Abstract
Background
Curcumin has been reported to have anti-inflammatory and anti-nociceptive effects. The present study was designed to explore the potential therapeutic effects of curcumin on visceral hyperalgesia and inflammation in a rat model of ulcerative colitis. We observed the effects of orally administered curcumin on the disease activity index, histological change in colon, colorectal distension-induced abdominal withdrawal reflex, the expression of transient receptor potential vanilloid 1 (TRPV1) and phosphorylated TRPV1 in dextran sulfate sodium-induced colitis rats. In addition, a HEK293 cell line stably expressing human TRPV1 (hTRPV1) was used to examine the effects of curcumin on the change in membrane expression of TRPV1 induced by phorbol myristate acetate (a protein kinase C activator).
Results
Repeated oral administration of curcumin inhibited the increase in abdominal withdrawal reflex score induced by dextran sulfate sodium without affecting dextran sulfate sodium-induced histological change of colon and the disease activity index. A significant increase in colonic expression of TRPV1 and pTRPV1 was observed in dextran sulfate sodium-treated rats and this was reversed by oral administration of curcumin. TRPV1 expression in L6-S1 dorsal root ganglion was increased in the small- to medium-sized isolectin B4-positive non-peptidergic and calcitonin gene-related peptide-positive peptidergic neurons in dextran sulfate sodium-treated rats and oral administration of curcumin mitigated such changes. In the HEK293 cell line stably expressing hTRPV1, curcumin (1, 3 µm) inhibited phorbol myristate acetate-induced upregulation of membrane TRPV1.
Conclusion
Oral administration of curcumin alleviates visceral hyperalgesia in dextran sulfate sodium-induced colitis rats. The anti-hyperalgesic effect is partially through downregulating the colonic expression and phosphorylation of TRPV1 on the afferent fibers projected from peptidergic and non-peptidergic nociceptive neurons of dorsal root ganglion.
Keywords: Curcumin, visceral hyperalgesia, transient receptor potential vanilloid 1, ulcerative colitis
Background
Inflammatory bowel diseases (IBDs) are chronic recurrent inflammatory disorders of the gastrointestinal tract.1 Ulcerative colitis (UC), being one type of IBDs, is characterized by contiguous inflammation of the colonic lamina propria with subsequent injury and disruption of the mucosal barrier.2 The clinical symptoms, such as abdominal discomfort and pain, are usually present in patients with UC. Most of the clinical treatments for UC are unsatisfactory.
Curcumin (diferuloylmethane, or (E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is a low-molecular-weight hydrophobic polyphenol extracted from turmeric (dry rhizomes of curcuma longa Linn), which is commonly used as a food colorant and spice. In traditional Chinese medicine, turmeric is an important medical ingredient used in many compound prescriptions for the treatment of rheumatism, inflammatory pain, neuropathic pain, diabetes, cancer, and many other diseases.3,4 Recent studies have shown that curcumin exhibits a wide range of biological activities including anti-inflammation,5 anti-oxidation,6 and anti-tumor7 actions.
An analgesic effect has been shown for curcumin in various animal pain models, including chronic neuropathic pain following peripheral nerve injury, postoperative pain, diabetic peripheral neuropathic pain, and acetic acid-induced visceral pain.8–12 In addition, pre-emptive intraperitoneal application of curcumin mitigated dextran sulfate sodium (DSS)-induced colonic inflammation in mice.13 However, it is unknown whether curcumin has a therapeutic effect on visceral hyperalgesia associated with UC.
Transient receptor potential vanilliod 1 (TRPV1) is considered as a polymodal sensory transducer since it can be activated by multiple noxious stimuli including heat, low pH, endogenous lipid derivatives, such as anandamide, as well as exogenous substances which can cause pain, inflammation, and hyperalgesia.14 Phosphorylation of TRPV1, which leads to enhanced function and membrane translocation of TRPV1, is a potential mechanism underlying inflammation-mediated hyperalgesia.15–17 Our previous work showed that in the mouse jejunum, the mesenteric afferent nerve responses to ramp distension and capsaicin were attenuated by curcumin and such effects disappeared in TRPV1-knockout mice.18 Martelli et al.19 reported that oral administration of curcumin ameliorated dinitrobenzene sulfonic acid-induced colitis in mice and such effect was completely abolished by TRPV1 antagonist capsazepine. These results indicate that TRPV1 might be the target through which curcumin exerted its effects on visceral sensitivity and colonic inflammation. Whether curcumin attenuates visceral hyperalgesia and inflammation via inhibiting phosphorylation of TRPV1 in UC condition remains to be determined.
DSS-induced colonic inflammation is the most appropriate IBD model that resembles, in many facets, the human phenotype.20 In this study, we investigated the therapeutic effects of repetitive oral administration of curcumin on visceral hyperalgesia and inflammation in the rat model of DSS-induced UC. The potential effects of curcumin on the expression of TRPV1 and phosphorylation of TRPV1 in the inflamed colon and the primary afferent neurons were explored. In addition, we investigated whether curcumin affected surface translocation of TRPV1 in HEK293 cells stably expressing human TRPV1.
Methods
Animals
Male Sprague–Dawley rats (190–210 g, n = 57) were obtained from the animal facility of Shanghai Jiao Tong University School of Medicine. Rats were housed in plastic cages in a climate-controlled room under a 12/12-h light-dark cycle, with free access to food and water. All animal care and experimental protocols complied with the Guiding Principles in the Care and Use of Animals and the Animal Management Rule of The Health And Family Planning Committee, People’s Republic of China (documentation 545, 2001). All efforts were made to minimize animal suffering and the number of animals used.
Induction of colitis and assessment of disease activity index
UC was induced by adding 5% dextran sodium sulfate (DSS, MW 54,000; MP Inc., France) to drinking water for seven consecutive days. Rats given regular water for seven consecutive days served as the normal control (designated as the naive group). Disease activity index (DAI) was scored for each animal as described previously.21 Briefly, body weight, stool consistency, and stool blood were recorded every day throughout DSS administration. DAI was determined by combining scores of body weight loss, stool consistency, and stool blood. The average of the three values constituted the DAI score.
Experimental design
Curcumin (20, 60 mg/kg, dissolved in saline, n = 8 for each dose; Sigma-Aldrich, St Louis, MO, USA) was orally administered by gavage under anesthesia with isoflurane (2–4% in oxygen) once daily for 10 consecutive days, starting from three days after the initiation of DSS (designated as DSS-curcumin 20, DSS-curcumin 60 groups). Saline was given as control treatment (designated as DSS-saline group, n = 7). Behavioral tests were done 2 h after the last administration of curcumin/saline and then distal colons were collected immediately after behavioral tests under deep anesthesia with sodium pentobarbital (100 mg/kg) for Western blot (WB) assay of TRPV1 and phosphorylated (p) TRPV1. The same experimental paradigms were completed with rats of the naive group (n = 7). The expression of TRPV1 in different subtypes of DRG neurons was examined using immunofluorescent (IF) staining in rats from the naive group (n = 4), DSS-saline group (n = 6) and DSS-curcumin 60 group (n = 5). The histological changes of colon were examined using hematoxylin and eosin (H&E) staining in rats from the naive group (n = 3), the DSS-saline group (n = 4) and DSS-curcumin 60 group (n = 5).
Behavioral testing
Behavioral responses to colorectal distention (CRD) were assessed by measuring the abdominal withdrawal reflex (AWR) using a semiquantitative score as described previously.22 Briefly, distention balloons were placed in the descending colons of mildly sedated adult rats (Isoflurane, 2–4% in oxygen) and secured by taping the attached tubing to the rat’s tail. The rats were then housed in small Lucite cubicles (18 × 3.5 × 3.5 cm) on an elevated Plexiglas platform and allowed to wake up and adapt (1 h). The rats were given graded CRD (20, 40, 60, and 80 mm Hg) for 20 s every 4 min. To achieve an accurate measure, the distention was repeated five times for each intensity. Measurement of the AWR consisted of visual observation of the animal response to graded CRD by blinded observers and assignment of an AWR score: 0, no behavioral response to CRD; 1, brief head movement followed by immobility; 2, contraction of abdominal muscles; 3, lifting of abdomen; 4, body arching and lifting of pelvic structures. The AWR scores from the five distensions were averaged to obtain a single AWR score at each pressure for each animal.
H&E staining
The histological changes in colon were assessed using H&E staining. H&E staining was carried out on paraffin-embedded colon tissue sections (5 µm). The sections were deparaffinized with xylene and rehydrated with graded alcohol and then stained in hematoxylin solution for 5 min and differentiated in 1% acid alcohol for 30 s. The sections were counterstained in eosin solution for 30 s, and then dehydrated with graded alcohol and cleared with xylene.
H&E stained sections were given a colitis activity score in a blinded fashion. Scores ranged from 0 to 11 (total score), which represent the sum of scores from 0 to 4 for severity of crypt damage, inflammation (e.g., neutrophil infiltration) extent, and percent involvement (e.g., how much of each tissue section showed evidence of damage or inflammation), as described previously.23 For crypt damage: 0 = none, 1 = basal 1/3 damage, 2 = basal 2/3 damage, 3 = crypt lost, surface epithelium present, and 4 = crypt and surface epithelium lost. For inflammation extent: 0 = none, 1 = mucosa, 2 = submucosa, and 3 = transmural. For percent involvement: 0 = 0%, 1 = 1–25%, 2 = 26–50%, 3 = 51–75%, and 4 = 76–100%.
Western blot
Frozen tissue samples were homogenized in ice-cold lysis buffer containing 20 mmol/L Tris–HCl (pH 8.0), 150 mmol/L NaCl, 1 mmol/L EDTA, 1% NP-40, 1 mmol/L PMSF, protease inhibitor cocktail (Sigma-Aldrich) and phosphatase inhibitor cocktail (Thermo Scientific, Indianapolis, IN, USA) for 1 h at 4℃. The lysates were centrifuged at 10,000 g for 30 min at 4℃ and the concentration of protein in each supernatant was determined using a BCA assay (Pierce, Rackford, IL, USA). Thirty-microgram aliquots were separated on 10% Tris–glycine gels, the separated proteins were transferred from the gel to the surface of polyvinylidene fluoride (Millipore Immobilon, USA). The membranes were blocked with 5% fat-free dry milk or 1% bovine serum albumin (BSA) in Tris-buffered saline (TBS) containing 0.1% Tween-20 for 1 h and were then incubated overnight at 4℃ with primary rabbit anti-TRPV1 antibody (1:1000, Alomone, Zion, Israel) in 5% fat-free dry milk/TBST or rabbit anti-pTRPV1 antibody (1:500, Cosmo bio, Tokyo, Japan) in 5% BSA/TBST. Bound primary antibodies were detected with HRP-conjugated anti-rabbit antibody (1:2000, Bio-rad, Hercules, CA, USA). Immunoreactive bands were visualized using enhanced chemiluminescence (Thermo Scientific) and digital imaging was captured with Image Quant LAS 4000 mini (GE Healthcare, Life Science, USA). The density of specific bands was measured with NIH ImageJ (http://rsb.info.nih.gov/ij/) software and was normalized against a loading control (β-actin).
IF staining
Under deep anesthesia with pentobarbital, rats were transcardially perfused with saline followed by 4% paraformaldehyde and 0.14% picric acid in phosphate buffer (PB, 0.1 mol/L, pH 7.4). The L6-S1 DRGs were removed and post-fixed in the same fixative overnight at 4℃ and then cryoprotected with 30% sucrose in 0.1 mol/L PB overnight at 4℃. The samples were cut at 10 µm and the sections were first incubated with 0.05 mol/L phosphate-buffered saline (PBS) containing 10% normal goat serum and 0.5% Triton X-100 at room temperature for 1 h to block non-specific binding and this was followed by co-incubation with a combination of primary guinea pig anti-TRPV1 antibody (1:3000, Neuromics, Edina, MN, USA) and one of the following antibodies: (1) rabbit anti-CGRP antibody (1:2000, Abcam, Cambridge, MA, USA); (2) mouse anti-NF200 antibody (1:1000, Sigma-Aldrich); and (3) fluorescein isothiocyanate-labeled IB4 (IB4-FITC, 1:1000, Sigma) at 4℃ overnight. The sections were rinsed with PBS for four times and were then incubated with goat anti-guinea pig Alexa fluor 568 secondary antibody (1:1000; Molecular Probes-Invitrogen, Eugene, OR, USA) and goat anti-mouse/rabbit Alexa Fluro 488 (1:1000; Molecular Probes-Invitrogen) at room temperature for 1 h. After washing with PBS, the sections were mounted on glass slides and viewed under the fluorescent microscope (Leica DM2500, Leica Microsystems Limited, Buffalo Grove, IL, USA) and the digital images were analyzed using Leica application suite version 4.3 (Leica Microsystems Limited). The cell stained with moderate to strong density and clear visible nucleus was selected to minimize statistical error. The percentage of TRPV1-positive neurons in all the neurons, IB4-positive neurons, or CGRP-positive neurons was calculated and averaged from six randomized fields for each rat.
Cell culture and cell-surface biotinylation assay
HEK293 cells stably transfected with hTRPV1 (gift from Dr. Michael Zhu, The University of Texas Health Science Center) were cultured with Dulbecco’s modified Eagle’s medium containing 10% fatal bovine serum, 0.1% Hygromycine and 0.05% Blasticidin (Invitrogen). One trial in which the cells were incubated with curcumin (1, 3, or 10 µM) for 5 min and the other trial in which the cells were incubated with curcumin (1 or 3 µM) for 5 min following phorbol myristate acetate (PMA, 1 µM dissolved in 0.01% DMSO) treatment for 5 min were performed. The expression of either membrane (m) or total (t) TRPV1 was examined using cell-surface biotinylation assay and subsequent WB.
Surface biotinylation and isolation of membrane proteins were performed using a Pierce Cell Surface Protein Isolation Kit (Pierce, Rockford, IL, USA). HEK293 cells (1.5–2.0 × 106 cells/dish) were rinsed three times with ice-cold PBS (containing Ca2 + and Mg2+, pH 7.4) and biotinylated with 250 µg/ml EZlinksulfo-NHS-LC-Biotin (Thermo Scientific, Waltham, MA, USA) at 4℃ for 30 min. Unreacted biotin was quenched using PBS solution containing 0.1 M glycine at 4℃ for 20 min.Then the cells were subsequently lysed in 500 µL of radioimmunoprecipitation assay buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% deoxycholic acid) containing protease inhibitors cocktail (Sigma-Aldrich). After centrifugation of the lysates, 10% volume of the supernatant was saved for the determination of total protein. The remainder was incubated with NeutrAvidin plus Ultralink beads (Thermo Scientific) overnight at 4℃ to pull down all biotinylated proteins. Beads were washed five times and eluted with 6 × SDS loading buffer by boiling for 10 min and were analyzed by WB with a rabbit anti-TRPV1 antibody (1:1000, Alomone, Zion, Israel). Three independent experiments were performed.
Statistical analysis
Data were presented as mean ± SD for histological score, the expression of TRPV1 or pTRPV1 in colon, the expression of tTRPV1 or mTRPV1 in HEK293 cells, and presented as mean ± SEM for DAI and AWR score, respectively. Statistical analysis of the data sets was carried out using Graphpad Prism version 5.0 (Graphpad software, La Jolla, CA, USA). Two-way analysis of variance (ANOVA) with Bonferroni’s posttest was used to assess the difference between experimental groups for DAI, AWR scores, and the percentage of TRPV1-positive neurons. One-way ANOVA with Bonferroni’s posttest was used to assess the difference between experimental groups for histological score and WB data. P < 0.05 was regarded as significantly different.
Results
The effects of curcumin on DSS-induced inflammation
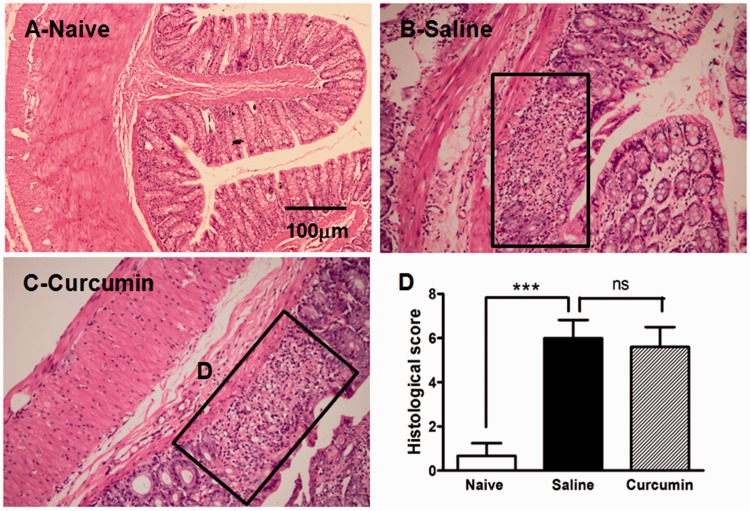
Rats treated with 5% DSS in drinking water showed gradual increases in DAI scores (Table 1) and marked histological changes in the colon, including either mucosal or submucosal erosion, ulceration, and inflammatory cell infiltration. The histological score was significantly increased in DSS-saline group compared to the naive group (6.00 ± 0.82 vs. 0.67 ± 0.58, P < 0.001; Figure 1). Therapeutic treatment with curcumin (60 mg/kg) did not alter the DSS-induced increases in DAI scores (Table 1). Likewise, curcumin treatment (60 mg/kg) did not affect DSS-induced increase in histological score (5.67 ± 0.89 vs. 6.00 ± 0.82, P > 0.05; Figure 1).
Table 1.
The effects of curcumin on the DSS-induced increase in DAI score.
| Group | N | Day 0 | Day 1 | Day 3 | Day 5 | Day 7 |
|---|---|---|---|---|---|---|
| Normal control | 7 | 0 | 0 | 0 | 0 | 0 |
| DSS + saline | 7 | 0 | 2.01 ± 0.31 | 2.50 ± 0.32 | 6.10 ± 1.00 | 8.80 ± 0.58 |
| DSS + curcumin | 8 | 0 | 1.99 ± 0.23 | 2.63 ± 0.41 | 5.25 ± 0.65 | 7.43 ± 0.46 |
Figure 1.
The effect of curcumin on histological change in DSS-induced colitis. Hematoxylin and eosin staining of colon from rats receiving water (a; normal control, n = 3), saline (b; DSS + saline, n = 4), and curcumin (c; DSS + curcumin, n = 5). Compared with that of normal control, colon of DSS-given rats showed destruction of epithelium with loss of crypts and inflammatory cells infiltration. The morphological damage of colon from rats treated with curcumin was similar to that of saline treatment. The area in the panel shown in (b) and (c) exhibited the morphological damage induced by DSS. The histological score was significantly increased by DSS compared with normal control (***P < 0.001, one-way ANOVA with Bonferroni’s posttest). However, curcumin did not alter the DSS-induced increase in histological score compared with saline treatment (P > 0.05, one-way ANOVA with Bonferroni’s posttest, (d)).
The effects of curcumin on DSS-induced visceral hyperalgesia
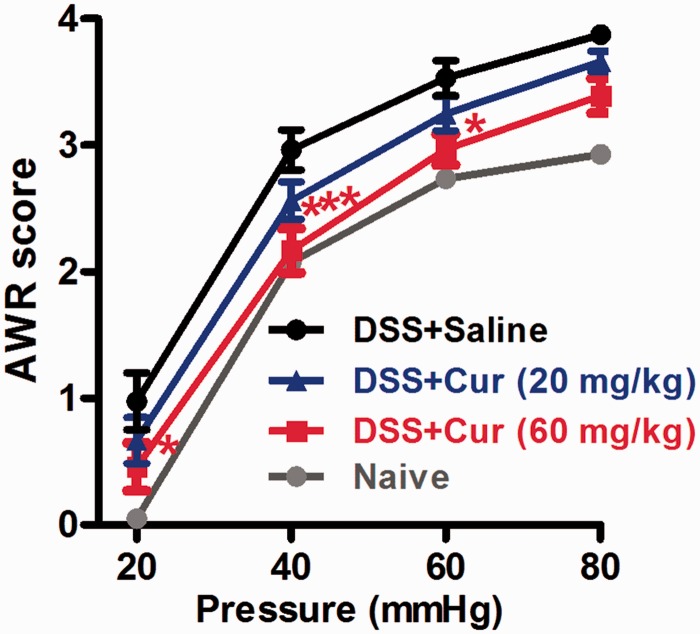
To investigate whether therapeutic curcumin has anti-nociceptive action, we examined the effects of curcumin on DSS-induced increase in AWR score. The AWR scores were measured in response to graded CRD (20, 40, 60, and 80 mm Hg). The rats treated by DSS showed significant increases in AWR scores for all intensities of CRD compared with naive rats. Curcumin (20, 60 mg/kg) dose dependently attenuated DSS-induced AWR scores. The higher dose of curcumin (60 mg/kg) produced a significant analgesic effect in that averaged AWR scores in responses to 20–60 mmHg CRD in DSS-curcumin 60 group were significantly lower compared with the DSS-saline group (Figure 2).
Figure 2.
The effects of curcumin on abdominal withdrawal reflex (AWR) to colorectal distension in rats treated with DSS. DSS produced increase in AWR score at all intensities of stimuli (20/40/60/80 mmHg). Repetitive oral administration of curcumin dose dependently decreased the AWR score, especially, higher dose of curcumin (60 mg/kg) significantly reduced AWR score at 20, 40, and 60 mmHg (*P < 0.05, ***P < 0.001 vs. saline treatment, two-way ANOVA with Bonferroni’s posttest). Naive: normal control group (n = 7); Saline: DSS + saline group (n = 7); Curcumin: DSS + curcumin (20, 60 mg/kg, n = 8 for each dose).
The effects of curcumin on the expression of TRPV1/pTRPV1 in the inflamed colon
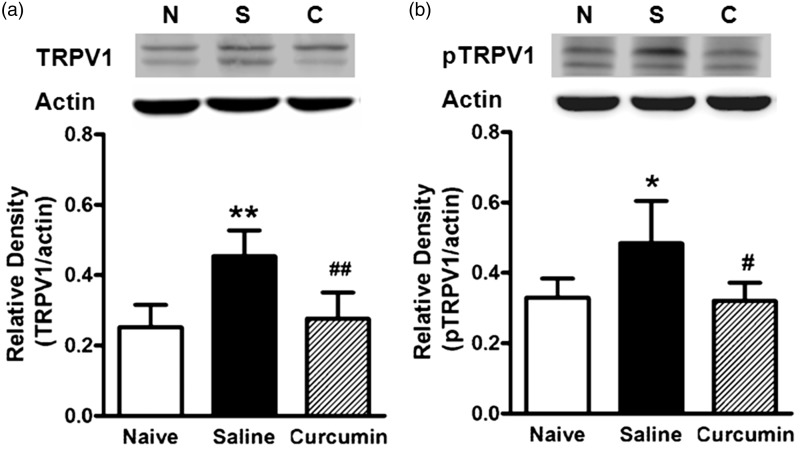
To investigate whether the anti-nociceptive action of curcumin was associated with suppressed expression of TRPV1 and the phosphorylation of TRPV1, we performed WB assay of the protein level of TRPV1/pTRPV1 in the colon of the three groups of rats (naive, DSS-saline, and DSS-curcumin 60). The data showed that the protein levels of TRPV1 (P < 0.01) and pTRPV1 (P < 0.05) both increased in DSS-saline group compared with the naive group (Figure 3). Notably, curcumin treatment (60 mg/kg) mitigated the increases in protein level of TRPV1 (P < 0.01) and pTRPV1 (P < 0.05) in the inflamed colon (Figure 3).
Figure 3.
The effects of curcumin on DSS-induced upregulation of TRPV1 (a) and pTRPV1 (b) in colon. (a) and (b) top: The examples of TRPV1 and pTRPV1 (MW: 98 KDa) expression in colon from naive (N: normal control), saline (S: DSS + saline), and curcumin (C: DSS + curcumin) groups. (a) and (b) bottom: The protein level of either TRPV1 or pTRPV1 was apparently higher in DSS + saline group than that in normal control group (**P < 0.01, *P < 0.05, one-way ANOVA with Bonferroni’s posttest). The protein level of either TRPV1 or pTRPV1 was much less in curcumin treatment group than that in saline treatment group (##P < 0.01, #P < 0.05, one-way ANOVA with Bonferroni’s posttest). n = 5–6 rats/group.
The effects of curcumin on DSS-induced change in TRPV1 expression in L6-S1 DRG neurons
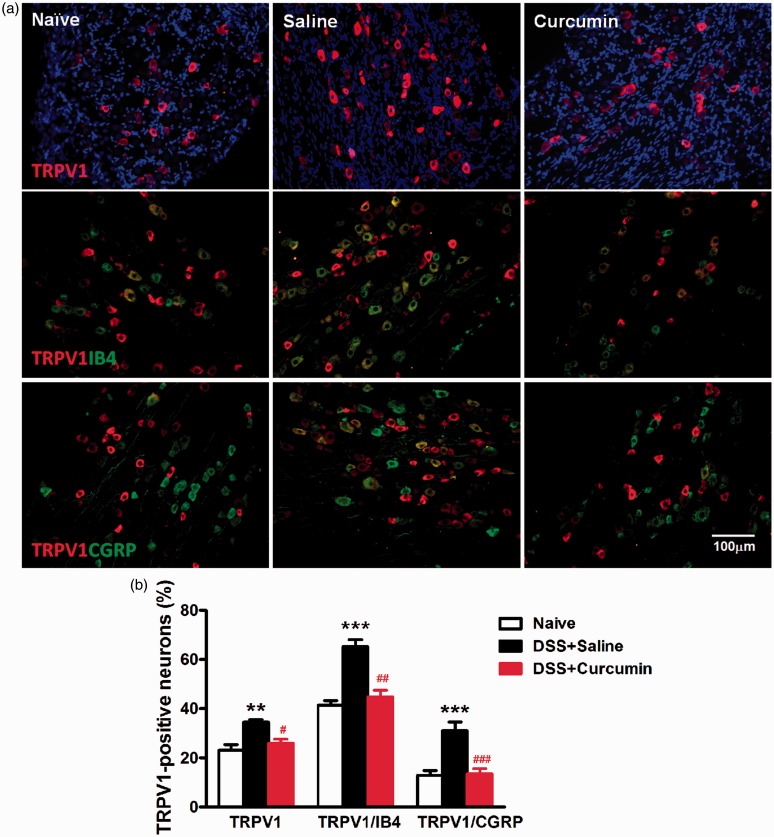
TRPV1 is mainly expressed in small- to medium-sized primary afferent neurons in the dorsal root, trigeminal, and vagal ganglia. These neurons form unmyelinated or thin myelinated nerve fibers that project to most organs and tissues including colon.24 We examined whether curcumin treatment would affect the expression of TRPV1 in L6-S1 DRG using IF staining. Indeed, we found TRPV1 immunofluorescence was confined to IB4 or CGRP-positive small- to medium-sized neurons (diameter ≤ 35 µm) and was absent in NF200-positive large-sized neurons (diameter ≥ 50 µm). In the naive group, the percentage of TRPV1-positive neurons was 23.1 ± 2.2%. For IB4-positive non-peptidergic and CGRP-positive peptidergic neurons, 41.5 ± 1.7% and 12.9 ± 1.8% expressed TRPV1, respectively. DSS treatment increased the expression of TRPV1 in L6-S1 DRG neurons (35.1 ± 2.2%, P < 0.01), in IB4-positive neurons (66.6 ± 3.7%, P < 0.001) and in CGRP-positive neurons (33.6 ± 3.8%, P < 0.001). Curcumin mitigated DSS-induced increase of TRPV1 expression in DRG neurons (26.9 ± 2.1%, P < 0.05), in IB4-positive neurons (44.2 ± 3.2%, P < 0.01), and in CGRP-positive neurons (12.7 ± 2.7%, P < 0.001) (Figure 4).
Figure 4.
The effects of curcumin on DSS-induced increase in TRPV1 expression in L6-S1 DRG. (a) Immunofluorescent (IF) staining of TRPV1 and double IF staining of TRPV1 with IB4 or CGRP as an example. Naive: normal control group; saline: DSS+saline group; curcumin: DSS+curcumin group. (b) Quantification analysis of DRG neurons stained with TRPV1 and doubly stained with TRPV1 and IB4 or CGRP. TRPV1 was expressed in both IB4-positive non-peptidergic and CGRP-positive peptidertic neurons. DSS significantly produced increase of TRPV1 expression in DRG neurons (**P < 0.01, two-way ANOVA with Bonferroni’s posttest), particularly in both IB4-positive neurons (***P < 0.001, two-way ANOVA with Bonferroni’s posttest) and CGRP-positive neurons (***P < 0.001, two-way ANOVA with Bonferroni’s posttest). Curcumin could inhibit DSS-induced increase in TRPV1 expression in DRG neurons (#P < 0.05, two-way ANOVA with Bonferroni’s posttest), especially in IB4-positive neurons (##P < 0.01, two-way ANOVA with Bonferroni’s posttest) and CGRP-positive neurons (###P < 0.001, two-way ANOVA with Bonferroni’s posttest).
The effects of curcumin on surface expression of TRPV1 in HEK293 cells
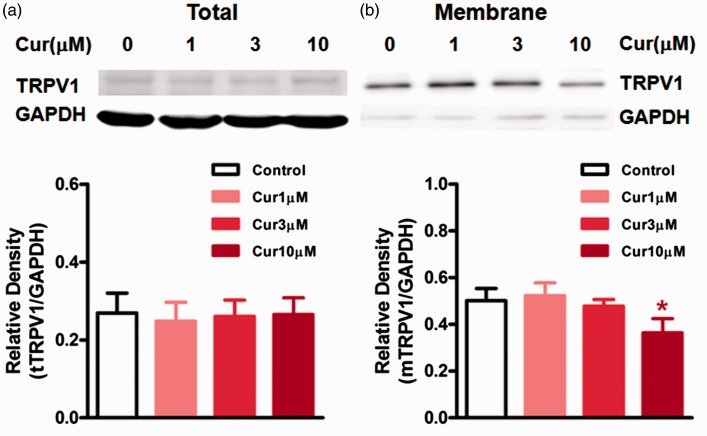
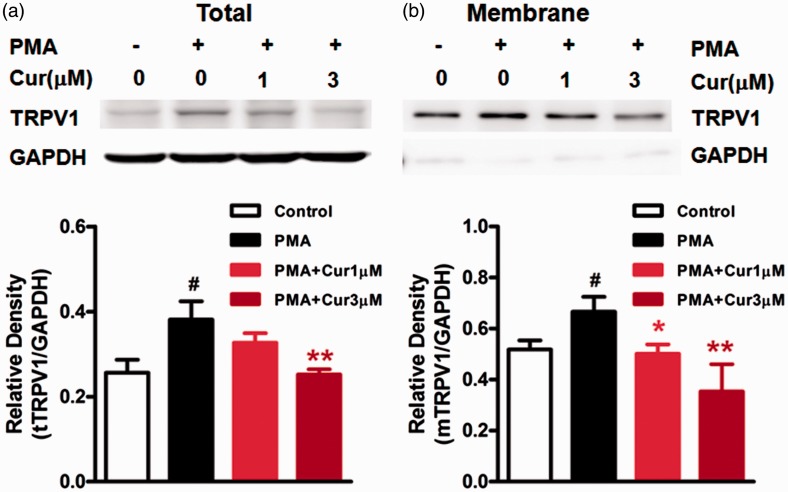
Phosphorylation of TRPV1 is known to promote the trafficking of TRPV1 to the membrane.25 To investigate whether curcumin influences the membrane translocation of TRPV1, we examined the surface expression of TRPV1 in HEK293 cells stably transfected with human TRPV1 following treatment with curcumin (1, 3, and 10 µM) for 5 min. We found that the highest dose (10 µM) of curcumin significantly reduced the expression of membrane TRPV1 compared to the control (P < 0.05) without significant effect on the expression of total TRPV1 (Figure 5). In addition, we observed the effect of curcumin on PMA-induced membrane translocation of TRPV1. As shown in Figure 6, cells treated with the PKC activator PMA (1 µM) for 10 min showed an increase in either total TRPV1 or membrane TRPV1 (P < 0.05). Curcumin (1, 3 µM) dose dependently inhibited the PMA-induced increase in expression of membrane TRPV1 (P < 0.05, P < 0.01, Figure 6(b)), while higher dose of curcumin (3 µM) attenuated the PMA-induced increase in expression of total TRPV1 (P < 0.05; Figure 6(a)).
Figure 5.
The effects of curcumin on the expression of total or membrane TRPV1 in HEK293 cells transfected with human TRPV1. (a) and (b) top: The examples of total TRPV1 (tTRPV1) and membrane TRPV1 (mTRPV1) expression in HEK293 cells. (a) bottom: All doses of curcumin did not affect the protein level of tTRPV1. (b) bottom: Only highest dose of curcumin (10 µM) reduced the protein level of mTRPV1 (*P < 0.05, one-way ANOVA with Bonferroni’s posttest). Three independent experiments were performed.
Figure 6.
The effects of curcumin on the PMA-induced increase in total or membrane TRPV1 in HEK293 cells transfected with human TRPV1. (a) and (b) top: The examples of total TRPV1 (tTRPV1) and membrane TRPV1 (mTRPV1) expression in HEK293 cells. A and B bottom: PMA produced increase in expression of either tTRPV1 or mTRPV1 (#P < 0.05 vs. control, one-way ANOVA with Bonferroni’s posttest). Curcumin dose dependently inhibited the PMA-induced increase in mTRPV1 expression (**P < 0.01, *P < 0.05 vs. non-treatment, one-way ANOVA with Bonferroni’s posttest), while only high dose of curcumin (3 µM) reduced tTRPV1 expression (*P < 0.05 vs. non-treatment, one-way ANOVA with Bonferroni’s posttest). Three independent experiments were performed.
Discussion
The current study investigated the anti-nociceptive and anti-inflammatory effects of curcumin on DSS-induced UC and visceral hyperalgesia. Our results demonstrated that repetitive treatment with curcumin orally, starting three days after DSS initial administration, attenuated DSS-induced visceral hyperalgesia. However, such treatment with curcumin did not alter the DSS-induced increases in DAI score and histological change of colon. In addition, the same treatment of curcumin also reduced the expression of TRPV1 and pTRPV1 in inflamed colon compared to saline treatment. Furthermore, in vitro data showed inhibitory effect of curcumin on the increase in surface translocation of TRPV1 induced by PMA in HEK 293 cells. Taken together, our data suggest that curcumin attenuates UC-associated pain potentially via inhibiting the expression of TRPV1, phosphorylation of TRPV1, and membrane translocation of TRPV1.
Abdominal pain is very common in patients with UC. Analgesic drugs currently in clinical use for pain relief often induce various side effects.26,27 The notion that curcumin has shown pleiotropic beneficial effects in various pathological conditions3,4 attracts our attention to address the possible therapeutic roles of curcumin on DSS-induced UC and visceral hyperalgesia in male SD rats. DSS induced an experimental model of UC that was well characterized morphologically. Here, we found that 5% DSS in drinking water resulted in significant weight loss, bloody stool, severe mucosal and submucosal erosion, ulceration, and inflammatory cell infiltration in the rat. These macroscopic and microscopic alterations were in agreement with many previous studies in rats and mice.13,28,29 We also found that DSS-induced colitis was accompanied by colorectal hyperalgesia such that CRD-induced exaggerated visceromotor responses in DSS-treated rats.
Curcumin has been reported to attenuate inflammatory pain,11 neuropathic pain,8,10 postoperative pain,9 and visceral pain,12 especially with repetitive treatment of curcumin. In agreement with these reports, the present study showed that repetitive oral treatment with curcumin dose dependently attenuated DSS-induced increase in AWR score.
The mechanisms underlying curcumin’s anti-nociceptive effects may be complicated. Previously, Zhao et al.30 reported that the brainstem descending monoamine system and opioid receptors are involved in curcumin-induced anti-nociceptive effects. Banafshe et al. reported that repetitive application of curcumin attenuated diabetic neuropathic pain and such effect was blocked by naloxone, which was supportive of the involvement of the opioid system in curcumin’s antinociceptive effects. Other studies demonstrated that curcumin abrogated diabetic neuropathic pain by downregulating tumor necrosis factor-α and inhibition of oxidative stress.31–33 Our previous work and study by Yeon et al.34 provided evidence that curcumin produces an antihyperalgesic effect via direct antagonism of TRPV1 function.18 Here, we extended the previous findings of the direct TRPV1 antagonism and found that curcumin also significantly decreased the expression of TRPV1 in the inflamed colon. Therefore, curcumin may attenuate visceral hyperalgesia via inhibiting the expression of TRPV1 as well as antagonizing TRPV1 activation.
Previous studies demonstrated that TRPV1 function is associated with DSS-induced inflammation since blockade of TRPV1 ameliorated inflammatory tissue injury.23,35,36 Here, we found that therapeutic application of curcumin was without significant effect on colonic inflammation, despite TRPV1 expression was significantly downregulated. A possible explanation for the lack of anti-inflammatory effect following curcumin treatment might be that 5% DSS-induced colitis was largely TRPV1 indepenent. This argument is supported by a previous report which showed 2% but not 5% DSS-induced colitis was significantly ameliorated in TRPV1 knockout mice.35
Ward et al.37 reported that TRPV1 was expressed not only on the nerve fibers in the mucosa and muscle layers but also on the epithelial lining of the mucosa in colon. Unfortunately, we failed to detect specific expression of TRPV1 in the colon using several antibodies (data not shown). However, TRPV1 is expressed in sensory neurons of small to medium size in DRG. These neurons form unmyelinated and lightly myelinated nerve fibers that project to most organs including colon and conduct nociceptive stimuli.24 Thus, we examined the change in TRPV1 expression in L6-S1 DRG using IF staining. We found that DSS produced an increase in TRPV1 expression in both small- to medium-sized peptidergic and non-peptidergic neurons. Likewise, previous studies showed that chronic inflammatory nociception produced by intraplantar injection of complete Freund’s adjuvant was associated with increased expression of TRPV1 in both peptidergic and non-peptidergic neurons in DRG.38 Importantly, we found that therapeutic application of curcumin abrogated the increase in TRPV1 expression in both peptidergic and non-peptidergic neurons of L6-S1 DRG.
It is well known that TRPV1 is a non-selective nociceptive cationic channel that can be activated by polymodal stimuli to cause pain.39 Under pathophysiological conditions, multiple inflammatory mediators activate several kinases that phosphorylate TRPV1 and enhance its functionality. Among the protein kinases, protein kinase C (PKC) is one of major players in TRPV1 sensitization.39 PKC-induced TRPV1 phosphorylation enhances response to capsaicin, acid, and heat.40,41 Phosphorylation of TRPV1 by cyclin-dependent kinase 5 promotes TRPV1 surface translocation which leads to inflammatory thermal hyperalgesia.25 In the present study, the higher dose of curcumin significantly decreased the expression of pTRPV1 in inflamed colon, suggesting that the anti-nociceptive effect of curcumin was mediated partially via inhibiting the phosphorylation of TRPV1. Thus, we further tested whether curcumin can inhibit membrane translocation of TRPV1 induced by phosphorylation of TRPV1. The in vitro data showed that only higher dose (10 µM) of curcumin significantly decreased the expression of membrane TRPV1, while lower doses (1 and 3 µM) of curcumin apparently reduced the increased expression of membrane TRPV1 induced by PMA, an activator of PKC.
In summary, this study demonstrated that oral repetitive therapeutic curcumin effectively attenuated visceral hyperalgesia associated with DSS-induced colitis through inhibiting the expression of TRPV1 and pTRPV1 in the inflamed colon and the afferent neurons of L6-S1 DRG. In addition, in vitro data indicated that curcumin might inhibit the phosphorylation and subsequent membrane translocation of TRPV1. These findings support the idea that curcumin may be of therapeutic value for visceral hyperalgesia.
Author Contributions
MY, JW, and CY performed the study and drafted the manuscript; HH and WR analyzed the experimental data and reviewed the manuscript; GZ analyzed the experimental data, designed the study, and reviewed the manuscript. All authors read and approved the final manuscript. MY, JW, and CY contributed equally to this work.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the grants from the National Natural Science Foundation of China (No. 81070891, 81271245 to GZ) and Shanghai Municipal Education Commission (No. 13ZZ085 to GZ).
References
- 1.Abraham C, Cho J. Inflammatory bowel disease. N Engl J Med 2009; 361: 2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahuja V, Tandon RK. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011; 474: 298–306. [DOI] [PubMed] [Google Scholar]
- 3.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer 2005; 41: 1955–1968. [DOI] [PubMed] [Google Scholar]
- 4.Kumar G, Mittal S, Sak K, et al. Molecular mechanisms underlying chemopreventive potential of curcumin: Current challenges and future perspectives. Life Sci 2016; 148: 313–328. [DOI] [PubMed] [Google Scholar]
- 5.Sandur SK, Pandey MK, Sung B, et al. Curcumin, demethoxy curcumin, bisdemethoxy curcumin, tetrahydro curcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007; 28: 1765–1773. [DOI] [PubMed] [Google Scholar]
- 6.Lee KH, Ab Aziz FH, Syahida A, et al. Synthesis and biological evaluation of curcumin-like diarylpentanoid analogues for anti-inflammatory, antioxidant and anti-tyrosinase activities. Eur J Med Chem 2009; 44: 3195–3200. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Chen H, Xu C, et al. Curcumin inhibits tumor epithelial-mesenchymal transition by downregulating the Wnt signaling pathway and upregulating NKD2 expression in colon cancer cells. Oncol Rep 2016; 35: 2615–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeon Y, Kim CE, Jung D, et al. Curcumin could prevent the development of chronic neuropathic pain in rats with peripheral nerve injury. Curr Ther Res Clin Exp 2013; 74: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Q, Sun Y, Yun X, et al. Antinociceptive effects of curcumin in a rat model of postoperative pain. Sci Rep 2014; 4: 4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banafshe HR, Hamidi GA, Noureddini M, et al. Effect of curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol 2014; 723: 202–206. [DOI] [PubMed] [Google Scholar]
- 11.Chen JJ, Dai L, Zhao LX, et al. Intrathecal curcumin attenuates pain hypersensitivity and decreases spinal nueroinflammation in rat model of monoarthritis. Sci Rep 2015; 5: 10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tajik H, Tamaddonfard E, Hamzeh-Gooshchi N. The effect of curcumin (active substance of turmeric) on the acetic acid-induced visceral nociception in rats. Pak J Biol Sci 2008; 11: 312–314. [DOI] [PubMed] [Google Scholar]
- 13.Arafa HM, Hemeida RA, El-Bahrawy AI, et al. Prophylactic role of curcumin in dextran sulfate sodium (DSS)-induced ulcerative colitis murine model. Food Chem Toxicol 2009; 47: 1311–1317. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y. The functional regulation of TRPV1 and its role in pain sensitization. Neurochem Res 2008; 33: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 15.Xing BM, Yang YR, Du JX, et al. Cyclin-dependent kinase 5 controls TRPV1 membrane trafficking and the heat sensitivity of nociceptors through KIF13B. J Neurosci 2012; 32: 14709–14721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laínez S, Valente P, Ontoria-Oviedo I, et al. GABAA receptor associated protein (GABARAP) modulates TRPV1 expression and channel function and desensitization. FASEB J 2010; 24: 1958–1970. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Du J, Yang Y, et al. Phosphorylation of TRPV1 by cyclin-dependent kinase 5 promotes TRPV1 surface localization, leading to inflammatory thermal hyperalgesia. Exp Neurol 2015; 273: 253–262. [DOI] [PubMed] [Google Scholar]
- 18.Zhi L, Dong L, Kong D, et al. Curcumin acts via transient receptor potential vanilloid-1 receptors to inhibit gut nociception and reverses visceral hyperalgesia. Neurogastroenterol Motil 2013; 25: e429–e440. [DOI] [PubMed] [Google Scholar]
- 19.Martelli L, Ragazzi E, di Mario F, et al. A potential role for the vanilloid receptor TRPV1 in the therapeutic effect of curcumin in dinitrobenzene sulphonic acid-induced colitis in mice. Neurogastroenterol Motil 2007; 19: 668–674. [DOI] [PubMed] [Google Scholar]
- 20.Kawada M, Arihiro A, Mizoguchi E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J Gastroenterol 2007; 13: 5581–5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzpatrick LR, Wang J, Le T. In vitro and in vivo effects of gliotoxin, a fungal metabolite: Efficacy against dextran sodium sulfate-induced colitis in rats. Dig Dis Sci 2000; 45: 2327–2336. [DOI] [PubMed] [Google Scholar]
- 22.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology 2000; 119: 1276–1285. [DOI] [PubMed] [Google Scholar]
- 23.Kihara N, de la Fuente SG, Fujino K, et al. Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut 2003; 52: 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bevan S, Quallo T, Andersson DA. TRPV1. Handb Exp Pharmacol 2014; 222: 207–245. [DOI] [PubMed] [Google Scholar]
- 25.Jiao Liu, Junxie Du, Yanrui Yang, et al. Phosphorylation of TRPV1 by cyclin-dependent kinase 5 promotes TRPV1 surface localization, leading to inflammatory thermal hyperalgesia. Exp Neurol 2015; 273: 253–262. [DOI] [PubMed] [Google Scholar]
- 26.Sehgal N, Smith HS, Manchikanti L. Peripherally acting opioids and clinical implications for pain control. Pain Physician 2011; 14: 249–258. [PubMed] [Google Scholar]
- 27.Lee Y, Hong S, Cui M, et al. Transient receptor potential vanilloid type 1 antagonists: a patent review (2011–2014). Expert Opin Ther Pat 2015; 25: 291–318. [DOI] [PubMed] [Google Scholar]
- 28.Chung HL, Yue GG, To KF, et al. Effect of scutellariae radix extract on experimental dextran-sulfate sodium-induced colitis in rats. World J Gastroenterol 2007; 13: 5605–5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damiani CR, Benetton CA, Stoffel C, et al. Oxidative stress and metabolism in animal model of colitis induced by dextran sulfate sodium. J Gastroenterol Hepatol 2007; 22: 1846–1851. [DOI] [PubMed] [Google Scholar]
- 30.Zhao X, Xu Y, Zhao Q, et al. Curcumin exerts antinociceptive effects in a mouse model of neuropathic pain: descending monoamine system and opioid receptors are differentially involved. Neuropharmacology 2012; 62: 843–854. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Zhang Y, Liu DB, et al. Curcumin attenuates diabetic neuropathic pain by downregulating TNF-α in a rat model. Int J Med Sci 2013; 10: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banafshe HR, Hamidi GA, Noureddini M, et al. Effect of curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol 2014; 723: 202–206. [DOI] [PubMed] [Google Scholar]
- 33.Zhao WC, Zhang B, Liao MJ, et al. Curcumin ameliorated diabetic neuropathy partially by inhibition of NADPH oxidase mediating oxidative stress in the spinal cord. Neurosci Lett 2014; 560: 81–85. [DOI] [PubMed] [Google Scholar]
- 34.Yeon KY, Kim SA, Kim YH, et al. Curcumin produces an antihyperalgesic effect via antagonism of TRPV1. J Dent Res 2010; 89: 170–174. [DOI] [PubMed] [Google Scholar]
- 35.Szitter I, Pozsgai G, Sandor K, et al. The role of transient receptor potential vanilloid 1 (TRPV1) receptors in dextran sulfate-induced colitis in mice. J Mol Neurosci 2010; 42: 80–88. [DOI] [PubMed] [Google Scholar]
- 36.Kimball ES, Wallace NH, Schneider CR, et al. Vanilloid receptor 1 antagonists attenuate disease severity in dextran sulphate sodium-induced colitis in mice. Neurogastroenterol Motil 2004; 16: 811–818. [DOI] [PubMed] [Google Scholar]
- 37.Ward SM, Bayguinov J, Won K, et al. Distribution of the Vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol 2003; 465: 121–135. [DOI] [PubMed] [Google Scholar]
- 38.Yu L, Yang F, Luo H, et al. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete freund’s adjuvant. Mol Pain 2008; 4: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung MK, Jung SJ, Oh SB. Role of TRP channels in pain sensation. Adv Exp Med Biol 2011; 704: 615–636. [DOI] [PubMed] [Google Scholar]
- 40.Bhave G, Hu HJ, Glauner KS, et al. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid (TRPV1). Proc Natl Acad Sci USA 2003; 11: 12480–12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vellani V, Mapplebeck S, Moriondo A, et al. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol 2001; 534: 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]