Abstract
The evaluation of the leishmanicidal and trypanocidal activity of the hydroalcoholic extract of the bark of Stryphnodendron rotundifolium Mart. (EHCSR) was carried out to find an alternative treatment for parasitic diseases. EHCSR was prepared and used at four different concentrations (1000, 500, 250, 125 μg/mL) in in vitro assays for activity against Leishmania promastigotes using the species Leishmania brasiliensis and Leishmania infantum and for trypanocidal activity using the epimastigotes of Trypanosoma cruzi. We also tested EHCSR for cytotoxicity against adhered cultured Murine J774 fibroblasts. The tests were performed in triplicate, and the percent mortality of parasites, IC50 and percent toxicity were determined. With regard to anti-leishmania activity against L. infantum, there was a mean mortality of 45% at all concentrations, and against L. brasiliensis, a substantial effect was seen at 1000 μg/mL with 56.38% mortality, where the IC50 values were 1338.76 and 987.35 μg/mL, respectively. Trypanocidal activity was notably high at 1000 μg/mL extract with 82.31% mortality of epimastigotes. Cytotoxicity at the highest extract concentrations of 500 and 1000 μg/mL was respectively 75.12% and 94.14%, with IC50 = 190.24 μg/mL. Despite that the extract has anti-parasitic activity, its substantial cytotoxicity against fibroblasts cells makes its systemic use nonviable as a therapeutic alternative.
Keywords: Stryphnodendron rotundifolium Mart, Trypanocidal, Leishmanicidal, Cytotoxicity
1. Introduction
Leishmaniasis and Chagas disease are known as serious public health problems due to their high mortality rate in countries such as Brazil, since they are directly linked to socioeconomic issues (Lindoso and Lindoso, 2009).
Leishmaniasis is caused by a blood parasite, where its transmission is through the bite of an infected sandfly, where during blood feeding, parasites are inoculated into the skin of the host. Domestic animals serve as reservoirs, especially the house dog and wild ones, but humans have become a possible reservoir due to the organizational changes in cities, and thus, leishmaniasis is no longer just a rural disease (Monteiro et al., 2005). American cutaneous leishmaniasis has 3 clinical forms, cutaneous, mucocutaneous and diffuse cutaneous. The mucocutaneous one is characterized by lesions that destroy cartilage and mucosal tissues. Visceral leishmaniasis, known as kala azar, is considered the most severe and has symptoms such as hepatosplenomegaly and pancytopenia, and can lead to death depending on the progress of the disease. These 3 forms are caused respectively by Leishmania brasiliensis and Leishmania donovani, Leishmania infantum and Leishmania chagasi (Aleixo et al., 2006, Maia-Elkhoury et al., 2008).
A major problem with this disease is the lack of vaccines and medicines that are safe and effective, especially for dogs, which have become the main host and cause of major difficulties in controlling the disease, because this animal is found in homes and because they are cherished by humans (Monteiro et al., 2005).
Chagas disease is caused by the protozoan Trypanosoma cruzi, which is transmitted by the triatomine known as the kissing bug, during blood feeding, when it penetrates the skin leaving urine or feces infected with metacyclic trypomastigotes, which infect the host, usually mammals. But this is not the only transmission route, as others have been demonstrated, such as contamination by blood transfusion and organ transplantation and orally by ingestion of the vector, which happens when it is triturated during the preparation of any food (Schofield et al., 2006). The symptomatology is diverse, with the most serious problems in the chronic phase with the occurrence of cardiac and hepatic complications, among others (Rassi et al., 2010). The treatment of the latter has several limitations, such as being more effective in the acute phase of the disease and causing many side effects (Coura, 2009). Because of these factors, the importance of developing new drugs is evident, especially those derived from medicinal plants, due to their wide variety and easy access for people with less purchasing power (Izumi et al., 2011).
Because of the tradition of using medicinal plants in Brazil, their pharmacological properties have been arduously explored by Brazilian investigators and more recently by the pharmaceutical industry because of the need to develop new drugs (de Oliveira et al., 2014).
The genus Stryphnodendron belongs to the subfamily Mimosoideae and family Leguminosae, which encompasses approximately 64 genera. Stryphnodendron rotundifolium Mart is species popularly known as ‘barbatimão, barba-de-timão, charãozinho-roxo, casca-da-virgindade, casca-da-mocidade and barbatimão-vermelho” (Sanches et al., 2007). The species S. rotundifolium Mart., endemic to Chapada do Araripe, is used by much of the population of the Cariri where the bark of this tree is used to treat wounds, sores, gastritis, vaginal inflammation and infections (Oliveira et al., 2011).
Phytochemical prospecting of S. rotundifolium bark extract has been performed, showing the presence of tannin pyrogallics, flavones, flavonols, flavononols, xanthones, chalcones, flavonones and steroids as secondary metabolites in this species (Oliveira et al., 2011), along with antibacterial and antifungal (Rodrigues et al., 2008), antioxidant (Costa et al., 2012) and antibiotic-modifying (Oliveira et al., 2011) activity.
Accordingly, the aim of the study was to evaluate the trypanocidal and leishmanicidal activity of the hydroalcoholic extract of the bark of S. rotundifolium to determine its therapeutic potential in these conditions, for a possible alternative treatment for patients.
2. Materials and methods
2.1. Botanical material
The plant material of S. rotundifolium was collected in Crato, Ceará, Brazil, in March 2011. A voucher specimen of the species is deposited in the Herbarium Dárdaro de Andrade Lima-URCA under No. 4661.
2.2. Preparation of hydroalcoholic extract of the bark of S. rotundifolium Mart. (EHCSR)
The plant material (dry bark of S. rotundifolium) was subjected to cold extraction with water and 99.99% ethanol (1:1), and the solvent was removed using a rotary evaporator, resulting in a dry crude extract after lyophilization (EHCSR).
2.3. Preparation of the solution EHCSR
EHCSR was dissolved in phosphate buffered saline (PBS, pH 7.2) at a concentration of 1 mg/mL, subsequently giving test concentrations of 500, 250 and 125 μg/mL. The assays were performed in triplicate.
2.4. Quantification of compounds by HPLC-DAD
All chemical agents were of analytical grade. Methanol, acetic acid, gallic acid and caffeic acid were purchased from Merck (Darmstadt, Germany). Catechin, rutin and kaempferol were acquired from Sigma Chemical Co. (St. Louis, MO, USA). High performance liquid chromatography (HPLC-DAD) was performed with the Shimadzu HPLC system (Shimadzu, Kyoto, Japan) and Prominence Auto Sampler (SIL-20A), equipped with Shimadzu LC-20AT reciprocating pumps connected to the degasser DGU 20A5 with integrator CBM 20A, UV–vis detector DAD (diode) SPD-M20A and Software LC solution 1.22 SP1.
Reverse-phase chromatographic analyses were carried out under gradient conditions using a C18 column (4.6 mm × 250 mm) packed with 5-μm diameter particles; the mobile phase was water containing 2% acetic acid (A) and methanol (B), and the composition gradient was: 5% of B until 2 min and changed to 25, 40, 50, 60, 70 and 100% B at 10, 20, 30, 40, 50 and 80 min, respectively, following the method described by da Silva et al. (2014) with slight modifications. EHCSR was dissolved in ethanol at a concentration of 5 mg/mL and analyzed. The presence of five phenolic compounds was determined, namely catechin, gallic and caffeic acids and the flavonoids rutin and kaempferol. Identification of these compounds was performed by comparing their retention time and UV absorption spectrum with those of commercial standards. The flow rate was 0.6 mL/min and injection volume 40 μL, and the wavelengths used for detection were 257 nm for gallic acid, 280 nm for catechin, 325 nm for caffeic acid and 365 nm for rutin and kaempferol. All samples and mobile phase were filtered through a 0.45-μm membrane filter (Millipore) and then degassed with an ultrasonic bath prior to use. Stock solutions of reference standards were prepared in the HPLC mobile phase at concentrations of 0.020–0.200 mg/mL for kaempferol and rutin and 0.030–0.250 mg/mL for catechin, caffeic and chlorogenic acids. The chromatographic peaks were confirmed by comparing their retention times with those of reference standards and by DAD spectra (200–400 nm). The calibration curves were: gallic acid, Y = 12575x + 1253.8 (r = 0.9999); caffeic acid, Y = 11407x + 1359.8 (r = 0.9996); catechin, Y = 11355x − 1047.1 (r = 0.9968); rutin, Y = 12492x − 1065.7 (r = 0.9997); and kaempferol, Y = 14923x − 1238.9 (r = 0.9997).
2.5. Obtaining parasites and cells
We used blood cultures of T. cruzi epimastigotes and promastigotes of L. brasiliensis and L. infantum. The epimastigotes were obtained in the exponential growth phase in liver infusion tryptose (LIT) and washed twice in sterile PBS, pH 7.2, with centrifugation at 1500 rpm for 10 min at 4 °C, and the number of parasites was determined in a Neubauer chamber. Afterward, the parasites were suspended in LIT medium supplemented with 10% fetal bovine serum (FBS) (inactivated at 56 °C), adjusted to 5 × 106 epimastigotes/mL, and kept at 4 °C until use.
The promastigotes of L. brasiliensis and L. infantum were in the exponential growth phase in Schneider medium supplemented with 5% FBS and 2% urine. They were washed twice in sterile PBS, pH 7.2, with centrifugation at 1500 rpm for 10 min at 4 °C, and the number of parasites was determined in a Neubauer chamber. The parasites were then suspended in Schneider medium plus 5% FCS and 2% of urine, adjusted to 5 × 106 promastigotes/mL, and kept at 4 °C until use.
Fibroblasts were grown in minimal essential medium (MEM), supplemented with 10% heat-inactivated FBS, penicillin G (100 U/mL) and streptomycin (100 μg/mL). Cultures were maintained at 37 °C in a humidified 5% CO2 atmosphere until use.
2.6. In vitro leishmanicidal evaluation
For the evaluation of leishmanicidal activity against the promastigote form, assays were performed in triplicate in 96-well culture plates containing 20 μL of EHCSR at concentrations in descending order, diluted in sterile PBS, pH 7.2, plus 180 μL of the suspension of promastigotes. In the controls, the parasites were incubated in the absence of extract or in the presence of 100 μg/mL pentamidine. After the addition of the natural products, the samples were shaken and incubated at 24 °C for 72 h. The plates were then evaluated visually under a microscope to check for motility of parasites. The results were expressed as a percentage. The assays were carried out in triplicate with the objective of assessing the stability of EHCSR activity and the reproducibility of the results. Pentamidine was used as the reference drug.
2.7. In vitro evaluation of trypanocidal
The trypanocidal assays against epimastigotes were performed in triplicate in 96-well culture plates containing 20 μL of EHCSR at concentrations in descending order, diluted in sterile PBS, pH 7.2, plus 180 μL of epimastigote suspension. In the control, the parasites were incubated in the absence of extract or in the presence of 100 μg/L Nifurtimox. The plates were then shaken and incubated at 24 °C for 72 h and assessed visually under a microscope to check for motility of parasites. The activity was determined by counting in a Neubauer chamber, followed by statistical evaluation. The results were expressed as a percentage. The assays were carried out in triplicate to assess the stability of the extract activity and reproducibility of the results.
2.8. Evaluation of cytotoxicity
Murine J477 fibroblasts were adhered to 96-well microdilution plates at a final concentration of 3 × 104 cells/well and incubated at 37 °C in an atmosphere of 5% CO2. Subsequently, MEM was removed and 200 μL of EHCSR in different concentrations were added and the plates incubated for another 24 h. Afterward, 20 μL of 2 mM resazurin was added to each well. Plates were incubated for 3 h and the reduction of resazurin was determined by absorbance at 490 and 595 nm. The value of the control (blank) was subtracted. Each concentration was tested in triplicate.
2.9. Statistical analysis
All assays were performed in triplicate and repeated at least once. Half-maximal growth inhibitory concentration (IC50) was determined by linear regression using GraphPad Prism Software.
3. Results and discussion
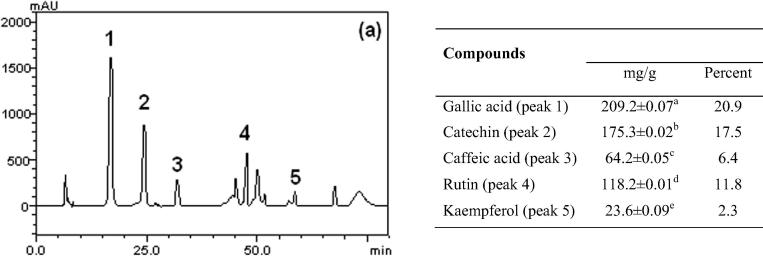
HPLC fingerprinting of EHCSR revealed the presence of gallic acid (tR = 17.01 min; peak 1), catechin (tR = 24.88 min; peak 2), caffeic acid (tR = 31.62 min; peak 3), rutin (tR = 47.14 min; peak 4) and kaempferol (tR = 58.49 min; peak 5) (Fig. 1). Thus, HPLC analysis revealed the EHCSR contained flavonoids (rutin and kaempferol), phenolics acids (caffeic acids) and catechin.
Figure 1.
Representative high performance liquid chromatography profile of phenolics and flavonoid composition of the Stryphnodendron rotundifolium extract, detection UV was at 325 nm. Results were expressed as mean ± standard deviations (SD) of three determinations. Averages followed by different letters differ by Tukey test at p < 0.005.
An ethnopharmacological study has shown that S. rotundifolium is used as a popular medicine by traditional communities in Northeast Brazil. The stem bark of this plant is used especially in the form of a decoction (de Oliveira et al., 2014). Phytochemical prospecting of hydroalcoholic extract of S. rotundifolium (HESR) has led to the identification of tannins, pyrogallics, flavones, flavonoids, flavononols, xanthones, chalcones, flavonones and steroids (Oliveira et al., 2011). Data from the literature show that bark extracts of species of this genus contain tannins and flavonoids (Lopes et al., 2009, Siqueira et al., 2012).
The results in Table 1 show the effect of the reference drug, pentamidine, on Leishmania sp., where IC50 = 5.69 μg/mL. Table 2, Table 3 show the results for the anti-promastigote activity against Leishmania sp. Against L. infantum (Table 2), the four concentrations of EHCSR tested caused a similar percentage of parasite inhibition, around 45%, with an estimated IC50 = 1300 μg/mL. L. brasiliensis (Table 3) was substantially inhibited only at 1000 μg/mL, where the percentage of the inhibition was 56.38%, yielding an IC50 = 987.35 μg/mL.
Table 1.
Results of pentamidine on Leishmania infantum promastigotes in the parasitic form (IC50 = 5.69 μg/mL).
| Concentration (μg/mL) | % Anti-promastigote (L. infantum) | % ± standard deviation |
|---|---|---|
| 100 | 93.9 | 0.3 |
| 50 | 93.9 | 0.1 |
| 25 | 89.2 | 0.6 |
| 12.5 | 80.6 | 0.2 |
| 6.25 | 54.2 | 0.3 |
| 3.125 | 15.5 | 1.1 |
Table 2.
Results of EHCSR, on Leishmania infantum promastigotes in the parasitic form (IC50 = 1338.76 μg/mL).
| Concentration (μg/mL) | % Anti-promastigote (L. infantum) | % ± standard deviation |
|---|---|---|
| 1000 | 47.14 | 0.69 |
| 500 | 45.63 | 2.52 |
| 250 | 40.67 | 1.12 |
| 150 | 43.47 | 2.72 |
Table 3.
Result of EHCSR, on Leishmania brasiliensis in parasitic promastigote form (IC50 = 987.35 μg/mL).
| Concentration (μg/mL) | % Anti-promastigote (L. infantum) | % ± standard deviation |
|---|---|---|
| 1000 | 56.38 | 5.49 |
| 500 | 4.68 | 2.06 |
| 250 | 2.85 | 1.79 |
| 150 | 2.85 | 1.07 |
According to Rosas et al. (2007), when more than 50% parasite inhibition is achieved at an extract concentration of 500 μg/mL, the test substance is considered a good inhibitor of parasites. Although EHCSR did not show this level of activity, its potential cannot be ruled out, since its activity came close to the above benchmark, which can be explained by the EHCSR components present, including tannins, flavonoids and phenolic compounds, the main secondary metabolites present in this species (Vilar et al., 2010).
The anti-Leishmania effect was the target of a study by Kolodziej and Kiderlen (2005), who demonstrated that condensed tannins, polyphenols, simple phenolics and tannins possess this activity. In previous studies the antileishmanial activity of extracts of the fruit of Cocos nucifera L., rich in polyphenols was evaluated, where a satisfactory effect was obtained with a minimum inhibitory concentration of 10 μg/mL (Mendonça-Filho et al., 2004).
According to Paula-Junior et al. (2006), the hydroethanolic extract of Caryocar brasiliense leaves has antileishmanial activity against the promastigote forms and strong antioxidant activity, corroborating the findings of the present study, because extracts of the genus Stryphnodendron have antioxidant activity as already mentioned (Lopes et al., 2005), and this was also shown for S. rotundifolium by Costa et al. (2012). Williams et al. (2003) reported that antioxidant compounds such as flavonoids may have an effect on promastigotes of Leishmania sp., where viability was measured by reduction of a tetrazolium salt. Sen et al. (2008) reported that treatment with the flavonoid quercetin combined with serum albumin showed increased bioavailability of the flavonoid, proving to be major advantage in promoting its effectiveness against L. donovani.
According to Bezerra et al. (2006) crude extracts from plants have a greater leishmanicidal effect against promastigotes, Reis et al. (2013) showed that the hydroalcoholic extract of Chenopodium ambrosioides leads to a decrease in parasite load in infection with L. amazonian in vivo, demonstrating that the extract used in our study possibly possesses these leishmanicidal characteristics due to these factors, and because this is a crude hydroalcoholic extract, as previously used.
Table 4 shows the effect of the reference drug chosen, Nifurtimox, on motility of T. cruzi. epimastigotes, showing an IC50 = 2.20 μg/mL. The trypanocidal activity of EHCSR against epimastigotes (Table 5) showed relevancy at a concentration of 1000 μg/mL, at which there was 82.31% death of parasitic forms. Lower extract concentrations of 250 and 500 μg/mL showed low toxicity, respectively 2.31% and 7.07%, while the lowest concentration of 150 μg/mL had no effect on epimastigotes, and IC50 = 748.97 μg/mL was determined. In studies by Herzog-Soares et al. (2002) using the crude extract of the bark of the species Stryphnodendron adstringens, which belongs to the same genus of the extract used in this study proved to be effective against the parasite T. Cruzi in vivo, almost totally eliminating all parasites found in the blood of animals, demonstrating its effectiveness against it.
Table 4.
Result of Nifurtimox, on Trypanosoma cruzi epimastigote form in the parasite (IC50 = 20.02 μg/mL).
| Concentration (μg/mL) | % Anti-epimastigote (T. cruzi) | % ± standard deviation |
|---|---|---|
| 100 | 100 | 00.46 |
| 50 | 93 | 0.66 |
| 10 | 84 | 0.62 |
| 1 | 43 | 0.93 |
| 0.5 | 13 | 2.50 |
| 0.1 | 0 | 1.54 |
Table 5.
Result of EHCSR, against Trypanosoma cruzi epimastigote form in the parasite (IC50 = 748.97 μg/mL).
| Concentration (μg/mL) | % Anti-epimastigote (T. cruzi) | % ± standard deviation |
|---|---|---|
| 1000 | 82.31 | 12.57 |
| 500 | 7.07 | 12.55 |
| 250 | 2.13 | 1.21 |
| 150 | 0 | 12.35 |
Ribeiro et al. (1997) isolated flavanones from Trixis vauthieri L. and found that they eliminated about 99% of the parasites in infected blood. Grael et al. (2005) isolated a number of substances from Lychnophora pohlii, where flavonoids showed considerable trypanocidal activity. Takeara et al. (2003) demonstrated that flavonoids present in other species of Lychnophora were effective against T. cruzi in contaminated blood in blood banks. Schinor et al. (2004) observed the same activity but in vivo. These data are of paramount importance for the present study because of the presence of flavonoids in EHCSR, which shows one of the probable reasons for the trypanocidal effect observed. Abe et al. (2004) showed that a xanthone isolated from Garcinia subellíptica was active against epimastigote and trypomastigote forms and protozoa of T. cruzi; xanthones were also found in EHCSR.
Previous findings support the potential anti-parasitic activity of EHCSR, but one cannot dismiss the importance of the results demonstrating potential host cytotoxicity (Table 6). Concentrations of 500 and 1000 μg/mL, which were the most representative of anti-parasitic activity, also showed very high toxicity in cultured fibroblasts cells, 75.12% and 94.14% respectively, with an IC50 = 190.24 μg/mL.
Table 6.
EHCSR cytotoxic activity on fibroblasts (IC50 = 190.24 μg/mL).
| Concentration (μg/mL) | % Cytotoxicity | % ± standard deviation |
|---|---|---|
| 1000 | 94.14 | 1.04 |
| 500 | 75.12 | 1.63 |
| 250 | 62.42 | 1.01 |
| 150 | 43.39 | 2.03 |
Our results demonstrated a dose-dependent effect, but limited because bioavailability was not considered. Several studies have shown the contribution of in vitro assays for in vivo applicability (Henning et al., 2005). These methods will therefore be highly useful for future preclinical and clinical studies. These results then provide important knowledge to explore the potential of EHCSR in the treatment of parasitic infections as T. cruz or Leishmania.
4. Conclusion
We found that the hydroalcoholic extract of S. rotundifolium bark (EHCSR) had better activity against T. cruzi in comparison with two Leishmania species. However, the results did not show clinical significance with regard to anti-parasitic activity. EHCSR showed a significant cytotoxicity against fibroblast cells, indicating limits to its use in a systemic way. This plant appears to be promising for the development of herbal products and/or new drugs with antiparasitary actions, but, in vivo assay is necessary for drug evaluation.
Acknowledgments
The authors are grateful to the Brazilian research agencies CNPq and FUNCAP. Dr. A. Leyva helped with English editing of the manuscript.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Henrique D.M. Coutinho, Email: hdmcoutinho@gmail.com.
Irwin R.A. Menezes, Email: irwinalencar@yahoo.com.br.
References
- Abe F., Nagafuji S., Okabe H., Akahane H., Estrada-Muñiz E., Huerta-Reyes M., Reyes-Chilpa R. Trypanocidal constituents in plants 3. Leaves of Garcinia intermedia and heartwood of Calophyllum brasiliense. Biol. Pharm. Bull. 2004;27:141–143. doi: 10.1248/bpb.27.141. [DOI] [PubMed] [Google Scholar]
- Aleixo J.A., Nascimento E.T., Monteiro G.R., Fernandes M.Z., Ramos A.M.O., Wilson M.E., Pearson R.D., Jeronimo S.M.B. Atypical American visceral leishmaniasis caused by disseminated Leishmania amazonensis infection presenting with hepatitis and adenopathy. Trans. R. Soc. Trop. Med. Hyg. 2006;100:79–82. doi: 10.1016/j.trstmh.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Bezerra J.L., Costa G.C., Lopes T.C., Carvalho I.C.D.S., Patrício F.J., Sousa S.M., Amaral F.M.M., Rebelo J.M.M., Guerra R.N.M., Ribeiro M.N.S. Evaluation of the in vitro leishmanicidal activity of medicinal plants. Rev. Bras. Farmacogn. 2006;16:631–637. [Google Scholar]
- Costa J.G.M., Leite G.O., Dubois A.F., Seeger R.L., Boligon A.A., Athayde M.L., Campos A.R., Rocha J.B.T. Antioxidant effect of Stryphnodendron rotundifolium Martius extracts from Cariri-Ceará state (Brazil): potential involvement in its therapeutic use. Molecules. 2012;17:934–950. doi: 10.3390/molecules17010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coura J.R. Present situation and new strategies for Chagas disease chemotherapy: a proposal. Mem. Inst. Oswaldo Cruz. 2009;104:549–554. doi: 10.1590/s0074-02762009000400002. [DOI] [PubMed] [Google Scholar]
- Da Silva A.R.H., Moreira L.R., Brum E.S., Freitas M.L., Boligon A.A., Athayde M.L., Roman S.S., Mazzanti C.M., Brandão R. Biochemical and hematological effects of acute and sub-acute administration to ethyl acetate fraction from the stem bark Scutia buxifolia Reissek in mice. J. Ethnopharmacol. 2014;153:908–916. doi: 10.1016/j.jep.2014.03.063. [DOI] [PubMed] [Google Scholar]
- de Oliveira D.R., Júnior W.S.F., Bitu V.C.N., Pinheiro P.G., Menezes C.D.A., Brito Junior F.E., Albuquerque U.P., Kerntopf M.R., Coutinho H.D.M., Fachinetto R., Menezes I.R.A. Ethnopharmacological study of Stryphnodendron rotundifolium in two communities in the semi-arid region of northeastern Brazil. Rev. Bras. Farmacogn. 2014;2:124–132. [Google Scholar]
- Grael C.F.F., Albuquerque S., Lopes J.L.C. Chemical constituents of Lychnophora pohlii and trypanocidal activity of crude plant extracts and of isolated compounds. Fitoterapia. 2005;76:73–82. doi: 10.1016/j.fitote.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Henning S.M., Niu Y., Liu Y., Lee N.H., Hara Y., Thames G.D., Minutti R.R., Carpenter C.L., Wang H., Heber D. Bioavailability and antioxidant effect of epigallocatechin gallate administered in purified form versus as green tea extract in healthy individuals. J. Nutr. Biochem. 2005;16(10):610–616. doi: 10.1016/j.jnutbio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Herzog-Soares J.D., Alves R.K., Isac E., Bezerra J.C.B., Gomes M.H., Santos S.C., Ferri P.H. Atividiade tripanocida in vivo de Stryphnodendron adstringens (barbatimão verdadeiro) e Caryocar brasiliensis (pequi) Rev. Bras. Farmacogn. 2002;12:1–2. [Google Scholar]
- Izumi E., Ueda-Nakamura T., Dias Filho B.P., Veiga V.F., Júnior, Nakamura C.V. Natural products and Chagas’ disease: a review of plant compounds studied for activity against Trypanosoma cruzi. Nat. Prod. Rep. 2011;28:809–823. doi: 10.1039/c0np00069h. [DOI] [PubMed] [Google Scholar]
- Kolodziej H., Kiderlen A.F. Antileishmanial activity and immune modulatory effects of tannins and related compounds on Leishmania parasitised RAW 264.7 cells. Phytochemistry. 2005;66:2056–2071. doi: 10.1016/j.phytochem.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Lindoso J.A.L., Lindoso A.A.B.P. Neglected tropical diseases in Brazil. Rev. Inst. Med. Trop. São Paulo. 2009;51:247–253. doi: 10.1590/s0036-46652009000500003. [DOI] [PubMed] [Google Scholar]
- Lopes G.C., Sanches A.C.C., Nakamura C.V., Dias Filho B.P., Hernandes L., Mello J.C.P. Influence of extracts of Stryphnodendron polyphyllum Mart. and Stryphnodendron obovatum Benth. on the cicatrisation of cutaneous wounds in rats. J. Ethnopharmacol. 2005;99:265–272. doi: 10.1016/j.jep.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Lopes G.C., Sanches A.C.C., Toledo C.E.M., Isler A.C., Mello J.C.P. Determinação quantitativa de taninos em três espécies de Stryphnodendron por cromatografia líquida de alta eficiência. Braz. J. Pharm. Sci. 2009;45:135–143. [Google Scholar]
- Maia-Elkhoury A.N.S., Alves W.A., Sousa-Gomes M.L., Sena J.M., Luna E.A. Visceral leishmaniasis in Brazil: trends and challenges. Cad. Saúde Pública. 2008;24:2941–2947. doi: 10.1590/s0102-311x2008001200024. [DOI] [PubMed] [Google Scholar]
- Mendonça-Filho R.R., Rodrigues I.A., Alviano D.S., Santos A.L.S., Soares R., Alviano C.S., Lopes A.H.C.S., Rosa M.S.S. Leishmanicidal activity of polyphenolic-rich extract from husk fiber of Cocos nucifera Linn. (Palmae) Res. Microbiol. 2004;155:136–143. doi: 10.1016/j.resmic.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Monteiro E.M., França-Silva J.C., Costa R.T., Costa D.C., Barata R.A., Paula E.V., Machado-Coelho G.L.L., Rocha M.F., Fortes-Dias C.L., Dias E.S. Leishmaniose visceral: estudo de flebotomíneos e infecção canina em Montes Claros, Minas Gerais. Rev. Soc. Bras. Med. Trop. 2005;38:147–152. doi: 10.1590/s0037-86822005000200004. [DOI] [PubMed] [Google Scholar]
- Oliveira D.R., Brito F.E., Bento E.B., Matias E.F.F., Sousa A.C.A., Costa J.G.M., Coutinho H.D.M., Kerntopf M.R., Menezes I.R.A. Antibacterial and modulatory effect of Stryphnodendron rotundifolium. Pharm. Biol. 2011;49:1265–1270. doi: 10.3109/13880209.2011.589857. [DOI] [PubMed] [Google Scholar]
- Paula-Junior W., Rocha F.H., Donatti L., Fadel-Picheth C.M.T., Weffort-Santos A.M. Leishmanicidal, antibacterial, and antioxidant activities of Caryocar brasiliense Cambess leaves hydroethanolic extract. Rev. Bras. Farmacogn. 2006;16:625–630. [Google Scholar]
- Rassi A., Jr., Rassi A., Marin-Neto J.A. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- Reis A.S., Rios C.E.P., Melo L.P., Costa G.C., Silva L.A., Patrício F.J.B., Amaral F.M.M., Nascimento F.R.F. Atividade Leishmanicida in vitro de frações do extrato hidroalcoólico das folhas de Chenopodium ambrosioides L. Rev. Cienc. Saúde. 2013;14:119–126. [Google Scholar]
- Ribeiro A., Piló-Veloso D., Romanha A.J., Zani C.L. Trypanocidal flavonoids from Trixis vauthieri. J. Nat. Prod. 1997;60:836–838. doi: 10.1021/np970196p. [DOI] [PubMed] [Google Scholar]
- Rodrigues F.G., Cabral B.S., Coutinho H.D.M., Cardoso A.H., Campos A.R., Costa J.G.M. Antiulcer and antimicrobial activities of Stryphnodendron rotundifolium Mart. Pharmacogn. Mag. 2008;4:193. [Google Scholar]
- Rosas L.V., Cordeiro M.S.C., Campos F.R., Nascimento S.K.R., Januário A.H., França S.C., Nomizo A., Toldo M.P.A., Albuquerque S., Pereira P.S. In vitro evaluation of the cytotoxic and trypanocidal activities of Ampelozizyphus amazonicus (Rhamnaceae) Braz. J. Med. Biol. Res. 2007;40:663–670. doi: 10.1590/s0100-879x2007000500009. [DOI] [PubMed] [Google Scholar]
- Sanches A.C.C., Lopes G.C., Toledo C.E.M., Sacramento L.V.S., Sakuragui C.M., Mello J.C.P. Estudo Morfológico Comparativo das Cascas e Folhas de Stryphnodendron adstringens, S. polyphyllum e S. obovatum-Leguminosae. Latin Am. J. Pharm. 2007;26:362. [Google Scholar]
- Sen G., Mukhopadhyay S., Ray M., Biswas T. Quercetin interferes with iron metabolism in Leishmania donovani and targets ribonucleotide reductase to exert leishmanicidal activity. J. Antimicrob. Chemother. 2008;61(5):1066–1075. doi: 10.1093/jac/dkn053. [DOI] [PubMed] [Google Scholar]
- Schinor E.C., Salvador M.J., Ito I.Y., Albuquerque S., Dias D.A. Trypanocidal and antimicrobial activities of Moquinia kingii. Phytomedicine. 2004;11:224–229. doi: 10.1078/0944-7113-00342. [DOI] [PubMed] [Google Scholar]
- Schofield C.J., Jannin J., Salvatella R. The future of Chagas disease control. Trends Parasitol. 2006;22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Siqueira C.F.Q., Cabral D.L.V., Peixoto Sobrinho T.J.S., Amorim E.L.C., Melo J.G., Araújo T.A.S., Albuquerque U.P. Levels of tannins and flavonoids in medicinal plants: evaluating bioprospecting strategies. ECAM. 2012;2012:1–7. doi: 10.1155/2012/434782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeara R., Albuquerque S., Lopes N.P., Lopes J.L.C. Trypanocidal activity of Lychnophora staavioides Mart. (Vernonieae, Asteraceae) Phytomedicine. 2003;10:490–493. doi: 10.1078/094471103322331430. [DOI] [PubMed] [Google Scholar]
- Vilar J.B., D’Oliveira M.I.P., Santos S.C., Chen L.C. Cytotoxic and genotoxic investigation on barbatimão [Stryphnodendron adstringens (Mart:) Coville, 1910] extract. Braz. J. Pharm. Sci. 2010;46:687–694. [Google Scholar]
- Williams C., Espinosa O.A., Montenegro H., Cubilla L., Capson T.L., Ortega-Barría E., Romero L.I. Hydrosoluble formazan XTT: its application to natural products drug discovery for Leishmania. J. Microbiol. Methods. 2003;55:813–816. doi: 10.1016/j.mimet.2003.08.013. [DOI] [PubMed] [Google Scholar]