Abstract
Preeclampsia is a serious medical complication during pregnancy. In response to an increasing number of preeclamptic cases and scarcity of data concerning the interrelation between trace element levels and preeclampsia, we carried out a hospital based case–control study in Riyadh, Saudi Arabia to study the correlation between levels of serum trace elements and risk of preeclampsia. One hundred and twenty pregnant women were enrolled in this study and divided into three groups of 40 each—Control group, HR group (women at high risk of preeclampsia) and PET group (Preeclampsia group). Serum trace element levels were estimated by inductively coupled plasma optical emission spectrophotometer. The analysis found that mean values of Ca, Mg and Zn were 90.08 ± 6.38, 19.33 ± 3.32 and 1.30 ± 0.83 mg/L respectively in normotensive control and 77.85 ± 4.47, 15.44 ± 1.43 and 0.98 ± 0.63 mg/L respectively in the HR group. The mean values of Ca, Mg and Zn in the preeclamptic group were 70.37 ± 4.66, 13.58 ± 1.98 and 0.67 ± 0.59 mg/L, respectively. Interelement analysis reflected a negative correlation between Ca and Mg and between Mg and Zn whereas positive correlation between Ca and Zn in preeclamptic women. However the correlation was not statistically significant. In conclusion, our study suggests that decreased levels of these trace elements in serum may act as predisposing factors in pathogenesis of Preeclampsia.
Keywords: Preeclampsia, Risk factors, Calcium, Magnesium, Zinc
1. Introduction
Preeclampsia is a common medical complication of pregnancy and is characterized by the hypertension, edema and proteinuria (Pallavi et al., 2012). It has a worldwide prevalence of 2–10% of pregnancies and one of the major causes of increase in maternal and perinatal morbidity and mortality. In Saudi Arabia, it accounts for 13,876 out of a population of 25,795,938 (Statistic by country, 2014). Preeclampsia is hemodynamically characterized by peripheral vasoconstriction which occurs due to imbalance of vasodilating and vasoconstrictor activity (Conrad et al., 1993). It is characterized by hypertension and proteinuria after 20 weeks of gestation.
Women of reproductive age are susceptible to both macro and micro nutritional deficiencies and the risk is increased in pregnant women due to increased requirements of nutrients like zinc, copper, calcium and vitamins etc., to fulfill the needs of the growing fetus (King, 2000). Essential trace elements are involved in various biochemical pathways. Their specific and the most important functions are the catalytic role in chemical reactions and in structural function in large molecules such as enzymes and hormones. Alterations in concentrations and homeostasis of each of these micronutrients in body are well-known contributors in pathophysiology of various disorders and diseases (Ulmer, 1977).
In muscle contraction and regulation of water balance in cells, calcium physiologically plays an important role. Changes in calcium levels of plasma lead to alteration of blood pressure (Manual for HIV-1 diagnosis, 2002). Magnesium is another important trace element; it acts as cofactor for many enzymes, required in various enzymatic processes, in proper bone formation and as an essential element to fetal development. Magnesium deficiency may possibly result in preeclampsia and pre-term delivery which can lead to low birth weight. It is reported that during gestation, magnesium deficiency increases chances of neonatal mortality and morbidity (Sarma and Gambhir, 1995). Magnesium plays a significant role in peripheral vasodilation and in neurochemical transmission (Gibson, 1994). One of the potential causes of preeclampsia could be alteration of calcium and magnesium metabolism during pregnancy however, this role in pregnant women is still being discussed.
Zinc is associated with a number of biochemical pathways; as a co-factor in the synthesis of DNA, RNA and numerous enzymes (Pathak and Kapil, 2004). The deficiency of Zinc has been connected with fetal growth retardation, congenital abnormalities, complications of pregnancy and delivery. It is observed that during pregnancy there is reduction in levels of circulating zinc and as pregnancy progresses further reduction occurs, this may be due to less number of zinc binding proteins and enhanced transfer of zinc from mother to fetus.
Even though numerous studies have been carried out on preeclampsia, still the etiology of preeclampsia is not clear. Some of the studies reported that changes in metal levels of blood observed in preeclamptic patients may be associated with pathogenesis of preeclampsia, whereas, other studies have failed to show such association (Bringman et al., 2006, Caughey et al., 2005). This study was undertaken keeping in view the disparity in the findings of trace elements role in preeclampsia and additionally the scarcity of data on the preeclamptic women residing in Riyadh, Saudi Arabia. The present study is an extension of our previous work in which we reported abnormal kidney function tests in preeclamptic women (Noura et al., 2014). This study was carried out to add to better understanding of trace elements like calcium, magnesium and zinc and their role in etiology of preeclampsia and their correlation with basic clinical characteristics of the preeclamptic patients. The high risk group was included in the present work to study the scenario of levels of trace elements in this group. We thus hypothesize, that the altered homeostasis of these trace elements’ levels in the high risk group of patients could help in prediction of preeclampsia during pregnancy.
2. Materials and methods
2.1. Study population
This study was carried out in collaboration with the Department of Clinical laboratory Sciences, King Saud University and Section of Obstetrics and Gynecology, King Saud Medical City Hospital, Riyadh from September 2012 to March 2014. The hospital’s ethics committee has approved the study and informed consent was obtained from patients before blood sampling.
A total of one hundred and twenty pregnant women were enrolled in this study and divided into three groups of forty each: Control group – normal healthy pregnant women, HR group – pregnant women at high risk of preeclampsia and PET group – women with preeclampsia. All patients were attending antenatal care unit or labor room in their third trimester of pregnancy.
2.2. Inclusion criteria
Control group – Pregnant women with normal BP, absence of proteinuria, normal renal function and without any other systemic or endocrine disorder. All subjects included were in their third trimester (gestational age of ⩾24 weeks).
High risk group – Women in the high risk group were included based on the following criteria: pregnant women with body mass index (BMI) of 35 or more, with mild hypertension or those with preeclampsia, gestational diabetes, IUGR (intrauterine growth restriction) or pre-term delivery in previous pregnancies and those with family history of preeclampsia.
PET group – Selection of the pre eclamptic group was according to the definition of American College of Obstetrics and Gynecologists (ACOG practice bulletin, 2002). Patients with renal dysfunction were also included.
2.3. Exclusion criteria
Patients with obesity, severe anemia or suffering from any hepatic dysfunction were excluded from the study.
2.4. Collection of blood samples and preliminary biochemical analysis
On admission, five milliliter of blood was drawn from each subject participated in the study in metal free sterile vacutainers. Blood samples obtained were then kept at room temperature for 30 min and centrifuged at 3000 rpm for 15 min to extract the serum. The serum samples were transferred in eppendorf tubes and stored at −80 °C until analysis. Basic biochemical tests including Complete Blood Count and Hematocrit (Hct) concentration were measured in auto analyzer Cell Dyne 3700 and platelet count was obtained using automatic reader, (STA compact, Mediserv, UK). Urine protein was measured and graded on a scale of 0–4+ (0, none; 1+, 30 mg/dl; 2+, 100 mg/dl; 3+, 300–1999 mg/dl; 4+, at least 2000 mg/dl) by the dipstick method.
2.5. Analysis of trace elements in serum
Serum trace elements – magnesium and zinc were analyzed by ICP-OES (Inductively coupled plasma optical emission spectrometer, ACTIVA-S, HORIBA JOBIN, France) and calcium was determined in COBAS INTEGRA Autoanalyzer 800 using O-cresolphthalein complexone. Serum samples were filtered prior to analysis. 300 μl of serum was appropriately diluted with 1% HNO3 and 0.01% Triton X 100 (HPLC grade, Sigma Aldrich) as diluents. Different concentrations of standards (100, 500, 1000 ppb for Ca, Mg and 30, 500, 1000 ppb for Zinc) of trace elements were prepared from a stock solution of 1000 ppm for calibration of standard graphs. Absorbances were taken at 393.3, 279.5 and 213.5 nm for Ca, Mg and Zn respectively. All measurements were conducted in duplicate. The concentrations of trace elements analyzed were expressed in mg/dl equivalent to μg/ml.
2.6. Statistical analysis
Data were analyzed using SPSS software and the levels of trace element were expressed as mean ± S.D. Clinical characteristics and biochemical parameters of cases were compared with control among the groups by one way ANOVA followed by Holm–Sidak test. Pearson’s correlation was done to know the effect of trace element on gestational age, BMI, Systolic and diastolic blood pressure.
3. Results
3.1. Analysis of trace elements in serum
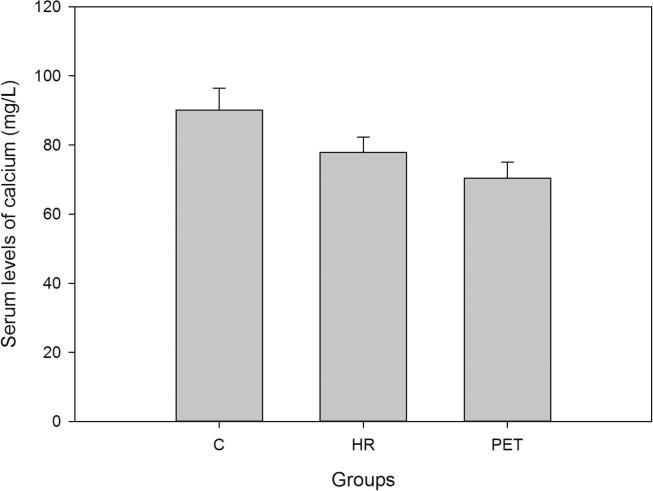
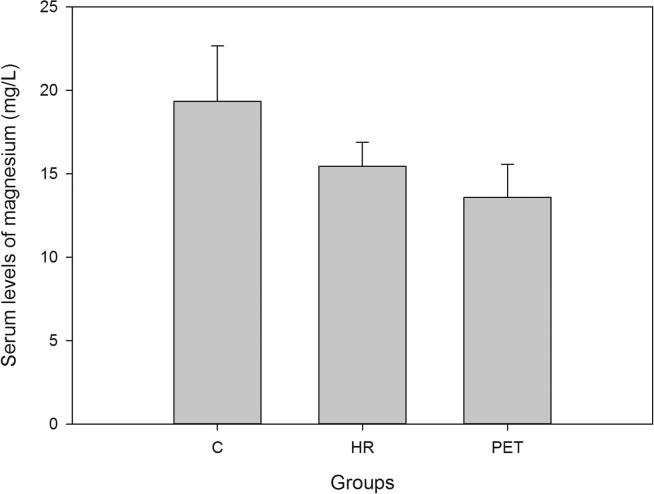
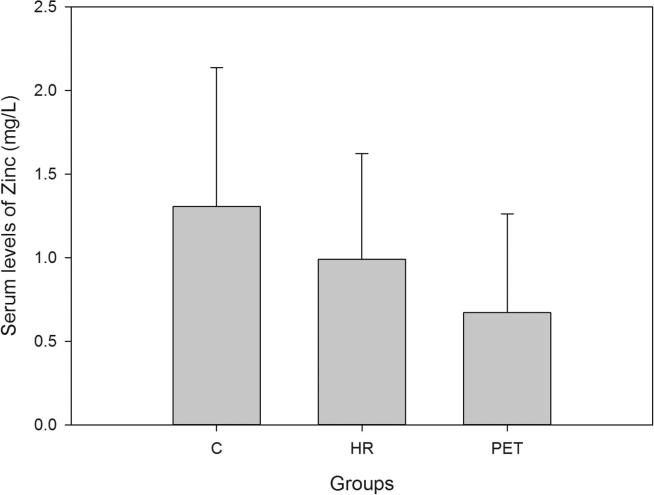
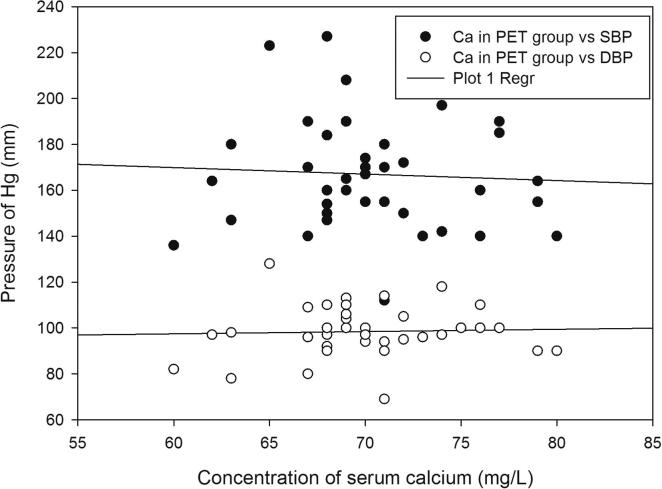
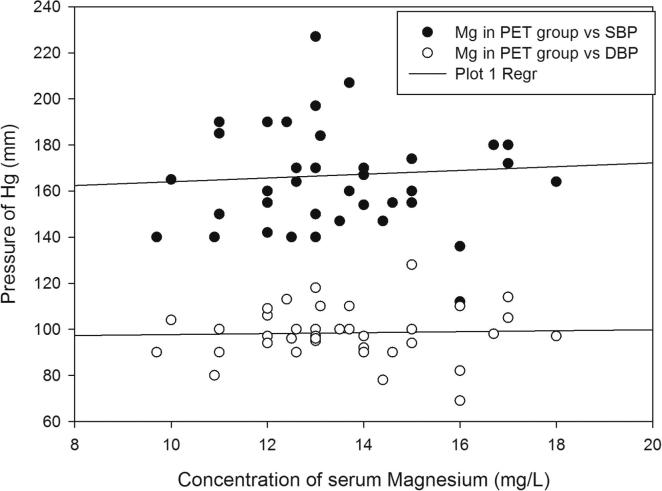
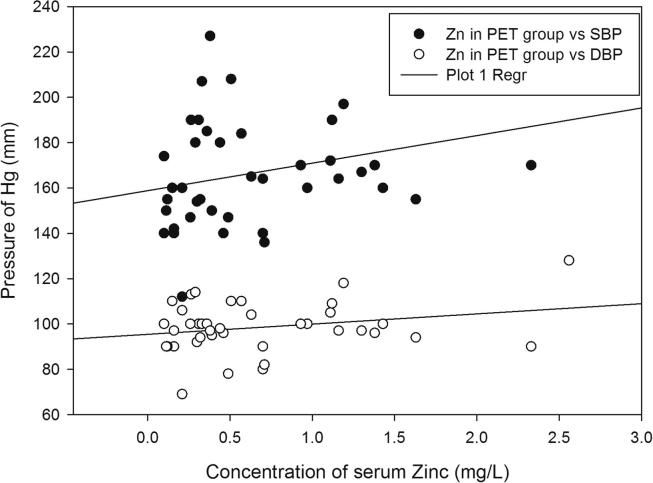
Demographic data and the serum levels of trace elements in control, HR and Preeclamptic groups are shown in Table 1, Table 2. The mean values of Ca, Mg and Zn were 90.08 ± 6.38, 19.33 ± 3.32 and 1.30 ± 0.83 mg/L respectively in normotensive control and 77.85 ± 4.47, 15.44 ± 1.43 and 0.98 ± 0.63 mg/L respectively in the HR group. In preeclamptic cases, the levels of Ca, Mg and Zn were 70.37 ± 4.66, 13.58 ± 1.98 and 0.67 ± 0.59 mg/L respectively (Figure 1, Figure 2, Figure 3). The levels of Ca were found to decrease significantly in HR and preeclamptic cases in comparison with control (p < 0.001). Similarly, levels of magnesium were found to decrease significantly in HR and PET groups compared to control. Like serum Ca and Mg, levels of Zn decreased significantly in the PET group (p < 0.001) compared to control and between HR and PET groups at p < 0.05 level of significance. One way ANOVA showed overall significance of p < 0.001 in changes of Ca, Mg and Zn among the control and the cases. Data were further analyzed in order to determine the effect of maternal age, gestational age, BMI, systolic and diastolic blood pressure on serum trace elements in the preeclamptic group (Table 3) by Pearson’s correlation. BMI was positively correlated with decreased concentrations of calcium, magnesium and zinc. Positive correlation was observed between systolic and diastolic blood pressure (SBP and DBP) and decreased levels of calcium, magnesium and zinc except the negative correlation of calcium with SBP in the preeclamptic group. The correlation of calcium, magnesium and zinc with SBP and DBP is shown in Figure 4, Figure 5, Figure 6.
Table 1.
Demographic data and serum levels of calcium, magnesium and zinc in the study population.
| Control group (n = 40) | High risk (HR) group (n = 40) | Preeclamptic group (n = 40) | |
|---|---|---|---|
| Age (years) | 31.20 ± 5.84 | 34.26 ± 6.69 | 31.55 ± 6.14 |
| BMI (kg/m2) | 29.94 ± 6.05 | 37.36 ± 9.00 | 35.12 ± 6.06 |
| Gestational age (weeks) | 31.17 ± 5.33 | 30.55 ± 6.33 | 33.72 ± 3.70 |
| Hematocrit (%) | 34.75 ± 4.30 | 34.48 ± 3.55 | 32.76 ± 3.71 |
| Platelet count (103/μl) | 266.17 ± 84.83 | 209.82 ± 47.64 | 156.65 ± 52.21 |
| SBP (mmHg) | 113.56 ± 13.93 | 124.7 ± 16.21 | 167. 0 ± 24.43 |
| DBP (mmHg) | 67.66 ± 9.38 | 74.45 ± 19.14 | 98.51 ± 11.16 |
| Serum calcium (mg/L) | 90.088 ± 6.389 | 77.850 ± 4.478 | 70.375 ± 4.661 |
| Serum magnesium (mg/L) | 19.330 ± 3.321 | 15.448 ± 1.438 | 13.585 ± 1.987 |
| Serum zinc (mg/L) | 1.306 ± 0.830 | 0.989 ± 0.633 | 0.671 ± 0.592 |
Values are expressed as mean ± SD.
Table 2.
Comparison of the clinical characteristics between control and cases.
| Control with high risk group |
High risk group with preeclampsia |
Control group with preeclampsia |
||||
|---|---|---|---|---|---|---|
| t | p | t | p | t | p | |
| BMI (kg/m2) | 4.626 | <0.001⁎ | 3.23 | 0.003⁎⁎ | 1.395 | 0.16 |
| Gestational age (weeks) | 0.698 | 0.48 | 2.85 | <0.05⁎⁎ | 2.15 | 0.06 |
| Hematocrit (%) | 0.31 | 0.75 | 1.98 | 0.096 | 2.30 | 0.06 |
| Platelet count (103/μl) | 3.964 | <0.001⁎ | 3.741 | <0.001⁎ | 7.705 | <0.001⁎ |
| SBP (mmHg) | 2.63 | 0.01⁎⁎ | 10.07 | <0.001⁎ | 12.64 | <0.001⁎ |
| DBP (mmHg) | 2.16 | 0.033⁎⁎ | 7.66 | <0.001⁎ | 9.762 | <0.001⁎ |
| Serum albumin (g/L) | 4.04 | <0.001⁎ | 3.94 | <0.001⁎ | 7.96 | <0.001⁎ |
| Serum calcium (mg/L) | 10.43 | <0.001⁎ | 6.37 | <0.001⁎ | 16.80 | <0.001⁎ |
| Serum magnesium (mg/L) | 7.28 | <0.001⁎ | 3.49 | <0.001⁎ | 10.78 | <0.001⁎ |
| Serum zinc (mg/L) | 2.05 | 0.08 | 2.04 | 0.04⁎⁎ | 4.09 | <0.001⁎ |
p < 0.001.
p < 0.05.
Figure 1.
Serum levels of calcium in all the groups.
Figure 2.
Serum levels of magnesium in all the groups.
Figure 3.
Serum levels of zinc in all the groups.
Table 3.
Correlation of gestational age, BMI, systolic and diastolic blood pressure with trace elements in preeclamptic group.
| Calcium |
Magnesium |
Zinc |
|
|---|---|---|---|
| r (p value) | r (p value) | r (p value) | |
| Age (years) | 0.019(0.90) | −0.11 (0.49) | −0.24(0.13) |
| Gestational age (weeks) | −0.15(0.35) | 0.16 (0.31) | 0.07(0.66) |
| BMI (kg/m2) | 0.145(0.37) | 0 (0.99) | 0.18(0.24) |
| SBP (mmHg) | −0.05(0.74) | 0.06 (0.68) | 0.29(0.064) |
| DBP (mmHg) | 0.041(0.79) | 0.03 (0.82) | 0.24(0.13) |
Figure 4.
Regression graphs showing correlation between calcium with systolic and diastolic blood pressure in preeclamptic group.
Figure 5.
Regression graphs showing correlation between magnesium with systolic and diastolic blood pressure in preeclamptic group.
Figure 6.
Regression graphs showing correlation between zinc with systolic and diastolic blood pressure in preeclamptic group.
3.2. Inter-element correlations in preeclamptic group
Inter-element correlation for the analyzed elements in the preeclamptic group was performed using Pearson’s correlation and represented in Table 4. Inter-element analyses reflected a negative correlation between Ca and Mg and between Mg and Zn whereas positive correlation between Ca and Zn in preeclamptic women. However, the correlation was not statistically significant.
Table 4.
Interrelationship between trace elements in preeclamptic group.
| Correlation parameters | Control group |
PET group |
||
|---|---|---|---|---|
| r | p Value | r | p Value | |
| Ca and Mg | 0.009 | 0.95 | −0.06 | 0.69 |
| Ca and Zn | 0.094 | 0.56 | 0.09 | 0.56 |
| Mg and Zn | −0.092 | 0.57 | −0.08 | 0.60 |
4. Discussion
The levels of calcium, magnesium, and zinc in maternal serum decrease progressively during pregnancy. Lower consumption of these minerals and accelerated metabolism may also contribute to decreased concentration of these elements in serum (Golmohammed et al., 2008). Preeclampsia is the commonest obstetrical complication in pregnant women. Despite several studies on preeclampsia, its etiology has not been clearly understood. Fewer studies have observed that alteration in values of trace elements in preeclampsia cases could be responsible in pathophysiology of preeclampsia (Bringman et al., 2006). The present study was designed to analyze the potential alterations of the serum levels of Ca, Zn and Mg in preeclamptic pregnancies.
Preeclampsia, a multi factorial disease results on account of generation of oxidative stress in pregnant women. Enhanced production of free radicals and reduced levels of some trace elements necessary for antioxidant defense mechanisms are the important contributors to oxidative stress. In the present study on Saudi women, levels of calcium were found to decrease significantly (p < 0.001) in HR and preeclamptic groups in comparison with normotensive control. Similarly, serum zinc concentration was found to decrease significantly in the preeclamptic group compared to normotensive pregnant women. Our results are consistent with earlier reports (Akinloye et al., 2010, Jain et al., 2010, Nourmohammadi et al., 2008). During pregnancy, there is enhanced secretion of parathyroid hormone and renin on account of hypocalcemia, which in turn results in increased concentration of intracellular calcium in vascular smooth muscle. These increased levels of calcium in smooth muscles cause vasoconstriction and thereby increased vascular resistance that results in boosting up the blood pressure in preeclamptic mother. Therefore, lowered levels of calcium in serum and increased levels of cellular calcium may be responsible in elevation of blood pressure seen in preeclamptic mothers.
Hypozincemia in the preeclamptic group observed in this study may also result in generation of oxidative stress. Decreased levels of zinc and other trace elements were reported in an earlier study on pregnant women of Bangladesh and India, which indicates that trace element status in preeclamptic pregnant women, is not altered on geographical variation (Jain et al., 2010, Sarwar et al., 2013). Zinc, is an important part of antioxidant enzymes required by the antioxidant defense system to protect cells from free radicals injury. It is an integral part of the antioxidant enzyme-superoxide dismutase (SOD). Decreased concentration of zinc in serum, may lead to decrease in activity of this enzyme. (Ross and Moldeus, 1991). Deficiency of these elements may withdraw the effect of antioxidant potential of cells leading to an increase in blood pressure (Akinloye et al., 2010, Powell, 2000). Decreased levels of zinc observed in the preeclamptic group could be due to dilution of blood, transfer of this mineral from mother to the growing fetus and increased excretion of this mineral in urine. Also the increased lipid peroxidation causes diminution in concentrations of transporter proteins and estrogen hormone that result in lower concentrations of zinc in pregnant women with preeclampsia. (Kumru et al., 2003). Like calcium and zinc, serum levels of magnesium decreased in preeclamptic women compared to normotensive control. There was significant change of serum magnesium between normotensive control and the high risk group. Serum Mg has a profound effect on excitability of cardiac muscles and on tone and contractility of vascular smooth muscle cells. Low serum concentrations of Ca and Mg induce constriction of vascular smooth muscles and increase vascular resistance and thus increase blood pressure. Increase in blood pressure may be due to decrease vasodilating action of serum magnesium. Moreover, in our study we observed that levels of serum calcium, magnesium and zinc decreased significantly in patients at high risk when compared to normotensive pregnant women. Therefore through this study, we prove the hypothesis that calcium and zinc are good indicators of underlying hypertension in patients with abnormality in risk factors such as raised blood pressure and proteinuria that gradually leads to preeclampsia. Through our study, we found that decreased levels of these trace elements along with increased proteinuria may act as markers in prediction of preeclampsia in early stages of pregnancy.
On further analysis, of the effect of maternal age, gestational age, BMI, systolic and diastolic blood pressure on serum trace elements in the preeclamptic group, the study reveals that systolic and diastolic blood pressure were positively correlated with decreased levels of calcium, zinc and magnesium in the preeclamptic group. Based on results obtained in the present study, we support the hypothesis that decreased levels of serum calcium, zinc and magnesium may contribute for generation of oxidative stress, increased vascular resistance and high blood pressure. Apart from these factors, many other parameters play a role to increase the blood pressure observed in the preeclamptic women. Interelement analysis found that calcium was negatively correlated with magnesium in the preeclamptic group. Interaction between these trace elements may be responsible for development of raised blood pressure in preeclamptic patients.
Calcium is responsible for contraction of blood vessels and magnesium on the other hand, has antagonistic action i.e. magnesium acts as a calcium channel blocker, restricting the calcium dependent constriction in arterial smooth muscles ultimately leading to vasodilation (Thakur et al., 2004). In addition, there is an increase in the response of large number of neurohormonal agents like angiotensin-II, serotonin, bradykinin, epinephrine nor epinephrine, and acetylcholine that cause increased vasoconstriction leading to high blood pressure (Altura and Altura, 1984). Thus, the decreased concentration of intracellular magnesium and defect in magnesium channels in membranes, might play an essential role in pathogenesis of vasoconstriction. Also, there was positive correlation observed between calcium and zinc. Increased vasoconstriction due to decreased serum calcium and decreased vasodilating action of zinc are responsible for development of preeclampsia in pregnant women. However, besides the disturbances in levels of these trace element concentrations, disturbances in endothelial function and in sympathetic tone could be the additional factors that contribute to the pathogenesis of hypertension in pregnancy (Schobel et al., 1996).
5. Conclusion
The preeclamptic Saudi women, have lower serum levels of calcium, magnesium and zinc compared to normal pregnant women. Results obtained in our data support the earlier hypothesis that hypocalcemia and hypomagnesemia are possible etiologies of preeclampsia. Although these findings provide a role of Ca, Zn and Mg in the development and pathogenesis of preeclampsia, the present study has some limitations: we did not study the dietary intake of preeclamptic women to find out whether the reduced levels of trace elements were from nutritional deficiencies or not. Further study investigating the roles of dietary supplementation of these elements needs to be undertaken.
Disclosure
All the authors of this study declare the absence of any potential conflicts of interest.
Authors’ contribution
N. Al-jameil and H. Tabassum contributed to conception and design of the study, H. Tabassum and F.A. Khan in collection of samples and analyzing trace elements, M.N. Ali and M.A. Qadeer have performed statistical analysis; H. Tabassum, M.N. Ali and May Al-Rashed have been involved in compilation of data and drafting the manuscript in a final version.
Acknowledgements
The authors are thankful to Research Center of the ‘Center for Female Scientific and Medical Colleges’, Deanship of Scientific Research, King Saud University for the grant and King Saud Medical City Hospital, Riyadh, for providing samples and facilities for completion of the study.
Footnotes
Peer review under responsibility of King Saud University.
References
- ACOG practice bulletin Diagnosis and management of preeclampsia and eclampsia. Obstet. Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- Akinloye O., Oyewale O.J., Oguntibeju O.O. Evaluation of trace elements in pregnant women with preeclampsia. Afr. J. Biotechnol. 2010;9(32):5196–5202. [Google Scholar]
- Altura B.M., Altura B.T. Magnesium, electrolyte transport and coronary vascular tone. Drug. 1984;28:120–142. doi: 10.2165/00003495-198400281-00013. [DOI] [PubMed] [Google Scholar]
- Bringman J., Gibbs C., Ahokas R. Differences in serum calcium and magnesium between gravidas with severe preeclampsia and normotensive controls. Am. J. Obstet. Gynecol. 2006;195:148. [Google Scholar]
- Caughey A.B., Stotland N.E., Washington A.E., Escobar G.J. Maternal ethnicity, paternal ethnicity and parental ethnic discordance: predictors of preeclampsia. Obstet. Gynecol. 2005;106:156–161. doi: 10.1097/01.AOG.0000164478.91731.06. [DOI] [PubMed] [Google Scholar]
- Conrad K.P., Joffe G.M., Kruszyna H., Kruszyna R., Rochelle L.G., Smith R.P. Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB. 1993;7:566–571. [PubMed] [Google Scholar]
- Gibson R.S. Zinc nutrition in developing countries. Nutr. Res. Rev. 1994;7:151–173. doi: 10.1079/NRR19940010. [DOI] [PubMed] [Google Scholar]
- Golmohammed S., Amirabilou A., Yazdian M., Pashapour N. Evaluation of serum calcium, magnesium, copper, and zinc levels in women with preeclampsia. Iran J. Med. Sci. 2008;33:231–234. [Google Scholar]
- Jain S., Sharma P., Kulshreshtha S., Mohan G., Singh S. The Role of calcium, magnesium and zinc in preeclampsia. Biol. Trace Elem. Res. 2010;133:162–170. doi: 10.1007/s12011-009-8423-9. [DOI] [PubMed] [Google Scholar]
- King J.C. Physiology of pregnancy and nutrient metabolism. Am. J. Clin. Nutr. 2000;71:1218–1225. doi: 10.1093/ajcn/71.5.1218s. [DOI] [PubMed] [Google Scholar]
- Kumru S., Aydin S., Simsek M., Sahim K., Yaman M., Ay G. Comparison of serum copper, zinc, calcium, and magnesium levels in preeclamptic and healthy pregnant women. Biol. Trace Elem. Res. 2003;94:105–112. doi: 10.1385/BTER:94:2:105. [DOI] [PubMed] [Google Scholar]
- Manual for HIV-1 diagnosis, 2002. Ethiopian Health and Nutrition Research Institute, Addis Ababa, Ethiopia.
- Noura A.J., Tabassum H., Huda A.M., Latifa A.O., Amal A.S., Khan F.A. Identification of predictive markers of pre-renal damage in pregnant women with preeclampsia and women at high risk: a prospective study conducted in Riyadh, Saudi Arabia. Int. J. Med. Sci. Public Health. 2014;3:182–186. [Google Scholar]
- Nourmohammadi I., Akbaryan A., Fatemi S. Serum zinc concentration in Iranian preeclamptic and normotensive pregnant women. Middle East J. Fam. Med. 2008;6(4):30–32. [Google Scholar]
- Pallavi P.C., Pranay A.J., Jasmin H.J. Changes in serum calcium and magnesium level in preeclampsia vs normal pregnancy. Int. J. Biomed. Adv. Res. 2012;3(6):511–513. [Google Scholar]
- Pathak P., Kapil U. Role of trace elements-zinc, copper, magnesium in pregnancy and its outcome. Indian J. Pediatr. 2004;71:1003–1005. doi: 10.1007/BF02828116. [DOI] [PubMed] [Google Scholar]
- Powell S.R. The antioxidant properties of zinc. J. Nutr. 2000;130:1447–1454. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- Ross D., Moldeus P. CRC Press; Boca Raton: 1991. Antioxidant Defense Systems and Oxidative Stress in Vigo-Pelfrey C. Membrane Lipid Oxidation. [Google Scholar]
- Sarma P.C., Gambhir S.S. Therapeutic uses of magnesium. Indian J. Pharmacol. 1995;27:7–13. [Google Scholar]
- Sarwar M.S., Ahmed S., ShahidUllah M., kabir H., Mustafizur Rahman G.K.M., Husnat A., Safiqul Islam M. Comparative study of serum zinc, copper, manganese and iron in preeclamptic pregnant women. Biol. Trace Elem. Res. 2013;154:14–20. doi: 10.1007/s12011-013-9721-9. [DOI] [PubMed] [Google Scholar]
- Schobel H.P., Fischer T., Heuszer K. Preeclampsia – a state of sympathetic overactivity. N. Engl. J. Med. 1996;335:1480–1485. doi: 10.1056/NEJM199611143352002. [DOI] [PubMed] [Google Scholar]
- Statistic by country. <http://www.rightdiagnosis.com/p/preeclampsia/stats-country.htm>. [Cited January, 2014].
- Thakur S., Gupta N., Kakkar P. Serum copper and zinc concentrations and their relation to superoxide dismutase in severe malnutrition. Eur. J. Pediatr. 2004;163:742–744. doi: 10.1007/s00431-004-1517-7. [DOI] [PubMed] [Google Scholar]
- Ulmer D.D. Trace elements. N. Engl. J. Med. 1977;297(6):318–321. doi: 10.1056/NEJM197708112970607. [DOI] [PubMed] [Google Scholar]