Abstract
The aim of the present study was to investigate histological alterations of rat thyroid gland after short-term treatment with supraphysiological doses of thyroid hormones. Rats from experimental groups were treated with triiodothyronine (T3) or thyroxine (T4) during five days. In both treated groups, thyrocyte height was reduced and follicular lumens were distended. Progressive involutive changes of the thyroid parenchyma were apparent, including follicular remodeling (fusion) and death of thyrocytes. Morphological changes confirmed by quantitative analysis were more pronounced in the T4-treated group. Our results demonstrate that thyrotoxicosis, whether induced by T3 or T4, leads to different grades of thyroid tissue injury, including some irreversible damages. These changes might be explained at least in part by lack of trophic and cytoprotective effects of the thyroid stimulating hormone. Since the period required for morphophysiological recovery may be unpredictable, findings presented here should be taken into consideration in cases where the thyroid hormones are used as a treatment for thyroid and non-thyroid related conditions.
Abbreviations: T3, triiodothyronine; T4, thyroxine; TSH, thyroid stimulating hormone; TRH, TSH-releasing hormone; PI, propidium iodide
Keywords: Light microscopy, Electron microscopy, Thyroid gland, Thyroid hormones, Wistar rats
1. Introduction
Thyroid gland is specialized for production, storage and release of thyroid hormones thyroxine (T4) and triiodothyronine (T3). T4 is a quantitatively dominant hormone released from the thyroid gland, while T3 is biologically more active and originates mainly from peripheral deiodination of T4 (Boelaert and Franklyn, 2005). Thyroid hormones are involved in regulation of metabolic rate and energy expenditure in homeothermic animals (Cavalieri, 1997) and they are necessary for normal cell growth and development (Silva, 1995).
Biosynthesis and secretion of thyroid hormones are regulated by hypothalamus-pituitary-thyroid axis, with the negative feedback loop at the level of both hypothalamus and pituitary gland (Norman and Litwack, 1987, Williams and Bassett, 2011). Namely, thyroid gland activity is positively regulated by thyroid stimulating hormone (TSH) synthesized and secreted from pituitary thyrotrophs, which activity is in turn controlled by hypothalamic TSH-releasing hormone (TRH). Under physiological conditions, circulating thyroid hormones suppress the release of TSH and TRH thus providing the so-called long negative feedback loop regulating thyroid function. In addition, there exist short and ultra-short feedback loops represented by suppressive activity of TSH on both TRH and TSH release (Prummel et al., 2004). A failure at any step of this complex regulatory mechanism leads to dysregulation of thyroid function, which is manifested as either hyper- or hypoproduction of hormones and can cause serious health problems in humans and companion animals. Normal production of thyroid hormones also depends on an adequate supply of iodine (Kelly, 2000).
Thyroid hormones are frequently used in human and veterinary medicine as replacement therapy for thyroid deficiency (Dixon et al., 2002, Escobar-Morreale et al., 2005, Wiersinga, 2001) and, in doses slightly above physiological, in therapy of differentiated thyroid carcinoma (Brabant, 2008). Supraphysiological doses of thyroid hormones are also in use as supplemental therapy for some diseases and conditions that are not associated with thyroid dysfunction such as prophylaxis-resistant affective disorders (Bauer et al., 2002) or Wilson’s temperature syndrome (Friedman et al., 2006). Besides, some promising investigations regarding use of supraphysiological doses of thyroid hormones for heart repair after myocardial infarctation are in progress (Pantos et al., 2007, Pantos et al., 2008, Pantos et al., 2009).
Data on possible side effects of such therapies in humans are still inconsistent and vary depending on tissue, organ or function examined. Despite the fact that hyperthyroidism is commonly associated with insomnia, high doses of T4 used for treatment of mood disorders did not cause sleep impairment in otherwise healthy patients (Kraemer et al., 2011). Also, it seems that supraphysiological doses of T4 are not necessarily associated with bone mineral density loss even after a very long period of treatment (Ricken et al., 2012; but see also Chen et al., 2004). From the animal studies it is known that experimentally induced thyrotoxicosis causes impairment of some cognitive functions (Taşkin et al., 2011) and activates hypothalamic–pituitary–adrenal axis thus potentially compromising adrenal function (Johnson et al., 2005).
Given the wide use of thyroid hormones as therapeutics in a number of diseases and conditions and rather good common knowledge on their undesired effects on various organs, there is a surprising paucity of studies dealing with the effects of supraphysiological doses of thyroid hormones on the thyroid gland.
Bearing in mind that the structure of any organ closely reflects the state of its function, the aim of the present study was to investigate the effects of treatment with supraphysiological doses of T3 or T4 on histological and cytological characteristics of thyroid gland in euthyroid animals. The obtained results should contribute to better understanding of the possible side-effects and safety of therapy with high-doses of thyroid hormones.
2. Materials and methods
2.1. Animals
The experiment was performed on a total of 18 male Wistar rats, weighing 180–250 g. Animals were caged individually, at room temperature (22 ± 1 °C), in 12:12 h light–dark cycle and had free access to food (commercial rat food, Subotica, Serbia) and tap water. Animal handling and treatment were carried out in accordance with The Serbian Laboratory Animal Protection Law proposed guidelines and protocols approved by The Ethics Committee of the Faculty of Biology, University of Belgrade.
2.2. Experimental design
The rats were divided into three equal groups and treated once a day, for 5 days, as follows: T3-treated rats received injections of T3 (200 μg/kg b.w.) dissolved in 9 mM NaOH; T4-treated animals received T4 (300 μg/kg b.w.) dissolved in 9 mM NaOH; control (euthyroid) animals were injected with vehicle only (9 mM NaOH, 1 ml/kg b.w.). Body temperature was measured at the beginning and at the end of the experiment. In the course of the experiment, all animals were in good health and condition. After the last injection, body mass was measured and rats were sacrificed by decapitation using a guillotine (Harvard Apparatus, Holliston, MA, USA).
2.3. Determination of thyroid hormones in the circulation
For determination of T3 and T4 concentration in the serum, blood samples were collected from the trunk during sacrificing. Total serum T3 and T4 concentrations were determined by the RIA method, at the laboratory of The Institute for the Application of Nuclear Energy (INEP, Belgrade, Serbia).
2.4. Processing of thyroids for light microscopy
After isolation and weighing, the left lobe of each thyroid gland was routinely processed for light and the right one for electron microscopy. For light microscopy, each thyroid lobe was fixed in 3.7% phosphate-buffered formalin (pH = 7.2), dehydrated through an ethanol series and xylol and embedded in paraffin. For general histological analysis, as well as for stereological measurements, 5 μm thick paraffin sections stained with hematoxylin/eosin method, taken from the anterior, medial and posterior part of the thyroid lobe (five non-serial sections per each chosen part of a lobe), were analyzed on Leica DMLB light microscope (Wetzlar, Germany).
2.5. Detection of cell death
Cell death was demonstrated by propidium iodide (PI) staining method (Markelic et al., 2011, Scaglia et al., 1997). Briefly, deparaffinized and rehydrated 5 μm thick sections were stained in 1% PI solution, for 10 min. Glycerol-mounted sections were examined with a Zeiss Observer.Z1 fluorescent microscope and photographed with AxioCam MR3 camera, using AxioVision Rel4.7 software. The occurrence of cell death was estimated by counting normal and apoptotic nuclei in the follicular wall, using three non-serial sections per each chosen part of the thyroid lobe (as described above). Results are presented as percentage of apoptotic nuclei.
2.6. Processing of the thyroids for electron microscopy
After initial fixation in 3.7% phosphate-buffered formalin, the right lobe of each thyroid gland was thoroughly rinsed in tap water, diced into small pieces (∼1 mm3) and fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer, at pH 7.4, for 1 h. This was followed by postfixation in 1% osmium-tetroxide in 0.1 M phosphate buffer at pH 7.4, for 1 h. After dehydration through a series of cold alcohols and propylene oxide, tissue samples were embedded in Araldite. Ultrathin sections, gained after selection from semi-fine (1 μm), toluidine blue stained sections, were cut on Leica EM UC6 (Leicamicrosystem, Wetzlar, Germany), contrasted with uranyl acetate and lead-citrate and examined with a Philips CM12 electron microscope (Eindhoven, The Netherlands).
2.7. Stereological analysis
Stereological analysis was performed by the point-counting method, using M42 multipurpose test grid, at a final magnification of 400×. Data obtained from 20 test fields of each sectioned part of the thyroid lobe (paraffin embedded samples stained with hematoxylin/eosin) served to calculate volume density of particular phases (colloid, follicular epithelium, interfollicular tissue) using standard equation Vvph = Pph/Ptot, where Pph is number of points on a particular phase and Ptot is the total number of points over tested area) (Weibel, 1979). Thyroid activation index (Ia) was expressed as the epithelial to colloid volume density ratio (Ia = Vve/Vvc) (Kališnik, 1972). All fully visible thyroid follicles seen on examined slides were included in the determination of mean diameter of thyroid follicles, which was calculated as (max transverse diameter + max diameter perpendicular to the first one)/2. Follicular cell height was determined from toluidine blue-stained semi-thin sections of plastic embedded tissue. Measurement was carried out on 300 cells per animal, having a clearly defined nucleus, by the use of a micrometer scale inserted into the ocular, at a magnification of 1000×.
2.8. Statistical analysis
Statistical analysis for differences between control and each of two experimental groups was performed using Student’s t-test. Data were expressed as mean ± SEM. The level of significance was set at p < 0.05, 0.01 and 0.001.
3. Results
3.1. General effects of thyroid hormones
Results for thyroid hormone level, body temperature and absolute and relative mass of the thyroid gland in control, T3- and T4-treated rats are presented in Table 1.
Table 1.
Effect of treatment with T3 or T4 on thyroid hormone serum levels, body temperature and absolute and relative mass of thyroid gland.
| Control | T3-treated | T4-treated | |
|---|---|---|---|
| Serum T3 concentration (nmol/l) | 1.19 ± 0.074 | 1.69 ± 0.070⁎⁎ | 1.87 ± 0.130⁎⁎ |
| Serum T4 concentration (nmol/l) | 133.7 ± 8.27 | 20.6 ± 3.10⁎⁎⁎ | 289.3 ± 23.16⁎⁎⁎ |
| Body temperature change (°C) | 0.2 ± 0.10 | 0.4 ± 0.20 | 1.0 ± 0.26⁎ |
| Absolute thyroid gland mass (mg) | 34.3 ± 8.46 | 28.6 ± 2.80 | 28.2 ± 3.90 |
| Relative thyroid gland mass (mg/100 g b.w.) | 13.7 ± 1.12 | 11.6 ± 0.95 | 11.3 ± 1.36 |
p < 0.05 vs. control.
p < 0.01 vs. control.
p < 0.001 vs. control.
After T3 treatment, level of T3 in circulation was increased compared to the control (p < 0.01), while the level of T4 was extremely reduced (p < 0.001). Treatment with T4 significantly increased both T3 and T4 concentrations in circulation (p < 0.01 and p < 0.001, respectively).
Mean body temperature of rats was elevated after both treatments but with significance recorded only in the T4-group.
Absolute and relative thyroid gland masses remained statistically unchanged in both treated groups.
3.2. Thyroid hormone effects on histological organization of the thyroid gland
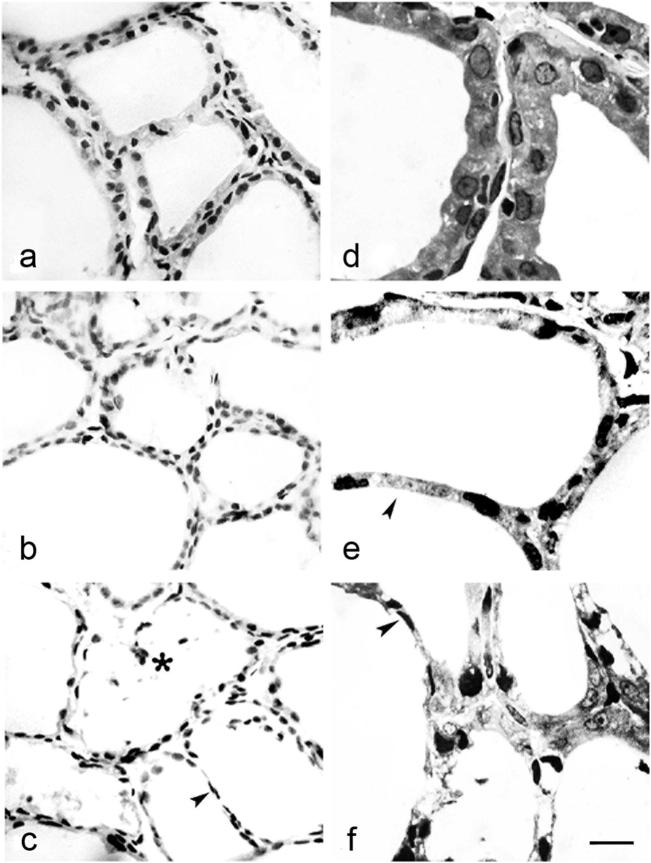
Thyroid glands from control animals (Fig. 1a and d) showed normal histological appearance, with cuboidal to low-columnar epithelium lining small and medium-sized follicles, and lower epithelium lining large follicles (arbitrary determined). Interstitium contained connective tissue with blood capillaries and individual or clustered parafollicular cells. In T3- and T4-treated groups (Fig. 1b and e and Fig. 1c and f, respectively), thyroid parenchyma appeared less well ordered. In both treated groups, thyroid follicles were enlarged and seemed distended due to colloid accumulation. Follicles were lined mostly with flattened thyrocytes containing oval nuclei with increased chromatin density. Epithelium of some large follicles was extremely low, especially in T4-treated animals. Large follicles of irregular shape suggesting disruption of follicular walls and fusion of neighboring follicles (Fig. 1c, asterisk), as well as an interfollicular barrier consisting of a single row of thyrocytes (Fig. 1c, e and f, arrowhead), were also apparent. Inside the lumen of some irregular follicles desquamated thyrocytes were noticed (Fig. 1c, asterisk). The amount of interfollicular connective tissue was reduced, and capillaries were collapsed.
Figure 1.
Thyroid follicles from control (a and d), T3- (b and e) and T4-treated rats (c and f), paraffin (a–c, hematoxyline/eosin) and plastic (d–f, toluidine-blue) embedded sections. Note thinning of the interfollicular barrier (arrowheads in c, e and f) and desquamated thyrocytes within the follicular lumen (asterisk in c). Bar 10 μm (a–c) and 4 μm (d–f).
3.3. Demonstration of cell death
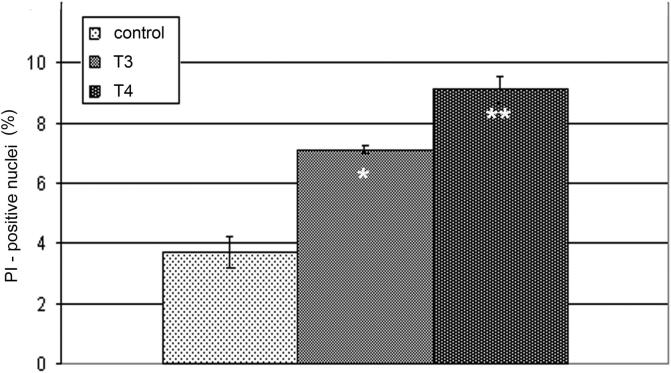
Paraffin sections stained with PI served for demonstration of apoptotic cell death. PI is a fluorescent dye that binds to nucleic acids in dead cells. Thus, all nuclei on tissue section are stained, but the nuclei of cells that were dead at the moment of fixation appear opaque and show strong fluorescence (referred as PI-positive nuclei), and can be readily discriminated from slightly transparent nuclei of cells that were alive immediately before fixation. Statistical analysis showed that the percentage of dead cell nuclei was significantly increased after treatments with T3 and T4 (Fig. 2). In the control group, PI-positive reaction was noted only sporadically, inside the follicular lumen where desquamated cells were present (Fig. 3a). Apoptotic cells, mainly thyrocytes, observed individually or in small groups in the wall of follicles subjected to remodeling in both T3- and T4-treated groups, were more prominent in the latter (Fig. 3b and c, respectively).
Figure 2.
Percentage of PI-positive nuclei in thyroids of control and TH treated animals. Percentages of PI-positive nuclei in thyroids of T3-treated rats (7.1 ± 0.12, *p < 0.05) and T4-treated rats (9.1 ± 0.44, **p < 0.01) were significantly higher than in control animals (3.7 ± 0.53).
Figure 3.
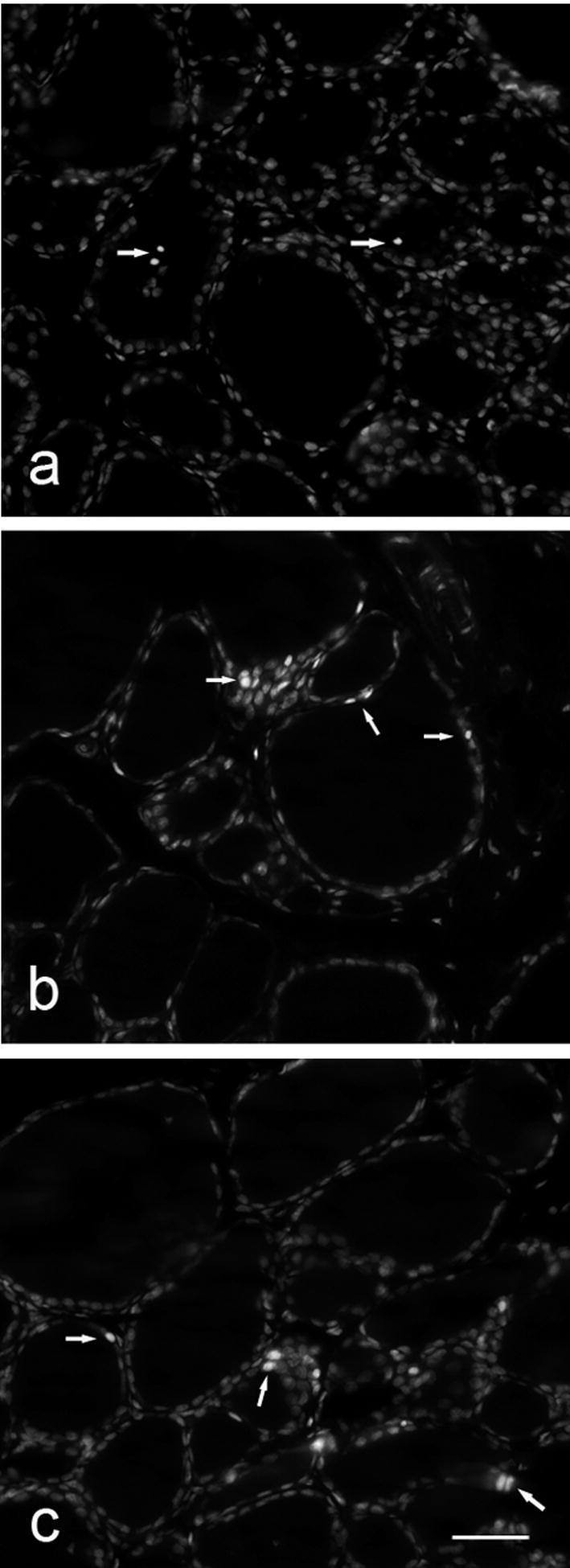
PI-labeled nuclei (arrows) in the follicular lumen of control rats (a) and within follicular wall of T3- and T4-treated rats (b and c). Bar 40 μm (a–c).
3.4. Electron microscopical study
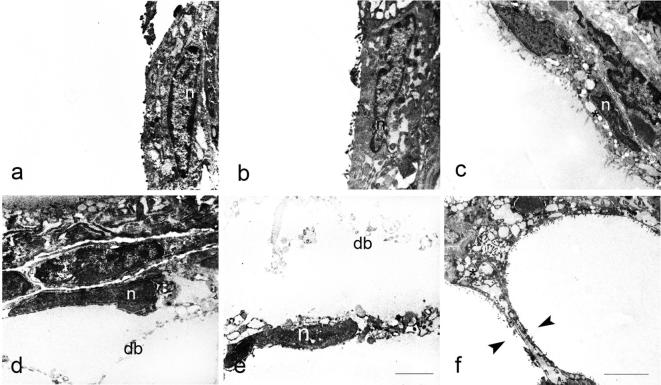
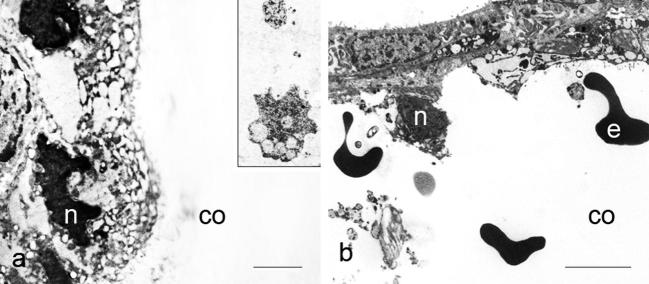
Electron microscopy (Fig. 4) revealed that thyrocytes from the control group in addition to a prominent nucleus, possessed endoplasmic reticulum, Golgi complex placed at the lateral margin of the cell and occasionally small dense granules usually identified as lysosomes. Apically, thyrocytes had numerous long, regular microvilli projecting into the follicular lumen (Fig. 4a). After T3 treatment, thyrocytes became slightly flattened with irregular and fuzzy-like microvilli (Fig. 4b). Treatment with T4 resulted in further flattening of follicular epithelium and shortening or even loss of microvilli (Fig. 4c). Progressive stages of thyrocyte injury leading to gradual dissolution of interfollicular wall might be seen in places (Fig. 5a–f). Some thyrocytes had irregularly shaped nuclei with condensed chromatin, and vesiculated cisterns of endoplasmic reticulum located in the apical region of the cell (Fig. 6a). Increased quantity of cell debris (Fig. 6a, inset) as well as individual erythrocytes within some follicles from T3- and T4-treated animals were also noted (Fig. 6 b).
Figure 4.
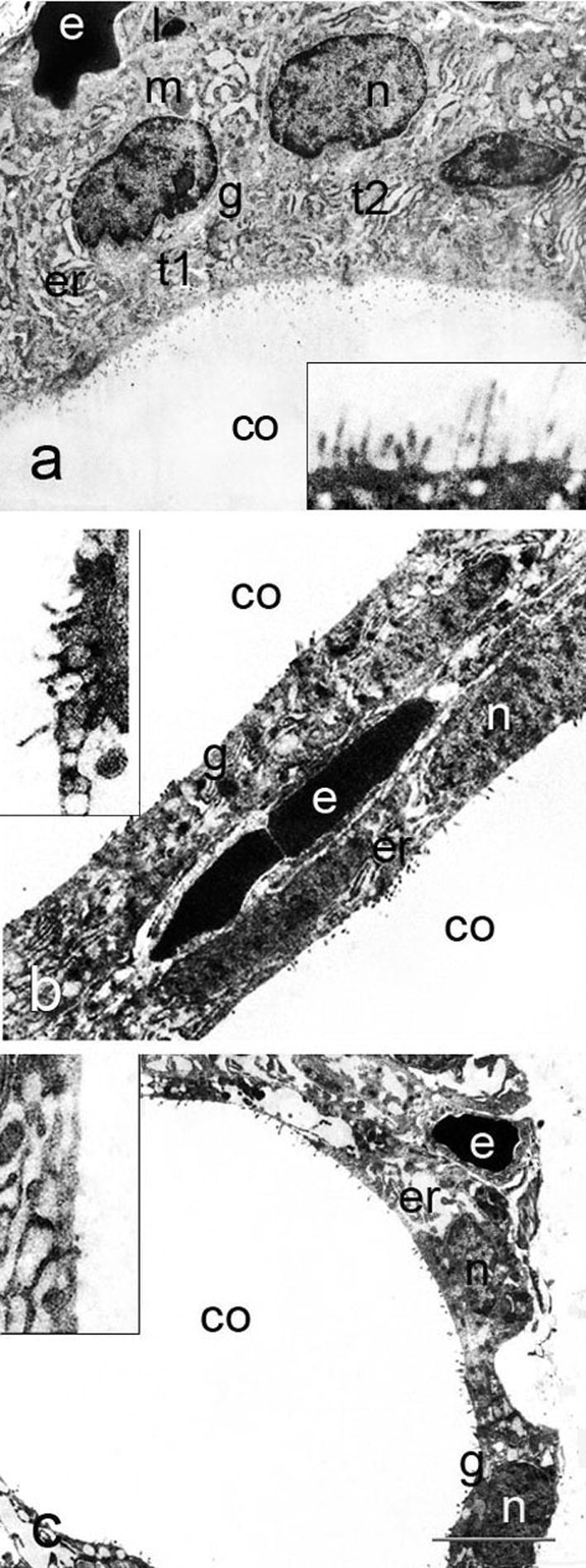
Follicular epithelium of control, T3- and T4-treated rats (a, b and c); t – thyrocyte, n – nucleus, m – mitochondrion, l – lysosome, g – Golgi complex, er – endoplasmic reticulum, co – colloid, e – erythrocyte in the capillary lumen. Insets show apical pole of thyrocytes, with reduced and disorganized microvilli in T3- and T4-treated rats. Bar 4 μm (a–c) and 1.5 μm (insets).
Figure 5.
Gradual injury of thyrocytes leading to dissolution of the interfollicular barrier. Note nuclear changes (n on a–e), intracytoplasmic vesiculation (asterisk on c) and cellular debris (db) in the follicular lumen (d and e); arrowheads show thinning and dissolution of interfollicular barrier. Bar 2 μm (a–e) and 5 μm (f).
Figure 6.
Thyrocytes from animals treated with thyroid hormones, with deformed nuclei and vesiculated endoplasmic reticulum; inset shows cell debris in the follicular lumen (a); erythrocytes in the colloid observed after treatments with T3 or T4 (b); n – nucleus, co – colloid, e – erythrocyte. Bar 2 μm (a) and 5 μm (b).
3.5. Results of stereological analysis
Stereological analysis confirmed described visual observations (Table 2). The volume density of colloid was increased in both T3- and T4-treated groups, while the volume density of the follicular epithelium was decreased. At the same time, the volume density of follicles and interstitium remained nearly the same, although some tendency toward enlargement of follicles on the account of interstitium was noticed in the T4-treated group. Height of follicular cells was reduced in both hormone-treated groups. Finally, the thyroid activation index was significantly reduced in groups under treatment.
Table 2.
Results of stereological analysis of thyroid gland after treatment with thyroid hormones.
| Control | T3-treated | T4-treated | |
|---|---|---|---|
| Vv colloid (μm0) | 0.450 ± 0.0329 | 0.542 ± 0.0217⁎ | 0.615 ± 0.0258⁎⁎ |
| Vv follicular epithelium (μm0) | 0.348 ± 0.0172 | 0.248 ± 0.0031⁎⁎ | 0.216 ± 0.0115⁎⁎⁎ |
| Vv follicles (μm0) | 0.723 ± 0.0633 | 0.785 ± 0.0185 | 0.834 ± 0.0098 |
| Vv interstitium (μm0) | 0.202 ± 0.0179 | 0.222 ± 0.0135 | 0.167 ± 0.0098 |
| Thyroid activation index (Ia) | 0.795 ± 0.0979 | 0.453 ± 0.0240⁎⁎ | 0.347 ± 0.0331⁎⁎ |
| Follicular cells height (μm) | 8.26 ± 0.452 | 4.66 ± 0.368⁎⁎⁎ | 4.51 ± 0.225⁎⁎⁎ |
p < 0.05 vs. control.
p < 0.01 vs. control.
p < 0.001 vs. control.
4. Discussion
The improper thyroid function is associated not only with general metabolic disturbance but also with a number of other apparently unrelated health issues such as cardiac diseases, lupus, rheumatoid arthritis, reproductive difficulties or diabetes (Duntas et al., 2011, Klein and Danzi, 2007, Krassas et al., 2010, Kumar et al., 2012). In addition, thyroid hormones disbalance may lead to emotional and behavioral disturbances and impair patients’ everyday life. Therefore, any research on the thyroid gland and its pathology has important medical as well as social implications.
In this experiment we used a previously established model of experimentally induced hyperthyroidism in rats (Petrovic et al., 2003). Animals were treated with T3 or T4 in doses which were highly above physiological replacement doses estimated at 3 μg/kg b.w. for T3 (Dillmann et al., 1983) and 20 μg/kg b.w. for T4 (Berstein, 1980).
Expectedly, treatment with T3 significantly elevated circulating T3. T4 was markedly decreased as the result of T3 suppressive effects on thyroid gland activity, mediated through feedback inhibition of TRH and TSH release, at levels of hypothalamus and pituitary, respectively (Belchetz et al., 1978, Maruta and Greer, 1988, Saleh et al., 1998). At the same time, it means that T3 in serum of T3-treated animals was mainly exogenous.
In rats treated with supraphysiological doses of T4, concentrations of both T3 and T4 were elevated but the elevation was not of the same magnitude. Namely, T3 increased for about 50%, while T4 concentration was more than doubled. This suggests that peripheral T4 to T3 deiodination mechanisms reached the level of saturation and could not be further intensified, even with excess of T4 as substrate. T4 concentration in the blood remained steadily increased due to the overloaded deiodination pathway.
Absolute and relative masses of the thyroid gland were unchanged after both treatments, while stereological analysis showed no differences in the relative abundance of main thyroid tissue compartments (parenchyma vs. interstitium) between control and experimental groups. These results are in disagreement with the data reported previously by Soukup et al. (2001) who found markedly atrophied thyroids in rats treated with high doses of thyroid hormones. However, considerably shorter duration of our experiment (5 days vs. 6 month) offers a good explanation for the absence of changes in mentioned parameters.
Within follicles, however, relative proportions of the thyroid epithelium and colloid were shifted in favor of colloid, while the height of follicular cells was markedly reduced in both treated groups. It is well known that the morphofunctional status of each follicle is controlled not only by the TSH level, but also by other factors including thyroglobulin contained within the follicle. Depending on the stage of the follicle physiological cycle, TSH may stimulate either expression of thyroid-specific genes involved in thyroglobulin synthesis, or reabsorption of colloid, its lysosomal degradation and release of thyroid hormones (Suzuki et al., 2011). Follicles containing large amounts of colloid, as in T3- and T4-treated groups, are lined with thyrocytes in which synthesis of thyroglobulin is low (Suzuki et al., 1998, Suzuki et al., 1999, Suzuki et al., 2011). Normally, under TSH-stimulation, such follicles should be engaged in colloid resorption and hormones release. Although we did not measure TSH levels, we estimated it indirectly, using the thyroid activation index, which is known to be positively correlated with the level of TSH in circulation (Kališnik, 1981, Rajkovic et al., 2003, Rajkovic et al., 2006). Thus, according to results obtained for thyroid activation index, thyroids in both hormone-treated groups were understimulated and in the state of arrest. Cytologically, the disorganization and/or reduction of microvilli, as well as significantly reduced epithelial height, provide further evidence for the functional quiescence of the thyroid gland after thyroid hormone treatment.
In the control group the rare occurrence of desquamated cells inside the follicles was noted after PI-staining. This finding in the absence of hormonal treatment is related to normal basal thyroid cells turnover since it is known that, as in many other organs, thyroid gland maintains its mass homeostasis by sustained basal proliferation and apoptosis (Dremier et al., 1994, Okayasu et al., 1995, Tamura et al., 1998). Apoptotic cells are shed into the follicular lumen, subsequently being removed by macrophages and the remaining thyrocytes (Matsunaga et al., 1988). More frequently observed presence of cell fragments and debris inside some low-epithelial and irregularly shaped follicles of T3- and T4-treated rats, together with PI-positive condensed nuclei within walls of such follicles, point to loss of thyrocytes and remodeling/fusion of follicles. Such intensified shedding of thyrocytes and their membrane-bounded fragments was seen previously during involution of the hyperplastic thyroid gland (Tachiwaki et al., 1990, Tachiwaki and Wollman, 1982). Electron microscopy provided more detailed insights on cell damages after treatments with T3 or T4. Some thyrocytes within the follicular wall exhibited signs of reversible cell injury, such as nuclear condensation and vesiculation of cytoplasmic organelles. More advanced destruction of thyrocytes sometimes even with abruption of the follicular epithelium after thyroid hormone treatments might be considered as the high grade tissue injury. Occasionally, progressive damage expanded to nearby capillaries, resulting in microhemorrhage indicated by the occurence of erythrocytes inside the follicular lumen.
As it has been proposed earlier and mentioned above herein, thyroglobulin accumulated inside the follicle is involved in the regulation of thyrocyte activity, counterbalancing the TSH action (Suzuki et al., 1998, Suzuki et al., 1999, Suzuki et al., 2011). We believe that excessive intrafollicular accumulation of thyroglobulin may produce increased pressure on thyrocytes, deforming their cytoskeleton and generating pro-apoptotic signals (Field, 2010, Janmey, 1998). Thus, beside attenuation of trophic TSH signals due to high circulating levels of thyroid hormones, thyrocytes in hyperthyroid rats may have received proapoptotic signals. The intensity of these signals, together with functional status of the particular follicle, determines the degree of epithelial damage ranging from mild degeneration of individual lining cells to total destruction of follicular wall and lumen fusion. Investigating the period needed for thyroid recovery after cessation of treatment was out of the scope of this study. However, it has been shown previously that morphofunctional alterations of the thyroid gland caused by noxious stimulation were not completely resolved even after three months of repairing period (Rajkovic et al., 2003).
In summary, results of the present work demonstrate that high doses of thyroid hormones cause serious damage of thyroid follicles in euthyroid rats, even after short-term treatment. Observed changes are most probably based on the absence of cytoprotective effects of TSH, together with reception of more pronounced proapoptotic signals. Given that structural alterations of the thyroid gland associated with dysfunction may require an unpredictable recovery period, current findings point to necessary caution needed when thyroid hormones are used as therapeuticals.
Acknowledgments
This work was carried out in cooperation with Center for Electron Microscopy, Faculty of Biology, University of Belgrade. The authors thank Mrs Maja Bogdanović and Mrs Anita Lazarević for their highly professional technical assistance. Financial support from the Libyan Ministry of Higher Education and Scientific Research for the first author is thankfully acknowledged.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Njia M. Ali Rajab, Email: bahaeddin_1974@yahoo.com.
Mirela Ukropina, Email: mirela@bio.bg.ac.rs.
Maja Cakic-Milosevic, Email: maja@bio.bg.ac.rs.
References
- Bauer M., Berghöfer A., Bschor T., Baumgartner A., Kiesslinger U., Hellweg R., Adli M., Baethge C., Müller-Oerlinghausen B. Supraphysiological doses of l-thyroxine in the maintenance treatment of prophylaxis-resistant affective disorders. Neuropsychopharmacology. 2002;27:620–628. doi: 10.1016/S0893-133X(02)00320-2. [DOI] [PubMed] [Google Scholar]
- Belchetz P.E., Gredley G., Bird D., Himsworth R.L. Regulation of thyrotropin secretion by negative feedback of tri-iodothyronine on the hypothalamus. J. Endocrinol. 1978;76:439–448. doi: 10.1677/joe.0.0760439. [DOI] [PubMed] [Google Scholar]
- Berstein L.M. The effect of physiological doses of thyroxine on the level of cyclic adenosine 3′,5′- monophosphate in pituitary and anterior hypothalamus of male rats of different age. Endocrinologie. 1980;75:29–34. [PubMed] [Google Scholar]
- Boelaert K., Franklyn J.A. Thyroid hormone in health and disease. J. Endocrinol. 2005;187:1–15. doi: 10.1677/joe.1.06131. [DOI] [PubMed] [Google Scholar]
- Brabant G. Thyrotropin suppressive therapy in thyroid carcinoma: what are the targets? J. Clin. Endocrinol. Metab. 2008;93:1167–1169. doi: 10.1210/jc.2007-2228. [DOI] [PubMed] [Google Scholar]
- Cavalieri M.M. Iodine metabolism and thyroid physiology: current concepts. Thyroid. 1997;7:177–181. doi: 10.1089/thy.1997.7.177. [DOI] [PubMed] [Google Scholar]
- Chen C.H., Chen J.F., Yang B.Y., Liu R.T., Tung S.C., Chien W.Y., Lu Y.C., Kuo M.C., Hsieh C.J., Wang P.W. Bone mineral density in women receiving thyroxine suppressive therapy for differentiated thyroid carcinoma. J. Formos. Med. Assoc. 2004;103:442–447. [PubMed] [Google Scholar]
- Dillmann W.H., Berry S., Alexander N.M. A physiological dose of triiodothyronine normalizes cardiac myosine adenosine triphosphatase activity and changes myosine isoenzyme distribution in semistarved rats. Endocrinology. 1983;112:2081–2087. doi: 10.1210/endo-112-6-2081. [DOI] [PubMed] [Google Scholar]
- Dixon R.M., Reid S.W., Mooney C.T. Treatment and therapeutic monitoring of canine hypothyroidism. J. Small Anim. Pract. 2002;43:334–340. doi: 10.1111/j.1748-5827.2002.tb00082.x. [DOI] [PubMed] [Google Scholar]
- Dremier S., Golstein J., Mosselmans R., Dumont J.E., Galand P., Robaye B. Apoptosis in dog thyroid cells. Biochem. Biophys. Res. Commun. 1994;200:52–58. doi: 10.1006/bbrc.1994.1412. [DOI] [PubMed] [Google Scholar]
- Duntas L.H., Orgiazzi J., Brabant G. The interface between thyroid and diabetes mellitus. Clin. Endocrinol. 2011;75:1–9. doi: 10.1111/j.1365-2265.2011.04029.x. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale H.F., Botella-Carretero J.I., Escobar del Rey F., Morreale de Escobar G. Treatment of hypothyroidism with combination of levothyroxine plus liothyronine. J. Clin. Endocrinol. Metabol. 2005;90:4946–4954. doi: 10.1210/jc.2005-0184. [DOI] [PubMed] [Google Scholar]
- Field J.M. The actin cytoskeleton and cell survival. In: Anninos P., Rossi M., Pham T.D., Falugi C., Bussing A., Koukkou M., editors. WSEAS Press; Cambridge UK: 2010. pp. 322–330. (Recent Advances in Clinical Medicine). [Google Scholar]
- Friedman M., Miranda-Massari J.R., Gonzalez M.J. Supraphysiological cyclic dosing of sustained release T3 in order to reset low basal body temperature. P. R. Health Sci. J. 2006;25:23–29. [PubMed] [Google Scholar]
- Janmey P.A. The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol. Rev. 1998;78:763–781. doi: 10.1152/physrev.1998.78.3.763. [DOI] [PubMed] [Google Scholar]
- Johnson E.O., Kamilaris T.C., Calogero A.E., Gold P.W., Chrousos G.P. Experimentally-induced hyperthyroidism is associated with activation of the rat hypothalamic-pituitary-adrenal axis. Eur. J. Endocrinol. 2005;153:177–185. doi: 10.1530/eje.1.01923. [DOI] [PubMed] [Google Scholar]
- Kališnik M. A histometric thyroid gland activation index. J. Microsc. 1972;95:345–348. doi: 10.1111/j.1365-2818.1972.tb03733.x. [DOI] [PubMed] [Google Scholar]
- Kališnik M. Morphometry of the thyroid gland. Stereol. Iugosl. 1981;3(Suppl 1):547–569. [Google Scholar]
- Kelly G. Peripheral metabolism of thyroid hormones: a review. Altern. Med. Rev. 2000;5:306–333. [PubMed] [Google Scholar]
- Klein I., Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725–1735. doi: 10.1161/CIRCULATIONAHA.106.678326. [DOI] [PubMed] [Google Scholar]
- Kraemer S., Danker-Hopfe H., Pilhatsch M., Bes F., Bauer M. Effects of supraphysiological doses of levothyroxine on sleep in healthy subjects: a prospective polysomnography study. J. Thyroid Res. 2011 doi: 10.4061/2011/420580. (article ID 420580, 7 pages) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krassas G.E., Poppe K., Glinoer D. Thyroid function and human reproductive health. Endocr. Rev. 2010;31:702–755. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- Kumar K., Kole A.K., Karmakar P.S., Ghosh A. The spectrum of thyroid disorders in systemic lupus erythematosus. Rheumatol. Int. 2012;32:73–78. doi: 10.1007/s00296-010-1556-5. [DOI] [PubMed] [Google Scholar]
- Markelic M., Velickovic K., Golic I., Otasevic V., Stancic A., Jankovic A., Vucetic M., Buzadzic B., Korac B., Korac A. Endothelial cell apoptosis in brown adipose tissue of rats induced by hyperinsulinaemia: the possible role of TNF-α. Eur. J. Histochem. 2011;55:187–193. doi: 10.4081/ejh.2011.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta S., Greer M.A. Evidence that thyroxine inhibits either basal or TRH-induced TSH secretion only after conversion to triiodothyronine. Proc. Soc. Exp. Biol. Med. 1988;187:391–397. doi: 10.3181/00379727-187-42679. [DOI] [PubMed] [Google Scholar]
- Matsunaga M., Eguchi K., Fukuda T., Tezuka H., Ueki Y., Kawabe Y., Shimomura C., Otsubo T., Ishikawa N., Ito K., Nagataki S. The effects of cytokines, antithyroidal drugs and glucocorticoids on phagocytosis by thyroid cells. Acta Endocrinol. (Copenh) 1988;119:413–419. doi: 10.1530/acta.0.1190413. [DOI] [PubMed] [Google Scholar]
- Norman A.W., Litwack G. Thyroid hormones. In: Norman A.W., Litwack G., editors. Hormones. Academic Press; San Diego: 1987. pp. 221–263. [Google Scholar]
- Okayasu I., Saegusa M., Fujiwara M., Hara Y., Rose R. Enhanced cellular proliferative activity and cell death in chronic thyroiditis and thyroid papillary carcinoma. J. Cancer Res. Clin. Oncol. 1995;121:746–752. doi: 10.1007/BF01213321. [DOI] [PubMed] [Google Scholar]
- Pantos C., Mourouzis I., Markakis K., Dimopoulos A., Xinaris C., Kokkinos A.D., Panagiotou M., Cokkinos D.V. Thyroid hormone attenuates cardiac remodeling and improves hemodynamics early after acute myocardial infarction in rats. Eur. J. Cardiothorac. Surg. 2007;32:333–339. doi: 10.1016/j.ejcts.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Pantos C., Mourouzis I., Markakis K., Tsagoulis N., Panagiotou M., Cokkinos D.V. Long-term thyroid hormone administration re-shapes left ventricular chamber and improves cardiac function after myocardial infarction in rats. Basic Res. Cardiol. 2008;103:308–318. doi: 10.1007/s00395-008-0697-0. [DOI] [PubMed] [Google Scholar]
- Pantos C., Mourouzis I., Tsagoulis N., Markakis K., Galanopoulos G., Roukounakis N., Perimenis P., Liappasi A., Cokkinos D.V. Thyroid hormone at supra-physiological dose optimizes cardiac geometry and improves cardiac function in rats with old myocardial infarction. J. Physiol. Pharmacol. 2009;60:49–56. [PubMed] [Google Scholar]
- Petrovic N., Cvijić G., Davidović V. Thyroxine and triiodothyronine differently affect uncoupling protein-1 content and antioxidant enzyme activities in rat interscapular brown adipose tissue. J. Endocrinol. 2003;176:31–38. doi: 10.1677/joe.0.1760031. [DOI] [PubMed] [Google Scholar]
- Prummel M.F., Brokken L.J., Wiersinga W.M. Ultra-short feedback loop control of thyrotropin secretion. Thyroid. 2004;14:825–829. doi: 10.1089/thy.2004.14.825. [DOI] [PubMed] [Google Scholar]
- Rajkovic V., Matavulj M., Gledic D., Lazetic B. Evaluation of rat thyroid gland morphophysiological status after three months exposure to 50 Hz electromagnetic field. Tissue Cell. 2003;35:223–231. doi: 10.1016/s0040-8166(03)00029-6. [DOI] [PubMed] [Google Scholar]
- Rajkovic V., Matavulj M., Johansson O. Light and electron microscopic study of the thyroid gland in rats exposed to power-frequency electromagnetic fields. J. Exp. Biol. 2006;209:3322–3328. doi: 10.1242/jeb.02375. [DOI] [PubMed] [Google Scholar]
- Ricken R., Bermpohl F., Schlattmann P., Bschor T., Adli M., Mönter N., Bauer M. Long-term treatment with supraphysiological doses of thyroid hormone in affective disorders – effects on bone mineral density. J. Affect. Disord. 2012;136:89–94. doi: 10.1016/j.jad.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Saleh D.M., Barrell G.K., Bailey C.I., Frampton C.M.A. Effects of exogenous triiodothyronine (T3) and a goitrogen, methylthiouracil (MTU), on thyroid gland function in sheep. Small Rum. Res. 1998;30:49–56. [Google Scholar]
- Scaglia L., Cahill C.J., Finegood D.T., Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rats. Endocrinology. 1997;138:1736–1741. doi: 10.1210/endo.138.4.5069. [DOI] [PubMed] [Google Scholar]
- Silva J.E. Thyroid hormone control of thermogenesis and energy balance. Thyroid. 1995;5:481–492. doi: 10.1089/thy.1995.5.481. [DOI] [PubMed] [Google Scholar]
- Soukup T., Zachařová G., Smerdu V., Jirmanová I. Body, heart, thyroid gland and skeletal muscle weight changes in rats with altered thyroid status. Physiol. Res. 2001;50:619–626. [PubMed] [Google Scholar]
- Suzuki K., Kawashima A., Yoshihara A., Akama T., Sue M., Yoshida A., Kimura H.J. Role of thyroglobulin on negative feedback autoregulation of thyroid follicular function and growth. J. Endocrinol. 2011;209:169–174. doi: 10.1530/JOE-10-0486. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Lavaroni S., Mori A., Ohta M., Saito J., Pietrarelli M., Kimura S., Katoh R., Kawaoi A., Kohn L.D. Autoregulation of thyroid-specific gene transcription by thyroglobulin. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8251–8256. doi: 10.1073/pnas.95.14.8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Mori A., Saito J., Moriyama E., Ullianich L., Kohn L.D. Follicular thyroglobulin suppresses iodide uptake by suppressing expression of the sodium/iodide symporter gene. Endocrinology. 1999;140:5422–5430. doi: 10.1210/endo.140.11.7124. [DOI] [PubMed] [Google Scholar]
- Tachiwaki O., Wollman S.H. Shedding of dense cell fragments into the follicular lumen early in the evolution of the hyperplastic thyroid gland. Lab. Invest. 1982;47:91–98. [PubMed] [Google Scholar]
- Tachiwaki O., Zeligs J.D., Wollman S.H. Ultrastructural changes in thyroid epithelium during involution of the hyperplastic thyroid gland. Am. J. Anat. 1990;189:45–56. doi: 10.1002/aja.1001890106. [DOI] [PubMed] [Google Scholar]
- Tamura M., Kimura H., Koji T., Tominaga T., Ashizawa K., Kiriyama T., Yokoyama N., Yoshimura T., Eguchi K., Nakane P.K., Nagataki S. Role of apoptosis of thyrocytes in a rat model of goiter. A possible involvement of Fas system. Endocrinology. 1998;139:3646–3653. doi: 10.1210/endo.139.8.6140. [DOI] [PubMed] [Google Scholar]
- Taşkin E., Artis A.S., Bitiktas S., Dolu N., Liman N., Süer C. Experimentally induced hyperthyroidism disrupts hippocampal long-term potentiation in adult rats. Neuroendocrinology. 2011;94:218–227. doi: 10.1159/000328513. [DOI] [PubMed] [Google Scholar]
- Weibel E.R. Vol. 1. Academic Press; London: 1979. Stereological methods. (Practical Methods for biological Morphometry). [Google Scholar]
- Wiersinga W.M. Thyroid hormone replacement therapy. Horm. Res. Suppl. 2001;1:74–81. doi: 10.1159/000048140. [DOI] [PubMed] [Google Scholar]
- Williams G.R., Bassett J.H. Local control of thyroid hormone action: role of type 2 deiodinase. Deiodinases: the balance of thyroid hormone. J. Endocrinol. 2011;209:261–272. doi: 10.1530/JOE-10-0448. [DOI] [PubMed] [Google Scholar]