Abstract
Ebitz and Moore [1] recently described that subthreshold electrical microstimulation of the macaque frontal eye fields (FEF) modulates the pupillary light reflex. This elegant study suggests that the influence of the FEF and prefrontal cortex on attentional modulation of cortical visual processing extends to the subcortical circuit that mediates a very basic reflex, the pupillary light reflex.
Keywords: Pupillary light reflex, Attention, Frontal Eye Fields, Prefrontal cortex
Main text
While we are most familiar with the constriction of the pupil that occurs with light, the pupil modulates due to other factors. For example, many studies have documented that pupil dilation accompanies mental effort or increased attention, while pupils constrict during times when we are sleepy [2]. Further, pupil dilation as a signal of heightened vigilance and arousal has been suggested to increase attractiveness (hence the use during the Italian Renaissance of the plant, Atropa belladonna – beautiful woman – whose active agent atropine is a pupil dilator). And as an extreme case, Richard Gregory, a renowned vision scientist of the mid-20th century, showed that the pupils of a talking parrot were modulated not by light, but during the attention required for vocalization or the recognition of known words from humans [3].
Light- and arousal related pupil responses are counted among the most basic behaviors in the repertoire of many diverse species. Their neural pathways are known in some detail, and involve a circuit from the retina thorough the mesencephalon (Olivary Pretectal Nucleus [OPN] encoding retinal illumination) to the pupilloconstrictor preganglionic neurons of the Edinger-Westphal nucleus, and a sympathetic component responsible for arousal effects [2]. As Ebitz and Moore [1] point out, there has recently been a renewed interest in pupillary responses in non-human primates related to orienting responses and task conflict [4, 5]. In addition, three laboratories working with human participants have shown a new kind of attentionally driven pupil behavior: without any change of light level, covertly attending to a brighter region of the visual field is sufficient to drive a pupillary constriction [6, 7] and, when a light increment does occur, the evoked pupillary constriction is enhanced when the light stimulus is attended vs. ignored [8].
The neural substrates of this attentional modulation of the pupillary light response were completely unknown until Ebitz and Moore [1] identified a key component of the underlying circuit: the Frontal Eye Field (FEF), a prefrontal cortical area implicated in the control of eye movements and attention [9]. Their main finding is that the amplitude of a pupil response depends on the coincidence between the light stimulus and subthreshold FEF electrical microstimulation – precisely as it depends on the coincidence between stimulus and attention in the experiments on human participants. While monkeys maintained fixation, a peripheral light stimulus was presented either inside or outside the stimulated site’s movement field, as previously defined by suprathreshold microstimulation; the light always evoked pupillary constriction, but the constriction was stronger when the stimulus was inside the stimulated site’s movement field; it was weaker when the stimulus was 180° to, and at the same eccentricity as, the stimulated site’s movement field. Further, the FEF microstimulation was only effective when it preceded the visual probe by 40 ms, and not 80 or 160 ms.
Given the spatial and temporal specificity of this response modulation, these effects cannot be dismissed as dependent on cognitive load or arousal, nor explained by a change in sympathetic tone. There are other known cortical influences on pupil size, but these also fail to explain this attentional modulation. For example, there is the “pupillary near response”, a pupillary constriction that accompanies the ocular convergence and change in focus required to view a near object, which involves pathways from regions of visual and frontal cortex to neurons in the midbrain near response region that then project to pupilloconstrictor neurons within the Edinger Westphal nucleus [2]. One might wonder if this response could explain the effects of attention or FEF stimulation, because both these manipulations might induce near viewing. However, if this were the case, FEF stimulation should induce pupil constriction irrespective of the presence or location of the light stimulus, which is not what Ebitz and Moore [1] found. Human data also suggest that the effect of attention cannot be explained by the pupillary near response. The key finding is that attention also enhances the pupillary dark response: increasing dilation in response to a luminance decrement [10].
Cortical input is also involved in another subtle, but consistent, pupil behavior: the transient pupil constriction at the onset of any equiluminant visual stimulus (i.e. stimuli that do not change luminance [2]). This “onset response” is likely included in all pupillary responses measured in attention studies as well as in Ebitz and Moore [1], and the neural circuits explaining the two effects are likely to be partially overlapping. Yet, again, the two are not identical: in humans, the effect of attention is not explained by adding an “onset” constriction component, but consists of a gain increase of the pupillary response to both light and dark: enhanced constriction in response to light increments and, symmetrically, enhanced pupillary dilation in response to light decrements [10]. Note that Ebitz and Moore [1] did not investigate whether FEF stimulation also enhances dilation in response to dark, as attention does; perhaps a future study will clarify this point.
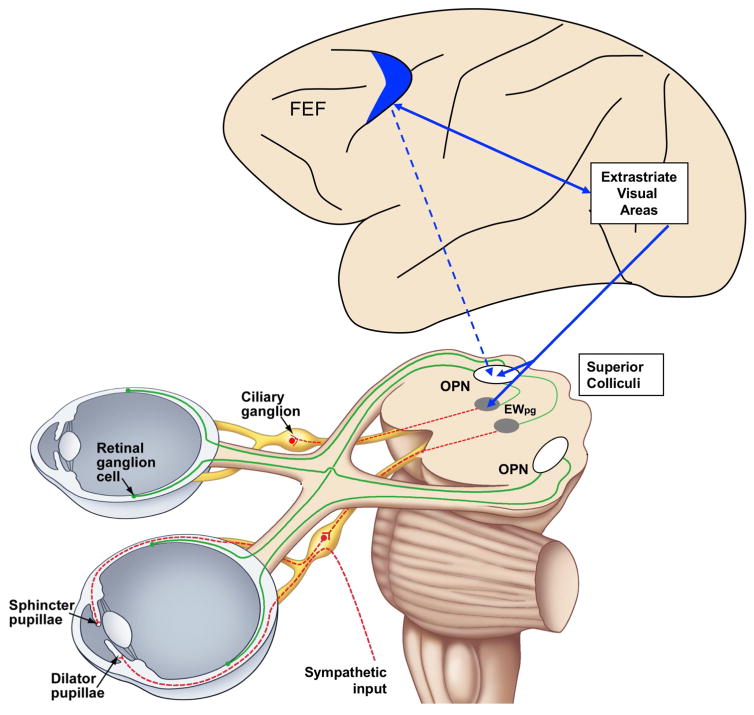
How, then, could FEF activity affect the subcortical reflex circuit mediating the pupillary light response? To account for the enhancement of pupillary light response, it is necessary that FEF stimulation enhances a neural encoding of brightness that, in turn, drives pupillary constriction. Figure 1 shows two possible pathways. FEF stimulation could directly modulate the Olivary Pretectal Nucleus, which encodes retinal illumination and directly activates the pupillo-constrictor pathway (Figure 1, dashed blue line). Alternatively, or in addition, FEF might act indirectly through occipital visual cortical areas, whose visual response is modulated by FEF [9] and might participate in the PLR (Figure 1, continuous blue lines) by projecting to the mesencephalic Pupil Light Reflex circuit, either to the Olivary Pretectal Nucleus, or to pupilloconstrictor neurons within the EW). Consistent with this former suggestion, Clarke and colleagues reported that some neurons in the macaque OPN receive apparent cortical inputs [2]. In a conceptually similar model, the role of occipital visual areas could be replaced or supplemented by the superior colliculus which and has all the necessary cortical and subcortical connectivity to modulate pupillary responses [5].
Figure 1.
Cortical and subcortical structures that might be involved in the attentional modulation of the pupillary light response. Green lines show the retinal input and red lines the pupillomotor output. Blue lines show hypothetical cortical projections. Luminance encoding elements are represented as white boxes. For simplicity, connections and projections are represented for one side only. Abbreviations: EWpg – Edinger-Westphal preganglionic subdivision; FEF – Frontal Eye Fields; OPN – Olivary pretectal nucleus.
As should be evident from this overview and the recent report of Ebitz and Moore [1], far from being a simple light-evoked reflex, pupillary responses are modulated in a well-defined fashion by attention, fatigue, arousal, ocular convergence and accommodation, etc. We still know little of the central mechanisms that control these responses, but renewed attention to the pupil light reflex in both humans and non-human primates will hopefully lead to other important discoveries.
Text Box. Pupil responses in blindsight.
Subthreshold FEF micro-stimulation as well as the actual execution of a saccade modulates pupil size. It is interesting to note, as Ebitz and Moore point out, that the dynamic of this response changes depending on the task and the stimulus set. Far from being just an artifact to be controlled for, peri-saccdic pupil modulations might represent a new and rich source of information for monitoring visual processing during saccade planning and execution – e.g. to understand the processing of intra-saccadic signals and their suppression from conscious awareness [11], following the seminal work of Sahraie and colleagues [12] who find that pupillary “onset responses” – objectively measured with relative ease – predict one of the most elusive phenomena: blindsight in patients with cortical lesions.
Acknowledgments
PB is supported by the European Research Council (n. 338866) and the MIUR “Futuro in Ricerca,” (n. RBFR1332DJ). PDG is supported by NIH Grants RO1 EY025555, RO1 EY014263, P30 EY03039, NSF Award #1539034, and Alabama EyeSight Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ebitz RB, Moore T. Selective Modulation of the Pupil Light Reflex by Microstimulation of Prefrontal Cortex. J Neurosci. 2017;37(19):5008–5018. doi: 10.1523/JNEUROSCI.2433-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDougal DH, Gamlin PD. Autonomic control of the eye. Compr Physiol. 2015;5(1):439–73. doi: 10.1002/cphy.c140014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregory R, Hopkins P. Pupils of a talking parrot. Nature. 1974;252(5485):637–8. doi: 10.1038/252637a0. [DOI] [PubMed] [Google Scholar]
- 4.Ebitz RB, Platt ML. Neuronal activity in primate dorsal anterior cingulate cortex signals task conflict and predicts adjustments in pupil-linked arousal. Neuron. 2015;85(3):628–40. doi: 10.1016/j.neuron.2014.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang CA, Munoz DP. A circuit for pupil orienting responses: implications for cognitive modulation of pupil size. Curr Opin Neurobiol. 2015;33:134–40. doi: 10.1016/j.conb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Binda P, et al. Attention to bright surfaces enhances the pupillary light reflex. J Neurosci. 2013;33(5):2199–204. doi: 10.1523/JNEUROSCI.3440-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathôt S, et al. The Pupillary Light Response Reveals the Focus of Covert Visual Attention. PLoS ONE. 2013;8(10):e78168. doi: 10.1371/journal.pone.0078168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naber M, et al. Tracking the allocation of attention using human pupillary oscillations. Front Psychol. 2013;4:919. doi: 10.3389/fpsyg.2013.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noudoost B, et al. Top-down control of visual attention. Current Opinion in Neurobiology. 2010;20(2):183–190. doi: 10.1016/j.conb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binda P, Murray SO. Spatial attention increases the pupillary response to light changes. J Vis. 2015;15(2):1. doi: 10.1167/15.2.1. [DOI] [PubMed] [Google Scholar]
- 11.Benedetto A, Binda P. Dissociable saccadic suppression of pupillary and perceptual responses to light. J Neurophysiol. 2016;115(3):1243–51. doi: 10.1152/jn.00964.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahraie A, et al. Pupil response as a predictor of blindsight in hemianopia. Proc Natl Acad Sci U S A. 2013;110(45):18333–8. doi: 10.1073/pnas.1318395110. [DOI] [PMC free article] [PubMed] [Google Scholar]