Abstract
Objective
To evaluate the potential link between systemic inflammation and impaired lung function in people with ataxia-telangiectasia (A-T), we hypothesized that serum levels of interleukin (IL)-6, a proinflammatory cytokine, would correlate inversely with lung function in subjects with A-T.
Study design
Consecutive subjects with A-T were recruited from the Johns Hopkins Outpatient A-T Clinical Center. Serum levels of IL-6 and 8 were measured by enzyme-linked immunosorbent assay. Spirometry was performed in subjects ≥6 years of age on the same day that serum was obtained for measurements of cytokines.
Results
Approximately 80% of subjects had elevated serum IL-6 levels (>1.0 pg/mL). No association was found between elevated IL-6 and age. Elevated IL-8 levels were found in 23.6% of subjects, and all subjects with elevated IL-8 levels had elevated IL-6 levels. Subjects with elevated IL-6 levels (mean: 6.14 ± 7.47 pg/mL) had significantly lower mean percent forced vital capacity (FVC%, 50.5% ± 17.8%) compared with subjects with normal serum IL-6 levels (FVC% of 66.2 ± 16.1, P = .018). Greater IL-6 levels were associated with lower FVC% even after adjustment for receiving gamma globulin therapy (P = .024) and supplemental nutrition (P = .055).
Conclusions
An association was found between elevated serum IL-6 levels and lower lung function in subjects with A-T. In addition, subjects with both elevated IL-6 and IL-8 had the lowest mean lung function. These findings indicate that markers for systemic inflammation may be useful in identifying individuals with A-T at increased risk for lower lung function and may help in assessing response to therapy.
The ataxia-telangiectasia mutated (ATM) pathway is involved intricately in the response to oxidative stress and injury repair. Individuals with ataxia-telangiectasia (A-T) have been shown to develop cerebellar degeneration, immunodeficiency, sensitivity to ionizing radiation, and increased risk of malignancies.1–3 Sinopulmonary infections, impaired airway clearance, restrictive lung disease, and aspiration have been reported widely in children and adults with A-T.4,5 In AT, pulmonary symptoms often are not identified early in the disease process, and untreated lung disease can lead to significant morbidity and mortality. Therapies for the treatment of lung disease in A-T currently are supportive and include timely treatment of respiratory infections, pulmonary clearance techniques, and oral steroids for interstitial lung disease. Identifying risk factors and biomarkers associated with lung disease would allow for earlier treatment and improved outcomes.
Immune deficiency is present in more than 50% of people with A-T and can contribute to a decline in lung function.6 Chronic interleukin (IL)-6 production, an inflammatory cytokine produced by monocytes, macrophages, and other cell lineages, has been found previously in diseases characterized by immune dysregulation.7,8 In some of these diseases, IL-6 blockade has been shown to modify disease development and severity. In rheumatoid arthritis specifically, symptomatic improvement has been demonstrated in people who received a humanized anti-IL-6R monoclonal antibody. In A-T, the role of immune dys-regulation and inflammation in disease presentation and progression currently is unclear.9,10
People with A-T have abnormal DNA damage responses, telomere shortening, and increased sensitivity to oxidative stress.11,12 Oxidative stress and telomere shortening have been associated with inflammation-related diseases, including pulmonary fibrosis, neurodegeneration, and cancer,13 conditions commonly seen in A-T. In an earlier retrospective study, we found that a subset of individuals with A-T had elevated levels of serum IL-8, a proinflammatory neutrophil chemoattractant.14 This finding suggested a link between systemic inflammation and A-T. Both IL-6 and IL-8 have been shown to be elevated during conditions of nutritionally mediated oxidative stress and in stress-related inflammatory diseases.15,16 Because IL-6 elevation has been reported in chronic diseases associated with immune dysregulation, we sought to determine in a prospective cross-sectional study whether serum IL-6 levels correlated with lung function in people with A-T.
In this study we hypothesized that serum levels of IL-6 are associated inversely with lung function in people with A-T. To evaluate the potential association between lung function and IL-6 levels, we chose to limit spirometry data to that obtained on the same day that blood was drawn for cytokine measurement.
Methods
All subjects met the diagnosis of A-T on the basis of clinical symptoms and laboratory findings of either elevated alpha-fetoprotein, diminished ATM protein, and/or increased chromosomal breakage after in vitro exposure to x-rays as previously established.17 Demographic information was obtained from chart review. The institutional review board of the Johns Hopkins Medical Institutions approved the study, and written informed consent was obtained from every participant and/or his/her guardian.
Venous blood was drawn from 61 individual subjects undergoing outpatient evaluation at the Johns Hopkins A-T clinic between 2012 and 2014. Three of the 61 subjects were seen twice during this time period, and the first clinic visit was used for analysis. Concentrations of IL-6 (n = 61) and IL-8 (n = 55) were determined by the use of commercially available EIA kits (R&D Systems, Minneapolis, Minnesota). These were quantitative sandwich enzyme immunoassays used according to the manufacturer’s instructions. The optical density of each sample was determined with a microplate reader set to 450 nm (Optimax, Molecular Devices, Sunnyvale, California). Data were calculated from a standard curve and the results reported in picograms (pg) of cytokine protein per milliliter for each cytokine. The mean minimum detectable dose for IL-6 was 0.7 pg/mL with the Normal Adult Reference Range (Cytokine Laboratory, Johns Hopkins University, Baltimore, Maryland) between 0 and 1.0 pg/mL (n = 63). The mean minimum detectable dose for IL-8 was 3.5 pg/mL, with the Normal Adult Reference Range (R&D Systems, Minneapolis, Minnesota) being <31.2 pg/mL (n = 34). Samples were assayed in duplicate, and values were expressed as ±SD. All sample testing was performed in a masked setting.
Subjects who were 6 years of age or older and able to follow directions (n = 49) underwent standard spirometry according to recommendations by the American Thoracic Society18 on the same day that serum was obtained for measurements of cytokines. During each visit, a minimum of 3 flow-volume curves were attempted per person (MedGraphics). The best flow volume loop per visit was selected for further evaluation. Wang-predicted values were used for children up to 16 years of age,19 and the Third National Health and Nutrition Examination Survey–predicted values were used for adolescents older than 16 years of age.20
Statistical Analyses
Group comparisons and study-wide correlations were made by the use of nonparametric tests (Mann-Whitney U, Kruskal-Wallis, Fisher exact, Spearman correlation) because of the non-normal distribution of IL-6. When appropriate, multivariable linear regressions were performed with percent forced vital capacity (FVC%) as the dependent variable and the log of IL-6 levels and other covariates as the independent variables; co-efficient P values <.05 were considered evidence of association. Intercooled Stata 11 (StataCorp LP., College Station, Texas) was used for all statistical analyses.
Results
Demographic characteristics of subjects with A-T are summarized in Table I. Male and female subjects were represented equally. No differences among sexes were found with respect to either high (>1.0 pg/mL) or normal (≤1.0 pg/mL) serum IL-6. Subjects who had high serum IL-6 levels (mean: 6.14 ± 7.47 pg/mL) had a mean FVC% of 50.5 ± 17.8, and subjects with normal serum IL-6 levels (mean: 0.71 ± 0.13 pg/mL) had a mean FVC% of 66.2 ± 16.1 (P = .018).
Table I.
Study demographics of subjects with serum IL-6 levels (n = 61)*
| Entire study population (n = 61) | Normal serum IL-6 level [0–1.0] (n = 12) | Elevated serum IL-6 level [>1.0] (n = 49) | P value | |
|---|---|---|---|---|
| Sex (% male) | 49.2% | 58.3% | 46.9% | .53 |
| Age, y | 13.5 ± 7.7 [1, 27] | 11.8 ± 7.5 [2, 23] | 13.9 ± 7.7 [1, 27] | .34 |
| FVC%, predicted | 53.7 ± 18.5 [17, 98] (n = 49) | 66.2 ± 16.1 [44, 96] (n = 10) | 50.5 ± 17.8 [17, 98] (n = 39) | .018 |
| FEV1%, predicted | 60.0 ± 19.7 [20, 107] (n = 49) | 73.3 ± 15.8 [51, 99] (n = 10) | 56.6 ± 19.4 [20, 107] (n = 39) | .010 |
| Precancer/cancer (% yes)† | 14.8% | 0.0% | 18.4% | .18 |
| Cutaneous granulomas (% yes) | 8.2% | 8.3% | 8.2% | 1.00 |
| Gamma globulin therapy (% yes) | 31.2% | 8.3% | 36.7% | .08 |
| Supplemental nutrition (% yes)‡ | 24.6% | 8.3% | 28.6% | .26 |
| Serum IL-6, pg/mL | 5.07 ± 7.03 [0.48, 36.26] | 0.71 ± 0.13 [0.48, 0.97] | 6.14 ± 7.47 [1.03, 36.26] | <.001 |
| Serum IL-8, pg/mL | 41.8 ± 72.0 [5.8, 467.8] (n = 55) | 16.0 ± 5.3 [10.2, 30.2] (n = 12) | 49.1 ± 80.0 [5.8, 467.8] (n = 43) | .042 |
Values are mean ± SD [range] unless otherwise stated.
Precancer conditions included myelodysplastic syndrome (n = 1).
Supplemental nutrition includes formula delivered by gastrostomy or jejunostomy feeding tubes and/or total parental nutrition delivered intravenously in the home setting.
Correlation between Serum IL-6 and Clinical Phenotypes
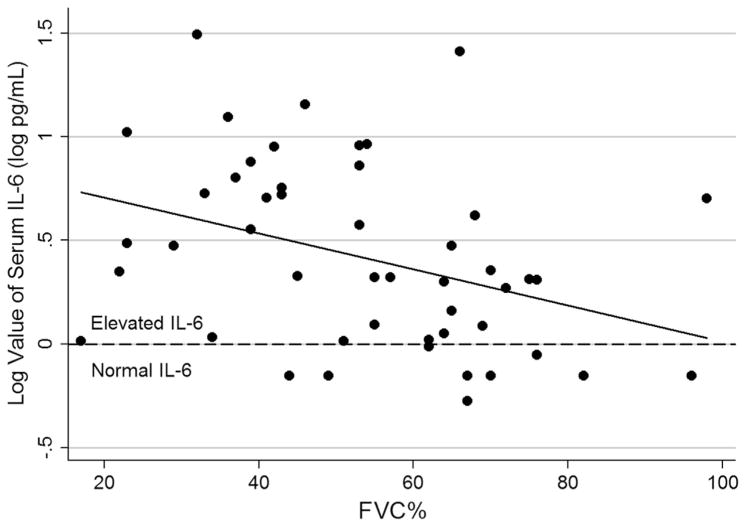
Because IL-6 levels were non-normally distributed in the study population, we used normally distributed log transformation of serum IL-6 levels to examine potential associations with clinical phenotypes. As described previously, we found that greater IL-6 levels correlated with lower FVC% and percent forced expiratory volume in 1 second (FEV1%) (Table II and Figure 1; Table II available at www.jpeds.com), such that a 10-fold increase in serum IL-6 level was associated with a 14.5% reduction in FVC% (P = .012) and a 15.8% reduction in FEV1% (P = .010). Elevated serum IL-6 levels also were associated with use of gamma globulin therapy and use of supplemental nutrition.
Table II.
Correlation between log of IL-6 levels and clinical phenotypes
| N | Spearman correlation (rho) | P value | |
|---|---|---|---|
| FVC%, predicted | 49 | −0.43 | .002 |
| FEV1%, predicted | 49 | −0.44 | .002 |
| Precancer/cancer (% yes) | 61 | 0.13 | .31 |
| Cutaneous granulomas (% yes) | 61 | 0.12 | .36 |
| Gamma globulin therapy (% yes) | 61 | 0.46 | <.001 |
| Supplemental nutrition (% yes) | 61 | 0.40 | .002 |
Figure 1.
Log of IL-6 serum level versus forced vital capacity (n = 49). By the use of linear regression, the log value of IL-6 was associated with lower FVC% such that a 10-fold increase in serum IL-6 level was associated with a 14.5% reduction in FVC% (P = .012; n = 49).
Because correlations between IL-6 and FVC% as well as between IL-6 and intravenous immunoglobulin (IVIG) were observed, we performed a post-hoc multivariable analysis accounting for FVC% vs IL-6 and IVIG or supplemental nutrition. In a multivariable regression adjusted for IVIG status for the 49 subjects who had measurements of FVC% (16 of whom have received IVIG), greater log levels of IL-6 remained associated with lower FVC% (P = .024), whereas IVIG was not associated with FVC% (P = .61). Similarly, in a multivariable regression adjusted for the presence of supplemental nutrition (15 of 49 subjects were receiving supplemental nutrition), greater log IL-6 levels tended to correlate with lower FVC% (P = .055), whereas supplemental nutrition was not associated with FVC% (P = .18).
No association between serum IL-6 levels and presence of cutaneous granulomas or history of myelodysplastic syndrome/cancer was found (Table II). There also was no correlation between IL-6 levels and age of the individual (r = 0.1360 and P = .29).
Elevated Serum IL-6 and IL-8 Levels in A-T
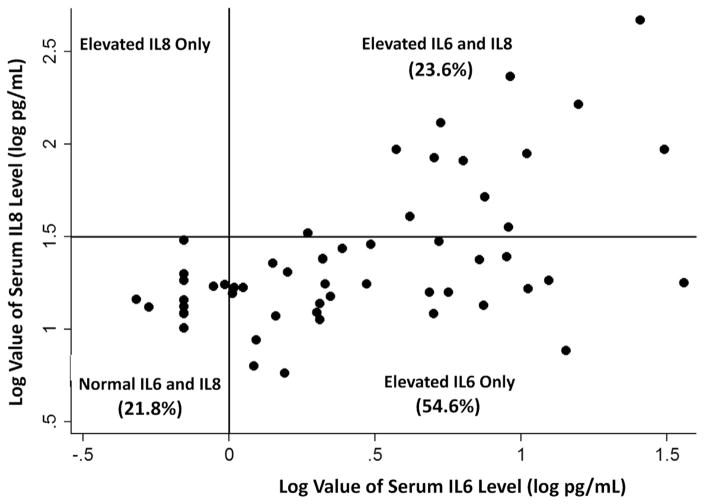
In a previous retrospective study, the neutrophil chemoattractant IL-8 was elevated in a subgroup of subjects with AT, suggesting an association between systemic inflammation and A-T.14 To determine whether serum IL-8 levels correlated with serum IL-6 levels, 55 subjects with A-T had IL-8 and IL-6 serum levels drawn concurrently. In the current study, elevated serum IL-8 levels (>31.2 pg/mL) were found in 23.6% of subjects tested, and subjects with high serum IL-8 levels had a mean IL-8 level of 122.9 ± 117.7 pg/mL. All subjects with high serum IL-8 levels also had high IL-6 levels (Figure 2). In addition, mean IL-6 serum levels were significantly greater in subjects with high IL-8 levels (10.41 ± 8.80 pg/mL) compared with subjects with normal IL-8 serum levels and elevated IL-6 levels (5.09 ± 6.89 pg/mL) (Mann-Whitney U test P = .004) (Table III). Subjects with both high IL-6 and high IL-8 serum levels had significantly lower mean FVC% than subjects with normal IL-6/normal IL-8 (Mann-Whitney U test, P = .024). Three subjects in the study had IL-6 and IL-8 levels drawn at 2 different time points approximately 2 years apart. In these subjects although variability occurred in IL-6 and IL-8 levels between the 2 time points, IL-6 and IL-8 levels trended in a similar directions (2 subjects had a decline in serum IL-6 and IL-8 levels, while 1 subject had an increase in IL-6 and IL-8, data not shown).
Figure 2.
Distribution of subjects by quadrants based on normal log values of serum IL-6 and IL-8 levels (n = 55).
Table III.
Study demographics of subjects with serum IL-6 and IL-8 levels (n = 55)*
| Normal IL-6 Normal IL-8 (n = 12) | Elevated IL-6 Normal IL-8 (n = 30) | Elevated IL-6 Elevated IL-8 (n = 13) | P value | |
|---|---|---|---|---|
| Sex, % male | 58.3 | 46.7 | 38.5 | .67 |
| Age, y | 11.8 ± 7.5 [2, 23] | 14.1 ± 7.9 [1, 27] | 14.7 ± 8.4 [3, 27] | .53 |
| FVC%, predicted | 66.2 ± 16.2 [44, 96] (n = 10) | 55.0 ± 17.5 [22, 98] (n = 22) | 47.6 ± 15.8 [23, 72] (n = 12) | .044† |
| FEV1%, predicted | 73.3 ± 15.8 [51, 99] (n = 10) | 62.0 ± 19.2 [23, 107] (n = 22) | 52.3 ± 15.9 [26, 76] (n = 12) | .028† |
| Precancer/cancer (% yes) | 0.0% | 26.7% | 7.7% | .10 |
| Granulomas (% yes) | 8.3% | 6.7% | 15.4% | .82 |
| Gamma globulin therapy (% yes) | 8.3% | 40.0% | 38.5% | .13 |
| Supplemental nutrition (% yes) | 8.3% | 23.3% | 46.2% | .09 |
| Serum IL-6, pg/mL | 0.71 ± 0.13 [0.48, 0.97] | 5.09 ± 6.89 [1.03, 36.26] | 10.41 ± 8.80 [1.86, 31.14] | .001‡ |
| Serum IL-8, pg/mL | 16.0 ± 5.3 [10.2, 30.2] | 17.0 ± 6.4 [5.8, 29.88] | 122.9 ± 117.7 [33.1, 467.8] | .001§ |
Values are mean ± SD [range] unless otherwise stated.
After Bonferroni correction, only the normal IL-6/normal IL-8 group was statistically different from the elevated IL-6/elevated IL-8 group.
After Bonferroni correction, all 3 groups were statistically different from each other.
After Bonferroni correction, the elevated IL-6/elevated IL-8 group was statistically different from the normal IL-6/normal IL-8 group and the elevated IL-6/normal IL-8 group.
Discussion
A-T has been characterized by defects in DNA damage repair (DDR), immune and telomere dysfunction, radiosensitivity, and increased risk of malignancy.21 Findings from this study suggest that A-T also may be a disorder of inflammation. We found that the majority of subjects with A-T had elevated levels of serum IL-6, with 23.6% having increased levels of both IL-6 and IL-8. Serum IL-6 levels were correlated inversely with FVC% and FEV1% in subjects with A-T. Although subjects with greater serum IL-6 levels were more likely to be receiving supplemental gamma globulin and supplemental nutrition, neither IVIG nor supplemental nutrition status were associated with FVC% after we accounted for IL-6. Subjects with both elevated IL-6 and IL-8 serum levels had the greatest mean serum IL-6 levels (10.41 ± 8.80 pg/mL) and lowest mean FVC% values, suggesting an association between systemic inflammation and lower lung function. Future studies are needed to examine the role of IL-6 and IL-8 in predicting pulmonary morbidity and to determine their utility as biomarkers in assessing treatment interventions.
Chronically elevated IL-6 levels have been shown to be associated with other pathologic lung conditions. In adults with systemic sclerosis, De Lauretis et al22 reported an association between serum IL-6 levels >7.67 pg/mL and lower FVC and worsening of interstitial lung disease. Greater levels of serum IL-6 also were reported in subjects with stable chronic obstructive pulmonary disease who had a history of greater smoking burden, bronchitis phenotype, and lower body mass index.23 In addition, lung transplant recipients with early lung allograft dysfunction had greater baseline IL-6 and IL-8 levels compared with lung transplant recipients without dysfunction.24 Studies that examine longitudinal measurements of serum IL-6 and IL-8 may help determine variability of serum cytokine levels over time, the impact of elevated inflammatory cytokines on disease outcomes, and response to treatment in people with A-T.
Cerebellar atrophy and extrapyramidal dysfunction is common in individuals with A-T; however, why these areas of the brain are targeted is unknown. IL-6 has been found to cross the blood–brain barrier and alter temperature regulation,25 and a study in mice found that brain IL-6 expression was greatest in the cerebellum of mice that received peripheral endotoxin.26 In a meta-analysis, depression was shown to be associated with greater IL-6 and C-reactive protein levels,27 and another study reported that greater serum IL-6 levels were associated with lower lung function and depression in patients with chronic obstructive pulmonary disease.28 Studies that examine whether an association exists between brain dysfunction and greater levels of IL-6 levels in people with A-T is warranted. In addition, induction of IL-6 through NF-κB and activation of Lin28 transcription has been shown to cause epigenetic changes associated with cellular transformation.29 Although it did not reach statistical significance, all of the subjects in our study with a history of malignancy or a premalignant condition had elevated serum IL-6 levels.
There are limitations to our study. Because the majority of individuals with A-T in this study had restrictive lung disease, the role of IL-6 in monitoring early/mild lung disease is unknown and will need to be addressed in future studies. Furthermore, this study was not designed to determine whether high serum levels of IL-6 were causal or merely associated with lower lung function. Indeed, elevated serum levels of IL-6 may be markers of greater overall disease severity in A-T, which may account for the lower lung function in subjects with high serum IL-6. In addition, because this was a cross-sectional study, data on the longitudinal variability of serum IL-6 and IL-8 levels were limited, and the relationship between serum cytokine levels and disease progression cannot be determined; however, 3 subjects had both IL-6 and IL-8 serum levels measured approximately 2 years apart, and although IL-6 and IL-8 levels varied over time, both IL-6 and IL-8 serum levels trended in similar directions among subjects, suggesting that these cytokines may be useful as biomarkers to assess response to treatment.
The mechanisms underlying the elevated serum levels of IL-6 and IL-8 in subjects with A-T were not explored in this study; however, there are several potential cellular pathways that may account for the increased systemic inflammation in people with A-T. Environmental stresses may activate DDR signaling through A-T and Rad3-related30 in individuals with ATM deficiency. This in turn may induce NF-κβ and the senescence-associated secretory phenotype pathway, increasing systemic levels of IL-6 and IL-8.30,31 Thus, the combination of A-T and Rad3-related activation through the DDR pathway and telomere dysfunction in people with A-T may account in part for the premature aging, susceptibility to ionizing irradiation, and increased incidence of malignancy in this population.21,32 Additional studies will be needed to examine these cellular pathways and their relationship to inflammation in people with A-T.
In summary, serum IL-6 levels were found to be increased in a majority of subjects with stable A-T and correlated inversely with lower FVC% and FEV1%. These findings suggest that serum IL-6 and/or IL-8 measurements may be useful in identifying individuals with A-T at increased risk for lower lung function and may be helpful in assessing responses to therapy.
Acknowledgments
Funded by Ataxia-Telangiectasia Children’s Project, Pediatric Clinical Research Center, The Johns Hopkins Hospital (RR00052), National Center for Research Resources, and the National Institutes of Health (RHL114800A [to S.M.]).
We thank Jennifer Wright for her help in collecting specimens and facilitating visits and all the people who participated in the study.
Glossary
- A-T
Ataxia-telangiectasia
- ATM
Ataxia-telangiectasia mutated
- DDR
DNA damage repair
- FEV1%
Percent Forced expiratory volume in 1 second
- FVC%
Percent forced vital capacity
- IL
Interleukin
- IVIG
Intravenous immunoglobulin
Footnotes
The authors declare no conflicts of interest.
References
- 1.Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol. 2013;14:197–210. [PubMed] [Google Scholar]
- 2.Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–53. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 3.Paull TT. Mechanisms of ATM activation. Annu Rev Biochem. 2015;84:711–38. doi: 10.1146/annurev-biochem-060614-034335. [DOI] [PubMed] [Google Scholar]
- 4.McGrath-Morrow SA, Gower WA, Rothblum-Oviatt C, Brody AS, Langston C, Fan LL, et al. Evaluation and management of pulmonary disease in ataxia-telangiectasia. Pediatr Pulmonol. 2010;45:847–59. doi: 10.1002/ppul.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroeder SA, Zielen S. Infections of the respiratory system in patients with ataxia-telangiectasia. Pediatr Pulmonol. 2014;49:389–99. doi: 10.1002/ppul.22817. [DOI] [PubMed] [Google Scholar]
- 6.Staples ER, McDermott EM, Reiman A, Byrd PJ, Ritchie S, Taylor AM, et al. Immunodeficiency in ataxia telangiectasia is correlated strongly with the presence of two null mutations in the ataxia telangiectasia mutated gene. Clin Exp Immunol. 2008;153:214–20. doi: 10.1111/j.1365-2249.2008.03684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi JF, Lu ZY, Jourdan M, Klein B. Interleukin-6 as a therapeutic target. Clin Cancer Res. 2015;21:1248–57. doi: 10.1158/1078-0432.CCR-14-2291. [DOI] [PubMed] [Google Scholar]
- 8.Desai GS, Mathews ST. Saliva as a non-invasive diagnostic tool for inflammation and insulin-resistance. World J Diabetes. 2014;5:730–8. doi: 10.4239/wjd.v5.i6.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniel LL, Daniels CR, Harirforoosh S, Foster CR, Singh M, Singh K. Deficiency of ataxia telangiectasia mutated kinase delays inflammatory response in the heart following myocardial infarction. J Am Heart Assoc. 2014;3:e001286. doi: 10.1161/JAHA.114.001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chessa L, Leuzzi V, Plebani A, Soresina A, Micheli R, D’Agnano D, et al. Intra-erythrocyte infusion of dexamethasone reduces neurological symptoms in ataxia teleangiectasia patients: results of a phase 2 trial. Orphanet J Rare Dis. 2014;9:5. doi: 10.1186/1750-1172-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward AJ, Olive PL, Burr AH, Rosin MP. Response of fibroblast cultures from ataxia-telangiectasia patients to reactive oxygen species generated during inflammatory reactions. Environ Mol Mutagen. 1994;24:103–11. doi: 10.1002/em.2850240205. [DOI] [PubMed] [Google Scholar]
- 12.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–21. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 13.Savage SA, Alter BP. The role of telomere biology in bone marrow failure and other disorders. Mech Ageing Dev. 2008;129:35–47. doi: 10.1016/j.mad.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGrath-Morrow SA, Collaco JM, Crawford TO, Carson KA, Lefton-Greif MA, Zeitlin P, et al. Elevated serum IL-8 levels in ataxia telangiectasia. J Pediatr. 2010;156:682–4. doi: 10.1016/j.jpeds.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–51. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 16.Munoz A, Costa M. Nutritionally mediated oxidative stress and inflammation. Oxid Med Cell Longev. 2013;2013:610950. doi: 10.1155/2013/610950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabana MD, Crawford TO, Winkelstein JA, Christensen JR, Lederman HM. Consequences of the delayed diagnosis of ataxia-telangiectasia. Pediatrics. 1998;102:98–100. doi: 10.1542/peds.102.1.98. [DOI] [PubMed] [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 20.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 21.Stanley SE, Rao AD, Gable DL, McGrath-Morrow S, Armanios M. Radiation Sensitivity and Radiation Necrosis in the Short Telomere Syndromes. Int J Radiat Oncol Biol Phys. 2015;93:1115–7. doi: 10.1016/j.ijrobp.2015.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Lauretis A, Sestini P, Pantelidis P, Hoyles R, Hansell DM, Goh NS, et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J Rheumatol. 2013;40:435–46. doi: 10.3899/jrheum.120725. [DOI] [PubMed] [Google Scholar]
- 23.de Moraes MR, da Costa AC, Correa KS, Junqueira-Kipnis AP, Rabahi MF. Interleukin-6 and interleukin-8 blood levels’ poor association with the severity and clinical profile of ex-smokers with COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:735–43. doi: 10.2147/COPD.S64135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen JG, Lee MT, Weiss ES, Arnaoutakis GJ, Shah AS, Detrick B. Preoperative recipient cytokine levels are associated with early lung allograft dysfunction. Ann Thorac Surg. 2012;93:1843–9. doi: 10.1016/j.athoracsur.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 25.Rummel C, Gerstberger R, Roth J, Hubschle T. Parthenolide attenuates LPS-induced fever, circulating cytokines and markers of brain inflammation in rats. Cytokine. 2011;56:739–48. doi: 10.1016/j.cyto.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Silverman HA, Dancho M, Regnier-Golanov A, Nasim M, Ochani M, Olofsson PS, et al. Brain region-specific alterations in the gene expression of cytokines, immune cell markers and cholinergic system components during peripheral endotoxin-induced inflammation. Mol Med. 2014;20:601–11. doi: 10.2119/molmed.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150:736–44. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Lu Y, Feng L, Feng L, Nyunt MS, Yap KB, Ng TP. Systemic inflammation, depression and obstructive pulmonary function: a population-based study. Respir Res. 2013;14:53. doi: 10.1186/1465-9921-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saha J, Wang M, Cucinotta FA. Investigation of switch from ATM to ATR signaling at the sites of DNA damage induced by low and high LET radiation. DNA Repair (Amst) 2013;12:1143–51. doi: 10.1016/j.dnarep.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Kang C, Xu Q, Martin TD, Li MZ, Demaria M, Aron L, et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015;349:aaa5612. doi: 10.1126/science.aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SS, Bohrson C, Pike AM, Wheelan SJ, Greider CW. ATM kinase is required for telomere elongation in mouse and human cells. Cell Rep. 2015;13:1623–32. doi: 10.1016/j.celrep.2015.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]