Advances in perinatal care have dramatically improved survival of extremely preterm infants and the incidence of bronchopulmonary dysplasia (BPD) has not changed over the past few decades, which likely reflects the impact of increased survival of extremely preterminfants.1 BPD remains the most common late morbidity of preterm birth, but many controversies persist regarding how to best define BPD, grade its severity, and prevent disease.2 Ongoing clinical care and research have largely focused on issues regarding the pathogenesis and prevention of BPD in preterm infants with the important goal of reducing the incidence of BPD at 36 weeks corrected age, with a focus on respiratory care related issues during the early neonatal intensive care unit (NICU) course.3 However, some preterm infants develop particularly severe chronic respiratory disease and have related comorbidities that persist throughout their NICU course and post-discharge, as reflected by the prolonged need for high levels of respiratory support, including mechanical ventilation and high inspired oxygen concentrations. The management of infants with severe BPD (sBPD) has received less attention regarding clinical studies and interventions when compared with preventive strategies, yet these infants constitute a critical population who remain at high risk for extensive morbidities and late mortality (Figure 1).
Figure 1.
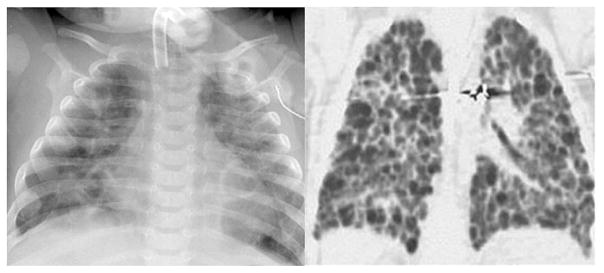
Imaging of sBPD: Chest radiograph (left) and image from computed tomography (CT) scan illustrate radiographic abnormalities of type 2 sBPD. Note the severe cystic disease present on the CT scan.
Based on consensus recommendations from a National Institutes of Health (NIH) workshop, BPD is commonly defined by the requirement for supplemental oxygen at 28 days in infants born at below 32 weeks gestation.4 Furthermore, BPD is divided into 3 severity grades (mild, moderate, or severe) based on respiratory support needs at 36 weeks postmenstrual age (PMA) (Table I). The incidence of sBPD is inversely correlated with gestational age and remains 16% for all infants born at <32 weeks (Table I).5 Unfortunately, high-quality evidence on which to base the care for infants and children with sBPD and consensus care guidelines are lacking. These knowledge gaps have contributed to marked variability in care within and between NICUs throughout the country. Current NIH definitions of BPD are almost exclusively focused on NICU-related issues, with limited discussion regarding long-term respiratory outcomes throughout infancy, childhood, and adulthood. In addition, the classification of sBPD is very broad and fails to differentiate between proposed phenotypes of infants with a persistent oxygen requirement and/or need for continuous positive airway pressure (CPAP) or high flow nasal cannula at 36 weeks postconceptual age who have relatively less severe respiratory disease (type 1 sBPD; Table I) from those with more extreme BPD (or type 2 sBPD), who remain ventilator-dependent and more often have severe complications, including pulmonary hypertension, poor growth, and neurodevelopmental problems. Understanding distinct antenatal, early postnatal, genetic, or epigenetic factors, or comorbidities that contribute to sBPD, especially the more severe phenotype (type 2 sBPD), are critical for enhancing late outcomes in this subgroup.
Table I.
BPD definition with severity
| BPD severity | Definition (Modified from Jobe and Bancalari4) | Relative incidence (Data from Ehrenkranz et al5) | Postdischarge mortality (Data from Ehrenkranzet al5) |
|---|---|---|---|
| None | O2 treatment <28 d and breathing room air at 36 wk PMA or discharge home, whichever comes first | 23.1% | 1.8% |
| Mild | O2 treatment at least 28 d and breathing room air at 36 wk PMA or discharge home, whichever comes first | 30.3% | 1.5% |
| Moderate | O2 treatment at least 28 d and receiving <30% O2 at 36 wk PMA or discharge home, whichever comes first | 30.2% | 2.0% |
| Severe (type 1) | O2 treatment at least 28 d and receiving ≥30% O2 or nasal CPAP/HFNC at ≥36 wk PMA | 16.4% | 4.8% |
| Severe (type 2) | O2 treatment at least 28 d and receiving mechanical ventilation at ≥36 wk PMA. |
HFNC, high flow nasal cannula; O2, oxygen.
Clinical needs of infants with type 2 sBPD who require ongoing respiratory support beyond term corrected age are diverse and complex, and management strategies that optimize survival and long-term outcomes are controversial and uncertain. Several factors contribute to increased morbidity and mortality in this population. First, there are few evidence-based strategies to improve outcomes. Second, patients with chronic ventilator-dependent BPD have historically been cared for in acute care settings. Management strategies for chronic disease differ considerably from acute respiratory failure, especially regarding approaches to mechanical ventilation. When compared with approaches toward acute respiratory failure, neonatal and pediatric intensivists may have less experience with ventilator management of infants with severe chronic lung disease. Most importantly, poor communication between providers, subspecialists, nursing staff, and families during prolonged hospitalization may lead to inconsistent care and adverse outcomes. High staff turnover and infrequent communication among the doctors and bedside staff as well as between parents and the medical team may contribute to these inconsistencies. These infants have complex clinical courses with multiple morbidities, including frequent hospitalizations throughout childhood, and often with poor continuity of care. Interdisciplinary care teams have the potential to alleviate many of these issues and improve outcomes for these infants.
Based on these concerns, a group of clinicians from interdisciplinary care programs for infants with sBPD at several major medical centers, including neonatologists, pulmonologists, critical care physicians, gastroenterologists, nurse specialists, and others, formed the “BPD Collaborative,” to address controversies and promote research to enhance the care of children with sBPD. In this review, we present an interdisciplinary approach to patients with sBPD and their families throughout the NICU and outpatient courses. Emphasis is placed on the rationale for developing the team approach to chronic care as well as highlighting key gaps in knowledge in our care for infants with sBPD, which would likely improve with multicenter investigations.
Incidence and Etiology of sBPD
Although marked improvements in care have led to milder respiratory courses for most preterm infants, sBPD remains a major problem. In the validation study from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network, the incidence of sBPD was 16% in infants born at <32 weeks and weighing <1000 g (Table I).5 Reports using birth cohorts from Scandinavia found that the incidence of sBPD was 25% in infants born at <27 weeks gestation in Sweden, and 20% in infants born at <28 weeks in Norway.6,7 The Children’s Hospital Neonatal Consortium reported a 16% incidence of sBPD of patients born at <32 weeks, of whom 91% survived to discharge, 66% were discharged on supplemental oxygen, 4% on mechanical ventilation, and 5% received tracheostomy.8 The BPD Collaborative reported a point prevalence of sBPD of 36.5% with a range across centers of 11%–58%, and 41% of the patients with sBPD had type 2 sBPD with a range of 0%–68%.9 Using an estimated incidence of sBPD of 16% for infants born at <32 weeks suggests that ~13 000 patients develop sBPD annually in the US alone.5
Epidemiologic data are limited, but estimates suggest that roughly 8000 children in the US receive mechanical ventilation at home.10 Based on 2011 data from the state of Pennsylvania’s Ventilator Assisted Children’s Home Program, 36% of ventilator-dependent children were diagnosed with chronic lung disease, 77% of these specifically with the diagnosis of sBPD.10 From these data, it can be extrapolated that ~2000 infants and children with sBPD are dependent on mechanical ventilation at home in the US.
The exact pathogenic mechanisms underlying the development of sBPD in preterm infants and factors that favor the development of sBPD are uncertain. Similarly, factors that contribute to the receipt of prolonged mechanical ventilation within the subgroup with type 2 sBPD are uncertain. The incidence of sBPD is inversely related to gestational age at birth.6,11,12 Male infants are more likely to develop sBPD and the need for mechanical ventilation. Patent ductus arteriosus, sepsis, and surgical necrotizing enterocolitis are strongly associated with the development of sBPD.11 A recent preclinical study suggests dose-related effects of antenatal endotoxin as a model for experimental chorioamnionitis that is sufficient to induce sBPD, and moderate oxygen treatment actually improved lung structure rather than worsening respiratory outcome, suggesting the complexity of pre- and postnatal events in driving BPD phenotypes.13
The genetics and epigenetic, or gene/environmental factors, underlying the risk for sBPD remain to be determined, however, recent twin studies have demonstrated a genetic component to the development of BPD in general,14,15 and there are an increasing number of gene-targeted studies finding specific mutations in genes associated with lung development, immunity, and oxidative stress associated with BPD in preterminfants.16–21 However, specific genes that increase the risk for sBPD remain unknown. In particular, data are lacking that specifically address whether distinct genetic, epigenetic, or other etiologic mechanisms contribute to the development of sBPD in comparison with milder forms of BPD. In addition, there is a clear need to develop biomarkers and other predictors for the early identification of preterm newborns who are at risk for BPD and more specifically for sBPD.
Clinical Features of sBPD
Although perhaps less common than in the past, infants with sBPD present persistent challenges, raise many questions regarding optimal strategies for enhancing outcomes, and may at times present significant ethical dilemmas. Furthermore, there is limited high quality evidence on which to base clinical management of the patient with sBPD. Evaluation of the patient with sBPD can include a variety of studies discussed herein, which should be guided by clinical presentation. In the extreme preterm infant with type 2 sBPD with the usual NICU course, there is little need to exclude other potential causes of chronic lung disease. In the infant with an atypical presentation or NICU course, potential causes of chronic neonatal respiratory failure include surfactant protein deficiency, gastroesophageal reflux disease (GERD), cystic fibrosis, immune deficiency, anatomic heart disease, pulmonary infection, H-type tracheoesophageal fistula, primary ciliary dyskinesia, and other developmental lung diseases. Evaluations may include bronchoscopy, echocardiogram, esophageal pH and impedance probes, genetic testing, chest computed tomography scan, lung biopsy, and other studies.
The NIH consensus statement that defined BPD severity4 did not address the wide range of disease severity within the group classified as sBPD. In 2003, the American Thoracic Society published a consensus statement pertaining to the care of children with BPD emphasizing the need for an interdisciplinary approach to address the multiorgan sequelae of premature birth.22 More specifically, infants with type 2 sBPD are at increased risk for adverse comorbidities including neurodevelopmental impairment, pulmonary hypertension (PH), GERD, feeding difficulties, airways disease, retinopathy of prematurity, and systemic hypertension. These comorbidities frequently complicate the course in sBPD, require the involvement of multiple subspecialists, and, when not integrated into the overall management plan, can further exacerbate gaps in communication and care. In centers that have adopted an interdisciplinary approach to care, survival for this subset of infants is over 75%–80% at discharge.9,23 Because of the low incidence of type 2 sBPD at any single center, this population remains understudied, and many gaps in knowledge (Table II24) and controversies exist regarding optimal clinical strategies to improve outcomes in this subgroup of infants.
Table II.
Identification of unique research needs to enhance our understanding of sBPD (Modified from Collaco et al24)
Epidemiology
|
Risk factors
|
Pathophysiology
|
Diagnosis
|
Management
|
Mechanical Ventilation in sBPD
The strategy for respiratory support in the acute phases of extreme prematurity is to avoid unnecessary intubation and mechanical ventilation to prevent secondary ventilator-associated lung injury. Persistent need for mechanical ventilator support at postnatal day 7 is associated with high risk for BPD,11 however, the link between BPD risk and sustained ventilator support at this time point may simply reflect the critical impacts of adverse antenatal and early postnatal events on lung structure and not merely be due to ventilator-associated lung injury alone. Recent multicenter trials no longer support intubation solely for the purpose of treatment with exogenous surfactant.25–27 Unfortunately, a significant proportion of infants cannot be stabilized and supported on nasal CPAP during their entire hospital course. Several recent trials have shown that infants randomized to immediate nasal CPAP were intubated secondary to apnea or respiratory failure in at least 50% of cases.25–27 Hence, the proportion of infants exposed to intubation and mechanical ventilation remains high along with a continued high incidence of chronic respiratory failure and the subsequent development of sBPD. In extremely low birth weight infants in the first week of life, attempts at extubation to and stabilization on nasal CPAP are still warranted, based on the potential impact of prolonged ventilator support on sBPD and adverse neurodevelopmental outcomes.28
However, at some point during the respiratory course of premature infants with evolving BPD, lung structure and function may become sufficiently abnormal that attempts at extubation to nasal CPAP are neither feasible nor desirable. Some indicators include sustained respiratory distress with retractions, recurrent cyanotic or bradycardic episodes, intolerance of respiratory and physical therapies or handling, poor growth, and the apparent use of or need for repeated steroid courses without sustained benefit. At this point, the goals of care need to be redirected toward optimizing management on mechanical ventilation to support adequate gas exchange, reduce the work of breathing, and optimize growth and healing of the injured lungs. Resolution of the lung disease and improvement in lung function depends on emphasizing nutritional strategies that enhance somatic and lung growth.
Matching Ventilator Strategies with Lung Mechanics in sBPD
Determining the specific type and level of respiratory support for infants with sBPD is challenging, as an optimal ventilator strategy required by these infants is frequently dramatically different than the strategy utilized in the first few weeks of life (Table III).29 The strategy must reflect the transition from lung mechanics in the first few days of life, which are dominated by low compliance, relative homogeneity, and normal airway resistance, to the mechanics that are seen in sBPD, dominated by high airway resistance, air trapping, and heterogeneous aeration (Figure 2).29 In the first week of life, lung mechanics suggest that a ventilator strategy aimed at the relatively uniform respiratory system with short time constants is reasonable and would include a fast rate, low tidal volume (Vt), short inspiratory time (Ti) strategy (Figure 2).29–31 In sBPD, the lung is characterized by different combinations of lung regions with different levels of airway resistance and altered distal lung compliance, which leads to highly diverse time constants (Figure 2). Thus, to enhance gas exchange, reduce the risk for atelectasis, decrease dead space ventilation, and perhaps lower pulmonary vascular resistance, the ventilator support strategy needs to change dramatically from a fast-rate, low Vt strategy during the early course to a slow-rate, high Vt and prolonged Ti strategy for chronic disease (Table III).
Table III.
Comparison of ventilator strategies and goals during progression of early disease to established sBPD (modified from Abman and Nelin29)
| Early (prevention) | Strategies to prevent acute lung injury | Low tidal volumes (3–5 mL/kg) |
| Short inspiratory times (0.2–0.3 seconds) | ||
| Increased PEEP for lung recruitment without overdistension | ||
| Strategies for gas exchange | Adjust FiO2 to target SpO2 (range: 91%–95%) | |
| Permissive hypercapnia | ||
| Late (established BPD) | Strategies for gas exchange | Marked regional heterogeneity |
| Larger tidal volumes (10–12 mL/kg) | ||
| Longer inspiratory times (≥0.6 s) | ||
| Airways obstruction | ||
| Slower rates allow for better emptying, especially with larger tidal volumes (10–20 bpm) | ||
| Complex roles for PEEP with dynamic airway collapse | ||
| Interactive effects of ventilator strategies | ||
| Changes in rate, tidal volume, inspiratory and expiratory times, and pressure support are highly interdependent | ||
| Overdistension can increase agitation and paradoxically worsen ventilation | ||
| Strategies for gas exchange | Adjust FiO2 to target higher SpO2 (92%–95%) | |
| Permissive hypercapnia to facilitate weaning |
Figure 2.
Illustration of A, 2-zone disease vs B, multizone (heterogeneous) disease. A, Chest CT with 2 zones in a patient with acute respiratory distress syndrome. B, Chest CT in a patient with type 2 sBPD and diffuse, heterogeneous disease. C, Schematic illustrating concept of multizone disease. Time constant, T = resistance x compliance. Reprinted with permission from Abman et al.29
As lung mechanics change over time in infants with sBPD who are on relatively low concentrations of supplemental oxygen and moderate or high rates, it is not uncommon to observe acute episodes (“spells”) of respiratory distress associated with high to fraction of inspired oxygen (FiO2) requirements, which are often difficult to address by simple maneuvers on the ventilator. At this stage, hypercarbia is often present with radiographic findings of hyperinflation alternating with areas of haziness and atelectasis. The timing of onset of these episodes in ventilated infants is variable, although these often develop during the first month of life. Some episodes of acute deteriorations in oxygenation may represent loss of volume during forced exhalation, leading to intrapulmonary shunt.32 These events are usually brief and self-limited, but may be frequent. These “breath-holding spells” are likely a separate phenomenon from the more prolonged events that may be associated with striking elevations of partial pressure of carbon dioxide (PaCO2), suggesting marked airways obstruction because of mucus plugging, aspiration, or central airway dynamic collapse. Approaches to severe spells include vigorous suctioning, airway cultures to diagnose ventilator-associated pneumonia, transitioning mechanical ventilator strategies to provide better support to maintain lung volume, and consideration of a trial of drug therapy, such as a steroid “burst,” bronchodilators, and/or diuretics. Although many infants get treated with bronchodilators and diuretics, their use between centers is highly variable.33,34
A single center study of preterm infants reported that infants on mechanical ventilation at 30 days with a respiratory severity score of >6 (defined as mean airway pressure × FiO2) were more likely to succumb to respiratory failure and were ventilated longer than infants with a respiratory severity score <6 at that time point.35 These data suggest that abnormal lung function develops well before 1 month of life and that with progressive respiratory instability, one should consider changing the respiratory support strategy to alter ventilator strategy to include a slow rate, larger Vt, and increased Ti to maintain minute ventilation and functional residual capacity.
Whether the use of volume targeted or pressure-limited ventilation is best with sBPD is uncertain, however, chronic respiratory support strategies require higher tidal volumes than those used with early “lung protective” strategies during the acute phases of disease.29,31 The targeted Vt for acute short time constant disease is typically in the range of 4–6 mL/kg, whereas for infants with developing obstructive airway disease the Vt may be 8–12 mL/kg. For pressure ventilation, the peak inflation pressure needed to generate this Vt can be well above 25 cm H2O, depending on the individual patient’s underlying airways resistance, lung compliance, and related respiratory system mechanics. These higher pressure settings reflect the need to provide sufficient Vt in lung disease that is generally characterized by high total respiratory system resistance and lower compliance. Furthermore, Ti are usually increased (0.5–0.8 seconds) to allow for better gas distribution into distal lung units, but longer Ti and increased Vt requires the use of a slow ventilator rate (often below 20 breaths per minute) to allow for sufficient emptying to avoid hyperinflation. Faster rates with increased Vt and prolonged Ti may worsen gas trapping and patient-ventilator asynchrony. Monitoring flow waveforms can aid in determining whether expiratory flow has been completed before the next breath is delivered.
Although generally counterintuitive in the setting of hyperinflation, positive end expiratory pressure (PEEP) should be relatively high (>6–8 cm H2O) to optimize gas exchange, maintain functional residual capacity, avoid regional atelectasis, and avoid the adverse effects of “inadvertent PEEP.” In some patients with significant airways closure especially with tracheomalacia or bronchomalacia, PEEP may need to be even higher to keep airways open during active exhalation. Flow-volume loops of patients with sBPD often show expiratory flow limitation at low lung volumes when lower PEEP levels are used, which is due to expiratory collapse of poorly supported small airways and air-trapping. With this pattern, PEEP can usually be titrated upward until this expiratory flow limitation is resolved by visual inspection of flow-volume loops. Questions regarding the use of this ventilator strategy for established sBPD include concerns for further inducing volutrauma because of additional lung overdistension, but when combined with lower ventilator rates and sufficient expiratory time, additional gas trapping is rare with this approach. Presently, there are no clinical trials to validate the strategy of low rate and large tidal volume ventilation, but this strategy is well-grounded with clinical experience and follows our current understanding of the pathophysiology of sBPD.29,31,36 For the very small number of patients with severe BPD who do not respond to this mechanical ventilation strategy, the practitioner must then consider rare but important causes of hypoxemia and ventilation-perfusion mismatch.
Structure-function studies in the nonresponsive patient with sBPD may identify other abnormalities such as predominantly restrictive lung disease, tracheobronchomalacia, and/or other rare causes of ventilation-perfusion mismatch.
In acute care, rapid weaning from mechanical ventilation is encouraged to avoid the potential adverse effects of prolonged intubation and ventilator support. In contrast, the goal of mechanical ventilation in the chronic phase of the ventilated infant with sBPD is to provide relatively stable respiratory support to reduce respiratory distress, improve gas exchange as reflected by lower FiO2, minimize intermittent “spells,” enhance tolerance of care and handling, and reduce the need for chronic sedation. Additional goals include preventing the development or progression of PH, providing optimal nutrition for quality growth, and encouraging developmental outcomes. These clinical variables of respiratory stability better reflect successful strategies than traditional metrics of PaCO2 or end-tidal carbon dioxide (CO2), which are unreliable in this setting. There is a slow but steady decrease in the amount of oxygen needed to provide stable saturations and sometimes even the Vt can be weaned. The focus should be on lowering the oxygen concentration in this phase of the healing disease and once infants are stable in less than 40% oxygen to achieve oxygen saturation (SpO2) values ≥ 92%, extubating to noninvasive respiratory support is considered and is usually successful even from relatively high Vt.
Considerations for Tracheostomy Placement and Commitment to Chronic Ventilator Support
The decision to commit an infant with sBPD to chronic ventilator support with the placement of a tracheostomy tube can be complex and difficult and must involve extensive discussions among care providers and family members. Many factors contribute to this decision, but the overall goal of chronic mechanical ventilator support is to reduce the severity of respiratory distress, including retractions, head-bobbing, dyspnea, and “spells,” to provide clinical stability that will enhance survival and optimize long-term developmental, neurocognitive, and growth-related outcomes. Tracheostomy placement is only part of an organized strategy to provide chronic ventilation and allows focus to shift toward enhancement of long-term outcomes. In some settings, tracheostomy represents a symbol of “futility” that may lead to some reluctance to perform tracheostomy and commit to ventilator support. Tracheostomy placement should not be viewed as a method for rapidly weaning of respiratory support but rather a means for sustained mechanical ventilation, relieving distress and providing the respiratory stability that is necessary to enhance neurocognitive, behavioral, and developmental outcomes. Nonetheless, determination of the efficacy and optimal timing of tracheostomy for infants with type 2 sBPD remains to be determined.
Beyond tracheostomy placement, an interdisciplinary team of collaborating subspecialists and therapists can provide continuity of care for these children. Teams of experienced personnel may coordinate care plans through regular rounds, family meetings, and collaboration with the attending service that will alleviate problems attributable to rapid turnover of staff in the intensive care unit setting. Thus, emphasis should be directed toward the role of sustained mechanical ventilation to promote growth and development, as supported by an interdisciplinary care team, and not on simply the placement of a tracheostomy.
Pulmonary Hypertension in sBPD
Our approach to PH in sBPD is to have a high index of suspicion and, when PH is found, to rigorously evaluate patients for underlying respiratory morbidities and to treat the lung disease aggressively (Table IV). PH complicates the course in up to 25% of patients with sBPD,38 therefore, screening echocardiograms should be performed in all patients with sBPD. Some cases of PH might be missed with the use of echocardiography alone;39 nevertheless, it remains the best non-invasive screening tool to assess PH in sBPD. Even though the accuracy of the echocardiogram may be limited regarding the ability to find a measurable and reproducible tricuspid regurgitant jet velocity to estimate right ventricular systolic pressure, other markers of PH, such as septal wall flattening and right ventricular dilation, may be clinically useful.24
Table IV.
Diagnoses
|
Pharmacologic treatments
|
ETRA, endothelin receptor antagonist; RV, right ventricular; V/Q, ventilation-perfusion.
If the screening echocardiogram is initially normal, subsequent echocardiograms should be performed in patients with sBPD at 1- to 2-month intervals until the respiratory status of the patient is significantly improved. If, on the other hand, the screening echocardiogram demonstrates PH, the initial clinical strategy for the management of PH in infants with BPD begins with optimizing the treatment of the underlying lung disease (Table IV).37 Periods of acute hypoxemia, whether intermittent or prolonged, contribute to the pathogenesis of late PH in sBPD and also exacerbate existing PH. Therefore, oxygen saturation limits may need to be changed to avoid intermittent or sustained hypoxemia with targets generally between 92% and 95%.37 Indeed, in patients with BPD and severe PH who also have significant respiratory distress and high levels of respiratory support, the presence of PH may provide a stronger rationale for long-termmechanical ventilatory support with tracheostomy. Changes in serial blood brain natriuretic peptide and N-terminal pro-brain natriuretic peptide levels may provide additional guides to aid the management of PH in sBPD.
Cardiac catheterization can be used to guide therapy, particularly in severe cases and in cases requiring long-term therapy.37 Although cardiac catheterization prior to the initiation of long-term therapy is encouraged, the risks and benefits of this procedure in sick newborns depend on local expertise with the procedure and severity of disease. The goals of cardiac catheterization are to assess the severity of PH; exclude or document the severity of associated anatomic cardiac lesions; define the presence of systemic-pulmonary collateral vessels, pulmonary venous obstruction, or left heart dysfunction; and to assess pulmonary vascular reactivity in patients who fail to respond to oxygen therapy alone.37 In particular, several key factors that may affect cardiopulmonary function include assessment of shunt lesions, especially atrial septal defects; the presence, size, and significance of bronchial or systemic collateral arteries; the presence of pulmonary vein stenosis; and structural assessments of the pulmonary arterial and venous circulation by angiography.
Despite the growing use of pulmonary vasodilator therapy for the treatment of PH in BPD, data demonstrating efficacy are extremely limited, and these agents should be used only after thorough diagnostic evaluations and aggressive management of the underlying lung disease (Table V).24,37 Current therapies used for PH therapy in infants with BPD generally include inhaled nitric oxide (iNO), phosphodiesterase type 5 inhibitors, endothelin receptor antagonists, and calcium channel blockers (CCBs). In a small number of patients with sBPD undergoing cardiac catheterization, iNO decreased pulmonary artery pressure and the ratio of pulmonary vascular resistance to systemic vascular resistance, which was further augmented with supplemental oxygen.40 Calcium channel blockers (such as nifedipine) benefit some patients with PH, and short-term effects of these agents in infants with BPD have been reported.41,42 However, CCBs can cause systemic hypotension and suppress myocardial function, especially in young infants with left ventricular dysfunction. In comparison with acute iNO reactivity in a small number of infants studied with sBPD, the acute response to CCBs was minimal.40
Table V.
Pharmacology of sBPD
| Categories | Medications | Dosing | Comments |
|---|---|---|---|
| Diuretics (Enteral dosing) | Furosemide | 1–2 mg/kg/dose q12–24h | Often PRN |
| Chlorothiazide | 20–40 mg/kg/d divided q12h | ||
| Spironolactone | 2–4 mg/kg/d divided q12–24h | ||
| Bronchodilators | Albuterol | 2.5 mg nebulized or 2 puffs q4–12h | Often PRN |
| Ipratropium | 0.5 mg q6–12h | Often PRN | |
| Levalbuterol | 0.31–1.25 mg or 2 puffs q4–12h | Often PRN | |
| Inhaled corticosteroids | Beclomethasone | 2 puffs q12h | 40 or 80 mcg MDIs |
| Budesonide | 1 neb q12–24h | 0.25 or 0.5 mg nebs | |
| Fluticasone | 2 puffs q12h | 44, 110, 220 mcg MDIs | |
| Pulmonary hypertensive agents | Bosentan | ½ tab BID PO | |
| Sildenafil | 0.25–0.5 mg/kg/dose q 6–8 h | ||
| Treprostinil | Starting dose: 2 ng/kg/min iv or SQ | ||
| Antireflux agents (enteral dosing) | Erythromycin | 2–4 mg/kg/dose q6–8h | |
| Lansoprazole | 0.5–1.0 mg/kg/dose BID | ||
| Metoclopramide | 0.1–0.2 mg/kg/dose q6–8 h | ||
| Omeprazole | 0.5–1.5 mg/kg/d | ||
| Ranitidine | 2–4 mg/kg/d divided q8–12 h |
MDI, metered dose inhaler; PRN, as needed.
Sildenafil or bosentan (an endothelin receptor antagonist) are frequently used for long-term therapy of PH in infants with BPD.43 Sildenafil, a highly selective type 5 phosphodiesterase inhibitor, augments cyclic guanosine monophosphate content in vascular smooth muscle and has been shown to benefit adults with PH as monotherapy and in combination with standard treatment regimens.44,45 In a study of infants with chronic lung disease and PH, prolonged sildenafil therapy was associated with improvement in PH by echocardiogram in most patients (88%) without significant adverse events.46 Although the time to improvement was variable, many patients were weaned from mechanical ventilator support and other PH therapies during the course of sildenafil treatment without worsening of PH.46 The recommended starting dose for sildenafil is 0.5 mg/kg/dose every 8 hours. If there is no evidence of systemic hypotension, this dose can be gradually increased over 2 weeks to achieve desired pulmonary hemodynamic effect or a maximum of 2 mg/kg/dose every 6–8 hours. Data on other agents, such as endothelin receptor antagonists and prostacyclin analogs, are limited in infants with BPD who fail to respond to other approaches.37 Recent studies suggest that chronic treprostinil infusion by subcutaneous delivery may provide additional benefit in infants with BPD and PH that is poorly responsive to other medications.47,48
Nutritional Management in sBPD
Infants with sBPD provide unique nutritional challenges partly because of periods of hypermetabolic states, increased work of breathing, growth suppression from chronic stress and inflammation, and chronic steroid or diuretic use. The goal of nutrition in BPD is to deliver adequate constituents that match the patient’s specific needs, but these needs change with time in infants with sBPD. Optimal nutrition requires an understanding of multiple factors that can affect somatic growth and also slow organ growth over time. Long-term pulmonary outcome studies highlight the persistence of abnormalities in lung development even into young adulthood, demonstrating the complexity of determining the nutritional state in these infants and defining their nutritional support requirements.
Unfortunately, data regarding optimal nutritional interventions in patients with sBPD are limited. It is known that infants with sBPD grow more poorly than preterm infants without BPD, and neonates with the slowest growth velocities are associated with a high risk for sBPD. Adequate nutrition, including micronutrient and vitamins A, D, and C supplementation, is likely critical for lung growth, development, function, and repair. The role of growth factors and their suppression by stress and inflammation have been shown to underlie the nutritional imbalance in sBPD, and monitoring of somatic growth by weight and length is essential. Despite improvements in nutritional intake and growth over the past decade, infants with sBPD often continue to demonstrate growth failure. Many studies only report weight gain, although there is strong recognition of the importance of linear growth. Better nutritional measures have also been shown to correlate with better neurodevelopmental outcomes in infants with BPD.49
Nutritional assessments should include a review of prenatal and past medical history, birth weight z scores, anthropometric data, medication exposure, biochemical data, and clinical status. Anthropometric data including measurements of weight, length, and head circumference are the most common methods used for monitoring postnatal growth and are compared with reference data. Individual patient data should be plotted and followed with an infant growth chart to better assess changes in growth trajectories. Although weight can be measured easily and accurately, it is influenced by fluid status and may not always reflect changes in lean body mass. Linear growth is widely regarded as the best measure of assessing adequacy of dietary intake and is associated with lean body mass accretion and organ growth and development. Measuring length in a precise and reproducible manner often poses a challenge. Use of a recumbent length board to measure crown-heel length should be a standard of practice in assessing linear growth in this patient population. Weight for length status should be assessed for proportionality to avoid developing overweight. Persistent weight gain at a rate that crosses growth chart percentiles without the same pattern demonstrated in linear growth warrants a re-evaluation in energy intake and is often associated with a change in the clinical status of the patient. Intrauterine growth retardation and/or small for gestational age are high-risk conditions for developing sBPD and may set the stage for persistent abnormal growth patterns in the postnatal period. Small for gestational age status has also been associated with an increased risk for PH in sBPD.50
Caloric demands are likely high to meet the metabolic requirements for growth and healing. This may be achieved with the use of highly fortified mother’s breast milk or calorically dense formula feedings. Consistent with American Academy of Pediatrics recommendations, mother’s breast milk should be used through the first 6 months of life. Energy intakes >130 kcal/kg/day may be required in these infants, particularly during the more unstable phase of BPD, whereas older infants in the more stable respiratory phase of BPD may require much less depending upon the level of activity and stability. Although excessive calories may increase CO2 production, past studies implicate a dietary imbalance of high carbohydrate to low fat that is particularly problematic in children and adults with chronic respiratory failure. Adequate protein intake is necessary to promote linear growth, lung growth, and repair of damaged tissue. Protein needs for the preterm infant are high, initially ranging from 3.5 to 4.2 g/kg/day. At present, there are no specific protein recommendations for infants with sBPD. Providing adequate amounts of protein for corrected gestational age and perhaps aiming toward the higher range may be beneficial to compensate for periods of catabolic, hyper-metabolic states when protein turnover is high. Medications, such as corticosteroids, also negatively impact protein accretion and body composition; however, no studies are available for nutritional guidance in this population. Individualized nutritional support with close monitoring and titration is important to avoid over- or underfeeding in this population.
Although high fluid intake early in the NICU course of preterm infants is associated with increased risk for BPD, there are no studies that directly address the role of fluid restriction in established BPD. Fluid intake for subjects with sBPD is usually in the range of 130–150 mL/kg/day, and it should be kept in mind that fluid needs decrease over the first year of life. It is reasonable to use conservative fluid intake in patients with sBPD, however, adequate delivery of nutrients and water to meet both nutritional and physiological needs must be assured. Adequate respiratory support is important from a nutritional standpoint in these infants because cyanotic spells, increased work of breathing, and air hunger place these infants at high risk for linear growth failure because of energy expenditure and stress-induced growth suppression.
Metabolic bone disease (MBD), also called “osteopenia” or “rickets of prematurity,” is a common finding in preterm infants, which is more common and severe in extremely preterm infants.51–58 After preterm birth several postnatal factors contribute to the risk for MBD, including parenteral and enteral supplements of calcium and phosphorus that cannot match in utero sources,59 placement of preterm infants in the NICU in physical environments that restrict movement,60 and exposure to multiple drug therapies that may affect overall bone health.61–63 In addition, severe respiratory insufficiency may exacerbate feeding intolerance in infants with sBPD, who then may require prolonged parental nutrition, which provides inadequate calcium and phosphorus and may also lead to aluminum accumulation that can have a negative effect on bone formation.64 The typical biochemical picture of MBD includes normal serum calcium, low serum phosphorus, and high serum alkaline phosphatase concentrations.65–67 Radiographs are an inefficient diagnostic tool for MBD and will not detect decreases of bone mineralization less than ~20%–30%.68 Bone density scans more accurately reflect bone demineralization, but a bone density scan involves radiation exposure, is not a portable device, and standards for preterm infants are lacking.69,70 Although short-term effects of poor bone health seem to resolve with time, long-term stunting effects have been documented in these infants into their teenage years.63
Aspiration is a potential risk factor for persistent lung injury in the infant with sBPD. Common sources of aspiration may include oral intake via dysfunctional swallowing,71 gastroesophageal reflux,72 a patient’s own oral secretions, and potentially increased upper respiratory secretions during infections. Limited recurrent aspiration may result in chronic respiratory symptoms, such as coughing, wheezing, or tachypnea (although aspiration in preterm infants can frequently be “silent”);73 desaturations or hypercarbia;74 or poor weight gain. More acute episodes of aspiration may result in pneumonia, tracheitis, or bronchitis, often accompanied by an escalation of respiratory support. Although aspiration in the preterm infant is often a function of delayed swallowing function maturation,71,75 other factors to consider include neurologic insults or congenital or acquired airway anomalies, such as vocal cord paresis or laryngeal clefts. Evaluation and ongoing assessments in the NICU by an experienced occupational or speech therapist is strongly recommended for the infant with sBPD. Diagnostic tests for dysphagia or reflux lack sensitivity and specificity for aspiration risk in the infant with sBPD,71 thus, there remains controversy regarding surgical treatment. However, in the appropriate clinical scenario, placement of gastrostomy tubes with or without fundoplication may be warranted.76
Independently, feeding tubes may need to be considered in infants who orally feed safely, but do not ingest enough calories for appropriate growth.
Pulmonary exacerbations because of respiratory syncytial virus (RSV) infections, reactive airways disease, and other problems may acutely alter swallowing function77 and baseline gastrointestinal motility in the preterm infant with sBPD. Gastric distension may potentially alter respiratory mechanics, thus, impairing ventilation. Impaired motility may increase GERD, and hence, the risk for aspiration. In addition, increased tachypnea can preclude oral intake in patients who orally feed. Strategies to consider during acute exacerbations include the use of intravenous fluids or enteral rehydration solutions for gastrointestinal rest, time-limited placement of a nasogastric tube for patients who typically eat/drink orally, and/or the use of continuous tube feeding.
Summary of Nutritional Management of sBPD
Nutritional success in patients with BPD is complicated by many factors including lack of prospective data in this population, likely changing needs of the patient over time, growth suppressive states of the patient, comorbidities, and high metabolic needs at times. However, certain goals and concepts should be employed to strive for the best outcomes. Linear growth should be measured accurately and followed closely using appropriate standardized growth curves. Weight for length should also be followed closely, seeking an ideal target goal of ~50%. Titrations in nutrition should be considered at least weekly in concert with a dietician familiar with this population. Strict attention should be given to the nonpharmacologic support and comfort of the infant to minimize stress. Furthermore, adequate respiratory support that allows for developmental activities and play must be provided because this encourages a “progrowth” state.
Pharmacologic Therapy for sBPD
Diverse respiratory system abnormalities contribute to altered lung function in different compartments: central airways, bronchioles, and distal lung airspace or parenchyma. In addition, respiratory system problems regarding central respiratory control, respiratory muscle function, chest wall compliance, and others also contribute to BPD severity. Each infant with sBPD may manifest a greater dysfunction in one part of the respiratory system than others, and the role of any given drug therapy depends in part on the physiologic target of that therapy.
However, there is a paucity of data on the effectiveness of medications for treating infants who have developed sBPD. The Cochrane Neonatal Systematic Reviews Library contains over 300 reviews with BPD prevention or treatment as the focus of ~35 of those reviews, the majority of which described studies with enrollment of infants early in the postnatal course before the development of sBPD. Evidence supporting the use of pharmacologic agents is limited and largely based on case reports, case series, and clinical experience, rather than randomized controlled studies. All should be considered in the broader context of respiratory support, including invasive and noninvasive ventilation, tracheostomy placement, and nutritional management. However, these medications have been formally assessed for only short-term surrogate outcomes, and marked center-to-center variability in drug use exists.9 This problem also leaves unanswered questions about the final risk-to-benefit ratio, which remains largely unknown for many agents. In Table V, we have outlined the medications used in sBPD with current dosing regiments described below, but these recommendations are based on anecdotal usage as there is currently no evidence to guide their use in sBPD.
Large Airway Disease
Some infants with sBPD have dynamic airways obstruction because of tracheomalacia or bronchomalacia, as well as possible fixed lesions, such as subglottic or tracheal stenosis, granulomas, complete cartilage rings, and other central airway problems.
Beyond manipulation of PEEP and surgical treatment of specific endobronchial lesions, effective therapies are largely lacking for infants with many central airway problems. Attempts to lower the work of breathing by using less dense gases such as helium-oxygen mixtures (heliox) or treatment of severe tracheomalacia by aortopexy have produced mixed results.
Small Airway Disease
The approach to reactive small airway disease is generally analogous to childhood asthma and includes systemic or inhaled corticosteroids (fluticasone or budesonide), bronchodilators (mainly albuterol, levalbuterol, or ipratropium), and mucolytic therapies. Bronchodilators can lower airway resistance and improve respiratory compliance, however, whether these effects provide sustained benefit in infants with sBPD is uncertain. The use of bronchodilators in sBPD is widely variable between centers.33 Albuterol and levalbuterol are adrenergic agonists that are used on various schedules to reduce bronchospasm. Clinically, there can be variability in response to bronchodilators, which may be a result of genetic variability in β2 adrenergic receptors as seen in children with asthma. However, this variability in response has not been systematically assessed in patients with sBPD. Ipratropium, an anticholinergic agent, is used by some practitioners for its putative effects in reducing bronchoconstriction and excess secretions. Inhaled anticholinergic agents may decrease gastrointestinal motility and result in dry/thickened secretions. Ipratropium is often used in combination with albuterol for synergism. However, no trials have been conducted to determine if combination therapy vs monotherapy is more efficacious. The use of inhaled dornase has been suggested for patients with sBPD with thick secretions, although never studied in randomized controlled trials. Acute exacerbation triggered by inflammation and infection at the lower and medium airways has been treated with cyclic inhaled tobramycin, but no definitive trials of its use in sBPD have been published. Other antibiotics, such as azithromycin, may also have putative anti-inflammatory benefits.
Systemic or inhaled steroid administration is commonly used for treating or preventing pulmonary exacerbations in sBPD, sometimes on a daily or every other day dosing schedule. Steroids may have a variety of beneficial effects as observed in other populations and diseases including enhanced surfactant production, decreased inflammation, decreased airway edema, decreased capillary leakage, and decreased lung fibrosis. Use of systemic steroids is not benign as chronic use at doses typically used in the population with sBPD may result in decreased growth, hypertension, bone demineralization, and developmental delay. The appropriate dose, time, and type of steroid need to be evaluated to maximize benefits and minimize risk. Individualizing steroid administration also will require better ability to predict the clinical course of sBPD to determine when and how to administer steroids in this population.
Other anti-inflammatory medications, such as the leukotriene receptor antagonists montelukast or zafirlukast, have been suggested as potentially beneficial in sBPD based on their benefits in asthma and allergies. Montelukast may have benefit in its ability to provide bronchodilation and to decrease inflammation, although data in sBPD are limited.78
Parenchymal or Distal Airspace Disease
Disease of the distal lung includes decreased alveolarization, dysmorphic vascular growth with hypertensive remodeling, dilated lymphatics, prominent intrapulmonary shunt vessels, and pulmonary edema. Diuretics (including furosemide, spironolactone, and chlorothiazide) either alone or in combination, are commonly used on the theory that they are combating the propensity of infants with BPD to develop pulmonary edema. Although short-term results of use of these medications have been favorable for improving lung compliance, no clinically significant outcomes (such as changes in mortality, duration of mechanical ventilation, oxygen dependence, length of hospital stay, etc) have been reported, and the use of diuretics in sBPD is highly variable between centers.34 Diuretics can have adverse effects, including hypercalciuria, nephrocalcinosis, metabolic alkalosis, and striking decreases in serum potassium, phosphate, and other electrolytes. Nebulized furosemide has been shown to provide short-term improvement in lung mechanics without systemic diuretic effects, but it has never been studied as a long-term therapy.79
Systemic Hypertension
Systemic hypertension can also occur in infants with sBPD, which may be related to corticosteroid therapy, marked neurohormonal stimulation, renal disease, or other factors.80 Echocardiograms can reveal left ventricular hypertrophy or asymmetric septal hypertrophy. Hypertension is treated empirically with antihypertensive medications and usually responds to therapy over time.
Antireflux Medications
Gastric propulsive agents and gastric acid secreting blockers are often used to reduce the risk of aspiration, but this use has not been confirmed in definitive trials. In fact, the routine use of these agents has been described as unnecessary and potentially harmful in neonatology.81
Respiratory Muscle Stimulants
The use of respiratory muscle stimulants, such as caffeine and doxapram, has been suggested by a few clinicians based on theoretical improvements in lung function that could potentially obviate the need for prolonged assisted ventilation or tracheostomy. However, there is no empirical evidence available addressing this theoretical use of respiratory muscle stimulants in established sBPD.
Summary of Pharmacology in sBPD
There are few proven treatments for the management of sBPD. Given the number of these infants and young children, the severity of their respiratory condition, and their guarded long-term pulmonary function outlook, phase 2 and eventually phase 3 clinical trials are warranted and desperately needed. Given the new combination therapies used for adults with chronic obstructive pulmonary disease, we suggest that trials of the most promising of these therapies be undertaken in combination with current therapies beginning at 36–40 weeks for infants with sBPD. The clinically relevant primary outcomes for these investigations are to reduce hospital days and reduce severity of pulmonary dysfunction, perhaps including reduced need for tracheotomy.
Transitional and Postdischarge Care of Infants with sBPD
The primary criteria, steps, and goals for discharge should be met so that the infant/child with sBPD can live and thrive at home in a safe environment while minimizing recurrent hospitalizations. Improving the outcomes of infants with sBPD involves well-coordinated, interdisciplinary teams that include links between inpatient and ambulatory care (Table VI). Infants with sBPD have longer lengths of initial hospitalizations and are at increased risk for re hospitalizations because of respiratory illnesses after discharge.82 Furthermore, BPD is associated with impaired postnatal lung growth,83 and in the absence of adequate recovery particularly during the first 2 years of life, these children are at increased risk for chronic obstructive pulmonary disease in later life. Comorbidities can further contribute to disease severity,84–88 and failure to recognize or adequately treat comorbidities can contribute to worse outcomes. Management of infants with sBPD in the home environment requires a healthcare provider who is accessible to the family, can coordinate patient care among the various subspecialists, and can recognize rapid changes in the health of the infant. Critical to outpatient care is a subspecialist with respiratory expertise who may be either a neonatologist or pulmonologist with expertise in the older child with BPD and comfortable with respiratory medication management, and oxygen and ventilator weaning and titration. This provider should be available for telephone consultation for families and other medical professionals for questions regarding respiratory management at all times. Subspecialty providers should be also comfortable counseling families on what to expect and how to avoid exposures that may worsen outcomes such as daycare or secondhand smoke.89–92 Outpatient clinic capacity should be sufficient to handle regular clinic visits with visit frequency dependent on the child’s needs, which could range from weekly to every 3 months especially during the first 2 years of life, a period of rapid postnatal lung growth and during which children with BPD are more likely to be hospitalized for respiratory illnesses.
Table VI.
Composition of interdisciplinary teams for the management of sBPD
| Disciplines | Inpatient (NICU/PICU) | Outpatient |
|---|---|---|
| Team leader/coordinator | Neonatologist, pulmonologist, critical care physician, hospitalist, or nurse practitioner | Primary care physician |
| Respiratory | Neonatologist or pulmonologist, and respiratory therapists | Neonatologist or pulmonologist |
| Cardiac | Cardiologist | Cardiologist |
| Airway | Otolaryngologist | Otolaryngologist |
| Gastrointestinal | Gastroenterologist and/or nutritionist | Gastroenterologist and/or nutritionist |
| Development | Neonatologist and therapists (also child life) | Neonatologist or developmental pediatrician, and therapists |
| Nursing | Nurses | Home nurses |
| Social | Social worker/and/or chaplain | Social worker |
PICU, pediatric intensive care unit.
Discharge Preparation
The risk of death after discharge from the NICU is likely highest in patients with more severe respiratory disease, patients with tracheostomies, and patients with a history of pulmonary hypertension. Preterm infants are at higher risk for death at home compared with full term infants, and infants with BPD may have abnormal responses to hypoxia, placing them at higher risk for adverse events at home.93 For preterm infants with PH, mortality has been reported to be as high as 12%–38%.24 In infants and children with sBPD, the risk of death in association with home mechanical ventilation has reported to be as high as 19%.94 In addition to mortality, infants with BPD are subject to significant morbidities. Various studies have reported that between 49% and 58% of infants required readmission within the first two years of life.95–97
Adequate caregiver training is essential to address outpatient needs of a child with sBPD and is perhaps the most important part of a safe discharge to home, as some of the morbidity and mortality following discharge may be preventable. Before discharge, the team should establish a BPD action plan (Table VII). Caregivers need to demonstrate proficiency in all aspects of care. The home caregiver must be able to recognize signs of respiratory distress and illness in their child and to address emergency health issues in the home environment, such as emergency tracheostomy changes, need for inhalational medications, and/or equipment failure.98 Poor adherence to respiratory medications was associated with an increased risk of emergency department visits and rescue inhaled beta-agonist use and increased activity limitations in infants with sBPD.99 The same study reported that medication non-adherence was associated with caregiver concerns regarding medication efficacy and side effects rather than sociodemographic or clinical factors, therefore, the risks and benefits of each therapy should be explained to the caregiver. Overall, it is highly advisable to have at least 2 caregivers trained for the appropriate cares, especially for an infant or child with a tracheostomy.98
Table VII.
sBPD action plan
| When to give PRN meds |
| When to suction or change the tracheostomy tube |
| When to seek telephone consultation |
| When to go to the emergency department |
| When to call 911 |
| Summary of previous and active medical issues |
| List of healthcare providers |
| List of medications including dosing and frequency |
| Current oxygen/ventilator settings |
| Summary of helpful information for caregivers and healthcare providers not familiar with the child |
In addition to family caregivers, some infants may require home nursing care as well. Determination of home nursing hours or visits should be finalized and discussed with the caregiver and payers prior to discharge. Continuous monitoring by trained caregivers 24 hours per day, 7 days per week is required, including substantial home nursing presence.98 Lastly, anticipatory support from social work should also address potential insurance concerns that may arise from medical costs in the outpatient setting, travel, and time commitments required to attend outpatient visits, as well as discussions of the adjustment process for caring for a chronically ill child at home, including issues of social isolation and management of communication issues with multiple health care professionals.100
Discharge of the Tracheostomy/Ventilator-Dependent Infant with sBPD
The child with chronic respiratory failure who is dependent on a tracheostomy and ventilator can create specific challenges for a successful transition to home. Infants and young children with tracheostomies with or without home ventilation are at increased risk for mortality because of their underlying lung disease and the potential for unobserved plugging of their tracheostomy tube or disconnection from the ventilator leading to cardiopulmonary arrest. In a retrospective study, Edwards et al101 reported 47 deaths (21% mortality rate) among 228 children on home ventilation with 49% of deaths being unexpected, including 19% of deaths being related to tracheostomy complications. Although there are no guidelines or consensus statements regarding the outpatient management of sBPD at the current time, the 2008 the American Academy of Pediatrics Committee on Fetus and Newborn updated a 1998 policy statement discussing the hospital discharge of the high-risk neonate, much of which is applicable to infants with sBPD.102 Approaches to the home care of ventilated infants was recently addressed by a consensus group of the American Thoracic Society.98
In keeping with the imperative to train family members as caregivers, a comprehensive checklist that addresses specific issues that may arise with tracheostomy and continue after discharge should be created and completed to address the personal needs of each child and caregiver prior to discharge. Lastly, a recent study has shown that a well-organized interdisciplinary team that includes inpatient and outpatient components can reduce duration of hospitalization and enhance long-term survival.103
Strategies for Home Ventilation
For infants who are ventilator dependent with type 2 sBPD, home monitoring with both a pulse oximeter and a cardiorespiratory monitor should be considered.98 The ability to monitor saturations is essential for adjusting ventilator and oxygen support in the outpatient setting, but continuous oxygen saturation monitoring is often not able to be maintained secondary to limitations on provision of pulse oximeter probes and by skin breakdown or burns when using nondisposable probes. However, use of a pulse oximeter is indicated when infants are not directly observed. Subspecialty providers should be comfortable with weaning children from chronic ventilation. Guidelines for weaning from chronic ventilation are uncertain, but recent recommendations from the American Thoracic Society suggest that children requiring chronic ventilation should have a comprehensive medical home comanaged by a generalist and a respiratory subspecialist.98 Polysomnography is more sensitive than clinic-based assessments for weaning support and may be useful in determining levels or types of respiratory support and determining optimal timing for weaning off of home ventilation.104 When polysomnography is not available, alternative approaches, such as pulse oximetry and end tidal CO2 monitoring, should be considered. In some cases, inpatient observation may be required for ventilator weaning, escalation of support, or capping of tracheostomies for safety purposes.
Strategies for Home Oxygen Therapy
Children with sBPD on oxygen should be monitored closely in the outpatient setting, and although the use of home pulse oximeters for children on oxygen is often necessary, it can cause many false alarms and stress caregivers. When weaning off supplemental oxygen, the basic tenet is that pulse oximetry should be used and oxygen saturations should be maintained at or above 92%, particularly if pulmonary hypertension is present.37 In the home setting, only 100% oxygen can be delivered via nasal cannula, and, therefore, flow rate is titrated during weaning in the outpatient setting. Few studies have been performed to determine the appropriate flow rate from which oxygen can be safely discontinued. In a small prospective study, Simoes et al105 demonstrated that infants on 20 mL/kg or less of oxygen were more successful at weaning to room air. A recent study of 20 pediatric pulmonary programs in the US found little consensus regarding weaning strategies from supplemental oxygen, but most used prolonged home assessments of oxygenation by nocturnal pulse oximetry.106 It is important to note that in addition to oxygen saturation, achieving adequate somatic growth and prevention or resolution of the signs of PH are important outcomes to consider prior to discontinuing supplemental oxygen therapy. When growth is poor, the use of nocturnal oxygenmay need to be extended. In summary, for many infants with sBPD, safe outpatient use of supplemental oxygen can allow infants to continue to progress in the home environment rather than in the NICU. Further research is needed to determine optimal weaning strategies.
Monitoring Lung Function
Infant pulmonary function testing (PFTs) can be useful in assessing severity of lung dysfunction and response to treatment. Limitations to infant PFTs include use of sedation and lack of availability at many tertiary care centers. All children with sBPD should undergo regular PFTs when they are able to follow directions because abnormalities in pulmonary function are often missed or underestimated in children with BPD. Small airway dysfunction manifested by hyperinflation and reactive and fixed small airway obstruction has been reported in school age children with a history of BPD.107 Oxygen desaturation with exercise or with lower respiratory tract illnesses can frequently occur in children with sBPD.108
Management of Respiratory Viruses and Lower Respiratory Illnesses
Respiratory infections can be life-threatening in children with sBPD and are more likely to result in hospitalization, including a high rate of pediatric intensive care unit admissions. In a cohort from the United Kingdom, 73% of infants with BPD required at least 1 readmission in the first 2 years of life and 27% had 3 or more hospitalizations.109 Historically, most admissions in children with BPD have been due to lower respiratory tract infections (LRTIs) secondary to RSV, but human metapneumovirus and rhinovirus LRTIs are common.110–112 The use of palivizumab, a monoclonal antibody against the F glycoprotein in RSV, has reduced the morbidity and mortality associated with RSV infection.111,112 Because respiratory infections can be more severe in children with BPD, these children require closer monitoring with the onset of viral symptoms. Infants who have recently weaned off supplemental oxygen are more likely to become hypoxic with viral exposures.113 Although management is focused on supportive care and monitoring to ensure that the child remains well saturated and well hydrated, a trial of inhaled bronchodilators or inhaled steroids may be helpful.
Although bronchiolitis usually is a self-limited illness, infants with BPD who require hospitalization for bronchiolitis are at increased risk for wheezing with subsequent viral exposures. Thus, the use of preventative measures is critical in this high-risk population. Vigilance in receiving all doses of palivizumab during the typical 6-month window of RSV infection reduces the risk of severe RSV disease, but efforts should be made to limit the impact of other viral pathogens. The use of hand hygiene is important, especially with siblings who enter the home from school or preschool exposures. The increased severity of LRTIs in infants with sBPD may also contribute to the increased prevalence of cough and wheezing that persists beyond the acute infection.
Neurodevelopmental Outcomes
Children with sBPD should be evaluated by experts in child development and be enrolled in an early infant intervention program that can provide home therapy visits (when possible) to address developmental issues common in pretermchildren. Nonoxygen-dependent children with BPD have been reported to catch up developmentally at 2 years of age, whereas those initially discharged home on oxygen have been reported to catch up by 4 years of age,114 but infants who required mechanical ventilation at 36 weeks PMA were 6 times more likely to have quadriparesis compared with infants who required only supplemental oxygen at 36 weeks PMA.115 Close neurodevelopmental follow-up can provide parents with reassurance and education on appropriate developmental milestone attainment, specific to the child’s chronologic age and degree of prematurity.
Conclusions
Despite major improvement in the care and outcomes of extremely preterminfants over the past few decades, some infants develop especially severe chronic lung disease that includes those who require prolonged ventilator support (or type 2 sBPD). More basic and translational research is needed to better understand the pathobiology of sBPD, including mechanisms that cause more severe disease than in other preterm infants, as well as epidemiologic and outcomes research to elucidate risk factors and patterns that may lead to earlier identification of at risk infants (Table II). Finally, organized approaches for clinical interventions and trials that involve multiple centers are necessary due to the relatively small numbers of sBPD at each site. Such approaches may help provide better information to develop new care guidelines to enhance the long-term outcomes of infants with sBPD.
Glossary
- BPD
Bronchopulmonary dysplasia
- CCBs
Calcium channel blockers
- CPAP
Continuous positive airway pressure
- FiO2
Fraction of inspired oxygen
- GERD
Gastroesophageal reflux disease
- iNO
Inhaled nitric oxide
- LRTIs
Lower respiratory tract infections
- MBD
Metabolic bone disease
- NICU
Neonatal intensive care unit
- NIH
National Institutes of Health
- PEEP
Positive end expiratory pressure
- PFTs
Pulmonary function testing
- PH
Pulmonary hypertension
- PMA
Postmenstrual age
- RSV
Respiratory syncytial virus
- sBPD
Severe BPD
- Ti
Inspiratory time
- Vt
Tidal volume
Appendix
Additional members of the BPD Collaborative include: Children’s Mercy, Kansas City, KC:
Charisse Lachica, MD, Winston Manimtim, MD, Michael F. Nyp DO MBA, Tamorah Lewis, MD, PhD; Children’s Hospital Colorado, Aurora, CO: Jody Thrasher, RN, Alicia Grenolds, PNP; Childrens Hospital of Philadelphia, Philadelphia, PA: Howard Panitch, MD; Erik A. Jensen, MD; David A. Munson, MD; William W. Fox, MD; Jason Z. Stoller, MD, Kathryn Maschhoff, MD; Kristen J. McKenna, MD; Nicolas Bamat, MD; Vanderbilt University, Nashville, TN: Steven Steele, BA; Brown University, Providence, RI: Shukla Khushbu, MD; Baylor University, Houston, TX: Sushma Nuthakki, MD; Nationwide Childrens Hospital, Columbus, OH: Carl H. Backes, MD; Sudarshan Jadcherla, MD; Chandar Ramanathan, MD; Kris Reber, MD; Margaret Holston, RN, BSN; Nick Foor; Daniel Malleske, MD; Joe DiMaggio Children’s Hospital, Hollywood, FL: Bruce Schulman, MD.
Footnotes
The other authors declare no conflicts of interest.
References
- 1.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314:1039–51. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poindexter BB, Feng R, Schmidt B, Aschner J, Ballard RA, Hamvas A, et al. Comparisons and limitations of current definitions of bronchopulmonary dysplasia for the prematurity and respiratory outcomes program. Ann Am Thorac Soc. 2015;12:1822–30. doi: 10.1513/AnnalsATS.201504-218OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McEvoy CT, Jain L, Schmidt B, Abman S, Bancalari E, Aschner JL. Bronchopulmonary dysplasia: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann Am Thorac Soc. 2014;11(Suppl 3):S146–53. doi: 10.1513/AnnalsATS.201312-424LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 5.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–60. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 6.EXPRESS Group. Incidence of and risk factors for neonatal morbidity after active perinatal care: extremely preterm infants study in Sweden (EXPRESS) Acta Paediatr. 2010;99:978–92. doi: 10.1111/j.1651-2227.2010.01846.x. [DOI] [PubMed] [Google Scholar]
- 7.Farstad T, Bratlid D, Medbo S, Markestad T Norwegian Extreme Prematurity Study Group. Bronchopulmonary dysplasia - prevalence, severity and predictive factors in a national cohort of extremely premature infants. Acta Paediatr. 2011;100:53–8. doi: 10.1111/j.1651-2227.2010.01959.x. [DOI] [PubMed] [Google Scholar]
- 8.Padula MA, Grover TR, Brozanski B, Zaniletti I, Nelin LD, Asselin JM, et al. Therapeutic interventions and short-termoutcomes for infants with severe bronchopulmonary dysplasia born at <32 weeks’ gestation. J Perinatol. 2013;33:877–81. doi: 10.1038/jp.2013.75. [DOI] [PubMed] [Google Scholar]
- 9.Guaman MC, Gien J, Baker CD, Zhang H, Austin ED, Collaco JM. Point prevalence, clinical characteristics, and treatment variation for infants with severe bronchopulmonary dysplasia. Am J Perinatol. 2015;32:960–7. doi: 10.1055/s-0035-1547326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boroughs D, Dougherty JA. Decreasing accidental mortality of ventilator-dependent children at home: a call to action. Home Healthc Nurse. 2012;30:103–11. doi: 10.1097/NHH.0b013e3182429243. quiz 12–3. [DOI] [PubMed] [Google Scholar]
- 11.Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183:1715–22. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Natarajan G, Pappas A, Shankaran S, Kendrick DE, Das A, Higgins RD, et al. Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev. 2012;88:509–15. doi: 10.1016/j.earlhumdev.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang JR, Seedorf GJ, Muehlethaler V, Walker DL, Markham NE, Balasubramaniam V, et al. Moderate postnatal hyperoxia accelerates lung growth and attenuates pulmonary hypertension in infant rats after exposure to intra-amniotic endotoxin. Am J Physiol Lung Cell Mol Physiol. 2010;299:L735–48. doi: 10.1152/ajplung.00153.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhandari V, Bizzarro MJ, Shetty A, Zhong X, Page GP, Zhang H, et al. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006;117:1901–6. doi: 10.1542/peds.2005-1414. [DOI] [PubMed] [Google Scholar]
- 15.Lavoie PM, Pham C, Jang KL. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics. 2008;122:479–85. doi: 10.1542/peds.2007-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rezvani M, Wilde J, Vitt P, Milaparambil B, Grychtol R, Krueger M, et al. Association of a FGFR-4 gene polymorphism with bronchopulmonary dysplasia and neonatal respiratory distress. Dis Markers. 2013;35:633–40. doi: 10.1155/2013/932356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koroglu OA, Onay H, Cakmak B, Bilgin B, Yalaz M, Tunc S, et al. Association of vitamin D receptor gene polymorphisms and bronchopulmonary dysplasia. Pediatr Res. 2014;76:171–6. doi: 10.1038/pr.2014.63. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen GL, Dahl M, Tan Q, Bendixen C, Holmskov U, Husby S. Surfactant protein-D-encoding gene variant polymorphisms are linked to respiratory outcome in premature infants. J Pediatr. 2014;165:683–9. doi: 10.1016/j.jpeds.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Li W, Liu W. GSTM1 and GSTT1 gene polymorphisms as major risk factors for bronchopulmonary dysplasia in a Chinese Han population. Gene. 2014;533:48–51. doi: 10.1016/j.gene.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Winters AH, Levan TD, Vogel SN, Chesko KL, Pollin TI, Viscardi RM. Single nucleotide polymorphism in toll-like receptor 6 is associated with a decreased risk for ureaplasma respiratory tract colonization and bronchopulmonary dysplasia in preterm infants. Pediatr Infect Dis J. 2013;32:898–904. doi: 10.1097/INF.0b013e31828fc693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali S, Hirschfeld AF, Mayer ML, Fortuno ES, Corbett N, Kaplan M, et al. Functional genetic variation in NFKBIA and susceptibility to childhood asthma, bronchiolitis, and bronchopulmonary dysplasia. J Immunol. 2013;190:3949–58. doi: 10.4049/jimmunol.1201015. [DOI] [PubMed] [Google Scholar]
- 22.Allen J, Zwerdling R, Ehrenkranz R, Gaultier C, Geggel R, Greenough A, et al. Statement on the care of the child with chronic lung disease of infancy and childhood. Am J Respir Crit Care Med. 2003;168:356–96. doi: 10.1164/rccm.168.3.356. [DOI] [PubMed] [Google Scholar]
- 23.Gien J, Kinsella JP, Grenolds A, Thrasher J, Abman SH, Baker CD. Retrospective analysis of an interdisciplinary care program intervention on survival of infants with ventilator-dependent BPD. Am J Perinatol. 2016 doi: 10.1055/s-0036-1584897. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collaco JM, Romer LH, Stuart BD, Coulson JD, Everett AD, Lawson EE, et al. Frontiers in pulmonary hypertension in infants and children with bronchopulmonary dysplasia. Pediatr Pulmonol. 2012;47:1042–53. doi: 10.1002/ppul.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Support Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362:1970–9. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358:700–8. doi: 10.1056/NEJMoa072788. [DOI] [PubMed] [Google Scholar]
- 27.Dunn MS, Kaempf J, de Klerk A, de Klerk R, Reilly M, Howard D, et al. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics. 2011;128:e1069–76. doi: 10.1542/peds.2010-3848. [DOI] [PubMed] [Google Scholar]
- 28.Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole WK, Tyson JE, et al. Extremely low birthweight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J Pediatr. 2005;146:798–804. doi: 10.1016/j.jpeds.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 29.Abman SH, Nelin LD. Management of the infant with severe bronchopulmonary dysplasia. In: Bancalari E, editor. The newborn lung: neonatology questions and controversies. Philadelphia (PA): Elsevier Saunders; 2012. pp. 407–25. [Google Scholar]
- 30.Jarriel WS, Richardson P, Knapp RD, Hansen TN. A nonlinear regression analysis of nonlinear, passive-deflation flow-volume plots. Pediatr Pulmonol. 1993;15:175–82. doi: 10.1002/ppul.1950150309. [DOI] [PubMed] [Google Scholar]
- 31.Castile RG, Nelin LD. Lung function, structure and the physiologic basis for mechanical ventilation of infants with established BPD. In: Abman SH, editor. Bronchopulmonary dysplasia. New York (NY): Informa Healthcare; 2010. pp. 328–46. [Google Scholar]
- 32.Bolivar JM, Gerhardt T, Gonzalez A, Hummler H, Claure N, Everett R, et al. Mechanisms for episodes of hypoxemia in preterm infants undergoing mechanical ventilation. J Pediatr. 1995;127:767–73. doi: 10.1016/s0022-3476(95)70171-0. [DOI] [PubMed] [Google Scholar]
- 33.Slaughter JL, Stenger MR, Reagan PB, Jadcherla SR. Inhaled bronchodilator use for infants with bronchopulmonary dysplasia. J Perinatol. 2015;35:61–6. doi: 10.1038/jp.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slaughter JL, Stenger MR, Reagan PB. Variation in the use of diuretic therapy for infants with bronchopulmonary dysplasia. Pediatrics. 2013;131:716–23. doi: 10.1542/peds.2012-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malkar MB, Gardner WP, Mandy GT, Stenger MR, Nelin LD, Shepherd EG, et al. Respiratory severity score on day of life 30 is predictive of mortality and the length of mechanical ventilation in premature infants with protracted ventilation. Pediatr Pulmonol. 2015;50:363–9. doi: 10.1002/ppul.23020. [DOI] [PubMed] [Google Scholar]
- 36.Shepherd EG, Knupp AM, Welty SE, Susey KM, Gardner WP, Gest AL. An interdisciplinary bronchopulmonary dysplasia program is associated with improved neurodevelopmental outcomes and fewer rehospitalizations. J Perinatol. 2012;32:33–8. doi: 10.1038/jp.2011.45. [DOI] [PubMed] [Google Scholar]
- 37.Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, et al. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation. 2015;132:2037–99. doi: 10.1161/CIR.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 38.Mourani PM, Sontag MK, Younoszai A, Miller JI, Kinsella JP, Baker CD, et al. Early pulmonary vascular disease in preterminfants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2014:191. doi: 10.1164/rccm.201409-1594OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics. 2008;121:317–25. doi: 10.1542/peds.2007-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mourani PM, Ivy DD, Gao D, Abman SH. Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2004;170:1006–13. doi: 10.1164/rccm.200310-1483OC. [DOI] [PubMed] [Google Scholar]
- 41.Johnson CE, Beekman RH, Kostyshak DA, Nguyen T, Oh DM, Amidon GL. Pharmacokinetics and pharmacodynamics of nifedipine in children with bronchopulmonary dysplasia and pulmonary hypertension. Pediatr Res. 1991;29:500–3. doi: 10.1203/00006450-199105010-00017. [DOI] [PubMed] [Google Scholar]
- 42.Brownlee JR, Beekman RH, Rosenthal A. Acute hemodynamic effects of nifedipine in infants with bronchopulmonary dysplasia and pulmonary hypertension. Pediatr Res. 1988;24:186–90. doi: 10.1203/00006450-198808000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Mourani PM, Abman SH. Pulmonary Hypertension and vascular abnormalities in bronchopulmonary dysplasia. Clin Perinatol. 2015;42:839–55. doi: 10.1016/j.clp.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–57. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 45.Sitbon O, Sattler C, Bertoletti L, Savale L, Cottin V, Jais X, et al. Initial dual oral combination therapy in pulmonary arterial hypertension. Eur Respir J. 2016;47:1727–36. doi: 10.1183/13993003.02043-2015. [DOI] [PubMed] [Google Scholar]
- 46.Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr. 2009;154:379–84. 384.e1–2. doi: 10.1016/j.jpeds.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaidi AN, Dettorre MD, Ceneviva GD, Thomas NJ. Epoprostenol and home mechanical ventilation for pulmonary hypertension associated with chronic lung disease. Pediatr Pulmonol. 2005;40:265–9. doi: 10.1002/ppul.20238. [DOI] [PubMed] [Google Scholar]
- 48.Ferdman DJ, Rosenzweig EB, Zuckerman WA, Krishnan U. Subcutaneous treprostinil for pulmonary hypertension in chronic lung disease of infancy. Pediatrics. 2014;134:e274–8. doi: 10.1542/peds.2013-2330. [DOI] [PubMed] [Google Scholar]
- 49.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–61. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 50.Check J, Gotteiner N, Liu X, Su E, Porta N, Steinhorn R, et al. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J Perinatol. 2013;33:553–7. doi: 10.1038/jp.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viswanathan S, Khasawneh W, McNelis K, Dykstra C, Amstadt R, Super DM, et al. Metabolic bone disease: a continued challenge in extremely low birth weight infants. JPEN J Parenter Enteral Nutr. 2014;38:982–90. doi: 10.1177/0148607113499590. [DOI] [PubMed] [Google Scholar]
- 52.Backstrom MC, Kuusela AL, Maki R. Metabolic bone disease of prematurity. Ann Med. 1996;28:275–82. doi: 10.3109/07853899608999080. [DOI] [PubMed] [Google Scholar]
- 53.Lyon AJ, McIntosh N, Wheeler K, Williams JE. Radiological rickets in extremely low birthweight infants. Pediatr Radiol. 1987;17:56–8. doi: 10.1007/BF02386596. [DOI] [PubMed] [Google Scholar]
- 54.Masel JP, Tudehope D, Cartwright D, Cleghorn G. Osteopenia and rickets in the extremely low birth weight infant–a survey of the incidence and a radiological classification. Australas Radiol. 1982;26:83–96. doi: 10.1111/j.1440-1673.1982.tb02282.x. [DOI] [PubMed] [Google Scholar]
- 55.Lee SM, Namgung R, Park MS, Eun HS, Park KI, Lee C. High incidence of rickets in extremely low birth weight infants with severe parenteral nutrition-associated cholestasis and bronchopulmonary dysplasia. J Korean Med Sci. 2012;27:1552–5. doi: 10.3346/jkms.2012.27.12.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitchell SM, Rogers SP, Hicks PD, Hawthorne KM, Parker BR, Abrams SA. High frequencies of elevated alkaline phosphatase activity and rickets exist in extremely low birth weight infants despite current nutritional support. BMC Pediatr. 2009;9:47. doi: 10.1186/1471-2431-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McIntosh N, Livesey A, Brooke OG. Plasma 25-hydroxyvitamin D and rickets in infants of extremely low birthweight. Arch Dis Child. 1982;57:848–50. doi: 10.1136/adc.57.11.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kovacs CS, Ho-Pao CL, Hunzelman JL, Lanske B, Fox J, Seidman JG, et al. Regulation of murine fetal-placental calcium metabolism by the calcium-sensing receptor. J Clin Invest. 1998;101:2812–20. doi: 10.1172/JCI2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rigo J, Senterre J. Nutritional needs of premature infants: current issues. J Pediatr. 2006;149:S80–8. [Google Scholar]
- 60.Sharp M. Bone disease of prematurity. Early Hum Dev. 2007;83:653–8. doi: 10.1016/j.earlhumdev.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 61.Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. 1997;18:832–72. doi: 10.1210/edrv.18.6.0319. [DOI] [PubMed] [Google Scholar]
- 62.Baveja R, Christou H. Pharmacological strategies in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol. 2006;30:209–18. doi: 10.1053/j.semperi.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 63.Bozzetti V, Tagliabue P. Metabolic bone disease in preterm newborn: an update on nutritional issues. Ital J Pediatr. 2009;35:20. doi: 10.1186/1824-7288-35-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferrone M, Geraci M. A review of the relationship between parenteral nutrition and metabolic bone disease. Nutri Clin Pract. 2007;22:329–39. doi: 10.1177/0115426507022003329. [DOI] [PubMed] [Google Scholar]
- 65.Ryan SW, Truscott J, Simpson M, James J. Phosphate, alkaline phosphatase and bone mineralization in preterm neonates. Acta Paediatr. 1993;82:518–21. doi: 10.1111/j.1651-2227.1993.tb12740.x. [DOI] [PubMed] [Google Scholar]
- 66.Lucas A, Brooke OG, Baker BA, Bishop N, Morley R. High alkaline phosphatase activity and growth in preterm neonates. Arch Dis Child. 1989;64:902–9. doi: 10.1136/adc.64.7_spec_no.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Portale A. Blood calcium, phosphorus, and magnesium. In: Favus M, editor. Primer on the metabolic bone disease and disorders of mineral metabolism. Philadelphia (PA): Lippincott Williams & Wilkins; 1999. pp. 115–8. [Google Scholar]
- 68.Ardran GM. Bone destruction not demonstrable by radiography. Br J Radiol. 1951;24:107–9. doi: 10.1259/0007-1285-24-278-107. [DOI] [PubMed] [Google Scholar]
- 69.Brunton JA, Bayley HS, Atkinson SA. Validation and application of dual-energy x-ray absorptiometry to measure bone mass and body composition in small infants. Am J Clin Nutr. 1993;58:839–45. doi: 10.1093/ajcn/58.6.839. [DOI] [PubMed] [Google Scholar]
- 70.Rigo J, Nyamugabo K, Picaud JC, Gerard P, Pieltain C, De Curtis M. Reference values of body composition obtained by dual energy X-ray absorptiometry in preterm and term neonates. J Pediatr Gastroenterol Nutr. 1998;27:184–90. doi: 10.1097/00005176-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 71.Lefton-Greif MA, McGrath-Morrow SA. Deglutition and respiration: development, coordination, and practical implications. Semin Speech Lang. 2007;28:166–79. doi: 10.1055/s-2007-984723. [DOI] [PubMed] [Google Scholar]
- 72.Radford PJ, Stillwell PC, Blue B, Hertel G. Aspiration complicating bronchopulmonary dysplasia. Chest. 1995;107:185–8. doi: 10.1378/chest.107.1.185. [DOI] [PubMed] [Google Scholar]
- 73.Uhm KE, Yi SH, Chang HJ, Cheon HJ, Kwon JY. Videofluoroscopic swallowing study findings in full-term and preterm infants with dysphagia. Ann Rehabil Med. 2013;37:175–82. doi: 10.5535/arm.2013.37.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee JH, Chang YS, Yoo HS, Ahn SY, Seo HJ, Choi SH, et al. Swallowing dysfunction in very low birth weight infants with oral feeding desaturation. World J Pediatr. 2011;7:337–43. doi: 10.1007/s12519-011-0281-9. [DOI] [PubMed] [Google Scholar]
- 75.Davis NL, Liu A, Rhein L. Feeding immaturity in preterm neonates: risk factors for oropharyngeal aspiration and timing of maturation. J Pediatr Gastroenterol Nutr. 2013;57:735–40. doi: 10.1097/MPG.0b013e3182a9392d. [DOI] [PubMed] [Google Scholar]
- 76.Jensen EA, Munson DA, Zhang H, Blinman TA, Kirpalani H. Anti-gastroesophageal reflux surgery in infants with severe bronchopulmonary dysplasia. Pediatr Pulmonol. 2015;50:584–7. doi: 10.1002/ppul.23052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khoshoo V, Edell D. Previously healthy infants may have increased risk of aspiration during respiratory syncytial viral bronchiolitis. Pediatrics. 1999;104:1389–90. doi: 10.1542/peds.104.6.1389. [DOI] [PubMed] [Google Scholar]
- 78.Rupprecht T, Rupprecht C, Harms D, Sterlacci W, Vieth M, Seybold K. Leukotriene receptor blockade as a life-saving treatment in severe bronchopulmonary dysplasia. Respiration. 2014;88:285–90. doi: 10.1159/000365488. [DOI] [PubMed] [Google Scholar]
- 79.Prabhu VG, Keszler M, Dhanireddy R. Pulmonary function changes after nebulised and intravenous frusemide in ventilated premature infants. Arch Dis Child Fetal Neonatal Ed. 1997;77:F32–5. doi: 10.1136/fn.77.1.f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abman SH. Monitoring cardiovascular function in infants with chronic lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed. 2002;87:F15–8. doi: 10.1136/fn.87.1.F15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ho T, Dukhovny D, Zupancic JA, Goldmann DA, Horbar JD, Pursley DM. Choosing wisely in newborn medicine: five opportunities to increase value. Pediatrics. 2015;136:e482–9. doi: 10.1542/peds.2015-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Landry JS, Chan T, Lands L, Menzies D. Long-term impact of bronchopulmonary dysplasia on pulmonary function. Can Respir J. 2011;18:265–70. doi: 10.1155/2011/547948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baker CD, Alvira CM. Disrupted lung development and bronchopulmonary dysplasia: opportunities for lung repair and regeneration. Curr Opin Pediatr. 2014;26:306–14. doi: 10.1097/MOP.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stuart BD, Sekar P, Coulson JD, Choi SE, McGrath-Morrow SA, Collaco JM. Health-care utilization and respiratory morbidities in preterminfants with pulmonary hypertension. J Perinatol. 2013;33:543–7. doi: 10.1038/jp.2012.170. [DOI] [PubMed] [Google Scholar]
- 85.Mizuno K, Nishida Y, Taki M, Hibino S, Murase M, Sakurai M, et al. Infants with bronchopulmonary dysplasia suckle with weak pressures to maintain breathing during feeding. Pediatrics. 2007;120:e1035–42. doi: 10.1542/peds.2006-3567. [DOI] [PubMed] [Google Scholar]
- 86.DeMauro SB, D’Agostino JA, Bann C, Bernbaum J, Gerdes M, Bell EF, et al. Developmental outcomes of very preterm infants with tracheostomies. J Pediatr. 2014;164:1303–10. e2. doi: 10.1016/j.jpeds.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duncan S, Eid N. Tracheomalacia and bronchopulmonary dysplasia. Ann Otol Rhinol Laryngol. 1991;100:856–8. doi: 10.1177/000348949110001013. [DOI] [PubMed] [Google Scholar]
- 88.Baker CD, Abman SH, Mourani PM. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Pediatr Allergy Immunol Pulmonol. 2014;27:8–16. doi: 10.1089/ped.2013.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McGrath-Morrow SA, Lee G, Stewart BH, McGinley BM, Lefton-Greif MA, Okelo SO, et al. Day care increases the risk of respiratory morbidity in chronic lung disease of prematurity. Pediatrics. 2010;126:632–7. doi: 10.1542/peds.2010-0844. [DOI] [PubMed] [Google Scholar]
- 90.Halterman JS, Lynch KA, Conn KM, Hernandez TE, Perry TT, Stevens TP. Environmental exposures and respiratory morbidity among very low birth weight infants at 1 year of life. Arch Dis Child. 2009;94:28–32. doi: 10.1136/adc.2008.137349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elder DE, Hagan R, Evans SF, Benninger HR, French NP. Recurrent wheezing in very preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;74:F165–71. doi: 10.1136/fn.74.3.f165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palta M, Sadek-Badawi M, Sheehy M, Albanese A, Weinstine M, McGuinness G, et al. Respiratory symptoms at age 8 years in a cohort of very low birth weight children. Am J Epidemiol. 2001;154:521–9. doi: 10.1093/aje/154.6.521. [DOI] [PubMed] [Google Scholar]
- 93.Fleming PJ, Blair PS. Sudden unexpected deaths after discharge from the neonatal intensive care unit. Semin Neonatol. 2003;8:159–67. doi: 10.1016/S1084-2756(02)00222-1. [DOI] [PubMed] [Google Scholar]
- 94.Cristea AI, Carroll AE, Davis SD, Swigonski NL, Ackerman VL. Outcomes of children with severe bronchopulmonary dysplasia who were ventilator dependent at home. Pediatrics. 2013;132:e727–34. doi: 10.1542/peds.2012-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smith VC, Zupancic JA, McCormick MC, Croen LA, Greene J, Escobar GJ, et al. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. J Pediatr. 2004;144:799–803. doi: 10.1016/j.jpeds.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 96.Furman L, Baley J, Borawski-Clark E, Aucott S, Hack M. Hospitalization as a measure of morbidity among very low birth weight infants with chronic lung disease. J Pediatr. 1996;128:447–52. doi: 10.1016/s0022-3476(96)70353-0. [DOI] [PubMed] [Google Scholar]
- 97.Chye JK, Gray PH. Rehospitalization and growth of infants with bronchopulmonary dysplasia: a matched control study. J Paediatr Child Health. 1995;31:105–11. doi: 10.1111/j.1440-1754.1995.tb00756.x. [DOI] [PubMed] [Google Scholar]
- 98.Sterni LM, Collaco JM, Baker CD, Carroll JL, Sharma GD, Brozek JL, et al. An official American Thoracic Society clinical practice guideline: pediatric chronic home invasive ventilation. Am J Respir Crit Care Med. 2016;193:e16–35. doi: 10.1164/rccm.201602-0276ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Collaco JM, Kole AJ, Riekert KA, Eakin MN, Okelo SO, McGrath-Morrow SA. Respiratory medication adherence in chronic lung disease of prematurity. Pediatr Pulmonol. 2012;47:283–91. doi: 10.1002/ppul.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McGrath-Morrow SA, Ryan T, Riekert K, Lefton-Greif MA, Eakin M, Collaco JM. The impact of bronchopulmonary dysplasia on caregiver health related quality of life during the first 2 years of life. Pediatr Pulmonol. 2013;48:579–86. doi: 10.1002/ppul.22687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Edwards JD, Kun SS, Keens TG. Outcomes and causes of death in children on home mechanical ventilation via tracheostomy: an institutional and literature review. J Pediatr. 2010;157:955–9. e2. doi: 10.1016/j.jpeds.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 102.American Academy of Pediatrics Committee on Fetus and Newborn. Hospital discharge of the high-risk neonate. Pediatrics. 2008;122:1119–26. doi: 10.1542/peds.2008-2174. [DOI] [PubMed] [Google Scholar]
- 103.Baker CD, Martin S, Thrasher J, Moore HM, Baker J, Abman SH, et al. A standardized discharge process decreases length of stay for ventilator-dependent children. Pediatrics. 2016:137. doi: 10.1542/peds.2015-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McGrath-Morrow SA, Ryan T, McGinley BM, Okelo SO, Sterni LM, Collaco JM. Polysomnography in preterm infants and children with chronic lung disease. Pediatr Pulmonol. 2012;47:172–9. doi: 10.1002/ppul.21522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simoes EA, Rosenberg AA, King SJ, Groothuis JR. Room air challenge: prediction for successful weaning of oxygen-dependent infants. J Perinatol. 1997;17:125–9. [PubMed] [Google Scholar]
- 106.Palm K, Simoneau T, Sawicki G, Rhein L. Assessment of current strategies for weaning premature infants from supplemental oxygen in the outpatient setting. Adv Neonatal Care. 2011;11:349–56. doi: 10.1097/ANC.0b013e318229be3d. [DOI] [PubMed] [Google Scholar]
- 107.Vom Hove M, Prenzel F, Uhlig HH, Robel-Tillig E. Pulmonary outcome in former preterm, very low birth weight children with bronchopulmonary dysplasia: a case-control follow-up at school age. J Pediatr. 2014;164:40–5. e4. doi: 10.1016/j.jpeds.2013.07.045. [DOI] [PubMed] [Google Scholar]
- 108.Northway WH, Jr, Moss RB, Carlisle KB, Parker BR, Popp RL, Pitlick PT, et al. Late pulmonary sequelae of bronchopulmonary dysplasia. N Engl J Med. 1990;323:1793–9. doi: 10.1056/NEJM199012273232603. [DOI] [PubMed] [Google Scholar]
- 109.Greenough A, Cox S, Alexander J, Lenney W, Turnbull R, Burgess S, et al. Health care utilisation of infants with chronic lung disease, related to hospitalisation for RSV infection. Arch Dis Child. 2001;85:463–8. doi: 10.1136/adc.85.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Broughton S, Roberts A, Fox G, Pollina E, Zuckerman M, Chautry S, et al. Prospective study of healthcare utilisation and respiratory morbidity due to RSV infection in prematurely born infants. Thorax. 2005;60:1039–44. doi: 10.1136/thx.2004.037853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Forbes ML, Kumar VR, Yogev R, Wu X, Robbie GJ, Ambrose CS. Serum palivizumab level is associated with decreased severity of respiratory syncytial virus disease in high-risk infants. Hum Vaccines Immunother. 2014;10:2789–94. doi: 10.4161/hv.29635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miller EK, Bugna J, Libster R, Shepherd BE, Scalzo PM, Acosta PL, et al. Human rhinoviruses in severe respiratory disease in very low birth weight infants. Pediatrics. 2012;129:e60–7. doi: 10.1542/peds.2011-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Greenough A, Alexander J, Burgess S, Chetcuti PA, Cox S, Lenney W, et al. Home oxygen status and rehospitalisation and primary care requirements of infants with chronic lung disease. Arch Dis Child. 2002;86:40–3. doi: 10.1136/adc.86.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moon NM, Mohay HA, Gray PH. Developmental patterns from 1 to 4 years of extremely preterm infants who required home oxygen therapy. Early Hum Dev. 2007;83:209–16. doi: 10.1016/j.earlhumdev.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 115.Newman JB, Debastos AG, Batton D, Raz S. Neonatal respiratory dysfunction and neuropsychological performance at the preschool age: a study of very preterm infants with bronchopulmonary dysplasia. Neuropsychology. 2011;25:666–78. doi: 10.1037/a0023895. [DOI] [PubMed] [Google Scholar]