Abstract
Dopamine D3 receptors (D3R) modulate neuronal activity in several brain regions including cortex, striatum, cerebellum, and hippocampus. A growing body of evidence suggests that aberrant D3R signaling contributes to multiple brain diseases, such as Parkinson’s disease, essential tremor, schizophrenia, and addiction. In line with these findings, D3R has emerged as a potential target in the treatment of neurological disorders. However, the mechanisms underlying neuronal D3R signaling are poorly understood, either in healthy or diseased brain. Here, I review the molecular mechanisms involved in D3R signaling via monomeric D3R and heteromeric receptor complexes (e.g., D3R-D1R, D3R-D2R, D3R-A2aR, and D3R-D3nf). I focus on D3R signaling pathways that, according to recent reports, contribute to pathological brain states. In particular, I describe evidence on both quantitative (e.g., increased number or affinity) and qualitative (e.g., switched signaling) changes in D3R that has been associated with brain dysfunction. I conclude with a description of basic mechanisms that modulate D3R signaling such as desensitization, as disruption of these mechanisms may underlie pathological changes in D3R signaling. Because several lines of evidence support the idea that imbalances in D3R signaling alter neural function, a better understanding of downstream D3R pathways is likely to reveal novel therapeutic strategies toward dopamine-related brain disorders.
Keywords: Dopamine D3 receptor, signaling, binding, affinity, heteromers, Parkinson’s disease, essential tremor, schizophrenia, addiction
Introduction
In the 1950s, after the role of dopamine (DA) as a signaling molecule in the brain was demonstrated,1 it was reported that DA levels dramatically decrease in Parkinson’s disease (PD).2 Shortly after that, the DA precursor l-DOPA (l-3,4-dihydroxyphenylalanine) was started to be used to increase DA concentration in the brain of patients with PD.2 The temporary relief of PD symptoms by l-DOPA indicated that loss of DA signaling is the pathognomonic feature of PD. Evidence from animal models and humans has shown that, in addition to PD, DA signaling also plays a central role in several brain disorders including schizophrenia, essential tremor, attention deficit hyperactivity disorder, depression, and addiction. This plethora of DA-associated diseases closely matches with the function of the DA system in multiple brain functions such as motor coordination, emotions, memory, reward mechanisms, and neuroendocrine regulation.
The brain DA system is constituted by DA-containing nuclei (i.e., substance nigra pars compacta, ventral tegmental area, and arcuate nucleus) and by the target areas (i.e., cortex, basal ganglia, thalamus, limbic structures, and pituitary gland).1 On target areas, DA acts through five DA receptors, which belong to the superfamily of seven-transmembrane G protein–coupled receptors (GPCRs). Based on structural, biochemical, and pharmacologic criteria, DA receptors have been grouped into two main families: the D1 (D1 and D5 subtypes) and D2 (D2, D3, and D4 subtypes) families.3 Structurally, the major difference between D3 receptor (D3R) and D1 family receptors is located in the third intracellular loop and carboxyl terminal tail: D3R has a long third loop and short carboxyl terminus, whereas D1 and D5 receptors display opposite features showing short third intracellular loops and long carboxyl terminal tails. Compared with D2 and D4 receptors, D3R has a distinctive third intracellular loop.3
A growing body of evidence suggests that aberrant D3R signaling contributes to several brain disorders. Consequently, D3R has emerged as a potential therapeutic target in the treatment of major neurological disorders such as schizophrenia,4 PD,5 and addiction. However, the mechanisms underlying D3R signaling are poorly understood, either in healthy or diseased brain. Here, I review recent reports on the molecular mechanisms involved in D3R signaling via monomeric D3R and heteromeric receptor complexes. Then, I illustrate examples of qualitative and quantitative changes in D3R signaling that may contribute to several brain diseases. I conclude with a description of potential targets for the modulation of D3R signaling. Unraveling the unknown downstream signaling pathways activated by D3R in both the healthy and the diseased brain is likely to reveal new therapeutic strategies toward DA-associated disorders.
Monomeric and Heteromeric D3R Signaling
Brain D3R messenger RNA (mRNA) is detected early in development (e.g., in the embryonic days 10 and 11 of rodents)6 and continually expressed during the postnatal period.7–10 In adult rats11 and mice,12 regions that affect emotional, cognitive, and endocrine functions express D3R mRNA (e.g., nucleus accumbens, islands of Calleja, hippocampus, prefrontal cortex, hypothalamus, and striatum). Interestingly, in the mouse brain, the D3R co-expressed with D1R and D2R with regional, sex, and age-dependent differences in the co-expression pattern.12 In the human brain, D3R mRNA has been detected in nucleus accumbens and in the islands of Calleja.13 Although D3R is widely expressed in the brain, elucidating D3R signal transduction mechanisms has been challenging because D3R and D2R have similar pharmacologic profiles due to the high sequence identity and homology shared by these receptors (e.g., homology of 52% overall and of 75% in the transmembrane domain of rat sequences).11 To specifically activate D3R, many studies have been performed in heterologous expression systems. These experimental systems have revealed that D3R can be linked to a wide array of intracellular signals via its coupling to multiple G protein α subunits, such as Giα3,14 Go,15,16 Gq,17,18 and Gs.19 Consistent with its ability to activate multiple G proteins, D3R can modulate several downstream effectors including adenylyl cyclase, cyclin-dependent kinase 5 (CDK5), creatine kinase 1 (CK-1), protein kinase A (PKA), protein kinase B (PKB)/Akt, protein kinase C (PKC), phospholipase C (PLC), phospholipase D (PLD), protein phosphatase 2B (PP2B), and extracellular signal–regulated kinase (ERK).14–16,18,20–27 Moreover, under particular conditions, D3R can mediate both opposite and synergistic interactions with signaling pathways related to the production of cyclic adenosine monophosphate (cAMP).21 Despite having similar sequences and pharmacologic profiles, D3R and D2R exhibit significant biochemical differences. For instance, D2R activates ERK via a Giα-dependent pathway, whereas D3R activates ERK by a mechanism that depends on Gβγ.26
In addition to the canonical signaling via monomeric GPCRs, several reports indicate that the interaction among GPCRs (forming dimers and higher-order entities) can influence GPCR signaling both quantitatively (i.e., heteromers exhibit increased affinity for the ligand) and qualitatively (i.e., heteromers use alternative signaling pathways).28,29 In particular, D3R functionally interacts with adenosine A2a receptors (A2aR),30 D1R,31,32 D2R,33 and nicotinic acetylcholine receptors.34 The D3R-D1R interaction increases the affinity of D1R for its ligand after D3R activation, whereas no change is observed in the D3R affinity to its ligand.31 The activation of A2aR in the D3R-A2aR macromolecular complex reduces both the affinity of D3R for DA and the D3R-mediated inhibition of adenylate cyclase, whereas A2aR signaling is inhibited by D3R activation.30 Moreover, D3R interacts with its own alternatively spliced variant, named D3nf, as demonstrated by co-immunoprecipitation and colocalization experiments.35–37 The expression of D3nf reduces the ligand-binding capacity of D3R, possibly due to D3R-D3nf mislocalization from the plasma membrane to intracellular compartments.37
The D3R gene contains 6 exons and 5 introns and produces at least 7 distinct alternatively spliced variants including the full-length D3R, a shorter receptor isoform (D3S) lacking 21 amino acids within the third intracellular loop (IL3), and the truncated isoform D3nf lacking transmembrane-spanning domains 6 and 7 by a premature stop codon.38 Interestingly, D3R and D3S bind DA with high affinity, whereas the 5 additional D3R variants including D3nf do not bind DA, but they may regulate receptor dimerization.39 Overall, this evidence supports the idea that receptor-receptor interactions at neuronal surface modulate D3R signaling.
Multiple D3R-associated pathways have been described in neurons.22,27 Indeed, D3R can modulate neural activity by acting on neurotransmitter receptors via PKA, as well as on ion channels via Gαi/o, as demonstrated by electrophysiological and imaging studies in isolated neurons and brain slices from rodents.17,40–42 These studies, however, did not clarify whether D3R effects are mediated by monomeric-canonical pathways or by heteromeric receptors. In medium spiny neurons (MSNs) of the striatum, D3R modulates Ca2+ channels via PLC and PP2B43 and can also activate the Akt/mTOR/p70S6/4E-BP1 pathway,44 which play a key role in protein synthesis, synaptic plasticity, and memory. Also, D3R suppresses synaptic transmission by reducing GABAA receptor current via PKA-mediated endocytosis of GABAA receptors in the nucleus accumbens40 and in CA1 pyramidal neurons from the hippocampus.41 Actions of D3R in the brain might be region specific7 and may be quantitative and qualitatively modified under disease states, as discussed in the following sections.
D3R Signaling in Brain Diseases
Essential tremor
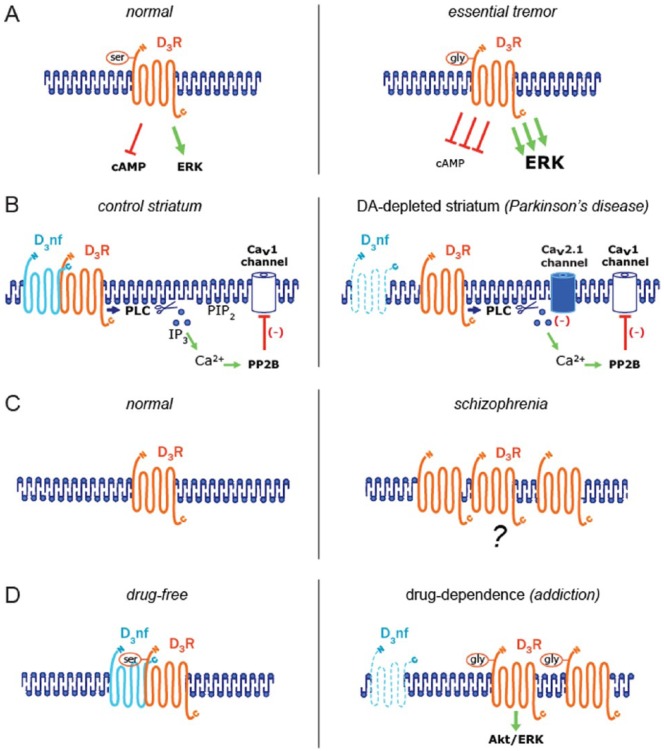
A critical insight into the role of D3R signaling in brain dysfunction was provided by studying the D3R Ser9Gly polymorphism in patients with essential tremor, the most commonly inherited movement disorder. In essential tremor, a subtle change in the D3R sequence (Ser9Gly polymorphism) increases D3R coupling to both the inhibition of cAMP formation and the activation of the MAPK pathway (Figure 1A). The enhanced D3R function, associated with the Gly-9 variant, was linked with the risk and age at onset for essential tremor.45 Further supporting the primary role of the gain of function of D3R in pathological DA signaling, the D3R-Gly-9 variant has been associated with impulsive behavior in PD, the second most prevalent neurodegenerative disease. Thus, these data suggest that an enhanced D3R signaling could impair reward-risk assessment in the mesolimbic system and contribute to the development of impulsive behavior, in carriers of this genotype. Overall, these data suggest that a gain of function in two D3R signaling pathways significantly contributes to brain dysfunction. It is noteworthy that the association of the D3R Ser9Gly polymorphism with essential tremor has been found to be significant in American, French,45 and Spanish46 but not in Italian47 or Asian48 populations.
Figure 1.
D3R in brain diseases. Based on recent reports, all models show molecular D3R changes/variants associated with specific pathological conditions. (A) The D3R-Gly-9 variant has been linked with the risk and age at onset for essential tremor. Relative to the D3R-Ser-9 variant, the D3R-Gly-9 is more efficient in both the inhibition of cAMP formation and the activation of ERK. (B) Under normal DA levels (control striatum), D3R interacts with D3nf and modulates CaV1 (L-type) channels through a PLC/IP3/Ca2+/PP2B signaling pathway, whereas after chronic DA depletion (as seen in PD), D3R-D3nf interaction is reduced as a result of the D3nf downregulation. Membrane redistribution of the “D3nf-free” D3R may situate it near the CaV2.1 channels, thus allowing channels to sense phosphatidylinositol-4,5-biphosphate depletion and reduce their opening after PLC activation by D3R. (C) Some postmortem studies suggest that brain D3R levels may be elevated in schizophrenia. (D) Long-lasting neuroadaptations following chronic drug use include an enhancement in both D3R expression and D3R-dependent signaling (Akt and ERK activation). Also, the functionally enhanced D3R-Gly-9 variant has been associated with drug-dependence. D3R indicates D3 receptor; DA, dopamine; ERK, extracellular signal–regulated kinase; PLC, phospholipase C.
Parkinson’s disease
Supporting the idea that a gain of function of D3R can impair brain physiology, recent evidence suggests that D3R activity is enhanced in PD,43,49 a neurodegenerative disease characterized by progressive loss of dopaminergic neurons in the substantia nigra pars compacta. Loss of dopaminergic neurons is accompanied by a dramatic reduction in DA levels in the striatum.50–52 Several reports have shown that DA depletion induces compensatory mechanisms (e.g., an increase in the number and the affinity of receptors), similar to that observed after ligand depletion in other receptor-ligand systems.53,54 For instance, positron emission tomography using [11C]raclopride has shown an increased density of D2-class receptors in the putamen nucleus in PD.55 Also, biochemical, electrophysiological, and behavioral data have shown that DA depletion increases the sensitivity (supersensitivity) of D2-class receptors in animals models of PD (e.g., DA depletion induced by reserpine,56 α-methyl-p-tyrosine,57 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine [MPTP],58 and 6-hydroxydopamine [6-OHDA]).49,59,60 In the 6-OHDA model, electrophysiological recordings in striatal MSNs have shown that D3R signaling contributes to the D2-class supersensitivity,43,49 thus suggesting that DA depletion may increase D3R expression and activity.
The enhancement of D3R signaling by DA depletion may be reflecting changes in pharmacologic parameters (potency, affinity, and receptor number). At present, however, there is no consensus on which pharmacologic parameter is mainly affected.10,11,61–64 Although unilateral lesion of the substantia nigra with 6-OHDA increases the potency of the selective D3R agonist 7-OH-DPAT in striatum65 and cerebellum,66 pharmacologic depletion of catecholamines increases the affinity of putative D3 sites with no increase in the total number of sites in striatum.67 An alternative mechanism underlying the enhancement of D3R signaling following DA depletion may rely on a reduced interaction of D3R with its regulatory splice variant D3nf. Indeed, D3nf protein levels were found to be reduced in striatal homogenates from 6-OHDA lesioned rats.43 In line with a reduction in D3R-D3nf interaction after DA depletion, the D3R/D3nf mRNA ratio increases when D3R is pharmacologically blocked (i.e., mimicking “hypodopaminergic” states).68
According to recent reports, qualitative changes in D3R signaling may also contribute to the pathophysiology of PD. In particular, a switch in D3R signaling has been described in striatal MSNs and striatonigral terminals of hemiparkinsonian rats,43,69 a PD model based on the 6-OHDA–mediated lesion of substantia nigra pars compacta in one hemisphere. In striatal MSNs, while physiological D3R signaling inhibits CaV1 (L-type) Ca2+ channels by PP2B activation, D3R additionally modulates CaV2.1 (P/Q-type) channels via the hydrolysis of phosphatidylinositol-4,5-biphosphate (PIP2) after DA depletion (Figure 1B). This CaV2.1 modulation by the D3R-PIP2 pathway is not detected in neurons from control animals, thus suggesting that this pathway is induced by DA depletion.43 Because CaV2.1 channels mediate γ-aminobutyric acid (GABA) release from the terminals of striatal neurons, the PIP2-dependent D3R signaling may reduce GABA release and affect synaptic transmission between striatal neurons after DA depletion.
In striatonigral terminals, the switch in D3R signaling is related to the D3R-D1R interaction.69 Although D3R activation potentiates both the stimulation of GABA release and the production of cAMP by D1R in terminals from the control (nonlesioned) hemisphere, D3R inhibited both D1R actions in terminals from the denervated hemisphere.69 Overall, data from MSNs and striatonigral terminals support the notion that DA depletion switches D3R signaling in basal ganglia.
l-DOPA, the gold standard treatment for PD, reduces parkinsonian symptoms but in later stages induces dyskinesia, a side effect characterized by hyperkinetic involuntary movements.70 Notably, l-DOPA increases D3R expression in the caudate/putamen nuclei and striatonigral MSNs.62,63,71–73 Recent evidence indicates that D3R plays a major role in l-DOPA–induced dyskinesia, which can be reduced by blocking D3R73–75 and D1R75 in parkinsonian models. Interestingly, in MPTP parkinsonian primates, a partial block of D3R (using a partial agonist for D3R) reduces dyskinesia without affecting the therapeutic effects of l-DOPA, whereas no recovery of motor disturbances is observed if D3R is entirely blocked.74 These data emphasize that D3R mediates different actions, participating in both dyskinesia and motor recovery following l-DOPA treatment, thus highlighting the importance of fine-tuning D3R signals in PD-diseased brain.
Schizophrenia
Dopamine signaling is the main target in schizophrenia, a mental disorder characterized by hallucinations, bizarre delusions, and negative symptoms such as lack of motivation, reduction in spontaneous speech, and social withdrawal. Indeed, first-generation antipsychotics (e.g., haloperidol and chlorpromazine) bind to D2-class receptors.76,77 Since its cloning in 1990, D3R emerged as a potential target for antipsychotics.11,78 Although it is controversial whether D3R levels are affected in schizophrenia,79 postmortem studies suggest that D3R levels may be elevated in people with schizophrenia who are off antipsychotics (Figure 1C).4 In contrast, the parietal cortex of postmortem tissue from long-term hospitalized patients with chronic schizophrenia expresses low D3R levels.80 These data suggest that long-term antipsychotic medication may modify brain D3R expression in schizophrenia. Interestingly, low D3R mRNA levels in patients with schizophrenia have been associated with an enhanced D3nf-specific splicing of D3R pre-mRNA.81
Addiction
Chronic drug use induces long-lasting neuroadaptations which has been associated with dopaminergic abnormalities. Although studies of D3R expression in human cocaine-dependent subjects have had conflicting results,39 the use of the D3R-preferring radioligand [11C](+)PHNO has shown higher number of available D3R in the substantia nigra, hypothalamus, and amygdala of cocaine addicts, compared with healthy controls82 (Figure 1D). Notably, substantia nigra D3R levels correlated with years of cocaine use.82 Consistent with the idea that chronic cocaine exposure leads to adaptive increases in D3R expression, a 6-fold increase in D3R mRNA levels was found in the nucleus accumbens of cocaine overdose victims, as compared with age-matched and drug-free control subjects.83 Similarly, an increased [11C](+)PHNO binding, reflecting higher D3R levels, has also been observed in the substantia nigra of methamphetamine users,84 as well as in hypothalamus of alcohol-dependent patients.85 According to the heightened D3R signaling in drug users, the functionally enhanced D3R-Gly-9 variant was associated with the development of early-onset heroin dependence in Chinese population (Figure 1D).86
Studies in vitro and in animal models support the idea that drug use induces D3R signaling abnormalities. In vitro, cocaine increases dendritic arborization and soma area in cultured dopaminergic neurons from mouse via D3R-dependent activation of ERK and Akt.87 In rats, it has been suggested that D3R play an important role in mediating nicotine’s effects on the brain.88 In both adolescents and adult rats, nicotine upregulates D3R but reduces D3nf mRNA levels in the nucleus accumbens, thus increasing the D3R/D3nf ratio (Figure 1D).88 It has also been proposed that D3R and its alternatively spliced isoform D3nf may play a role in cocaine addiction (a risk factor for schizophrenia) and behavioral sensitization (the progressive and long-lasting augmentation of certain behaviors following repetitive stimulant drug administration).39 Supporting this idea, D3R has been found to enhance the reinforcing effect of cocaine,89 and blockade of D3Rs inhibits cocaine’s rewarding effects and relapse to drug-seeking behavior in rats.90 A cornerstone study demonstrated the crucial role of D3R signaling in the motivation to take drug induced by drug-related cues.91 In this study, rats were trained to self-administer cocaine by a lever pressing. Next, progressively, a light stimulus was associated with cocaine self-administration. Light stimulus, which then becomes the conditioned stimulus, gains reinforcing properties and finally maintains drug-seeking behavior even without drug delivery. Remarkably, the selective D3R partial agonist BP897 dose-dependently reduced cue-induced cocaine-seeking behavior in rats trained under this schedule of reinforcement.91
Overall, multiple lines of evidence support the idea that D3R plays a central role in addiction, thus driving a need to develop novel pharmacologic agents targeting this receptor. Since 2005, 110 patents or patent applications have been published on original compounds with D3R selectivity.79 In contrast to the initial lack of D3R-selective drugs, a list of compounds with relative high selectivity for D3R currently includes the following: BP 897, SB-277011A, S33084, ABT-925, GSK598809, and F17141.79 In particular, GSK598809 has proven to transiently alleviate craving in smokers after overnight abstinence, thus providing the first clinical evidence for a usefulness of D3R antagonist for the treatment of addiction.92
Emerging perspectives for improving the treatment of brain disorders focus on the understanding of pathophysiology mechanisms to identify disease pathways, which will finally facilitate the selection of therapeutic targets.93 In DA-related brain diseases, quantitative and qualitative changes in D3R signaling may be reflecting direct effects on basic mechanisms that control D3R activity at the cell surface, such as desensitization and internalization. A better understanding of these basic mechanisms in both the healthy and the diseased brain is likely to reveal new molecular targets for therapeutic.
Modulation of D3R Signaling
Dopamine receptors are modulated by several mechanisms, such as homologous and heterologous desensitization, a reversible reduction in signal transduction after acute activation. Experimental and theoretical data suggest that D3R desensitization (tolerance) can develop after its structural rearrangement following agonist binding, and that the magnitude of the second D3R response can be reduced by 60% compared with the first response.94 Also, D3R can be desensitized following phosphorylation by both PKC95 and Ca2+/calmodulin-dependent protein kinase II (CaMKII).96 Both PKC and CaMKII phosphorylate the 229-serine residue at the IL3, a region that exhibits high divergence between D2R and D3R, and may play a crucial role in the unique signaling signatures of each receptor subtype.97 Downregulation of D3R signaling by CaMKII depends on intracellular Ca2+ levels and, therefore, is associated with neuronal activity.96 Importantly, the CaMKII-mediated inhibition of D3R is not observed after DA depletion.69
Although D3R undergoes limited agonist-induced internalization,98 D1R-D3R heterodimers can be internalized in response to the paired stimulation of both D1R and D3R via a β-arrestin–dependent mechanism in human embryonic kidney 293 cells.32 Also, a recent report showed that PKC-mediated phosphorylation of D3R can induce clathrin-mediated D3R endocytosis and lysosomal D3R degradation.95 Similarly, via caveolin/clathrin-mediated D3R internalization, dysbindin-1 reduces the magnitude and potency of DA-induced cAMP production and phosphorylation of ERK1/2 and Akt.99 Notably, dysbindin-1 is a candidate gene for schizophrenia. Palmitoylation is another posttranslational modification that can regulate D3R activity. Compared with D2R, D3R undergoes a more extensive palmitoylation on its cysteine residues at the carboxyl terminus tail and, importantly, palmitoylation was found to be essential for cell surface expression, PKC-mediated endocytosis, agonist affinity, and agonist-induced tolerance of D3R.100 Also, DA receptor–interacting proteins, such as AIP1 (ALG-2 interacting protein 1) could be substantial in D3R stability and trafficking.101
Concluding Remarks and Perspectives
In the past few years, great progress has been made in understanding D3R signaling in heterologous systems. Although neural D3R signaling is incompletely understood, a growing body of evidence indicates that changes in D3R-induced pathways contribute to several neurological disorders. Future studies may delineate the relative contribution of monomeric-canonical versus heteomeric D3R signaling to pathological brain states. Another intriguing aspect that remains to be clarified is whether sex-specific differences influence D3R signaling in the diseased brain, as suggested by the association of the Gly allele of the D3R Ser9Gly polymorphism with schizophrenia in female but not male patients.102,103
According to several reports, changes in both D3R signaling and DA levels are common factors in PD and schizophrenia. Similarly, quantitative and qualitative changes in D3R signaling develop after DA depletion in the 6-OHDA hemiparkinsonian model.43,69 However, it remains to be clarified whether the altered D3R signaling is reflecting an adaptive mechanism to compensate changes in DA levels. Some properties of D3R strongly suggest that D3R is the main detector of changes in extracellular DA concentration and, consequently, D3R signaling may be mainly affected by pathological changes in DA levels, as those observed in PD and schizophrenia. In particular, presynaptic and postsynaptic sites can contain D3R,104,105 which exhibits high affinity for DA (~25 nM).11,97,106 Considering basal extracellular (5-10 nM) and synaptic (50 nM) concentrations of DA,107–110 a fraction of D3R may be constitutively activated, thus playing a crucial role in both tonic and phasic DA signaling.67 Interestingly, the occupancy of D3R by DA is not completely abolished by catecholamine depletion using reserpine,67,111 suggesting that even under severe reduction in DA level, D3R is partially activated and able to perceive changes in DA concentration. Thus, a tempting but simplified model for PD may rely on an enhancement in striatal D3R signaling as part of an adaptive mechanism to compensate the dramatic reduction in DA levels.
Although there is a clear role of D3R signaling in drug-seeking behaviors and relapse in animal models, it is uncertain whether these findings translate to humans. Studies in humans could evaluate the significance of D3R signaling in neurological diseases. However, direct evidence of molecular mechanisms in the human brain has been elusive, primarily due to methodological limitations. Notably, novel approaches allow the study of functional responses, including receptor signaling, in postmortem human tissue.112,113 Using these novel approaches directly in human brain tissue, future studies will help to identify mechanisms that underlie pathological D3R signaling, either induced by quantitative or qualitative changes.
In summary, many brain disorders are characterized by perturbations in D3R signaling. A better understanding of how D3R activity can be regulated may uncover causal factors and may even suggest novel treatments for DA-related diseases. In particular, elucidating the mechanisms that control expression, desensitization, and alternative splicing of D3R may identify novel opportunities to modulate D3R signaling.
Footnotes
Peer review:Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 897 words, excluding any confidential comments to the academic editor.
Funding:The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The author was supported by the Journal Disease Models & Mechanisms to study D3R signaling (Travel Fellowship by The Company of Biologists’ journals). Also, the author was the recipient of fellowships from the non-profit organization ‘Fundación Alberto y Dolores Andrade’, and from the International Brain Research Organization (IBRO).
Declaration of conflicting interests:The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: GAP: Wrote the first draft of the manuscript, developed the structure and arguments for the paper, made critical revisions, reviewed and approved the final manuscript.
References
- 1. Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004;61:641–644. [DOI] [PubMed] [Google Scholar]
- 2. Hornykiewicz O. Oleh Hornykiewicz. In: Squire LR, ed. The History of Neuroscience in Autobiography. Vol 4 San Diego, CA: Academic Press; 2004:240–281. [Google Scholar]
- 3. Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. [DOI] [PubMed] [Google Scholar]
- 4. Joyce JN, Millan MJ. Dopamine D3 receptor antagonists as therapeutic agents. Drug Discov Today. 2005;10:917–925. [DOI] [PubMed] [Google Scholar]
- 5. Scheller D, Ullmer C, Berkels R, Gwarek M, Lubbert H. The in vitro receptor profile of rotigotine: a new agent for the treatment of Parkinson’s disease. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:73–86. [DOI] [PubMed] [Google Scholar]
- 6. Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- 7. Araki KY, Sims JR, Bhide PG. Dopamine receptor mRNA and protein expression in the mouse corpus striatum and cerebral cortex during pre- and postnatal development. Brain Res. 2007;1156:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stanwood GD, McElligot S, Lu L, McGonigle P. Ontogeny of dopamine D3 receptors in the nucleus accumbens of the rat. Neurosci Lett. 1997;223:13–16. [DOI] [PubMed] [Google Scholar]
- 9. Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 2001;411:86–89. [DOI] [PubMed] [Google Scholar]
- 10. Gurevich EV, Himes JW, Joyce JN. Developmental regulation of expression of the D3 dopamine receptor in rat nucleus accumbens and islands of Calleja. J Pharmacol Exp Ther. 1999;289:587–598. [PubMed] [Google Scholar]
- 11. Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Kuzhikandathil EV. Molecular characterization of individual D3 dopamine receptor-expressing cells isolated from multiple brain regions of a novel mouse model. Brain Struct Funct. 2012;217:809–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Landwehrmeyer B, Mengod G, Palacios JM. Dopamine D3 receptor mRNA and binding sites in human brain. Brain Res Mol Brain Res. 1993;18:187–192. [DOI] [PubMed] [Google Scholar]
- 14. Pedrosa R, Gomes P, Hopfer U, Jose PA, Soares-da-Silva P. Gialpha3 protein-coupled dopamine D3 receptor-mediated inhibition of renal NHE3 activity in SHR proximal tubular cells is a PLC-PKC-mediated event. Am J Physiol Renal Physiol. 2004;287:F1059–F1066. [DOI] [PubMed] [Google Scholar]
- 15. Zaworski PG, Alberts GL, Pregenzer JF, Im WB, Slightom JL, Gill GS. Efficient functional coupling of the human D3 dopamine receptor to G(o) subtype of G proteins in SH-SY5Y cells. Br J Pharmacol. 1999;128:1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chio CL, Lajiness ME, Huff RM. Activation of heterologously expressed D3 dopamine receptors: comparison with D2 dopamine receptors. Mol Pharmacol. 1994;45:51–60. [PubMed] [Google Scholar]
- 17. Kuzhikandathil EV, Oxford GS. Activation of human D3 dopamine receptor inhibits P/Q-type calcium channels and secretory activity in AtT-20 cells. J Neurosci. 1999;19:1698–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cussac D, Newman-Tancredi A, Pasteau V, Millan MJ. Human dopamine D(3) receptors mediate mitogen-activated protein kinase activation via a phosphatidylinositol 3-kinase and an atypical protein kinase C-dependent mechanism. Mol Pharmacol. 1999;56:1025–1030. [DOI] [PubMed] [Google Scholar]
- 19. Ilani T, Fishburn CS, Levavi-Sivan B, Carmon S, Raveh L, Fuchs S. Coupling of dopamine receptors to G proteins: studies with chimeric D2/D3 dopamine receptors. Cell Mol Neurobiol. 2002;22:47–56. [DOI] [PubMed] [Google Scholar]
- 20. Hellstrand M, Danielsen EA, Steen VM, Ekman A, Eriksson E, Nilsson CL. The ser9gly SNP in the dopamine D3 receptor causes a shift from cAMP related to PGE2 related signal transduction mechanisms in transfected CHO cells. J Med Genet. 2004;41:867–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Griffon N, Pilon C, Sautel F, Schwartz JC, Sokoloff P. Two intracellular signaling pathways for the dopamine D3 receptor: opposite and synergistic interactions with cyclic AMP. J Neurochem. 1997;68:1–9. [DOI] [PubMed] [Google Scholar]
- 22. Beaulieu JM, Tirotta E, Sotnikova TD, et al. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27:881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Everett PB, Senogles SE. D3 dopamine receptor activates phospholipase D through a pertussis toxin-insensitive pathway. Neurosci Lett. 2004;371:34–39. [DOI] [PubMed] [Google Scholar]
- 24. Chen PC, Lao CL, Chen JC. The D(3) dopamine receptor inhibits dopamine release in PC-12/hD3 cells by autoreceptor signaling via PP-2B, CK1, and Cdk-5. J Neurochem. 2009;110:1180–1190. [DOI] [PubMed] [Google Scholar]
- 25. Bruins Slot LA, Palmier C, Tardif S, Cussac D. Action of novel antipsychotics at human dopamine D3 receptors coupled to G protein and ERK1/2 activation. Neuropharmacology. 2007;53:232–241. [DOI] [PubMed] [Google Scholar]
- 26. Beom S, Cheong D, Torres G, Caron MG, Kim KM. Comparative studies of molecular mechanisms of dopamine D2 and D3 receptors for the activation of extracellular signal-regulated kinase. J Biol Chem. 2004;279:28304–28314. [DOI] [PubMed] [Google Scholar]
- 27. Zhang L, Lou D, Jiao H, et al. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Franco R. Neurotransmitter receptor heteromers in neurodegenerative diseases and neural plasticity. J Neural Transm (Vienna). 2009;116:983–987. [DOI] [PubMed] [Google Scholar]
- 29. Ferre S, Baler R, Bouvier M, et al. Building a new conceptual framework for receptor heteromers. Nat Chem Biol. 2009;5:131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Torvinen M, Marcellino D, Canals M, et al. Adenosine A2A receptor and dopamine D3 receptor interactions: evidence of functional A2A/D3 heteromeric complexes. Mol Pharmacol. 2005;67:400–407. [DOI] [PubMed] [Google Scholar]
- 31. Marcellino D, Ferre S, Casado V, et al. Identification of dopamine D1-D3 receptor heteromers. Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J Biol Chem. 2008;283:26016–26025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol Pharmacol. 2008;74:59–69. [DOI] [PubMed] [Google Scholar]
- 33. Scarselli M, Novi F, Schallmach E, et al. D2/D3 dopamine receptor heterodimers exhibit unique functional properties. J Biol Chem. 2001;276:30308–30314. [DOI] [PubMed] [Google Scholar]
- 34. Bontempi L, Savoia P, Bono F, Fiorentini C, Missale C. Dopamine D3 and acetylcholine nicotinic receptor heteromerization in midbrain dopamine neurons: relevance for neuroplasticity. Eur Neuropsychopharmacol. 2017;27:313–324. [DOI] [PubMed] [Google Scholar]
- 35. Nimchinsky EA, Hof PR, Janssen WG, Morrison JH, Schmauss C. Expression of dopamine D3 receptor dimers and tetramers in brain and in transfected cells. J Biol Chem. 1997;272:29229–29237. [DOI] [PubMed] [Google Scholar]
- 36. Elmhurst JL, Xie Z, O’Dowd BF, George SR. The splice variant D3nf reduces ligand binding to the D3 dopamine receptor: evidence for heterooligomerization. Brain Res Mol Brain Res. 2000;80:63–74. [DOI] [PubMed] [Google Scholar]
- 37. Karpa KD, Lin R, Kabbani N, Levenson R. The dopamine D3 receptor interacts with itself and the truncated D3 splice variant d3nf: d3-D3nf interaction causes mislocalization of D3 receptors. Mol Pharmacol. 2000;58:677–683. [DOI] [PubMed] [Google Scholar]
- 38. Liu K, Bergson C, Levenson R, Schmauss C. On the origin of mRNA encoding the truncated dopamine D3-type receptor D3nf and detection of D3nf-like immunoreactivity in human brain. J Biol Chem. 1994;269:29220–29226. [PubMed] [Google Scholar]
- 39. Richtand NM. Behavioral sensitization, alternative splicing, and D3 dopamine receptor-mediated inhibitory function. Neuropsychopharmacology. 2006;31:2368–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen G, Kittler JT, Moss SJ, Yan Z. Dopamine D3 receptors regulate GABAA receptor function through a phospho-dependent endocytosis mechanism in nucleus accumbens. J Neurosci. 2006;26:2513–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Swant J, Stramiello M, Wagner JJ. Postsynaptic dopamine D3 receptor modulation of evoked IPSCs via GABA(A) receptor endocytosis in rat hippocampus. Hippocampus. 2008;18:492–502. [DOI] [PubMed] [Google Scholar]
- 42. Mizuno T, Schmauss C, Rayport S. Distinct roles of presynaptic dopamine receptors in the differential modulation of the intrinsic synapses of medium-spiny neurons in the nucleus accumbens. BMC Neurosci. 2007;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Prieto GA, Perez-Burgos A, Palomero-Rivero M, Galarraga E, Drucker-Colin R, Bargas J. Upregulation of D2-class signaling in dopamine-denervated striatum is in part mediated by D3 receptors acting on Ca V 2.1 channels via PIP2 depletion. J Neurophysiol. 2011;105:2260–2274. [DOI] [PubMed] [Google Scholar]
- 44. Salles MJ, Herve D, Rivet JM, et al. Transient and rapid activation of Akt/GSK-3beta and mTORC1 signaling by D3 dopamine receptor stimulation in dorsal striatum and nucleus accumbens. J Neurochem. 2013;125:532–544. [DOI] [PubMed] [Google Scholar]
- 45. Jeanneteau F, Funalot B, Jankovic J, et al. A functional variant of the dopamine D3 receptor is associated with risk and age-at-onset of essential tremor. Proc Natl Acad Sci U S A. 2006;103:10753–10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Garcia-Martin E, Martinez C, Alonso-Navarro H, et al. Dopamine receptor D3 (DRD3) genotype and allelic variants and risk for essential tremor. Mov Disord. 2009;24:1910–1915. [DOI] [PubMed] [Google Scholar]
- 47. Vitale C, Gulli R, Ciotti P, et al. DRD3 Ser9Gly variant is not associated with essential tremor in a series of Italian patients. Eur J Neurol. 2008;15:985–987. [DOI] [PubMed] [Google Scholar]
- 48. Tan EK, Prakash KM, Fook-Chong S, et al. DRD3 variant and risk of essential tremor. Neurology. 2007;68:790–791. [DOI] [PubMed] [Google Scholar]
- 49. Prieto GA, Perez-Burgos A, Fiordelisio T, et al. Dopamine D(2)-class receptor supersensitivity as reflected in Ca2+ current modulation in neostriatal neurons. Neuroscience. 2009;164:345–350. [DOI] [PubMed] [Google Scholar]
- 50. Hornykiewicz O. The mechanisms of action of L-dopa in Parkinson’s disease. Life Sci. 1974;15:1249–1259. [DOI] [PubMed] [Google Scholar]
- 51. Chesselet MF, Delfs JM. Basal ganglia and movement disorders: an update. Trends Neurosci. 1996;19:417–422. [DOI] [PubMed] [Google Scholar]
- 52. Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. [DOI] [PubMed] [Google Scholar]
- 53. Benson DM, Blitzer RD, Haroutunian V, Landau EM. Functional muscarinic supersensitivity in denervated rat hippocampus. Brain Res. 1989;478:399–402. [DOI] [PubMed] [Google Scholar]
- 54. Cangiano A. Denervation supersensitivity as a model for the neural control of muscle. Neuroscience. 1985;14:963–971. [DOI] [PubMed] [Google Scholar]
- 55. Rinne JO, Laihinen A, Ruottinen H, et al. Increased density of dopamine D2 receptors in the putamen, but not in the caudate nucleus in early Parkinson’s disease: a PET study with [11C]raclopride. J Neurol Sci. 1995;132:156–161. [DOI] [PubMed] [Google Scholar]
- 56. Trugman JM, James CL. Rapid development of dopaminergic supersensitivity in reserpine-treated rats demonstrated with 14C-2-deoxyglucose autoradiography. J Neurosci. 1992;12:2875–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Verhoeff NP, Hussey D, Lee M, et al. Dopamine depletion results in increased neostriatal D(2), but not D(1), receptor binding in humans. Mol Psychiatry. 2002;7:233, 322–328. [DOI] [PubMed] [Google Scholar]
- 58. Chefer SI, Kimes AS, Matochik JA, et al. Estimation of D2-like receptor occupancy by dopamine in the putamen of hemiparkinsonian Monkeys. Neuropsychopharmacology. 2008;33:270–278. [DOI] [PubMed] [Google Scholar]
- 59. Hu XT, Wachtel SR, Galloway MP, White FJ. Lesions of the nigrostriatal dopamine projection increase the inhibitory effects of D1 and D2 dopamine agonists on caudate-putamen neurons and relieve D2 receptors from the necessity of D1 receptor stimulation. J Neurosci. 1990;10:2318–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cai G, Zhen X, Uryu K, Friedman E. Activation of extracellular signal-regulated protein kinases is associated with a sensitized locomotor response to D(2) dopamine receptor stimulation in unilateral 6-hydroxydopamine-lesioned rats. J Neurosci. 2000;20:1849–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hurley MJ, Stubbs CM, Jenner P, Marsden CD. D3 receptor expression within the basal ganglia is not affected by Parkinson’s disease. Neurosci Lett. 1996;214:75–78. [DOI] [PubMed] [Google Scholar]
- 62. St-Hilaire M, Landry E, Levesque D, Rouillard C. Denervation and repeated L-DOPA induce complex regulatory changes in neurochemical phenotypes of striatal neurons: implication of a dopamine D1-dependent mechanism. Neurobiol Dis. 2005;20:450–460. [DOI] [PubMed] [Google Scholar]
- 63. Morissette M, Goulet M, Grondin R, et al. Associative and limbic regions of monkey striatum express high levels of dopamine D3 receptors: effects of MPTP and dopamine agonist replacement therapies. Eur J Neurosci. 1998;10:2565–2573. [DOI] [PubMed] [Google Scholar]
- 64. Quik M, Police S, He L, Di Monte DA, Langston JW. Expression of D(3) receptor messenger RNA and binding sites in monkey striatum and substantia nigra after nigrostriatal degeneration: effect of levodopa treatment. Neuroscience. 2000;98:263–273. [DOI] [PubMed] [Google Scholar]
- 65. Sato K, Ueda H, Okumura F, Misu Y. 6-OHDA-induced lesion of the nigrostriatal dopaminergic neurons potentiates the inhibitory effect of 7-OHDPAT, a selective D3 agonist, on acetylcholine release during striatal microdialysis in conscious rats. Brain Res. 1994;655:233–236. [DOI] [PubMed] [Google Scholar]
- 66. Ishibashi T, Wakabayashi J, Ohno Y. 7-Hydroxy-N,N’-di-n-propyl-2-aminotetraline, a preferential dopamine D3 agonist, induces c-fos mRNA expression in the rat cerebellum. Jpn J Pharmacol. 2002;89:309–315. [DOI] [PubMed] [Google Scholar]
- 67. Levant B. Differential sensitivity of [3H]7-OH-DPAT-labeled binding sites in rat brain to inactivation by N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline. Brain Res. 1995;698:146–154. [DOI] [PubMed] [Google Scholar]
- 68. Richtand NM, Liu Y, Ahlbrand R, Sullivan JR, Newman AH, McNamara RK. Dopaminergic regulation of dopamine D3 and D3nf receptor mRNA expression. Synapse. 2010;64:634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Avalos-Fuentes A, Albarran-Bravo S, Loya-Lopez S, et al. Dopaminergic denervation switches dopamine D3 receptor signaling and disrupts its Ca(2+) dependent modulation by CaMKII and calmodulin in striatonigral projections of the rat. Neurobiol Dis. 2015;74:336–346. [DOI] [PubMed] [Google Scholar]
- 70. Mercuri NB, Bernardi G. The “magic” of L-dopa: why is it the gold standard Parkinson’s disease therapy? Trends Pharmacol Sci. 2005;26:341–344. [DOI] [PubMed] [Google Scholar]
- 71. Sanchez-Pernaute R, Jenkins BG, Choi JK, Iris Chen YC, Isacson O. In vivo evidence of D3 dopamine receptor sensitization in parkinsonian primates and rodents with L-DOPA-induced dyskinesias. Neurobiol Dis. 2007;27:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC. Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc Natl Acad Sci U S A. 1997;94:3363–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bordet R, Ridray S, Schwartz JC, Sokoloff P. Involvement of the direct striatonigral pathway in levodopa-induced sensitization in 6-hydroxydopamine-lesioned rats. Eur J Neurosci. 2000;12:2117–2123. [DOI] [PubMed] [Google Scholar]
- 74. Bezard E, Ferry S, Mach U, et al. Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nat Med. 2003;9:762–767. [DOI] [PubMed] [Google Scholar]
- 75. Monville C, Torres EM, Dunnett SB. Validation of the l-dopa-induced dyskinesia in the 6-OHDA model and evaluation of the effects of selective dopamine receptor agonists and antagonists. Brain Res Bull. 2005;68:16–23. [DOI] [PubMed] [Google Scholar]
- 76. Farde L, Nordstrom AL. PET examination of central D2 dopamine receptor occupancy in relation to extrapyramidal syndromes in patients being treated with neuroleptic drugs. Psychopharmacol Ser. 1993;10:94–100. [DOI] [PubMed] [Google Scholar]
- 77. van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–645. [DOI] [PubMed] [Google Scholar]
- 78. Sokoloff P, Diaz J, Le Foll B, et al. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5:25–43. [DOI] [PubMed] [Google Scholar]
- 79. Sokoloff P, Le Foll B. The dopamine D3 receptor, a quarter century later. Eur J Neurosci. 2016;45:2–19. [DOI] [PubMed] [Google Scholar]
- 80. Schmauss C, Haroutunian V, Davis KL, Davidson M. Selective loss of dopamine D3-type receptor mRNA expression in parietal and motor cortices of patients with chronic schizophrenia. Proc Natl Acad Sci U S A. 1993;90: 8942–8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Schmauss C. Enhanced cleavage of an atypical intron of dopamine D3-receptor pre-mRNA in chronic schizophrenia. J Neurosci. 1996;16:7902–7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Matuskey D, Gallezot JD, Pittman B, et al. Dopamine D(3) receptor alterations in cocaine-dependent humans imaged with [(1)(1)C](+)PHNO. Drug Alcohol Depend. 2014;139:100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Segal DM, Moraes CT, Mash DC. Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Brain Res Mol Brain Res. 1997;45:335–339. [DOI] [PubMed] [Google Scholar]
- 84. Boileau I, Payer D, Rusjan PM, et al. Heightened dopaminergic response to amphetamine at the D3 dopamine receptor in methamphetamine users. Neuropsychopharmacology. 2016;41:2994–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Erritzoe D, Tziortzi A, Bargiela D, et al. In vivo imaging of cerebral dopamine D3 receptors in alcoholism. Neuropsychopharmacology. 2014;39:1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kuo SC, Yeh YW, Chen CY, et al. DRD3 variation associates with early-onset heroin dependence, but not specific personality traits. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:1–8. [DOI] [PubMed] [Google Scholar]
- 87. Collo G, Bono F, Cavalleri L, et al. Pre-synaptic dopamine D(3) receptor mediates cocaine-induced structural plasticity in mesencephalic dopaminergic neurons via ERK and Akt pathways. J Neurochem. 2012;120:765–778. [DOI] [PubMed] [Google Scholar]
- 88. Smith LN, Bachus SE, McDonald CG, Smith RF. Role of the D3 dopamine receptor in nicotine sensitization. Behav Brain Res. 2015;289:92–104. [DOI] [PubMed] [Google Scholar]
- 89. Sokoloff P, Le Foll B, Perachon S, Bordet R, Ridray S, Schwartz JC. The dopamine D3 receptor and drug addiction. Neurotox Res. 2001;3:433–441. [DOI] [PubMed] [Google Scholar]
- 90. Song R, Bi GH, Zhang HY, et al. Blockade of D3 receptors by YQA14 inhibits cocaine’s rewarding effects and relapse to drug-seeking behavior in rats. Neuropharmacology. 2014;77:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pilla M, Perachon S, Sautel F, et al. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. [DOI] [PubMed] [Google Scholar]
- 92. Mugnaini M, Iavarone L, Cavallini P, et al. Occupancy of brain dopamine D3 receptors and drug craving: a translational approach. Neuropsychopharmacology. 2013;38:302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Millan MJ, Goodwin GM, Meyer-Lindenberg A, Ove Ogren S. Learning from the past and looking to the future: emerging perspectives for improving the treatment of psychiatric disorders. Eur Neuropsychopharmacol. 2015;25:599–656. [DOI] [PubMed] [Google Scholar]
- 94. Westrich L, Gil-Mast S, Kortagere S, Kuzhikandathil EV. Development of tolerance in D3 dopamine receptor signaling is accompanied by distinct changes in receptor conformation. Biochem Pharmacol. 2010;79:897–907. [DOI] [PubMed] [Google Scholar]
- 95. Zhang X, Sun N, Zheng M, Kim KM. Clathrin-mediated endocytosis is responsible for the lysosomal degradation of dopamine D3 receptor. Biochem Biophys Res Commun. 2016;476:245–251. [DOI] [PubMed] [Google Scholar]
- 96. Liu XY, Mao LM, Zhang GC, et al. Activity-dependent modulation of limbic dopamine D3 receptors by CaMKII. Neuron. 2009;61:425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Robinson SW, Jarvie KR, Caron MG. High affinity agonist binding to the dopamine D3 receptor: chimeric receptors delineate a role for intracellular domains. Mol Pharmacol. 1994;46:352–356. [PubMed] [Google Scholar]
- 98. Kim KM, Valenzano KJ, Robinson SR, Yao WD, Barak LS, Caron MG. Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and beta-arrestins. J Biol Chem. 2001;276:37409–37414. [DOI] [PubMed] [Google Scholar]
- 99. Schmieg N, Rocchi C, Romeo S, Maggio R, Millan MJ, Mannoury la, Cour C. Dysbindin-1 modifies signaling and cellular localization of recombinant, human D(3) and D(2) receptors. J Neurochem. 2016;136:1037–1051. [DOI] [PubMed] [Google Scholar]
- 100. Zhang X, Le HT, Zhang X, Zheng M, Choi BG, Kim KM. Palmitoylation on the carboxyl terminus tail is required for the selective regulation of dopamine D2 versus D3 receptors. Biochim Biophys Acta. 2016;1858:2152–2162. [DOI] [PubMed] [Google Scholar]
- 101. Zhan L, Liu B, Jose-Lafuente M, et al. ALG-2 interacting protein AIP1: a novel link between D1 and D3 signalling. Eur J Neurosci. 2008;27:1626–1633. [DOI] [PubMed] [Google Scholar]
- 102. Aksenova MG, Shestakova Iu N, Abramova LI, et al. [D3 dopamine receptor gene Ser9Gly polymorphism in Russian patients with schizophrenia]. Zh Nevrol Psikhiatr Im S S Korsakova. 2004;104:57–61. [PubMed] [Google Scholar]
- 103. Godlewska BR, Olajossy-Hilkesberger L, Limon J, Landowski J. Ser9Gly polymorphism of the DRD3 gene is associated with worse premorbid social functioning and an earlier age of onset in female but not male schizophrenic patients. Psychiatry Res. 2010;177:266–267. [DOI] [PubMed] [Google Scholar]
- 104. Stanwood GD, Artymyshyn RP, Kung MP, Kung HF, Lucki I, McGonigle P. Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [(125)I]7-OH-PIPAT: evidence for the presence of D3 receptors on dopaminergic and nondopaminergic cell bodies and terminals. J Pharmacol Exp Ther. 2000;295:1223–1231. [PubMed] [Google Scholar]
- 105. Diaz J, Pilon C, Le Foll B, et al. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci. 2000;20:8677–8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Freedman SB, Patel S, Marwood R, et al. Expression and pharmacological characterization of the human D3 dopamine receptor. J Pharmacol Exp Ther. 1994;268:417–426. [PubMed] [Google Scholar]
- 107. He M, Shippenberg TS. Strain differences in basal and cocaine-evoked dopamine dynamics in mouse striatum. J Pharmacol Exp Ther. 2000;293:121–127. [PubMed] [Google Scholar]
- 108. Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. [DOI] [PubMed] [Google Scholar]
- 109. Yao WD, Spealman RD, Zhang J. Dopaminergic signaling in dendritic spines. Biochem Pharmacol. 2008;75:2055–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ross SB. Synaptic concentration of dopamine in the mouse striatum in relationship to the kinetic properties of the dopamine receptors and uptake mechanism. J Neurochem. 1991;56:22–29. [DOI] [PubMed] [Google Scholar]
- 111. Zhang K, Weiss NT, Tarazi FI, Kula NS, Baldessarini RJ. Effects of alkylating agents on dopamine D(3) receptors in rat brain: selective protection by dopamine. Brain Res. 1999;847:32–37. [DOI] [PubMed] [Google Scholar]
- 112. Prieto GA, Trieu BH, Dang CT, et al. Pharmacological rescue of long-term potentiation in Alzheimer diseased synapses. J Neurosci. 2017;37:1197–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]