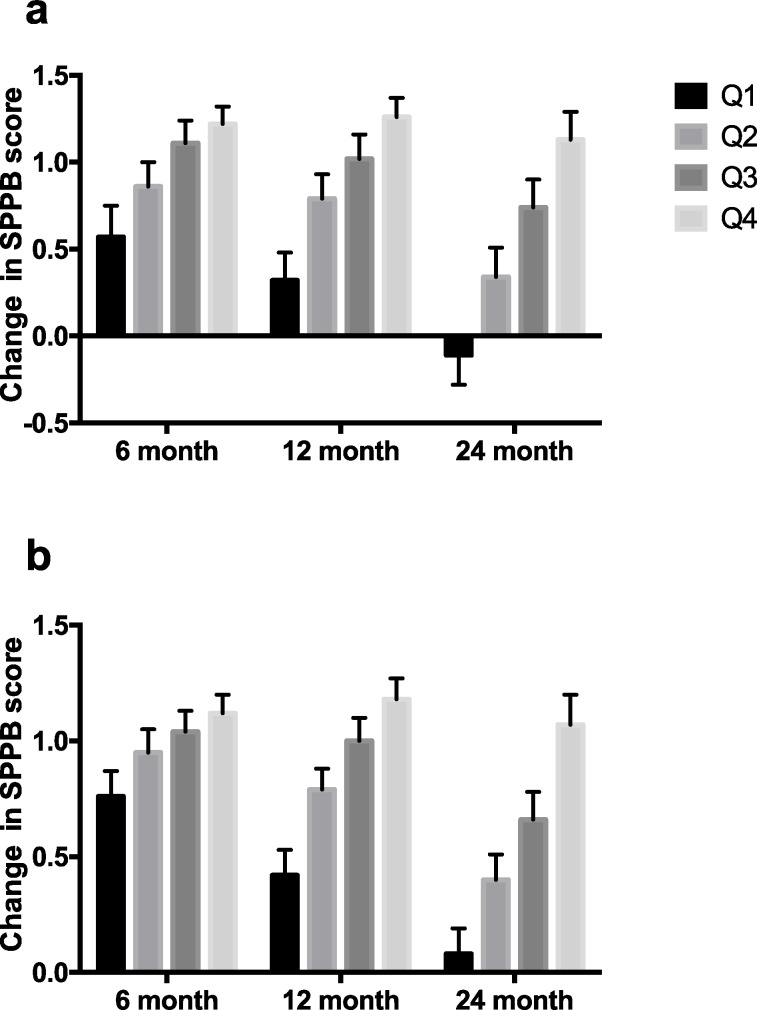
The mixed model analyses also revealed a statistically significant and clinically meaningful difference of the effect of quartile of change in accelerometry-based measures of activity from baseline to 24 month in SPPB score for the physical activity arm over time (Fig 3A) (P < 0.001). The difference in SPPB change from lowest to the highest quartiles was between 0.5 and 1.5. Again, this held true when the entire sample was combined (Fig 3B) (P < 0.001). These relationships remained when hospitalization was not included in the models (data not shown).
Fig 3.
Change in SPPB score compared to baseline SPPB score at 6, 12, and 24 months according to quartiles of change in physical activity by accelerometry from baseline to 24 months, (a.) physical activity arm alone and (b.) entire group combined (least square means ± SE). Overall effect P < 0.0001. Effects within each time point all P < 0.01(physical activity arm); Effects within each time point all P < 0.06 (month 6, P = 0.06, months 12 and 24, P < 0.0001) (entire group).
The changes in physical activity by self-reported minutes of walking plus weight training from baseline to 24 months were also associated with significant changes in 400 m gait speed (P < 0.001) and SPPB scores (P < 0.001) (Table 4) in the physical activity arm and when the entire sample was combined (Table 5). Again, the results were stable when models were run excluding hospitalizations (data not shown).
Table 4. Association with change in gait speed and SPPB in physical activity arm participants, using quartile of change from baseline to 24 months of self-reported walking plus weight training.
| Variable | visit | Q1a | Q2 | Q3 | Q4 | p-value | Overall p-value |
|---|---|---|---|---|---|---|---|
| Change in 400 m gait speed | 6 | -.001±0.010 | -.007±0.007 | 0.023±0.006 | 0.034±0.006 | <0.001 | <0.001 |
| 12 | -.045±0.010 | -.024±0.007 | 0.003±0.006 | 0.012±0.006 | <0.001 | ||
| 24 | -.062±0.012 | -.054±0.008 | -.040±0.008 | -.001±0.007 | <0.001 | ||
| Change in SPPB | 6 | 0.68±0.15 | 0.89±0.11 | 1.16±0.09 | 1.21±0.09 | 0.005 | <0.001 |
| 12 | 0.66±0.16 | 0.67±0.11 | 0.96±0.10 | 1.16±0.10 | 0.002 | ||
| 24 | 0.24±0.18 | 0.36±0.14 | 0.54±0.13 | 0.79±0.12 | 0.025 |
aQ1: < 0; Q2: 0–105; Q3: 106–225; Q4: > 225 Self-reported time (minutes/week) walking and weight training; least square means ± standard errors.
Table 5. Association with change in 400 m gait speed and SPPB in the entire sample, using quartile of change of self-reported walking plus weight training from baseline to 24 months.
| Variable | visit | Q1a | Q2 | Q3 | Q4 | p-value | Overall p-value |
|---|---|---|---|---|---|---|---|
| Change in 400 m gait speed | 6 | -.011±0.005 | -.004±0.004 | 0.011±0.004 | 0.016±0.004 | <0.001 | <0.001 |
| 12 | -.038±0.005 | -.024±0.004 | -.005±0.004 | -.003±0.005 | <0.001 | ||
| 24 | -.069±0.007 | -.055±0.005 | -.044±0.006 | -.024±0.007 | <0.001 | ||
| Change in SPPB | 6 | 0.64±0.09 | 0.90±0.07 | 0.99±0.08 | 1.11±0.08 | 0.002 | <0.001 |
| 12 | 0.61±0.10 | 0.71±0.07 | 0.92±0.08 | 0.98±0.09 | 0.004 | ||
| 24 | 0.12±0.11 | 0.49±0.08 | 0.54±0.10 | 0.74±0.10 | <0.001 |
aQ1: < 0; Q2: 0–105; Q3: 106–225; Q4: > 225 Self-reported time (minutes/week) walking and weight training; least square means ± standard errors.
We examined the relationship between quartiles of change in accelerometry-based activity and change in both self-reported walking plus weight training with the onset of major mobility disability for the physical activity arm and entire sample from baseline to 24 months. The relationships were statistically significant in the physical activity arm (P ≤ 0.003) and in the entire sample overall (P ≤ 0.001). For the physical activity arm alone, the onset of major mobility disability was reduced by 24% (P = 0.412), 22% (P = 0.489), and 77% (P = 0.001) in quartiles 2, 3, and 4 of change in accelerometry-based activity respectively and increased by 7% (P = 0.802), and reduced 57% (P = 0.007), and 75% (P < 0.001) in quartiles 2,3, and 4 of change in self-reported walking plus weight training respectively (Table 6). The hazard ratios were very similar for the entire sample (Table 7) and when hospitalization was removed as a covariate (data not shown).
Table 6. Hazard ratio for major mobility disability in the physical activity arm.
| Variable | Quartile | HR (95% CI)a | p-value | Overall p-value (3df) |
|---|---|---|---|---|
| Quartile of change in physical activity by accelerometry | 1 | 1.00 | 0.003 | |
| 2 | 0.76 (0.39, 1.48) | 0.412 | ||
| 3 | 0.78 (0.40, 1.55) | 0.484 | ||
| 4 | 0.23 (0.10, 0.52) | 0.001 | ||
| Quartile of change in self-reported walking and weight training | 1 | 1.00 | <0.001 | |
| 2 | 1.07 (0.62, 1.84) | 0.802 | ||
| 3 | 0.43 (0.23, 0.79) | 0.007 | ||
| 4 | 0.25 (0.13, 0.49) | <0.001 |
aAdjusted for baseline adherence measure, hospitalization, and quartile of adherence change.
Table 7. Hazard ratio for major mobility disability in the entire sample.
| Variable | Quartile | HR (95% CI)a | p-value | Overall p-value (3df) |
|---|---|---|---|---|
| Quartile of change in physical activity by accelerometry | 1 | 1.00 | ||
| 2 | 0.68 (0.43, 1.06) | 0.091 | <0.001 | |
| 3 | 0.65 (0.40, 1.06) | 0.085 | ||
| 4 | 0.29 (0.16, 0.51) | <0.001 | ||
| Quartile of change of self-reported walking plus weight training | 1 | 1.00 | <0.001 | |
| 2 | 0.90 (0.64, 1.27) | 0.558 | ||
| 3 | 0.57 (0.38, 0.85) | 0.006 | ||
| 4 | 0.29 (0.18, 0.46) | <0.001 |
aAdjusted for baseline adherence measure, randomization, hospitalization, and quartile of adherence change
The major results from this study were that changes in physical activity measured objectively by accelerometry as well as by self-report were associated with improvements in physical functioning over 24 months as well as a reduction in the onset of major mobility disability in a dose-dependent manner. These associations were stable when examined in the physical activity arm alone or when the physical activity and health education arms were combined. We observed similar results of physical activity participation on physical functioning and major mobility disability whether using accelerometry or self-report measures.
The magnitude of the increase in physical function in the physical activity arm was related to accelerometry-determined changes in physical activity and was progressively greater across all four quartiles of change. Observational studies have reported significant relationships between self-reported physical activity measures [38–41] and objective measures of physical functioning in older adults[42–44]. McDermott et al. reported that the decline in physical performance in patients with peripheral artery disease is slower in individuals who report walking 3 or more times per week, compared to those who walk for exercise less frequently or not at all[45]. In contrast to these observational studies, results reported here are from a randomized controlled trial and show greater improvements in physical functioning in individuals who participate in more physical activity. The difference in gait speed change from lowest to the highest quartiles was between 0.05 to 0.10 m/s, a magnitude which has been associated with reductions in mortality risk [46] and patient-reported outcomes[47]. The difference in SPPB score change from lowest to highest quartiles was between 0.5 and 1.0 and is within the range of a clinically meaningful difference[48].
Greater differences in both the change in self-reported physical activity and objectively measured activity were related to prevention of major mobility disability. The present analysis demonstrated a robust decline in major mobility disability in the highest quartile of change in accelerometer-determined physical activity. The change in physical activity observed in the highest quartile translated to approximately 43 minutes per week. This level of physical activity is approximately the dose of a single LIFE study physical activity session[28]. These data suggest that an increase in the dose of physical activity equivalent to a single LIFE physical activity session per week was sufficient to dramatically reduce disability risk and result in meaningful improvements in physical functioning.
The dose effect of physical activity on physical function and major mobility disability was also observed when we combined data from both study arms. This finding highlights that participants randomized to physical activity and whose adherence to the intervention was relatively low achieved smaller benefits, however, any participant randomized to health education who spontaneously increased their physical activity achieved significant and meaningful improvements in their function and disability risk. These effects were observed even when accounting for hospitalizations during the trial to adjust for the burden of inter-current illness.
The strengths of this investigation are the objective measurement of physical activity, the substantially greater length of the follow-up than in prior studies, and the study population, which consisted of individuals at high risk for mobility disability. Furthermore, the results of our analysis when both intervention arms were combined indicate that if participants increased or decreased their levels of activity over the course of the trial, irrespective of group assignment, their change in physical functioning or mobility disability was related to this change in activity. In addition to these strengths, there are several study limitations. Among them are the fact that the LIFE Study was not designed to specifically address varying doses of physical activity on physical functioning and disability risk. Differences in health status and inter-current illness may influence physical activity behavior and therefore may be potential confounders in these analyses. In addition, since both independent and dependent variables were assessed over the same time intervals it is not possible to establish temporal precedence. However, our models were stable even when inter-current hospitalizations were included in the analyses.
Conclusion
In conclusion, greater changes in physical activity behavior measured objectively by accelerometry and self-report were associated with clinically meaningful improvements in physical functioning and reductions in the development of major mobility disability. The dose of change in physical activity associated with the greatest benefit was greater than 48 minutes per week of physical activity. These data have important public health implications for the benefits of physical activity in mobility-limited older adults. These data support that beneficial effects of physical activity can be realized with substantially less physical activity than is currently recommended for most inactive older adults.
Supporting information
(DOC)
(PDF)
Acknowledgments
The LIFE Study Group Includes the Following (lead author: Marco Pahor, mpahor@ufl.edu):
Administrative Coordinating Center, University of Florida, Gainesville, FL
Marco Pahor, MD–Principal Investigator of the LIFE Study
Jack M. Guralnik, MD, PhD–Co-Investigator of the LIFE Study (University of Maryland School of Medicine, Baltimore, MD)
Christiaan Leeuwenburgh, PhD
Connie Caudle
Lauren Crump, MPH
Latonia Holmes
Jocelyn Lee, PhD
Ching-ju Lu, MPH
Data Management, Analysis and Quality Control Center, Wake Forest University, Winston Salem, NC
Michael E. Miller, PhD–DMAQC Principal Investigator
Mark A. Espeland, PhD–DMAQC Co-Investigator
Walter T. Ambrosius, PhD
William Applegate, MD
Daniel P. Beavers, PhD, MS
Robert P. Byington, PhD, MPH, FAHA
Delilah Cook, CCRP
Curt D. Furberg, MD, PhD
Lea N. Harvin, BS
Leora Henkin, MPH, Med
John Hepler, MA
Fang-Chi Hsu, PhD
Laura Lovato, MS
Wesley Roberson, BSBA
Julia Rushing, BSPH, MStat
Scott Rushing, BS
Cynthia L. Stowe, MPM
Michael P. Walkup, MS
Don Hire, BS
W. Jack Rejeski, PhD
Jeffrey A. Katula, PhD, MA
Peter H. Brubaker, PhD
Shannon L. Mihalko, PhD
Janine M. Jennings, PhD
Shyh-Huei Chen, PhD
June J. Pierce, AB
National Institutes of Health, Bethesda, MD
Evan C. Hadley, MD (National Institute on Aging)
Sergei Romashkan, MD, PhD (National Institute on Aging)
Kushang V. Patel, PhD (National Institute on Aging)
National Heart, Lung and Blood Institute, Bethesda, MD
Denise Bonds, MD, MPH
Field Centers
Northwestern University, Chicago, IL
Mary M. McDermott, MD–Field Center Principal Investigator
Bonnie Spring, PhD–Field Center Co-Investigator
Joshua Hauser, MD–Field Center Co-Investigator
Diana Kerwin, MD–Field Center Co-Investigator
Kathryn Domanchuk, BS
Rex Graff, MS
Alvito Rego, MA
Pennington Biomedical Research Center, Baton Rouge, LA
Timothy S. Church, MD, PhD, MPH–Field Center Principal Investigator
Steven N. Blair, PED (University of South Carolina)
Valerie H. Myers, PhD
Ron Monce, PA-C
Nathan E. Britt, NP
Melissa Nauta Harris, BS
Ami Parks McGucken, MPA, BS
Ruben Rodarte, MBA, MS, BS
Heidi K. Millet, MPA, BS
Catrine Tudor-Locke, PhD, FACSM
Ben P. Butitta, BS
Sheletta G. Donatto, MS, RD, LDN, CDE
Shannon H. Cocreham, BS
Stanford University, Palo Alto, CA
Abby C. King, PhD–Field Center Principal Investigator
Cynthia M. Castro, PhD William L. Haskell, PhD
Randall S. Stafford, MD, PhD Leslie A. Pruitt, PhD
Kathy Berra, MSN, NP-C, FAAN
Veronica Yank, MD
Tufts University, Boston, MA
Roger A. Fielding, PhD–Field Center Principal Investigator
Miriam E. Nelson, PhD–Field Center Co-Investigator
Sara C. Folta, PhD–Field Center Co-Investigator
Edward M. Phillips, MD
Christine K. Liu, MD
Erica C. McDavitt, MS
Kieran F. Reid, PhD, MPH
Dylan R. Kirn, BS
Evan P. Pasha, BS
Won S. Kim, BS
Vince E. Beard, BS
Eleni X. Tsiroyannis, BS
Cynthia Hau, BS, MPH
University of Florida, Gainesville, FL
Todd M. Manini, PhD–Field Center Principal Investigator
Marco Pahor, MD–Field Center Co-Investigator
Stephen D. Anton, PhD
Susan Nayfield, MD
Thomas W. Buford, PhD
Michael Marsiske, PhD
Bhanuprasad D. Sandesara, MD
Jeffrey D. Knaggs, BS
Megan S. Lorow, BS
William C. Marena, MT, CCRC
Irina Korytov, MD
Holly L. Morris, MSN, RN, CCRC (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Margo Fitch, PT (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Floris F. Singletary, MS, CCC-SLP (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Jackie Causer, BSH, RN (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Katie A. Radcliff, MA (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
University of Pittsburgh, Pittsburgh, PA
Anne B. Newman, MD, MPH–Field Center Principal Investigator
Stephanie A. Studenski, MD, MPH–Field Center Co-Investigator
Bret H. Goodpaster, PhD
Nancy W. Glynn, PhD
Oscar Lopez, MD
Neelesh K. Nadkarni, MD, PhD
Kathy Williams, RN, BSEd, MHSA
Mark A. Newman, PhD
George Grove, MS
Janet T. Bonk, MPH, RN
Jennifer Rush, MPH
Piera Kost, BA (deceased)
Diane G. Ives, MPH
Wake Forest University, Winston Salem, NC
Stephen B. Kritchevsky, Ph.D.–Field Center Principal Investigator
Anthony P. Marsh, PhD–Field Center Co-Investigator
Tina E. Brinkley, PhD
Jamehl S. Demons, MD
Kaycee M. Sink, MD, MAS
Kimberly Kennedy, BA, CCRC
Rachel Shertzer-Skinner, MA, CCRC
Abbie Wrights, MS
Rose Fries, RN, CCRC
Deborah Barr, MA, RHEd, CHES
Yale University, New Haven, CT
Thomas M. Gill, MD–Field Center Principal Investigator
Robert S. Axtell, PhD, FACSM–Field Center Co-Investigator (Southern Connecticut State University, Exercise Science Department)
Susan S. Kashaf, MD, MPH (VA Connecticut Healthcare System)
Nathalie de Rekeneire, MD, MS
Joanne M. McGloin, MDiv, MS, MBA
Karen C. Wu, RN
Denise M. Shepard, RN, MBA
Barbara Fennelly, MA, RN
Lynne P. Iannone, MS, CCRP
Raeleen Mautner, PhD
Theresa Sweeney Barnett, MS, APRN
Sean N. Halpin, MA
Matthew J. Brennan, MA
Julie A. Bugaj, MS
Maria A. Zenoni, MS
Bridget M. Mignosa, AS
Cognition Coordinating Center, Wake Forest University, Winston Salem, NC
Jeff Williamson, MD, MHS–Center Principal Investigator
Kaycee M Sink, MD, MAS–Center Co-Investigator
Hugh C. Hendrie, MB, ChB, DSc (Indiana University)
Stephen R. Rapp, PhD
Joe Verghese, MB, BS (Albert Einstein College of Medicine of Yeshiva University)
Nancy Woolard
Mark Espeland, PhD
Janine Jennings, PhD
Valerie K. Wilson, MD
Electrocardiogram Reading Center, University of Florida, Gainesville, FL
Carl J. Pepine MD, MACC
Mario Ariet, PhD
Eileen Handberg, PhD, ARNP
Daniel Deluca, BS
James Hill, MD, MS, FACC
Anita Szady, MD
Spirometry Reading Center, Yale University, New Haven, CT
Geoffrey L. Chupp, MD
Gail M. Flynn, RCP, CRFT
Thomas M. Gill, MD
John L. Hankinson, PhD (Hankinson Consulting, Inc.)
Carlos A. Vaz Fragoso, MD
Cost Effectiveness Analysis Center
Erik J. Groessl, PhD (University of California, San Diego and VA San Diego Healthcare System)
Robert M. Kaplan, PhD (Office of Behavioral and Social Sciences Research, National Institutes of Health)
Data Availability
The data underlying this study are third party data and are available to all interested researchers after receiving LIFE P&P approval via the LIFE website (www.thelifestudy.org). To submit a proposal contact the LIFE Administrative Coordinating Center at LIFEACC@ufl.aging.edu. The authors do not have any special access privileges to these data and confirm that interested researchers may apply for access to these data in the manner described.
Funding Statement
The Lifestyle Interventions and Independence for Elders Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement #UO1 AG22376 and a supplement from the National Heart, Lung and Blood Institute 3U01AG022376-05A2S, and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH. The research is partially supported by the Claude D. Pepper Older Americans Independence Centers at the University of Florida (1 P30 AG028740), Wake Forest University (1 P30 AG21332), Tufts University (1P30AG031679), University of Pittsburgh (P30 AG024827), and Yale University (P30AG021342) and the NIH/NCRR CTSA at Stanford University (UL1 RR025744). Tufts University is also supported by the Boston Rehabilitation Outcomes Center (1R24HD065688-01A1). Dr. Roger Fielding (Tufts University) is partially supported by the U.S. Department of Agriculture, under agreement No. 58-1950-4-003. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Dept. of Agriculture.
References
- 1.Buchner DM, Beresford SA, Larson EB, LaCroix AZ, Wagner EH. Effects of physical activity on health status in older adults. II. Intervention studies. Annu Rev Public Health. 1992;13:469–88. doi: 10.1146/annurev.pu.13.050192.002345 . [DOI] [PubMed] [Google Scholar]
- 2.Stuck AE, Walthert JM, Nikolaus T, Bula CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999. February;48(4):445–69. . [DOI] [PubMed] [Google Scholar]
- 3.Gardner MM, Robertson MC, Campbell AJ. Exercise in preventing falls and fall related injuries in older people: a review of randomised controlled trials. Br J Sports Med. 2000. February;34(1):7–17. doi: 10.1136/bjsm.34.1.7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillespie LD, Gillespie WJ, Robertson MC, Lamb SE, Cumming RG, Rowe BH. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev. 2001. (3):CD000340 doi: 10.1002/14651858.CD000340 . [DOI] [PubMed] [Google Scholar]
- 5.Gillespie LD, Gillespie WJ, Robertson MC, Lamb SE, Cumming RG, Rowe BH. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev. 2003. (4):CD000340 doi: 10.1002/14651858.CD000340 . [DOI] [PubMed] [Google Scholar]
- 6.Tinetti ME, Baker DI, McAvay G, Claus EB, Garrett P, Gottschalk M, et al. A multifatorial intervention to reduce risk of falling among elderly people living in the community. N Engl J Med. 1994;331:821–7. doi: 10.1056/NEJM199409293311301 [DOI] [PubMed] [Google Scholar]
- 7.Feskanich D, Willett W, Colditz G. Walking and leisure-time activity and risk of hip fracture in postmenopausal women. Jama. 2002. November 13;288(18):2300–6. . [DOI] [PubMed] [Google Scholar]
- 8.Uusi-Rasi K, Kannus P, Cheng S, Sievanen H, Pasanen M, Heinonen A, et al. Effect of alendronate and exercise on bone and physical performance of postmenopausal women: a randomized controlled trial. Bone. 2003. July;33(1):132–43. . [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez BL, Curb JD, Burchfiel CM, Abbott RD, Petrovitch H, Masaki K, et al. Physical activity and 23-year incidence of coronary heart disease morbidity and mortality among middle-aged men. The Honolulu Heart Program. Circulation. 1994. June;89(6):2540–4. . [DOI] [PubMed] [Google Scholar]
- 10.Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. Jama. 1999. October 20;282(15):1433–9. . [DOI] [PubMed] [Google Scholar]
- 11.Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH. A prospective study of exercise and incidence of diabetes among US male physicians. Jama. 1992. July 1;268(1):63–7. . [PubMed] [Google Scholar]
- 12.Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991. September 28;338(8770):774–8. . [DOI] [PubMed] [Google Scholar]
- 13.LaCroix AZ, Guralnik JM, Berkman LF, Wallace RB, Satterfield S. Maintaining mobility in late life. II. Smoking, alcohol consumption, physical activity, and body mass index. Am J Epidemiol. 1993. April 15;137(8):858–69. . [DOI] [PubMed] [Google Scholar]
- 14.Leveille SG, Guralnik JM, Ferrucci L, Langlois JA. Aging successfully until death in old age: opportunities for increasing active life expectancy. Am J Epidemiol. 1999. April 1;149(7):654–64. . [DOI] [PubMed] [Google Scholar]
- 15.Messier SP, Royer TD, Craven TE, O'Toole ML, Burns R, Ettinger WH Jr. Long-term exercise and its effect on balance in older, osteoarthritic adults: results from the Fitness, Arthritis, and Seniors Trial (FAST). J Am Geriatr Soc. 2000. February;48(2):131–8. . [DOI] [PubMed] [Google Scholar]
- 16.Nelson ME, Layne JE, Bernstein MJ, Nuernberger A, Castaneda C, Kaliton D, et al. The effects of multidimensional home-based exercise on functional performance in elderly people. J Gerontol A Biol Sci Med Sci. 2004. February;59(2):154–60. . [DOI] [PubMed] [Google Scholar]
- 17.Pahor M, Blair SN, Espeland M, Fielding R, Gill TM, Guralnik JM, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006. November;61(11):1157–65. . [DOI] [PubMed] [Google Scholar]
- 18.Rejeski WJ, Brubaker PH, Goff DC Jr., Bearon LB, McClelland JW, Perri MG, et al. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011. May 23;171(10):880–6. doi: 10.1001/archinternmed.2010.522 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDermott MM, Liu K, Guralnik JM, Criqui MH, Spring B, Tian L, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA. 2013. July 3;310(1):57–65. doi: 10.1001/jama.2013.7231 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin KA, Sinden AR. Who will stay and who will go? A review of older adults' adherence to randomized controlled trials of exercise. J Aging and Phys Activ. 2001;9:91–114. [Google Scholar]
- 21.Fielding RA, Katula J, Miller ME, Abbott-Pillola K, Jordan A, Glynn NW, et al. Activity adherence and physical function in older adults with functional limitations. Med Sci Sports Exerc. 2007. November;39(11):1997–2004. doi: 10.1249/mss.0b013e318145348d . [DOI] [PubMed] [Google Scholar]
- 22.Fielding RA, Rejeski WJ, Blair S, Church T, Espeland MA, Gill TM, et al. The Lifestyle Interventions and Independence for Elders Study: design and methods. J Gerontol A Biol Sci Med Sci. 2011. November;66(11):1226–37. doi: 10.1093/gerona/glr123 . Pubmed Central PMCID: 3193523. Epub 2011/08/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014 June 18;311(23):2387–96. doi: 10.1001/jama.2014.5616 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh AP, Lovato LC, Glynn NW, Kennedy K, Castro C, Domanchuk K, et al. Lifestyle interventions and independence for elders study: recruitment and baseline characteristics. J Gerontol A Biol Sci Med Sci. 2013. December;68(12):1549–58. doi: 10.1093/gerona/glt064 . Pubmed Central PMCID: 3814232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guralnik JM, Ferrucci K, Simonnick EM, Salive ME, Wallace RB. Lower extremity function over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000. April;55(4):M221–31. . [DOI] [PubMed] [Google Scholar]
- 27.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987. August;48(8):314–8. . [PubMed] [Google Scholar]
- 28.Rejeski WJ, Axtell R, Fielding R, Katula J, King AC, Manini TM, et al. Promoting physical activity for elders with compromised function: the lifestyle interventions and independence for elders (LIFE) study physical activity intervention. Clin Interv Aging. 2013;8:1119–31. doi: 10.2147/CIA.S49737 . Pubmed Central PMCID: 3775623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.United States. Dept. of Health and Human Services. Physical Activity guidelines Advisory Committee., United States. Dept. of Health and Human Services. Physical Activity Guidelines Advisory Committee report, 2008 to the Secretary of Health and Human Services Washington, DC: U.S. Dept. of Health and Human Services; 2008. Available from: http://www.health.gov/paguidelines/committeereport.aspx http://www.health.gov/paguidelines/Report/pdf/CommitteeReport.pdf [DOI] [PubMed]
- 30.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007. August 28;116(9):1094–105. doi: 10.1161/CIRCULATIONAHA.107.185650 . [DOI] [PubMed] [Google Scholar]
- 31.King AC, Rejeski WJ, Buchner DM. Physical activity interventions targeting older adults. A critical review and recommendations. Am J Prev Med. 1998. November;15(4):316–33. . [DOI] [PubMed] [Google Scholar]
- 32.Guralnik JM, Simonsick EM, Ferucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality in nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 33.Bann D, Hire D, Manini T, Cooper R, Botoseneanu A, McDermott MM, et al. Light Intensity physical activity and sedentary behavior in relation to body mass index and grip strength in older adults: cross-sectional findings from the Lifestyle Interventions and Independence for Elders (LIFE) study. PloS one. 2015;10(2):e0116058 doi: 10.1371/journal.pone.0116058 . Pubmed Central PMCID: 4315494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Copeland JL, Esliger DW. Accelerometer assessment of physical activity in active, healthy older adults. J Aging Phys Act. 2009. January;17(1):17–30. . [DOI] [PubMed] [Google Scholar]
- 35.Matthews CE. Calibration of accelerometer output for adults. Medicine and Science in Sports and Exercise. 2005. November;37(11):S512–S22. English. [DOI] [PubMed] [Google Scholar]
- 36.Rejeski WJ, Marsh AP, Brubaker PH, Buman M, Fielding RA, Hire D, et al. Analysis and Interpretation of Accelerometry Data in Older Adults: The LIFE Study. J Gerontol A Biol Sci Med Sci. 2015. October 29 doi: 10.1093/gerona/glv204 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001. July;33(7):1126–41. . [DOI] [PubMed] [Google Scholar]
- 38.Ringsberg KA, Gardsell P, Johnell O, Josefsson PO, Obrant KJ. The impact of long-term moderate physical activity on functional performance, bone mineral density and fracture incidence in elderly women. Gerontology. 2001. Jan-Feb;47(1):15–20. doi: 52765 . [DOI] [PubMed] [Google Scholar]
- 39.Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB, Health A, et al. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J Am Geriatr Soc. 2004. April;52(4):502–9. doi: 10.1111/j.1532-5415.2004.52154.x . [DOI] [PubMed] [Google Scholar]
- 40.Martin HJ, Syddall HE, Dennison EM, Cooper C, Sayer AA. Relationship between customary physical activity, muscle strength and physical performance in older men and women: findings from the Hertfordshire Cohort Study. Age Ageing. 2008. September;37(5):589–93. doi: 10.1093/ageing/afn148 . [DOI] [PubMed] [Google Scholar]
- 41.Chale-Rush A, Guralnik JM, Walkup MP, Miller ME, Rejeski WJ, Katula JA, et al. Relationship between physical functioning and physical activity in the lifestyle interventions and independence for elders pilot. J Am Geriatr Soc. 2010. October;58(10):1918–24. doi: 10.1111/j.1532-5415.2010.03008.x . Pubmed Central PMCID: 2952066. Epub 2010/08/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDermott MM, Greenland P, Ferrucci L, Criqui MH, Liu K, Sharma L, et al. Lower extremity performance is associated with daily life physical activity in individuals with and without peripheral arterial disease. J Am Geriatr Soc. 2002. February;50(2):247–55. . [DOI] [PubMed] [Google Scholar]
- 43.Morie M, Reid KF, Miciek R, Lajevardi N, Choong K, Krasnoff JB, et al. Habitual physical activity levels are associated with performance in measures of physical function and mobility in older men. J Am Geriatr Soc. 2010. September;58(9):1727–33. doi: 10.1111/j.1532-5415.2010.03012.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corcoran MP, Chui KK, White DK, Reid KF, Kirn D, Nelson ME, et al. Accelerometer Assessment of Physical Activity and Its Association with Physical Function in Older Adults Residing at Assisted Care Facilities. J Nutr Health Aging. 2016;20(7):752–8. doi: 10.1007/s12603-015-0640-7 . [DOI] [PubMed] [Google Scholar]
- 45.McDermott MM, Liu K, Ferrucci L, Criqui MH, Greenland P, Guralnik JM, et al. Physical performance in peripheral arterial disease: a slower rate of decline in patients who walk more. Ann Intern Med. 2006. January 3;144(1):10–20. . [DOI] [PubMed] [Google Scholar]
- 46.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. Jama. 2011. January 5;305(1):50–8. doi: 10.1001/jama.2010.1923 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwon S, Perera S, Pahor M, Katula JA, King AC, Groessl EJ, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging. 2009. June;13(6):538–44. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006. May;54(5):743–9. doi: 10.1111/j.1532-5415.2006.00701.x . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
Data Availability Statement
The data underlying this study are third party data and are available to all interested researchers after receiving LIFE P&P approval via the LIFE website (www.thelifestudy.org). To submit a proposal contact the LIFE Administrative Coordinating Center at LIFEACC@ufl.aging.edu. The authors do not have any special access privileges to these data and confirm that interested researchers may apply for access to these data in the manner described.