Abstract
Telomere dysfunction has been associated with chromosomal instability in colorectal carcinoma, but the consequences of telomere-dependent instability for chromosome integrity and clonal evolution have been little explored. We show here that abnormally short telomeres lead to a wide spectrum of mitotic disturbances in colorectal cancer cell lines, including anaphase bridging, whole-chromosome lagging, and mitotic multipolarity. These abnormalities were found in both the presence and absence of microsatellite instability. The mean telomere length varied extensively between cells from the same tumor, allowing the establishment of tumor cell subpopulations with highly different frequencies of mitotic disturbances. Anaphase bridging typically resulted in either inter-centromeric chromatin fragmentation or centromere detachment, leading to pericentromeric chromosome rearrangements and loss of whole chromosomes, respectively. There was a strong correlation between anaphase bridges and multipolar mitoses, and the induction of dicentric chromosomes by gamma irradiation and telomerase inhibition led to an elevated frequency of multipolar mitotic spindles, suggesting that multipolarity could result from polyploidization triggered by anaphase bridging. Chromatid segregation in multipolar mitoses was close to random, resulting in frequent nullisomies and nonviable daughter cells. In contrast, there was a high clonogenic survival among cells having gone through anaphase bridging in bipolar mitoses. Bridging of telomere-deficient chromosomes could thus be a major mutational mechanism in colorectal cancer, whereas mitotic multipolarity appears to be a secondary phenomenon that rarely, if ever, contributes to clonal evolution.
Keywords: chromosome instability, telomere dysfunction, colorectal cancer, cytogenetics, telomerase
Two major modes of mutation have been demonstrated in colorectal carcinomas, microsatellite instability (MIN) and chromosomal instability (CIN). MIN is typically caused by mutations in mismatch repair genes, whereas several different molecular mechanisms have been associated with CIN, including mutations in mitotic checkpoint genes (1, 2), mutation and/or loss of the APC gene (3, 4), microtubule spindle defects (4), and viral infection (5). Also telomere dysfunction has been implicated in colorectal carcinogenesis and CIN (6, 7). Transgenic APC Min Terc–/– mice with short telomeres show an increased rate of colorectal adenoma initiation (8), and significant telomere shortening has been detected at the earliest stages of invasive colorectal cancer in human material (9). However, the precise consequences of telomere shortening for chromosomal integrity remain unclear. The present study demonstrates that telomere dysfunction does not lead to chromosome aberrations through a single mechanism. Instead, it causes a spectrum of mitotic defects with anaphase bridges triggering both numerical and structural chromosome changes, and with mitotic multipolarity leading to more dramatic genomic changes that may prove deleterious to the cell.
Materials and Methods
Cell Culture and Cloning. The cell lines LoVo (10), DLD1 (11), HCT116 (12), HT29 (13), and SW480 (14) were obtained from the American Type Culture Collection. The first two cell lines contain MSH2 mutations, the third cell line contains a MLH1 mutation, and the remaining cell lines show no evidence of MIN (15). Dermal fibroblasts with a normal chromosome complement were obtained from a healthy 26-year-old male. Cell culture, harvest, and chromosome preparation for banding and FISH were according to standard methods (16). Unless otherwise specified, at least 200 cells were included in each separate FISH analysis. Monoclonal HCT116 populations were created by trypsinization into single-cell suspension followed by plating in a culture dish at low density. Individual colonies were observed after 14–21 days of culture and transferred to 48-well plates. Confluent wells were then transferred to 25-cm2 flasks for later harvesting. For isolation of single mitotic cells, SW480 cells grown in a Petri dish were identified by using an inverted microscope, excised with a heat-extended glass pipette, and transferred into a sterile 0.9% saline droplet on a Petri dish lid. Here, the excised material was checked for bystander cells having mistakenly been aspirated, and the target cell was subsequently transferred to a 10-mm-diameter microwell. Colony-forming capacity, defined as the outgrowth of >50 cells, was evaluated after 30 days. Microwells with more than one colony were excluded from the analysis.
Cytogenetic Analyses. For analysis of chromosome dynamics at mitosis, cells were harvested without metaphase arrest, stained with hematoxylin and eosin, and analyzed in a blinded fashion. Unless otherwise specified, at least 100 mitotic cells were included in each analysis. Anaphase bridges were defined as strings of chromatin either connecting the two poles or stretching from one pole in the direction of the other pole and spanning >2/3 of the interpolar distance. The frequency of anaphase bridges was calculated as the ratio between cells exhibiting such bridges and the total number of anaphase cells. Centrosomes were visualized by anti-γ-tubulin antibodies as described (17). Probes used for FISH analyses, including painting probes, chromosome-specific centromeric probes, and the LSI D7S486 probe for 7q31, were from Vysis (Downers Grove, IL).
Analysis of Telomere Length and Telomerase. TTAGGG repeats were visualized by FISH with fluorescein-conjugated (CCCTAA)3 peptide nucleic acid probes (18), and the number of negative chromosome termini was scored in metaphase cells of the lowest ploidy level. Although a negative terminus may still contain up to 500 bp of TTAGGG repeat sequences, this method has previously been shown to yield a valid assessment of the protective capacity of telomeres (16). Telomere signal fluorescence intensity was quantified by using trf-telo software, kindly provided by P. Landsdorp (BC Cancer Research Centre, Vancouver) (18). At least 15 cells were evaluated in each case. Human telomerase reverse transcriptase (hTERT) expression was detected by RT–PCR as described (16). Telomerase activity was inhibited by growing cells for 12 days with the MST-312 hTERT inhibitor at a noncytotoxic concentration of 0.5 μg/ml (ref. 19; Calbiochem/EMD Biosciences, Madison, WI). The cells were washed, and the medium was changed to standard medium 1 day before harvesting.
Localization of DNA Breaks. DNA double-strand breaks were visualized by incorporation of fluorescein–dUTP by terminal deoxynucleotidyl transferase (In Situ Cell Death Detection Kit, Roche Molecular Biochemicals) as described (16), and labeled cells were hybridized with Cy3-conjugated pan-alpha satellite DNA probes (Cambio, Cambridge, UK). At analysis, the localization of green fluorescence (double-strand breaks) was compared with that of alphoid sequences (red fluorescence).
Irradiation and Polyploidization. To avoid irradiation of mitotic cells, cells were grown in serum-free RPMI medium 1640 with l-glutamin, penicillin, and streptomycin until no mitotic figures could be discerned. This process was followed by exposure to 6 Gy of γ-irradiation from a 137Cs source. Immediately after irradiation, the medium was exchanged for fresh medium containing 17% bovine serum. After 24 h, a first passage to chamber slides was made for later harvesting. Further passages were made after two population doublings. Polyploid cells were obtained by growth in 0.03 μg/ml Colcemid for 4 days.
Results
Short Telomeres and MIN. Previous investigations have suggested that CIN and MIN are rarely present together in the same cell population (20). Hence, CIN caused by short dysfunctional telomeres should be rare in MIN+ cell lines compared with MIN– cell lines. On the other hand, at least one previous study has shown a positive correlation between telomere shortening and MIN (6). To clarify these matters, the telomere status of individual chromosome arms was evaluated in three colorectal cancer cell lines with MIN (LoVo, DLD1, and HCT116) and two without MIN (HT29 and SW480). Telomerase activity, reflected by human telomerase reverse transcriptase expression levels measured by semiquantitative RT-PCR, was three to five times higher in the MIN+ than in the MIN– cell lines (Table 1). However, all of the cell lines contained cells with an elevated number of TTAGGG-negative ends, with LoVo showing the lowest numbers and SW480 the highest number (Fig. 1A). Apart from these two, the numbers of negative ends overlapped between the cell lines. In contrast, fibroblasts showed signals at all chromosome termini. To estimate telomere length in individual chromosome arms, the mean telomere fluorescence intensity (TFI) was measured in LoVo, HT29, and SW480. These cell lines showed mean TFIs that differed from each other and were lower than in normal fibroblasts (P < 0.0001; two-sided t test). The TFI rank order of the cell lines corresponded perfectly to the rank obtained by scoring TTAGGG-negative chromosome ends. When the mean TFIs for individual cells were compared, there was a clear overlap between LoVo and HT29 on the one hand, and HT29 and SW480 on the other, similar to the overlap observed for the number of TTAGGG-negative ends (Fig. 2A). G-banding analyses of the cell lines revealed that the MIN+ telomere-defective cell lines DLD1 and HCT116 were both polyclonal with eight and five cytogenetically related clones, respectively (Table 2, which is published as supporting information on the PNAS web site). In fact, the MIN+ cell line HCT116 exhibited a subclone with more than three times as many changes as the stem-line karyotype (13 vs. 4). In contrast, the MIN+ cell line LoVo with very few TTAGGG-negative chromosome ends was monoclonal, whereas the MIN– cell lines HT29 and SW480 showed such an extensive cytogenetic heterogeneity that it was not meaningful to define separate clones. Thus, although telomerase expression was stronger in MIN+ than in MIN– cell lines, there was a considerable overlap in the degree of telomere shortening and cytogenetic polyclonality, indicating that telomere-dependent CIN can be present in both MIN+ and MIN– cells.
Table 1. Cytogenetic abnormalities, telomere dysfunction, and telomerase expression.
| Cell line | Clonal changes* | No. of clones | Chromosome nos. | TTAGGG-negative ends, mean (range) | Mean telomere fluorescence intensity | ABF, % | hTERT expression† |
|---|---|---|---|---|---|---|---|
| LoVo | 5 | 1 | 48-49 | 1 (0-3) | 302 | 5 | 1.4 |
| DLD1 | 1 | 8 | 45-47/91-94 | 2 (0-5) | ND | 10 | 0.84 |
| HCT116 | 3 | 5 | 44-45/51-63 | 3 (0-7) | ND | 11 | 1.0 |
| HT29 | 21 | >10 | 68-72/136-144 | 3 (0-9) | 202 | 11 | 0.23 |
| SW480 | 22 | >10 | 52-59/88-97 | 15 (5-30) | 95 | 29 | 0.27 |
hTERT, human telomerase reverse transcriptase; ND, not determined.
Annotations in stem line karyotype.
Relative to HCT116.
Fig. 1.
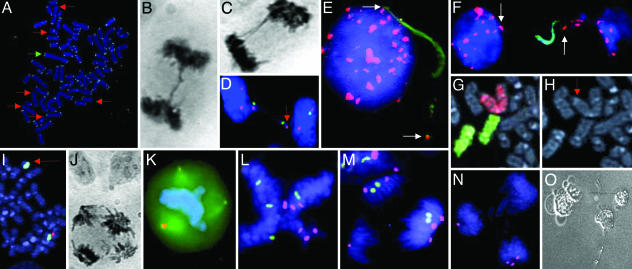
Cytogenetic analyses and single-cell cloning. (A) TTAGGG-negative ends (red arrows) and a dicentric chromosome (green arrow) in SW480. (B and C) Anaphase bridges in DLD1 (B) and SW480 (C). (D) Loss of chromosomes X (arrow; centromere in green) and 18 (centromere in violet) through bridge formation in SW480. (E and F) Fragmented DNA (green) flanked by alphoid repeats (arrows; red) in SW480. (G and H) A dicentric chromosome 7 derivative (red chromosome paint in G; red arrow in H) and two normal chromosomes 12 (green chromosome paint in G) in HT29. (I) A ring chromosome positive with the 7q31 probe (green) but not with the chromosome 7 alphoid probe (red) in the HCT116 subclone M7. (J and K) A tetrapolar anaphase cell in hematoxylin-eosin staining (J) and a tripolar metaphase cell with three centrosomes (K)(γ-tubulin in orange) in SW480. (L–N) FISH analyses with centromeric probes in SW480 showing a tetrapolar metaphase cell with four copies of the X chromosome (green) and six copies of chromosome 18 (violet) (L), a tripolar anaphase cell with 2 + 2 + 0 segregation of chromosome X and 3 + 2 + 1 segregation of chromosome 18 (M), and a tripolar anaphase cell with 3 + 1 + 0 segregation of chromosome 18 (N). (O) Degenerating daughter cells of an isolated tetrapolar mitotic cell from the same cell line. (Magnifications: ×1,000, A, E, and I; ×100, B–D, F, and J–N; ×2,000, G and H; and ×40, O.)
Fig. 2.
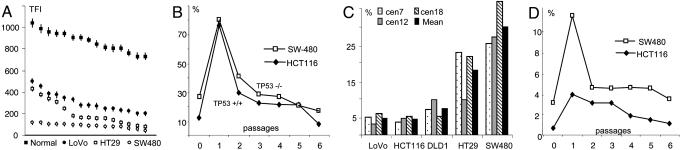
Telomere lengths, postirradiation development, and aneusomy. (A) Mean TFI measurements in normal lymphocytes and cells from three colorectal carcinoma cell lines; x axis represents rank order of cells. (B) Frequencies of cells with anaphase bridges before (passage 0) and after (passages 1–6) gamma irradiation of HCT116 cells with WT TP53 status and SW480 cells with homozygous TP53 mutation. (C) Interphase FISH assessment of the frequency of aneusomic cells using centromeric (cen) probes. (D) Frequencies of MMs before (passage 0) and after irradiation (passages 1–6) of HCT116 and SW480 cells.
Anaphase Bridging and MIN. Unprotected telomeres with few TTAGGG repeats can lead to the formation of dicentric chromosomes that may form bridges at anaphase. To analyze the level of telomere-dependent mitotic instability, we assessed the anaphase bridge frequency (ABF) in the cell lines. An elevated ABF compared with normal fibroblasts was found in all cell lines (Fig. 1 B and C). Two types of bridges were observed at approximately equal proportions. The first type consisted of one or two strings of decondensed chromatin anchored at both poles of the anaphase configuration. In the second type, the bridge had detached from either one or both of the anaphase poles (Fig. 1D). The ABF rank order of the cell lines correlated perfectly to the rank order of TTAGGG-negative chromosome ends (Table 1). Just as for the telomere lengths, there was an extensive overlap in ABF between cell lines with and without MIN. To evaluate whether differences in the elimination rate of unstable chromosomes had an impact on the ABF values, one culture each of HCT116 (ABF of 11%; retained WT TP53 alleles) and SW480 (ABF of 29%; homozygously mutated TP53; ref. 21) were exposed to ionizing radiation. After irradiation, both cell lines showed an expected increase in the frequency of cells with dicentric chromosomes, and the ABF values rose to ≈80% in each cell line (Fig. 2B). The ABF was then monitored for six passages in vitro, corresponding to ≈12 population doublings. The ABF decreased continuously in the cell lines, consistent with dicentric chromosomes being unstable and thus prone to loss or rearrangements into more stable derivatives. Both lines reached their preirradiation ABF values after five or six passages. There was thus no difference between the two lines in the elimination rate of unstable chromosomes. Taken together, our data indicate that anaphase bridging occurs in both the presence and absence of MIN and that telomere-length rather than DNA-damage checkpoint status is the primary determinant of anaphase bridging.
Chromatin Bridge Fragmentation and Chromosome Breakpoints. To characterize the breakage of anaphase bridges in detail, the terminal deoxynucleotidyl transferase (TDT) enzyme was used to incorporate fluorescently labeled nucleotides at sites of DNA fragmentation. No fragmentation was detected in anaphase cells by this method, but a population of interphase cells (0.1–3%) exhibited distinct regions of fragmented DNA. Typically, fragmented DNA was located in protrusions of the nuclear membrane (Fig. 1E) or in chromatin strings between two nuclei (Fig. 1F). Hybridization with a probe for all human centromeres to TDT-labeled slides from HT29 and SW480 demonstrated that the internuclear strings were in most cells flanked by centromeric signals, whereas the protrusions typically exhibited one signal at its base and occasionally another signal at its end. This finding was consistent with internuclear strings originating from decondensed, fragmented but not yet completely broken anaphase bridges and protrusions originating from bridges that had undergone fragmentation and complete breakage. In the vast majority (>90%) of strings and protrusions, the region of fragmented DNA began just next to the centromere and continued distally. Hence, DNA fragmentation in anaphase bridges appeared to result in breakage of chromosomes predominantly in the pericentromeric region. In the cell line karyotypes, 20 of the 54 breakpoints (37%) were in pericentromeric (p11-q11) bands, which make up 72 of the 344 bands (21%) in the male haploid genome (P = 0.005; χ2 test). To evaluate whether this breakpoint pattern corresponded to breakpoints occurring in vivo, we evaluated the karyotypes from the 338 colorectal adenocarcinoma cases listed in the Mitelman Database of Chromosome Aberrations in Cancer (http://cgap.nci.nih.gov/Chromosomes/Mitelman). Pericentromeric breaks were overrepresented also in this material (400/1,183 breakpoints; P < 0.0001; χ2 test) and were typically associated with isochromosomes and whole-arm translocations. In fact, the four most common breakpoints were all pericentromeric, including 17q10, 8q10, 17p11, and 13q10. The high frequency of pericentromeric changes in these tumor biopsies indicates that breakage of anaphase bridges not only leads to pericentromeric cytogenetic rearrangements in vitro, but also contributes to the generation of clonal changes in vivo.
Chromosome Loss Through Anaphase Bridging. The observed centromeric detachment of anaphase bridges indicated that bridging could contribute to aneuploidy by loss of chromosomes. When FISH with a centromeric probe for the X chromosome was performed in SW480 bipolar anaphase and telophase cells, 93% of the cells showed a 2 + 2 configuration, i.e., a balanced segregation leading to daughter cells with two chromosome copies each. The balanced configurations 4 + 4, 3 + 3, and 1 + 1 were observed in 1%, 1%, and 2% of the cells, respectively. In 3% of cells, the target chromosome was present in a bridge, which had detached from one of the anaphase/telophase poles (Fig. 1D). FISH with the same centromeric probe showed a similar pattern in the other four cell lines (60 cells analyzed). At least one-third of the anaphase cells with bridges also exhibited lagging chromatin material not physically connected to the bridge (33% in SW480; 38% in HCT116). Such lagging chromosomes were rarer in anaphase configurations without bridges (0 in SW480; 0.6% in HCT116; P < 0.001; χ2 test). One-third of the lagging chromosome fragments were positive with a pan-alpha satellite probe, indicating that they consisted of whole chromosomes. Thus, in the majority of bipolar cell divisions where chromosomes were lost, this phenomenon either occurred by involvement in a bridge or anaphase lagging in a cell where other chromosomes were involved in bridge formation.
Anaphase Bridging and Chromosome Copy Number. To evaluate copy-number variability of individual chromosomes in the cell lines, interphase FISH with a panel of three chromosome-specific centromeric probes, targeting the same chromosomes as in ref. 20, was performed, and the proportion of cells with copy numbers deviating from the modal value (aneusomic cells) was scored. This process demonstrated copy-number heterogeneity in cell lines both with and without MIN, with the heterogeneity being clearly larger in the MIN– than in the MIN+ cell lines (Fig. 2C). However, there was also a considerable difference in the frequency of aneusomy among individual chromosomes. In HT29, aneusomies of chromosomes 7 and 18 were twice as frequent as aneusomy of chromosome 12. To assess whether this finding had any relation to the tendency of these chromosomes to participate in bridging, anaphase bridges in HT29 were analyzed by centromeric probes for chromosomes 7 and 12. Bridges involving chromosome 7 were present in 10% of these cells, whereas no cell exhibited a bridge involving chromosome 12 (P < 0.05; χ2 test; 50 cells analyzed). Because bridging is known to trigger complex rearrangements of chromosome structure, we used chromosome-painting probes to assess the frequency of structural rearrangements involving chromosomes 7 and/or 12 in metaphase preparations from HT29. Besides the del(7) present in the HT29 stem line, structural rearrangements of chromosome 7 were observed in 8% of the cells, compared with only 1% of the cells showing structural rearrangements involving chromosome 12 (Fig. 1 G and H; P < 0.05; χ2 test; 80 cells analyzed). Comparison of TFI measurements in chromosomes 7 and 12 in HT29 showed that the values of 12pter(249), 12qter(242), and 7pter(219) did not differ significantly from each other, nor from the mean TFI in HT29. However, 7qter had a TFI value lower than the others (value 172; P = 0.01; two-sided t test). Thus, different telomere lengths, leading to different probabilities for involvement in anaphase bridging, could explain some of the observed differences in copy-number variability between chromosomes and also explain why some chromosomes with high numerical variability also show a high frequency of structural changes.
Single-Cell Subclones with Different Degrees of CIN. Because considerable intercellular heterogeneity in telomere length was found within each cell line, it was possible that the cell lines contained subclones showing different degrees of CIN. To address this issue, we created 10 single-cell subclones (M1–M10) from HCT116. In two of the clones, the ABF values differed significantly from the 11% seen in the original culture. In M7, the ABF was 21% and in M2 it was 5% (P < 0.04 for both; χ2 test). In M7, the mean number of TTAGGG-negative ends was more than seven times higher (mean = 15; range from 9 to 23) than in M2 (mean = 2; range from 0 to 6). In both M2 and M7, the majority of cells had 40–50 chromosomes. However, the number of cells outside of this range was significantly higher in M7 than in M2 (P < 0.0001; χ2 test). To compare the rate of novel chromosome changes in the two sublines, interphase FISH for centromere 7 was performed, demonstrating aneusomy in twice as many cells in M7 as in M2 (P = 0.001; χ2 test). To assess the frequency of structural rearrangements of chromosome 7, 50 metaphase cells in each subline were analyzed by using dual-color FISH with the alpha satellite probe and a 7q31 probe. This analysis showed structural rearrangements in 6% of cells in M7, whereas no rearrangements were found in M2 (Fig. 1I). The heterogeneity in telomere length and ABF among HCT116 cells thus appeared to reflect differences that were sufficiently stable for the creation of single-cell subclones with different degrees of CIN. In fact, although HCT116 has been reported as a cytogenetically stable MIN+ cell line (20), the M7 subline exhibited an ABF twice as high as that of the MIN– cell line HT29.
Multipolar Cell Divisions and Supernumerary Centrosomes. Besides anaphase bridges and lagging chromosomes, multipolar mitoses (MMs) were observed in all cell lines (Fig. 1J), but the frequency of these abnormalities was low (1–4%) and significant differences were not recognized between the lines. Detection of centrosomes by immunofluorescence demonstrated that MMs were invariably associated with the presence of supernumerary (>2) centrosomes (Fig. 1K). Typically, the number of centrosomes was equal to the number of spindle poles, but a minority of cells had additional centrosomes not participating in the mitotic spindle or two centrosomes colocalizing at one pole (data not shown). SW480 had the highest frequency of MM and was selected for a more detailed analysis. FISH analysis of multipolar metaphase and anaphase SW480 cells by centromeric probes for chromosomes X and 18 showed that only 7% of these cells contained the stem-line copy number (two for chromosome X and two for chromosome 18), whereas the remaining cells showed a higher copy number (Fig. 1L). The modal number of MM was four for the X chromosome and six for chromosome 18. FISH with the pan-alpha satellite probe corroborated that 90% of multipolar divisions were indeed highly polyploid (>100 centromere signals).
The polyploid state of the MM in SW480 suggested that these cells originated from bipolar cell divisions that had failed to undergo cytokinesis, possibly because of anaphase bridging. If this was the case, then any process that increased the frequency of mitoses with anaphase bridges should lead to a corresponding increase in the rate of multipolar cell divisions. Processes leading to polyploidization, on the other hand, should not necessarily lead to an increased ABF. When the irradiated HCT116 and SW480 cells were evaluated for MM, a dramatically elevated frequency was found in the first passage after irradiation, followed by decreasing frequencies of MM in successive passages (Fig. 2D). The elimination dynamics was highly similar to that of anaphase bridges previously observed in the same cells (Fig. 2B), and there was a positive correlation between ABF and the frequency of MM in irradiated cells (r = 0.71; P < 0.001; Pearson correlation). To further substantiate the possible causal connection between anaphase bridging and mitotic multipolarity, we compared the frequencies of MM in the HCT116 subclones M2 and M7. Only 6% of the mitoses were multipolar in M2, having few TTAGGG-negative chromosome ends, whereas 15% of the mitotic cells were multipolar in M7, having many negative ends (P < 0.001; χ2 test). To assess whether telomere shortening and anaphase bridging could actually induce MM, HCT116 cells were grown in the presence of the MST-312 telomerase inhibitor. After 6 days, the frequency of anaphase bridges had increased from 11% to 39%, but there was no difference in the frequency of MM. After another 6 days, 83% of the anaphase cells showed bridges, and the frequency of multipolar divisions had now also increased, from 2% to 37% of the mitotic figures (P < 0.0001; χ2 test; >30 cells analyzed). Finally, when HCT116 and SW480 cells were grown in the presence of the spindle-disrupting agent Colcemid for 4 days, ≈90% of cells became polyploid and the frequency of MM increased dramatically, from 1% to 26% in HCT116 and from 4% to 50% in SW480 (P < 0.0001; χ2 test). In contrast, the frequency of anaphase bridges did not change significantly after Colcemid exposure (P > 0.1; >90 cells analyzed). Taken together, these data show that an elevated frequency of anaphase bridges will lead to an increased frequency of MM, whereas the reverse scenario could not be demonstrated.
Survival After Multipolar Cell Division. To visualize the chromatid segregation pattern in MMs, FISH analysis with centromere probes for chromosomes X and 18 was performed in SW480 cells. To simplify the interpretation, only tripolar cell divisions with two copies of chromosome X or two copies of chromosome 18 without anaphase bridges were included. Assuming a symmetrical segregation of sister chromatids, a disomic tripolar mitosis should result in either a 2 + 1 + 1ora2 + 2 + 0 distribution of sister chromatids, where the former should be twice as common as the latter (Fig. 1M). However, FISH analysis of 54 tripolar anaphase cells demonstrated a high frequency of other patterns, including 3 + 1 + 0 (30%) and 4 + 0 + 0 (4%) segregations (Fig. 1N). These patterns implicate nondisjunction of sister chromatids, and the chromatid segregations in tripolar mitoses in SW480 did not, in fact, differ significantly from a random segregation of sister chromatids (P = 0.8; χ2 test). Of the 162 observed daughter cells of the tripolar divisions, 18% did not contain any copy of the X chromosome and 9% had no copy of chromosome 18, i.e., they were nullisomic for these chromosomes. Because nullisomy for any chromosome except for the Y is thought to be lethal, we decided to evaluate the survival of mitotic SW480 cells. Single mitotic cells were excised from cultures, isolated in microwells, and then monitored for 30 days. Of the 11 transferred bipolar anaphase cells with bridges, 8 formed colonies. Normal anaphase cells and interphase cells had similar colony-forming capacities (11/13 and 7/10 transferred cells, respectively). However, of the 29 isolated cells undergoing MM, 23 did not complete mitosis but remained single during the entire evaluation period. The remaining six cells divided into either three or four daughter cells that exhibited senescent morphology after ≈1 week and of which none had undergone cell division after 30 days (Fig. 1O). Clonogenic survival of cells having undergone multipolar cell division thus appeared to be low or even negligible.
Discussion
Telomere dysfunction and anaphase bridges have been associated with CIN in both sarcomas and carcinomas (8, 16). In ulcerative colitis, it has been shown that anaphase bridges may signal progression toward colorectal carcinoma (22). An elevated frequency of bridges can be a consequence of an increased formation rate of dicentrics, either through interstitial chromosome rearrangements or because of abnormal shortening of terminal TTAGGG repeats, leading to telomeric fusions. In addition, the frequency of bridges depends on the rate by which the mitotically unstable chromosomes are eliminated, and it has been demonstrated that tumor cells may have a slower elimination rate of dicentrics than nonneoplastic cells (23). In the present study, the frequency of bridges showed a perfect positive correlation to the number of abnormally short telomeres. We could also show that chromosomes with short telomeres were more frequently involved in structural and numerical changes, subclones with many TTAGGG-negative termini had more anaphase bridges than those with mostly intact telomeres, and the frequency of bridges increased dramatically after telomerase inhibition. Finally, there was no difference in the postirradiation elimination rate of anaphase bridges between cell lines with different degrees of instability and different TP53 checkpoint status. Taken together, our data strongly indicate that telomere shortening is the primary cause of anaphase bridging in colorectal cancer cells. However, our data do not preclude that telomere-independent mechanisms for CIN also are active in the cell lines. For instance, the large difference in cytogenetic heterogeneity between DLD1 and HCT116, on the one hand, and HT29 and SW480, on the other, indicates that the degree of genomic instability is also determined by other factors influencing chromosomal segregation, such as mutations in genes of the mitotic checkpoint machinery (1, 2).
A previous study has indicated that anaphase bridging may be statistically more common in the absence of mismatch repair deficiency (24). However, the present finding of a considerable overlap between MIN+ and MIN– cell lines in the frequency of bridges and the number of telomere-deficient chromosomes indicates that mismatch repair deficiency may occur together with telomere-dependent instability in some cells. This finding is consistent with another previous study, showing a positive correlation between telomere shortening and MIN in colorectal cancer biopsies (6). The vast intercellular heterogeneity in telomere lengths in the MIN+ lines and the fact that the two telomere-deficient MIN+ cell lines both were cytogenetically polyclonal further suggested that there could be subclones with a high degree of telomere-dependent instability. This finding was corroborated by isolation of single-cell subclones with different rates of instability from the MIN+ HCT116 line, one of which had an ABF value almost twice as high as the MIN– HT29 cell line. Notably, this heterogeneity in the rate of chromosome mutation between subclones could explain some of the discrepancies between different studies in the assessment of CIN. For example, LoVo has previously been classified as a cell line exhibiting both MIN and CIN (20), but in the present study, little evidence of CIN was found in LoVo by karyotyping and interphase FISH. Moreover, there were long telomeres and few anaphase bridges in this cell line.
Anaphase bridging was associated with the presence of DNA double-strand breaks in all of the cell lines, and a detailed analysis of chromosomes 7 and 12 in HT29 demonstrated a strong correlation between short telomeres and a high rate of structural rearrangements. Chromosomes that break at anaphase may enter breakage-fusion-bridge cycles, which may lead to further genetic abnormalities, such as deletion and amplification (25). In the present study, there was decondensation of bridged chromatin at anaphase and telophase, and extensive DNA fragmentation was detected at interphase. Whether the DNA fragmentation depended solely on mechanical strain on the DNA–protein scaffold or if other processes of dsDNA breakage such as nuclease digestion were involved remains to be established. However, the DNA fragmentation did not seem to have any significant impact on the cellular survival. The frequency of apoptotic cells by terminal deoxynucleotidyl transferase detection was <1% in all cell lines, and there was no difference in the colony-forming capacity of single-cell subclones from anaphase cells with bridges, compared with normal anaphase cells. The majority of bridges showed complete fragmentation of the chromatin beyond the centromere, which was reflected by a high number of pericentromeric breakpoints in the cell line karyotypes. A similar overrepresentation of centromeric breakpoints was found in published karyotypes from colorectal cancer biopsies. Our findings thus indicate that DNA fragmentation in chromosome bridges may significantly contribute to the accumulation of structural changes in colorectal cancers, particularly pericentromeric rearrangements such as whole-arm translocations and isochromosomes.
Anaphase bridging was also associated with detachment of centromeres from one or both spindle poles. Irrespective of whether the detachment occurs at the kinetochore or in the microtubule filaments, such a process should lead to whole-chromosome loss in one or both of the daughter cells. We indeed found a strong correlation between telomere deficiency and chromosomal copy-number alterations. In HT29, chromosome 7, with an extremely short q-arm telomere, had a higher frequency of copy-number alterations than the other observed chromosomes. In HCT116, the subclone M7 with short telomeres showed a higher proportion of aneuploid cells than did subclone M2 with long telomeres. Finally, lagging of chromosome material, including whole chromosomes, was strongly overrepresented in anaphase cells with bridges, suggesting that bridging may be associated with loss of genetic material also through mechanisms not directly related to the stretching of telomere-deficient chromosomes. Repeated chromosome loss in a diploid cell might lead to large, potentially lethal alterations in gene dosage. In contrast, a polyploid cell would have a higher chance of survival, allowing a gradual loss of heterozygosity through repeated chromosome losses. That the highly aneuploid clones in the present study were derived from polyploid cells was supported by the G-band analysis, showing that all clones with multiple losses or gains had hyperdiploid to hyperhexaploid modal numbers. However, even if clonal evolution through whole-chromosome loss might be limited to polyploid cells, anaphase bridging could still be a common mechanism for copy-number alterations in colorectal cancer, considering that as many as 20% of anaphase cells in tumor biopsies have been shown to contain bridges (8, 26).
Another potential source for numerical chromosome changes is multipolar cell division caused by supernumerary centrosomes. A previous study of colorectal cancer cells suggested that centrosomal abnormalities are restricted to highly aneuploid cell lines (27). In the present study, MMs and supernumerary centrosomes were also present in cell lines with a near-diploid stem line. MM occurred primarily in polyploid cells, and there was a strong positive correlation between the ABF and the frequency of MM in irradiated cells. In contrast, there was no increase in ABF when cells were polyploidized, indicating that the correlation of MM and bridges was not caused by a general laxity of the mitotic checkpoint machinery. The HCT116 subclone M7 with short telomeres had more than twice as high MM frequency as the M2 subclone with longer telomeres, and there was a significant increase of MM in HCT116 after telomerase inhibition. Taken together, these findings suggest that cytokinetic failure caused by anaphase bridging could, at least in part, contribute to the accumulation of cells with supernumerary centrosomes and MM, which would also explain why MM decreased gradually after irradiation, when bridges were no longer induced. A similar association between polyploidization and the formation of cells with extra centrosomes has been found in an animal model of pancreatic carcinoma (28) and a Tp53–/– in vitro model overexpressing Aurora A (29). However, whether such highly abnormal mitoses produce viable daughter cells has been little investigated. In SW480, the chromatid segregation pattern of MM was nearly random, leading to a high frequency of nullisomies. This finding was consistent with the failure to produce viable single-cell subclones from multipolar SW480 cell divisions, compared with a 70% colony formation rate for cells having gone through anaphase bridging. Finally, the nearly random multipolar chromatid segregation predicts that any daughter cell derived from a MM would be highly aneuploid, but with few structural rearrangements. Such karyotypes were not observed in any of the cell lines. This finding, taken together with the fact that only 1–4% of mitoses were multipolar in the cell lines, strongly suggests that multipolar cell division is an uncommon consequence of telomere shortening that has a minor, if any, role in the evolution of clonal cytogenetic changes. In contrast, anaphase bridging may trigger both structural and numerical changes in cells with a high probability of transmitting these mutations to later generations.
Supplementary Material
Acknowledgments
The Swedish Medical Society, the Swedish Cancer Society, the Sharon B. Lund Foundation of the American Cancer Society, and Lund University Hospital Donation Funds supported this study.
Author contributions: M.H., N.M., F. Mertens, and D.G. designed research; Y.S., L.G., T.J., N.L., and D.G. performed research; Y.S., L.G., T.J., M.H., N.M., F. Mertens, F. Mitelman, and D.G. analyzed data; and Y.S., F. Mitelman, and D.G. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ABF, anaphase bridge frequency; CIN, chromosomal instability; MIN, microsatellite instability; MM, multipolar mitosis; TFI, telomere fluorescence intensity.
References
- 1.Cahill, D. P., Lengauer, C., Yu, J., Riggins, G. J., Willson, J. K., Markowitz, S. D., Kinzler, K. W. & Vogelstein, B. (1998) Nature 392, 300–303. [DOI] [PubMed] [Google Scholar]
- 2.Rajagopalan, H., Jallepalli, P. V., Rago, C., Velculescu, V. E., Kinzler, K. W., Vogelstein, B. & Lengauer, C. (2004) Nature 428, 77–81. [DOI] [PubMed] [Google Scholar]
- 3.Fodde, R., Kuipers, J., Rosenberg, C., Smits, R., Kielman, M., Gaspar, C., van Es, J. H., Breukel, C., Wiegant, J., Giles, R. H. & Clevers, H. (2001) Nat. Cell Biol. 3, 433–438. [DOI] [PubMed] [Google Scholar]
- 4.Green, R. & Kaplan, K. B. (2003) J. Cell Biol. 163, 949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricciardiello, L., Baglioni, M., Giovannini, C., Pariali, M., Cenacchi, G., Ripalti, A., Landini, M. P., Sawa, H., Nagashima, K., Frisque, R. J., et al. (2003) Cancer Res. 63, 7256–7262. [PubMed] [Google Scholar]
- 6.Takagi, S., Kinouchi, Y., Hiwatashi, N., Nagashima, F., Chida, M., Takahashi, S., Negoro, K., Shimosegawa, T. & Toyota, T. (2000) Dis. Colon Rectum 43, S12–S17. [DOI] [PubMed] [Google Scholar]
- 7.Hastie, N. D., Dempster, M., Dunlop, M. G., Thompson, A. M., Green, D. K. & Allshire, R. C. (1990) Nature 346, 866–868. [DOI] [PubMed] [Google Scholar]
- 8.Rudolph, K. L., Millard, M., Bosenberg, M. W. & DePinho, R. A. (2001) Nat. Genet. 28, 155–159. [DOI] [PubMed] [Google Scholar]
- 9.Plentz, R. R., Wiemann, S. U., Flemming, P., Meier, P. N., Kubicka, S., Kreipe, H., Manns, M. P. & Rudolph, K. L. (2003) Gut 52, 1304–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drewinko, B., Romsdahl, M. M., Yang, L. Y., Ahearn, M. J. & Trujillo, J. M. (1976) Cancer Res. 36, 467–475. [PubMed] [Google Scholar]
- 11.Dexter, D. L., Spremulli, E. N., Fligiel, Z., Barbosa, J. A., Vogel, R., VanVoorhees, A. & Calabresi, P. (1981) Am. J. Med. 71, 949–956. [DOI] [PubMed] [Google Scholar]
- 12.Brattain, M. G., Fine, W. D., Khaled, F. M., Thompson, J. & Brattain, D. E. (1981) Cancer Res. 41, 1751–1756. [PubMed] [Google Scholar]
- 13.von Kleist, S., Chany, E., Burtin, P., King, M. & Fogh, J. (1975) J. Natl. Cancer Inst. 55, 555–560. [DOI] [PubMed] [Google Scholar]
- 14.Kyriazis, A. P., DiPersio, L., Michael, G. J., Pesce, A. J. & Stinnett, J. D. (1978) Cancer Res. 38, 3186–3190. [PubMed] [Google Scholar]
- 15.Branch, P., Hampson, R. & Karran, P. (1995) Cancer Res. 55, 2304–2309. [PubMed] [Google Scholar]
- 16.Gisselsson, D., Jonson, T., Petersén, A., Strömbeck, B., Dal Cin, P., Höglund, M., Mitelman, F., Mertens, F. & Mandahl, N. (2001) Proc. Natl. Acad. Sci. USA 98, 12683–12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gisselsson, D., Jonson, T., Yu, C., Martins, C., Jin, Y., Wiegant, J., Mandahl, N., Mertens, F. & Jin, C. (2002) Br. J. Cancer 87, 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landsdorp, P. M., Verwoerd, N. P., van de Rijke, F. M., Dragowska, W., Little, M.-T., Dirks, R. W., Raap, A. K. & Tanke, H. J. (1996) Hum. Mol. Genet. 5, 685–691. [DOI] [PubMed] [Google Scholar]
- 19.Seimiya, H., Oh-hara, T., Suzuki, T., Naasani, I., Shimazaki, T., Tsuchiya, K. & Tsuruo, T. (2002) Mol. Cancer Ther. 1, 657–665. [PubMed] [Google Scholar]
- 20.Lengauer, C., Kinzler, K. W. & Vogelstein, B. (1997) Nature 386, 623–627. [DOI] [PubMed] [Google Scholar]
- 21.Bosari, S., Marchetti, A., Buttitta, F., Graziani, D., Borsani, G., Loda, M., Bevilacqua, G. & Coggi, G. (1995) Diagn. Mol. Pathol. 4, 249–255. [DOI] [PubMed] [Google Scholar]
- 22.O'Sullivan, J. N., Bronner, M. P., Brentnall, T. A., Finley, J. C., Shen, W. T., Emerson, S., Emond, M. J., Gollahon, K. A., Moskovitz, A. H., Crispin, D. A., et al. (2002) Nat. Genet. 32, 280–284. [DOI] [PubMed] [Google Scholar]
- 23.Gisselsson, D., Pettersson, L., Höglund, M., Heidenblad, M., Gorunova, L., Wiegant, J., Mertens, F., Dal Cin, P., Mitelman, F. & Mandahl, N. (2000) Proc. Natl. Acad. Sci. USA 97, 5357–5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montgomery, E., Wilentz, R. E., Argani, P., Fisher, C., Hruban, R. H., Kern, S. E. & Lengauer, C. (2003) Cancer Biol. Ther. 2, 248–252. [DOI] [PubMed] [Google Scholar]
- 25.McClintock, B. (1940) Genetics 26, 234–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubio, C. A. (1991) Pathol. Res. Pract. 187, 508–513. [DOI] [PubMed] [Google Scholar]
- 27.Ghadimi, B. M., Sackett, D. L., Difilippantonio, M. J., Schröck, E., Neumann, T., Jauho, A., Auer, G. & Ried, T. (2000) Genes Chromosomes Cancer 27, 183–190. [PMC free article] [PubMed] [Google Scholar]
- 28.Levine, D. S., Sanchez, C. A., Rabinovitch, P. S. & Reid, B. J. (1991) Proc. Natl. Acad. Sci. USA 88, 6427–6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meraldi, P., Honda, R. & Nigg, E. A. (2002) EMBO J. 21, 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.