Abstract
Purpose
Seribantumab is a fully human immunoglobulin G2 monoclonal antibody that binds to human epidermal growth factor receptor (HER) 3 (ErbB3), blocking heregulin (HRG) –mediated ErbB3 signaling and inducing ErbB3 receptor downregulation. This open-label randomized phase II study evaluated progression-free survival (PFS) with seribantumab in combination with once-per-week paclitaxel compared with paclitaxel alone in patients with platinum-resistant or -refractory ovarian cancer. A key secondary objective was to determine if any of five prespecified biomarkers predicted benefit from seribantumab.
Patients and Methods
Patients with platinum-resistant or -refractory epithelial ovarian, fallopian tube, or primary peritoneal cancer were randomly assigned at a ratio of two to one to receive seribantumab plus paclitaxel or paclitaxel alone. Patients underwent pretreatment core needle biopsy; archival tumor samples were also obtained to support biomarker analyses.
Results
A total of 223 patients were randomly assigned (seribantumab plus paclitaxel, n = 140; paclitaxel alone, n = 83). Median PFS in the unselected intent-to-treat population was 3.75 months with seribantumab plus paclitaxel compared with 3.68 months with paclitaxel alone (hazard ratio [HR], 1.027; 95% CI, 0.741 to 1.425; P = .864). Among patients whose tumors had detectable HRG mRNA and low HER2 (n = 57 [38%] of 151 with available biomarker data), increased treatment benefit was observed in those receiving seribantumab plus paclitaxel compared with paclitaxel alone (PFS HR, 0.37; 95% CI, 0.18 to 0.76; P = .007). The HR in patients not meeting these criteria was 1.80 (95% CI, 1.08 to 2.98; P = .023).
Conclusion
The addition of seribantumab to paclitaxel did not result in improved PFS in unselected patients. Exploratory analyses suggest that detectable HRG and low HER2, biomarkers that link directly to the mechanism of action of seribantumab, identified patients who might benefit from this combination. Future clinical trials are needed to validate this finding and should preselect for HRG expression and focus on cancers with low HER2 levels.
INTRODUCTION
Ovarian cancer is a leading cause of cancer-related death among women, with approximately 240,000 cases diagnosed annually.1 Although most patients respond to initial treatment, most eventually develop resistance to platinum-based therapy. At this stage, single-agent therapies such as once-per-week paclitaxel confer a median progression-free survival (PFS) benefit of approximately 3 to 6 months.2-6
Patient outcomes could potentially be improved by identifying mechanisms of drug resistance and developing drugs that effectively combat such mechanisms. Many cancers become desensitized to therapy by upregulating autocrine or paracrine growth factors that activate antiapoptotic signaling pathways.7-9 Notably, heregulin (HRG) -driven ErbB3 signaling mediates insensitivity to a broad range of therapies by activating the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway.10-15 In ovarian cancer, ErbB3 promotes cell proliferation in preclinical models, and approximately 30% of patients show evidence of an HRG/ErbB3 autocrine loop in tumor cells derived from malignant ascites.16 Together, these data suggest that blocking HRG/ErbB3 may increase sensitivity to therapy when this pathway is active.
Seribantumab (MM-121; Merrimack Pharmaceuticals, Cambridge, MA) is a fully human immunoglobulin G2 antibody that targets ErbB3, blocks HRG from binding, and downregulates the receptor.17 Here, we describe the results of a phase II study in which women with advanced platinum-resistant or -refractory ovarian cancer were randomly assigned to receive once-per-week paclitaxel or paclitaxel in combination with seribantumab. Additionally, this study was designed to examine five potential biomarkers of ErbB3 activation that had been previously highlighted by computational modeling: HRG, ErbB3, human epidermal growth factor receptor 2 (HER2), epidermal growth factor receptor (EGFR), and betacellulin (BTC).15,17 In preclinical studies, the levels of these proteins predicted response to seribantumab in mouse xenograft models.15 The hypotheses underlying these biomarkers are summarized in Appendix Table A1 (online only). Because it was not known how these biomarkers are altered by disease progression, they were measured in both archived tissue and mandatory pretreatment biopsies.
PATIENTS AND METHODS
Study Design
This was a multicenter, open-label, randomized phase II study of seribantumab combined with paclitaxel versus paclitaxel alone in patients with advanced platinum-resistant or -refractory ovarian cancer. Patients were randomly assigned at a ratio of two to one to receive seribantumab with paclitaxel or paclitaxel alone, with two stratification factors: Eastern Cooperative Oncology Group (ECOG) performance status18 (0 to 1 v 2) and number of prior therapies (one to two v three or more). The primary end point was PFS as assessed by RECIST (version 1.1).19 Secondary objectives included correlation of PFS with each of five biomarkers, efficacy of combination treatment with regard to overall survival (OS) and objective response rate (ORR), assessment of treatment according to health-related quality-of-life (HRQOL) scores, further characterization of the safety profile of seribantumab, and examination of predictive biomarkers.
Patients
Eligibility criteria included confirmed advanced or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer that was platinum resistant or refractory per Gynecologic Oncology Group criteria.20,21 Patients underwent mandatory pretreatment core needle biopsy and also submitted archived tumor samples, as available. All patients supplied written informed consent, and local institutional review boards and/or ethics committees approved the study protocol.
Study Procedures
Patients were randomly assigned at a ratio of two to one to either the experimental (seribantumab plus paclitaxel) or control arm (paclitaxel alone). Seribantumab was administered intravenously (40 mg/kg loading dose, then 20 mg/kg once per week). Paclitaxel was administered intravenously (80 mg/m2 during cycle one, with optional modification in subsequent cycles to 80 mg/m2 once per week for 3 weeks followed by 1 week of rest). Disease status was assessed every 8 weeks. After discontinuation from study, patients were observed to determine OS. Data were collected every 4 months from the date of the 30-day follow-up visit.
Assessment of Response and Toxicity
The primary PFS end point was based on the intent-to-treat (ITT) and evaluable populations. The evaluable population was defined as all randomly assigned and treated patients who met all inclusion criteria and were evaluable for response (underwent one or more tumor evaluations while receiving treatment or exhibited early disease progression, including symptomatic deterioration or cancer-related death). Tumor assessment was per RECIST (version 1.1). HRQOL was assessed using the Functional Assessment of Cancer Therapy–Ovarian and –Taxane questionnaires. Safety analyses were performed on the safety population (all patients who received ≥ one dose of study drug), and adverse events (AEs) were graded according to National Cancer Institute Common Terminology Criteria for Advanced Events (version 4.0) and MedDRA system organ class.
Biomarker Assays
Five biomarkers (HRG, ErbB3, HER2, EGFR, and BTC) were analyzed using one or more of the following assays: fluorescence-based quantitative immunohistochemistry (IHC), chromogenic IHC, chromogenic RNA in situ hybridization (ISH), and reverse-transcriptase polymerase chain reaction. Detailed descriptions of assays, methods, and scoring criteria are provided in the Data Supplement.
Statistical Analyses
A total of 210 patients were needed to provide 164 PFS events for the primary efficacy analysis. With a two-sided α of 0.20, this provided 88% power to detect a hazard ratio (HR) of 0.67. The primary efficacy analysis was performed using a stratified log-rank test, adjusting for number of prior therapies but not for ECOG status, because there were too few patients with ECOG status of 2. Survival analyses were performed using Kaplan-Meier estimates, and HR estimates were calculated using a stratified Cox proportional hazards model.
Biomarker analyses were performed according to a prespecified plan, using data from pretreatment biopsies in the safety population (n = 220). Biomarkers were initially evaluated by fitting to a Cox proportional hazards model of biomarker-by-treatment interaction. Biomarkers showing a treatment interaction (P < .4) were subsequently evaluated using two-variable models. Additional details of the analysis are included in the Data Supplement.
RESULTS
Study Population
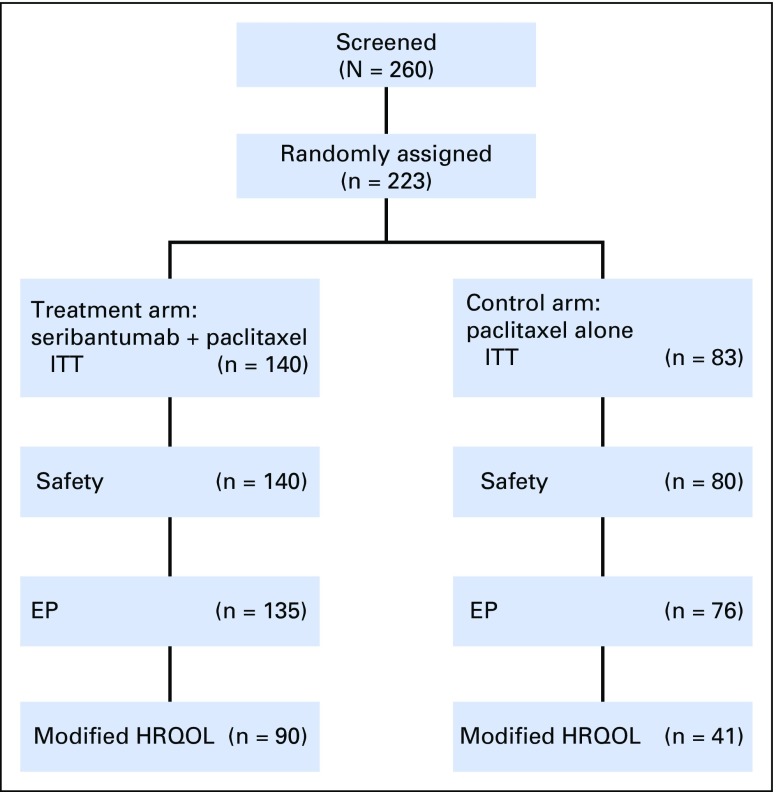
A total of 223 patients in North America and Europe were randomly assigned between February 28, 2012, and March 12, 2013, to receive seribantumab plus paclitaxel (n = 140) or paclitaxel alone (n = 83). Trial flow, ITT population, and subgroups for analysis are shown in Figure 1. Patient demographics and baseline characteristics were well balanced between treatment arms (Table 1).
Fig 1.
CONSORT diagram. EP, evaluable population; HRQOL, health-related quality of life; ITT, intent to treat.
Table 1.
Patient Demographic and Clinical Characteristics
Efficacy
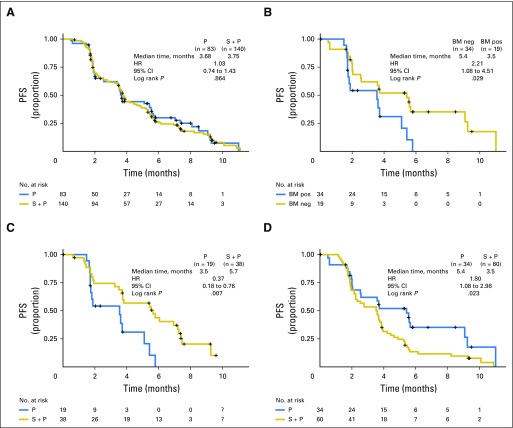
The data cutoff for the primary analysis was August 1, 2013. Median PFS in the ITT population was 3.75 months in the experimental arm compared with 3.68 months in the control arm (HR, 1.027; 95% CI, 0.741 to 1.425; stratified log-rank P = .864; events, 76.7%). The study therefore did not meet its primary end point of prolonging PFS in unselected patients. Median OS was 13.70 months in the experimental arm compared with 10.12 months in the control arm (HR, 0.991; 95% CI, 0.62 to 1.584; stratified log-rank P = .972; events, 35.4%). ORR was 13.6% (95% CI, 7.9% to 19.6%) in the experimental arm compared with 18.1% (95% CI, 9.8% to 26.4%) in the control arm, with no complete responses in either arm. HRQOL assessments detected no significant changes from baseline between treatment arms.
Safety
The overall safety profile was proportionate across study arms, with 140 patients (100%) in the experimental arm and 79 (98.8%) in the control arm reporting at least one treatment-emergent AEs (TEAEs). An overall increase in all-grade GI toxicities was observed; a majority of events, however, were reported as mild to moderate in severity (Table 2). Grade 3 or greater TEAEs occurred in 50 patients (35.7%) in the experimental arm and 24 (30.0%) in the control arm. Serious TEAEs occurred in 59 patients (42.1%) in the experimental arm and 25 (31.3%) in the control arm, whereas AEs leading to death were noted in 11 (7.9%) and two patients (2.5%) in the experimental and control arms, respectively. None of these deaths was related to study treatment, except in one patient who received seribantumab plus paclitaxel and died as a result of an infection or infestation.
Table 2.
TEAEs Occurring in ≥ 10% of Study Population
A review of venous thromboembolic events (VTEs) revealed a difference in the rate of occurrence of pulmonary embolism, but not overall VTEs, between the experimental and control arms. Eight patients (5.7%) in the experimental arm and six (7.5%) in the control arm had at least one treatment-emergent VTE. Among patients in the experimental arm, six pulmonary embolisms (4.3%) occurred, with five (3.6%) reported as grade 3. All these patients received corrective therapy and recovered. One nonserious AE (1.3%) of pulmonary embolism (grade 3) occurred on the control arm, which did not resolve. Overall, the safety profile was consistent with expected toxicities associated with ErbB inhibitors and combination with paclitaxel.
Biomarkers
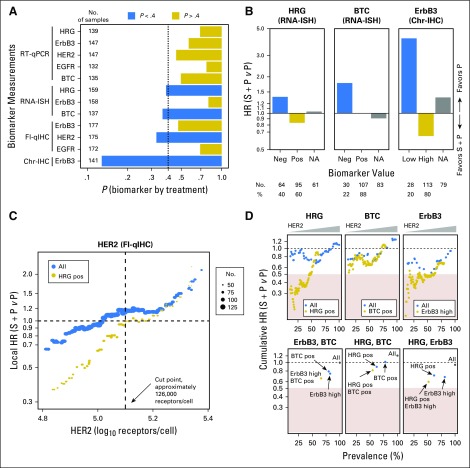
All biomarker analyses, unless otherwise noted, were performed using data from pretreatment biopsies. In total, five biomarkers were measured using 13 assays (Appendix Table A2, online only). Although pretreatment biopsies were mandated for all patients, some samples did not contain sufficient tumor material. The number of patients with available data for each assay is summarized in Figure 2A.
Fig 2.
Biomarker analyses of progression-free survival. (A) Significance testing of biomarkers and assays. Assays showing a treatment interaction (P < .4) are shown in blue. The number of patients for whom biomarker data were available for each assay is shown to the left. (B) Hazard ratio estimations by biomarker status for heregulin (HRG), betacellulin (BTC), and human epidermal growth factor receptor 3 (ErbB3) in all patients for whom biomarker data were available. HRG negative (neg), HRG score < 1; HRG positive (pos), HRG score ≥ 1; BTC neg, BTC score < 1; BTC pos, BTC score ≥ 1; ErbB3 low, ErbB3 score < 2; ErbB3 high, ErbB3 score ≥ 2. (C) Local hazard ratio (HR) scan of human epidermal growth factor receptor 2 (HER2) in all patients with available data (blue) or in the subset of these patients who were HRG pos (gold). Local HR was estimated by including all patients with HER2 levels within one standard deviation of the measurement error. The number of patients used for each HR calculation is indicated by the size of the dot. (D) Cumulative HR scans of two-way combinations of biomarkers that passed significance testing by univariable analysis. In the top three plots, cumulative HR was estimated as a function of HER2 threshold, either in all patients for whom biomarker data were available (blue) or in patients positive for the second biomarker (gold; HRG pos, BTC pos, or ErbB3 high [score ≥ 2]). In the bottom three plots, points represent the estimated HR for all patients with available biomarker data (gray), patients positive for one of the biomarkers (blue), or patients positive for both biomarkers (gold). In all six plots, the x-axis indicates the percentage of patients who meet the criteria. Yellow rectangles indicate HR < 0.5. Chr-IHC, chromogenic immunohistochemistry; Fl-qIHC, fluorescence-based quantitative immunohistochemistry; ISH, in situ hybridization; RT-qPCR, reverse-transcriptase quantitative polymerase chain reaction.
Univariable biomarker analysis.
The strength of biomarker-by-treatment interaction was used to rank the biomarkers, assays, and scoring methods (Fig 2A). On the basis of a predefined criterion of P < .4, four biomarkers and their associated assays were prioritized for further analysis: HRG chromogenic RNA ISH, ErbB3 chromogenic IHC, BTC chromogenic RNA ISH, and HER2 fluorescence-based quantitative IHC. Relationships between biomarker values and HRs are summarized in Figures 2B and 2C. Three of the biomarkers (HRG, BTC, and ErbB3) are semiquantitative, and cut points were chosen from a limited set of options based on prevalence (Data Supplement). Patients with detectable levels of HRG (score ≥ 1; 60% of 159 patients with evaluable samples) had a PFS HR that favored the experimental arm, whereas those with undetectable levels of HRG (score < 1; 40% of 159 patients) favored the control arm (Fig 2B). In contrast, patients with detectable levels of BTC (score of ≥ 1; 78% of 137 patients) showed no benefit from seribantumab (PFS HR, approximately 1), and those with undetectable levels (score < 1; 22% of 137 patients) favored the control arm (Fig 2B). For ErbB3, medium to high levels (score ≥ 2; 80% of 141 patients) favored the experimental arm, whereas low or undetectable levels (score < 2; 20% of 141 patients) favored the control arm (Fig 2B). For HER2, which was measured quantitatively, the relationship between HER2 levels and HR was visualized by plotting HR as a function of HER2 (Fig 2C, blue dots; Data Supplement). Low HER2 favored the experimental arm, with benefit from seribantumab observed at HER2 levels below approximately 5.0 on a log10 scale (100,000 receptors per cell).
Bivariable biomarker analysis.
Six two-biomarker models were constructed using the four biomarkers that passed significance testing. For each pair of biomarkers, cumulative HR (calculated using all patients with biomarker levels above or below a given cutoff) was plotted as a function of one biomarker and then repeated at different values of the other biomarker (Fig 2D; Data Supplement). On the basis of the tradeoff between HR and prevalence, HRG and HER2 were identified as the most favorable pair of biomarkers (Fig 2D). To explore this interaction further, local HR was plotted as a function of HER2 level for all patients with available HRG and HER2 data, as well as for the subpopulation of HRG-positive patients (Fig 2C). Notably, HR was consistently lower (ie, more favorable) in the HRG-positive subpopulation, particularly at low levels of HER2.
On the basis of the HR scan in HRG-positive patients, a cut point was chosen for HER2 as the level below which the experimental arm was favored relative to the control arm (HR < 1). This corresponded to an HER2 level of 5.1 on a log10 scale, or approximately 126,000 receptors per cell. Selection of this cut point was confirmed by performing five-fold cross validation (Data Supplement). Coincidentally, this closely matches the threshold separating a score of 1+ from 2+ on a standard HercepTest assay (Dako, Carpinteria, CA),22 suggesting a potentially standardized way to identify patients in future trials. For all additional analyses, a biomarker-positive subpopulation was defined as having HRG scores of 1 or greater and HER2 levels of 126,000 or fewer receptors per cell. This definition resulted in a biomarker-positive prevalence of 38%.
Characteristics of biomarker-positive subpopulation.
In total, HRG and HER2 data were available for 151 patients (biomarker-evaluable population). The PFS HR of this population was 1.09 (95% CI, 0.74 to 1.62; P = .719), which compared well with the safety population (HR, 1.06; 95% CI, 0.76 to 1.48; P = .801). Of these 151 patients, 57 (38%) were biomarker positive, and 94 (62%) were biomarker negative.
Kaplan-Meier PFS plots are shown in Figure 3. Corresponding analyses for OS and ORR are summarized in Appendix Table A3 (online only) and Appendix Figure A1 (online only). In the paclitaxel arm, the biomarkers seemed prognostic of rapid progression, with a PFS HR of 2.21 (biomarker positive v negative: 95% CI, 1.08 to 4.51; P = .029; Fig 3B). Comparing experimental and control arms, the biomarkers also seemed predictive of benefit from seribantumab; in the biomarker-positive subpopulation, the PFS HR was 0.37 (95% CI, 0.18 to 0.76; P = .007; Fig 3C). Notably, the control arm was favored in the biomarker-negative subpopulation, with a PFS HR of 1.80 (95% CI, 1.08 to 2.98; P = .023; Fig 3D).
Fig 3.
Kaplan-Meier estimated progression-free survival (PFS) curves by biomarker status. (A) PFS for unselected patients in the intent-to-treat population (seribantumab [S] + paclitaxel [P] v P). (B) PFS for control arm patients in the biomarker (BM) –evaluable population (P alone; BM positive [pos] v BM negative [neg]). (C) PFS for BM-pos patients in the BM-evaluable population (S + P v P). (D) PFS for BM-negative patients in the BM-evaluable population (S + P v P). BM pos, heregulin (HRG) score ≥ 1 and human epidermal growth factor receptor 2 (HER2) < 126,000 receptors per cell); BM neg, HRG score < 1 and/or HER2 ≥ 126,000 receptors per cell. HR, hazard ratio.
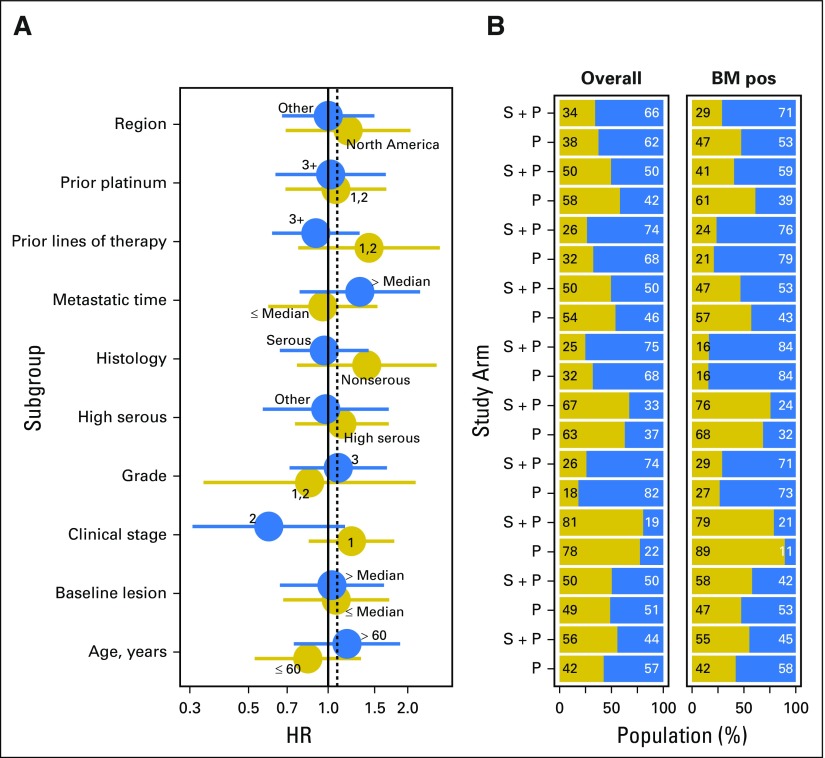
The robustness of these results was evaluated by determining how clinical variables affected PFS and how these variables were distributed between study arms in the biomarker-positive subpopulation compared with the safety population. Overall, similar distributions were observed for each covariate (Appendix Fig A2, online only).
Biomarker status in archived versus pretreatment samples.
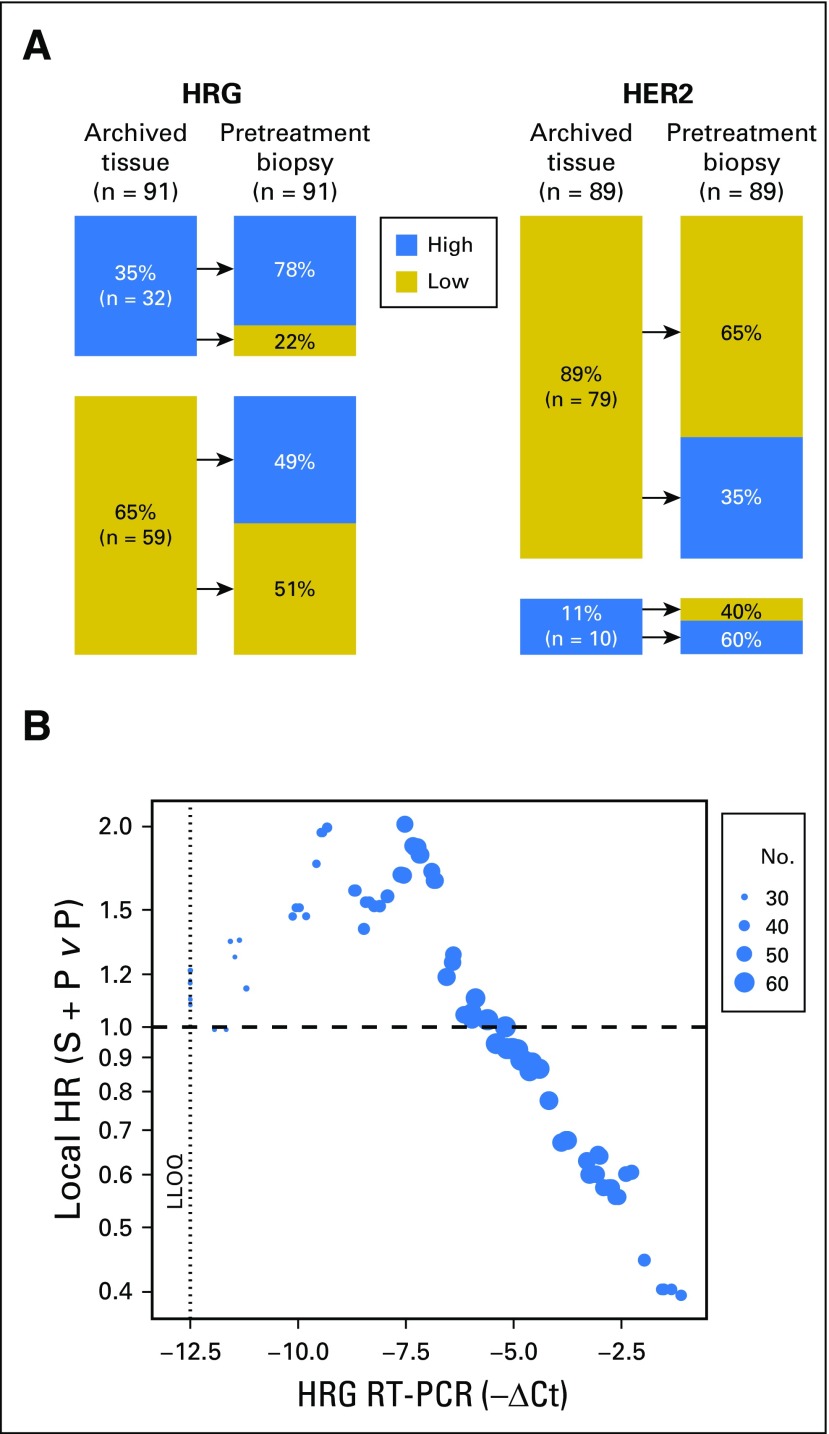
To determine if the biomarkers changed from time of initial diagnosis to time of study entry, HRG and HER2 were measured in archived tissue blocks and pretreatment biopsies (Fig 4A). Although 86% of patients were HER2 low (< 126,000 receptors per cell) at initial diagnosis, 35% of these patients had HER2 levels above this threshold in their pretreatment biopsies. Patients who were HRG positive in their archived tissue (35% of samples) tended to remain HRG positive (78%), whereas approximately 50% of patients who were HRG negative in their archived samples were HRG positive in their pretreatment biopsies.
Fig 4.
Biomarker analyses in archived tissue. (A) Concordance of heregulin (HRG) and human epidermal growth factor receptor 2 (HER2) status between archived tissue and pretreatment biopsies in patient-matched samples. HRG high, HRG score ≥ 1; HRG low, HRG score < 1; HER2 high, HER2 ≥ 126,000 receptors per cell; HER2 low, HER2 < 126,000 receptors per cell. (B) Relationship between local progression-free survival hazard ratio (HR; seribantumab [S] + paclitaxel [P] v P) and HRG reverse-transcriptase quantitative polymerase chain reaction (RT-PCR) values in archived tissue. HRs were estimated at different values of HRG by including all patients with HRG levels within one standard deviation of the measurement error. The number of patients used for each HR estimation is indicated by the size of the dot. LLOQ, lower limit of quantification. CT, cycle threshold.
Because patients with detectable HRG in their archived tissue tended to remain HRG positive, the effect of HRG level on PFS HR was assessed using results from the HRG reverse-transcriptase polymerase chain reaction assay performed on archived tissue (Fig 4B). Consistent with the RNA ISH results in pretreatment biopsies, favorable HRs were observed at high levels of HRG mRNA.
DISCUSSION
In the general population, the addition of seribantumab to paclitaxel did not result in an improvement in PFS, the primary study end point. A potential PFS and OS benefit was observed, however, in patients whose tumors were positive for HRG and low for HER2. Preclinical studies have shown that HRG induces a more aggressive and treatment-insensitive phenotype in tumor cells.10-12,23,24 Seribantumab inhibits HRG from binding to ErbB3, thereby reversing this aggressive phenotype. In our study, five potential biomarkers of ErbB3 activation were investigated.15 Univariable analyses revealed that three of these biomarkers (HRG, HER2, and ErbB3) differentially affected the experimental and control arms, and all three effects were directionally consistent with preclinical predictions (Appendix Table A1). Notably, the cut point for HER2 of approximately 126,000 receptors per cell agreed well with the HER2 level above which seribantumab exhibits decreased potency25 (Data Supplement). A relatively lenient criterion (P < .4) was used to identify biomarkers for bivariate analyses, because prior preclinical studies had indicated that more than one biomarker may be needed to accurately predict the activity of seribantumab.15 Although the importance of HRG was expected, we did not anticipate such a prominent role for HER2 expression in platinum-resistant ovarian cancer, based on its observed levels at the time of diagnosis.
The combination of HRG and HER2 as biomarkers provided the optimal balance between benefit and prevalence, with a PFS HR of 0.37 and prevalence of 38%. This biomarker pair also identified a subgroup of patients in the control arm who were particularly insensitive to paclitaxel. Interestingly, the PFS curve of biomarker-positive patients receiving seribantumab plus paclitaxel closely resembles that of biomarker-negative patients receiving paclitaxel alone (Figs 3B and 3C). This suggests that in this setting, seribantumab may act primarily by blocking resistance to therapy, rather than by inhibiting a driver of tumor growth.
Patient-matched pairs of biomarker measurements showed that pretreatment samples provided better predictive power than archived tissue. Additionally, patients with HRG-positive primary tumors tended to remain HRG positive, whereas approximately 50% of patients with HRG-negative primary tumors presented as HRG positive in their biopsies. This suggests that HRG expression increases in response to cytotoxic therapy and serves as a mechanism of acquired resistance.16
One unanticipated finding was that biomarker-negative patients experienced progression faster in the experimental compared with control arm. This effect is not unique to seribantumab; a similar effect was observed with other RTK inhibitors, including the anti–insulin-like growth factor-1 receptor antibodies figitumumab26 and dalotuzumab,27 the anti-EGFR antibodies cetuximab28 and panitumumab,29 the anti-Met antibody onartuzumab,30 and the anti-HER2/ErbB3 antibody pertuzumab31 (Appendix Table A4, online only). It is not currently known why these agents may accelerate progression when their target pathway is inactive. It is possible that some of these agents act as weak ligands, partially activating their target pathways. They may also trigger compensatory signaling through alternative pathways that are otherwise dormant. A third intriguing hypothesis is that these antibodies inhibit their target pathways in nontumor cells, including cells of the immune system. Whether any of these mechanisms occur with ErbB3 inhibitors remains to be determined.
One of the caveats of this study is that multiple biomarkers were analyzed retrospectively, raising the possibility of false discovery. This concern is mitigated by the mechanistic relevance of these findings, as well as by their consistency with preclinical predictions in terms of the direction of the treatment effect in this marker-specific subgroup. In addition, the findings in this study agree well with those from two other randomized studies of seribantumab: one in combination with exemestane in women with advanced hormone-refractory, HER2-negative breast cancer32,33 and one in combination with erlotinib in patients with EGFR wild-type advanced non–small-cell lung cancer.34 In both studies, HRG seemed prognostic of decreased PFS in the control arm and predictive of PFS benefit from the addition of seribantumab. Most patients in these studies had low or undetectable levels of HER2. Recently, Mendell et al35 found that HRG seemed predictive of benefit from patritumab, another ErbB3-directed antibody, when combined with erlotinib in non–small-cell lung cancer. In addition, Qian et al36 reported that HRG may be prognostic of decreased OS in oropharyngeal squamous cell carcinoma, further signifying its role in cancer biology. That several independent studies across different cancer types found HRG to be prognostic of poor outcome and predictive of benefit from ErbB3 inhibitors suggests that HRG-mediated resistance may be a broad phenomenon in cancer and that ErbB3 inhibition may be part of a general strategy to combat insensitivity to anticancer agents.
Appendix
Table A1.
Predefined Biomarker Hypotheses
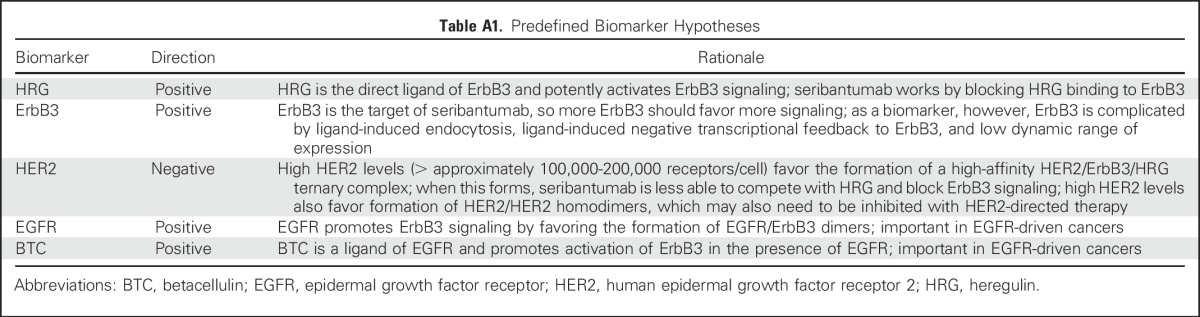
Table A2.
Biomarkers and Assays
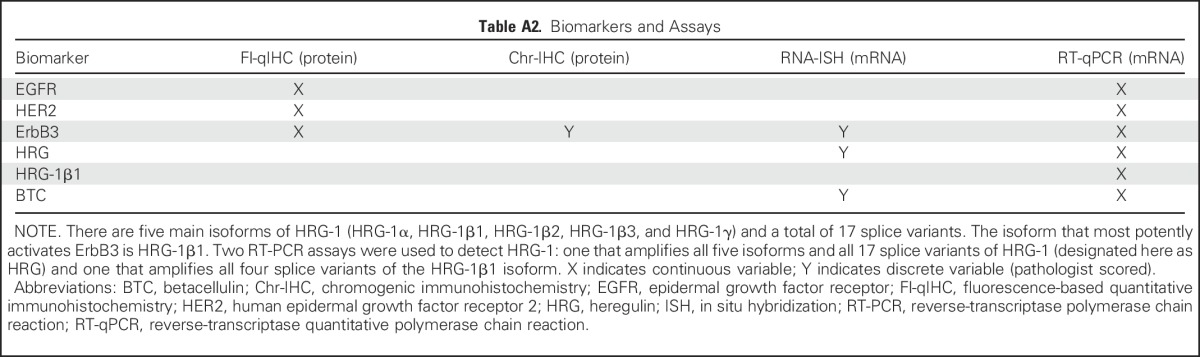
Table A3.
Best Response Rates by Treatment and Biomarker Status
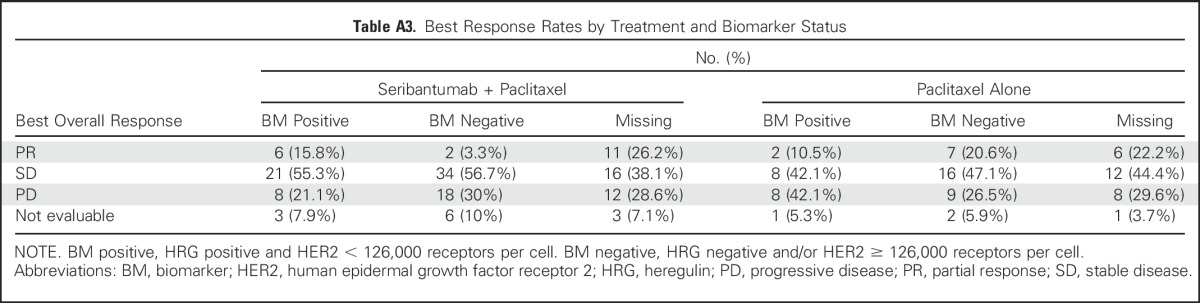
Table A4.
RTK-Directed Antibodies Seeming to Accelerate Progression in Patients Negative for Reported Biomarker
Fig A1.
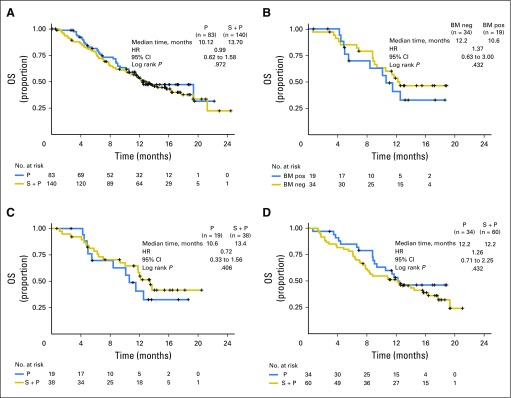
Kaplan-Meyer estimated overall survival (OS) curves by biomarker status. (A) OS for unselected patients in the intent-to-treat population (seribantumab [S] + paclitaxel [P] v P). (B) OS for control arm patients in the biomarker (BM) -evaluable population (P alone; BM positive [pos] v BM- negative [neg]). (C) OS for BM-positive patients in the BM-evaluable population (S + P v P). (D) OS for BM-negative patients in the BM-evaluable population (S + P v P). BM pos, heregulin (HRG) pos and human epidermal growth factor receptor 2 (HER2) < 126,000 receptors per cell); BM neg, HRG neg and/or HER2 ≥ 126,000 receptors per cell. HR, hazard ratio.
Fig A2.
Subgroup analyses by baseline clinical covariates. (A) Forest plot of progression-free survival by baseline clinical covariates in the overall safety population. (B) Number of patients in the treatment (seribantumab [S] + paclitaxel [P]) and control (P) arms by clinical covariates in the overall safety population and in the biomarker (BM) –positive (pos) subpopulation. BM pos, heregulin pos and human epidermal growth factor receptor 2 < 126,000 receptors per cell. HR, hazard ratio.
Footnotes
Supported by Merrimack Pharmaceuticals.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01447706.
See accompanying editorial on page 4312
AUTHOR CONTRIBUTIONS
Conception and design: Joyce F. Liu, Andrés M. Poveda, Isabelle Tabah-Fisch, Victor Moyo, Rachel Nering, William Kubasek, Akos Czibere, Ignace Vergote, Gavin MacBeath, Eric Pujade-Lauraine
Provision of study materials or patients: Joyce F. Liu, Isabelle Ray-Coquard, Frederic Selle, Hal Hirte, Felix Hilpert, Salvatore Siena, Robert L. Coleman, Eric Pujade-Lauraine
Collection and assembly of data: Joyce F. Liu, Isabelle Ray-Coquard, Frederic Selle, Andrés M. Poveda, David Cibula, Hal Hirte, Felix Hilpert, Francesco Raspagliesi, Laurence Gladieff, Philipp Harter, Salvatore Siena, Joseph Pearlberg, Kaveh Riahi, Rachel Nering, Bambang Adiwijaya, Akos Czibere, R. Wendel Naumann, Robert L. Coleman, Ignace Vergote, Gavin MacBeath, Eric Pujade-Lauraine
Data analysis and interpretation: Joyce F. Liu, Isabelle Ray-Coquard, Hal Hirte, Josep Maria del Campo, Isabelle Tabah-Fisch, Rachel Nering, William Kubasek, Bambang Adiwijaya, Akos Czibere, R. Wendel Naumann, Robert L. Coleman, Ignace Vergote, Gavin MacBeath
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase II Trial of Seribantumab in Combination With Paclitaxel in Patients With Advanced Platinum Resistant- or -Refractory Ovarian Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Joyce F. Liu
Consulting or Advisory Role: AstraZeneca, Genentech
Research Funding: Genentech (Inst), AstraZeneca (Inst), Merrimack Pharmaceuticals (Inst), Boston Biomedical (Inst), Atara Biotherapeutics (Inst), Acetylon Pharmaceuticals (Inst)
Isabelle Ray-Coquard
Honoraria: Roche, PharmaMar, AstraZeneca
Consulting or Advisory Role: Pfizer, AbbVie, Amgen
Frederic Selle
Consulting or Advisory Role: Genentech, PharmaMar, Merck Sharp & Dohme
Travel, Accommodations, Expenses: Genentech, PharmaMar, Merck Sharp & Dohme
Andrés M. Poveda
Consulting or Advisory Role: Roche, AstraZeneca, Novartis, Immunogen
Travel, Accommodations, Expenses: PharmaMar
David Cibula
No relationship to disclose
Hal Hirte
Honoraria: AstraZeneca, Roche
Consulting or Advisory Role: AstraZeneca
Felix Hilpert
Honoraria: Roche, Merck Sharp & Dohme, AstraZeneca, Johnson & Johnson
Consulting or Advisory Role: Roche, Merck Sharp & Dohme, AstraZeneca
Travel, Accommodations, Expenses: Roche, PharmaMar, Celgene, AstraZeneca
Francesco Raspagliesi
Consulting or Advisory Role: Roche, PharmaMar, AstraZeneca
Laurence Gladieff
Honoraria: PharmaMar, AstraZeneca
Travel, Accommodations, Expenses: Roche
Philipp Harter
Honoraria: AstraZeneca, Roche
Consulting or Advisory Role: AstraZeneca, Roche, PharmaMar
Travel, Accommodations, Expenses: Medac
Salvatore Siena
Consulting or Advisory Role: Amgen, Bayer HealthCare Pharmaceuticals, Eli Lilly, Merck, Roche, Sanofi
Josep Maria del Campo
Consulting or Advisory Role: PharmaMar, Merck Sharp & Dohme
Speakers’ Bureau: Merck Sharp & Dohme, PharmaMar, Roche
Isabelle Tabah-Fisch
Employment: Sanofi
Stock or Other Ownership: Sanofi
Joseph Pearlberg
Employment: Infinity Pharmaceuticals
Victor Moyo
Employment: Merrimack Pharmaceuticals
Leadership: L.E.A.F. Pharmaceuticals
Stock or Other Ownership: Merrimack Pharmaceuticals
Consulting or Advisory Role: Merrimack Pharmaceuticals, Diffusion Pharmaceuticals
Travel, Accommodations, Expenses: Merrimack Pharmaceuticals
Kaveh Riahi
Employment: Merrimack Pharmaceuticals, Genentech
Stock or Other Ownership: Merrimack Pharmaceuticals, Genentech
Rachel Nering
Employment: Merrimack Pharmaceuticals
Stock or Other Ownership: Merrimack Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Merrimack Pharmaceuticals
William Kubasek
Employment: Merrimack Pharmaceuticals
Stock or Other Ownership: Merrimack Pharmaceuticals, Gilead Sciences, Merck, Curis, Medivation, AMAG Pharmaceuticals, Threshold Pharmaceuticals, AbbVie, Juno Therapeutics, Epizyme, Galena Biopharma, Endocyte, Arena Pharmaceuticals, Silver Creek Pharmaceuticals, Exact Sciences
Consulting or Advisory Role: Merrimack Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Merrimack Pharmaceuticals
Bambang Adiwijaya
Employment: Merrimack Pharmaceuticals
Stock or Other Ownership: Merrimack Pharmaceuticals
Expert Testimony: Gilead Sciences
Akos Czibere
Employment: Merrimack Pharmaceuticals
Stock or Other Ownership: Merrimack Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Merrimack Pharmaceuticals
R. Wendel Naumann
Consulting or Advisory Role: Clovis Oncology, Janssen Oncology
Robert L. Coleman
Honoraria: National Comprehensive Cancer Network
Consulting or Advisory Role: Clovis Oncology, Genentech, Esperance Pharmaceuticals, National Comprehensive Cancer Network, Department of Defense Congressionally Directed Medical Research Program
Research Funding: AstraZeneca, Esperance Pharmaceuticals, OncoMed, Array BioPharma, Clovis Oncology, Amgen, Johnson & Johnson, Merck
Travel, Accommodations, Expenses: Millennium Pharmaceuticals, Merck, Amgen, AstraZeneca, Array BioPharma, Gradalis, Bayer HealthCare Pharmaceuticals, Clovis Oncology, Genentech, Research to Practice, University of California Irvine, New Mexico Cancer Center, University of Miami, University of Cincinnati Cancer Center
Ignace Vergote
No relationship to disclose
Gavin MacBeath
Employment: Merrimack Pharmaceuticals
Leadership: Merrimack Pharmaceuticals
Stock or Other Ownership: Merrimack Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Merrimack Pharmaceuticals
Eric Pujade-Lauraine
Honoraria: Roche, AstraZeneca, Pfizer
Consulting or Advisory Role: Roche, AstraZeneca, Pfizer
Travel, Accommodations, Expenses: Roche, AstraZeneca, Pfizer
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Naumann RW, Coleman RL. Management strategies for recurrent platinum-resistant ovarian cancer. Drugs. 2011;71:1397–1412. doi: 10.2165/11591720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Lortholary A, Largillier R, Weber B, et al. Weekly paclitaxel as a single agent or in combination with carboplatin or weekly topotecan in patients with resistant ovarian cancer: The CARTAXHY randomized phase II trial from Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens (GINECO) Ann Oncol. 2012;23:346–352. doi: 10.1093/annonc/mdr149. [DOI] [PubMed] [Google Scholar]
- 4.Poveda AM, Selle F, Hilpert F, et al. Bevacizumab combined with weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan in platinum-resistant recurrent ovarian cancer: Analysis by chemotherapy cohort of the randomized phase III AURELIA trial. J Clin Oncol. 2015;33:3836–3838. doi: 10.1200/JCO.2015.63.1408. [DOI] [PubMed] [Google Scholar]
- 5.Karlan BY, Oza AM, Richardson GE, et al. Randomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer. J Clin Oncol. 2012;30:362–371. doi: 10.1200/JCO.2010.34.3178. [DOI] [PubMed] [Google Scholar]
- 6.Monk BJ, Poveda A, Vergote I, et al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): A randomised, multicentre, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:799–808. doi: 10.1016/S1470-2045(14)70244-X. [DOI] [PubMed] [Google Scholar]
- 7.Holohan C, Van Schaeybroeck S, Longley DB, et al. Cancer drug resistance: An evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 8.Leto SM, Trusolino L. Primary and acquired resistance to EGFR-targeted therapies in colorectal cancer: Impact on future treatment strategies. J Mol Med (Berl) 2014;92:709–722. doi: 10.1007/s00109-014-1161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson TR, Fridlyand J, Yan Y, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–509. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amin DN, Campbell MR, Moasser MM. The role of HER3, the unpretentious member of the HER family, in cancer biology and cancer therapeutics. Semin Cell Dev Biol. 2010;21:944–950. doi: 10.1016/j.semcdb.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bezler M, Hengstler JG, Ullrich A. Inhibition of doxorubicin-induced HER3-PI3K-AKT signalling enhances apoptosis of ovarian cancer cells. Mol Oncol. 2012;6:516–529. doi: 10.1016/j.molonc.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakrabarty A, Sánchez V, Kuba MG, et al. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci USA. 2012;109:2718–2723. doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellyer NJ, Kim MS, Koland JG. Heregulin-dependent activation of phosphoinositide 3-kinase and Akt via the ErbB2/ErbB3 co-receptor. J Biol Chem. 2001;276:42153–42161. doi: 10.1074/jbc.M102079200. [DOI] [PubMed] [Google Scholar]
- 14.Ma J, Lyu H, Huang J, et al. Targeting of erbB3 receptor to overcome resistance in cancer treatment. Mol Cancer. 2014;13:105. doi: 10.1186/1476-4598-13-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoeberl B, Faber AC, Li D, et al. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer Res. 2010;70:2485–2494. doi: 10.1158/0008-5472.CAN-09-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheng Q, Liu X, Fleming E, et al. An activated ErbB3/NRG1 autocrine loop supports in vivo proliferation in ovarian cancer cells. Cancer Cell. 2010;17:298–310. doi: 10.1016/j.ccr.2009.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoeberl B, Pace EA, Fitzgerald JB, et al. Therapeutically targeting ErbB3: A key node in ligand-induced activation of the ErbB receptor-PI3K axis. Sci Signal. 2009;2:ra31. doi: 10.1126/scisignal.2000352. [DOI] [PubMed] [Google Scholar]
- 18.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Markman M, Bookman MA. Second-line treatment of ovarian cancer. Oncologist. 2000;5:26–35. doi: 10.1634/theoncologist.5-1-26. [DOI] [PubMed] [Google Scholar]
- 21.Thigpen JT, Blessing JA, Ball H, et al. Phase II trial of paclitaxel in patients with progressive ovarian carcinoma after platinum-based chemotherapy: A Gynecologic Oncology Group study. J Clin Oncol. 1994;12:1748–1753. doi: 10.1200/JCO.1994.12.9.1748. [DOI] [PubMed] [Google Scholar]
- 22.Onsum MD, Geretti E, Paragas V, et al. Single-cell quantitative HER2 measurement identifies heterogeneity and distinct subgroups within traditionally defined HER2-positive patients. Am J Pathol. 2013;183:1446–1460. doi: 10.1016/j.ajpath.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Frogne T, Benjaminsen R V, Sonne-Hansen K, et al. Activation of ErbB3, EGFR and Erk is essential for growth of human breast cancer cell lines with acquired resistance to fulvestrant. Breast Cancer Res Treat. 2009;114:263–275. doi: 10.1007/s10549-008-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Huang J, Lyu H, et al. Therapeutic targeting of erbB3 with MM-121/SAR256212 enhances antitumor activity of paclitaxel against erbB2-overexpressing breast cancer. Breast Cancer Res. 2013;15:R101. doi: 10.1186/bcr3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Onsum M, Yarar D, Paragas V, et al: Abstract 4945: Targeting ErbB3-addicted cancers across the HER2 spectrum. Cancer Res 72, 2012 (abstr 4945) [Google Scholar]
- 26.Langer CJ, Novello S, Park K, et al. Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non–small-cell lung cancer. J Clin Oncol. 2014;32:2059–2066. doi: 10.1200/JCO.2013.54.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sclafani F, Kim TY, Cunningham D, et al. A randomized phase II/III study of dalotuzumab in combination with cetuximab and irinotecan in chemorefractory, KRAS wild-type, metastatic colorectal cancer. J Natl Cancer Inst. 2015;107:djv258. doi: 10.1093/jnci/djv258. [DOI] [PubMed] [Google Scholar]
- 28.Bokemeyer C, Van Cutsem E, Rougier P, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: Pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48:1466–1475. doi: 10.1016/j.ejca.2012.02.057. [DOI] [PubMed] [Google Scholar]
- 29.Douillard J-Y, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 30.Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase II trial of onartuzumab in combination with erlotinib in patients with advanced non–small-cell lung cancer. J Clin Oncol. 2013;31:4105–4114. doi: 10.1200/JCO.2012.47.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makhija S, Amler LC, Glenn D, et al. Clinical activity of gemcitabine plus pertuzumab in platinum-resistant ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. J Clin Oncol. 2010;28:1215–1223. doi: 10.1200/JCO.2009.22.3354. [DOI] [PubMed] [Google Scholar]
- 32. Higgins MJ, Doyle C, Paepke S, et al: A randomized, double-blind phase II trial of exemestane plus MM-121 (a monoclonal antibody targeting ErbB3) or placebo in postmenopausal women with locally advanced or metastatic ER+/PR+, HER2-negative breast cancer. J Clin Oncol 32:5s, 2014 (suppl; abstr 587) [Google Scholar]
- 33. MacBeath G, Adiwijaya B, Liu J, et al: A meta-analysis of biomarkers in three randomized, phase 2 stuides of MM-121, a ligand blocking anti-ErbB3 antibody, in patients with ovarian, lung and breast cancers. Presented at the 39th Congress of the European Society for Medical Oncology, Madrid, Spain, September 26-30, 2014. [Google Scholar]
- 34. Sequist LV, Lopez-Chavez A, Doebele RC, et al: A randomized phase 2 trial of MM-121, a fully human monoclonal antibody targeting ErbB3, in combination with erlotinib in EGFR wild-type NSCLC patients. J Clin Oncol 32:5s, 2014 (suppl; abstr 8051) [Google Scholar]
- 35.Mendell J, Freeman DJ, Feng W, et al. Clinical translation and validation of a predictive biomarker for patritumab, an anti-human epidermal growth factor receptor 3 (HER3) monoclonal antibody, in patients with advanced non-small cell lung cancer. EBioMedicine. 2015;2:264–271. doi: 10.1016/j.ebiom.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian G, Jiang N, Wang D, et al. Heregulin and HER3 are prognostic biomarkers in oropharyngeal squamous cell carcinoma. Cancer. 2015;121:3600–3611. doi: 10.1002/cncr.29549. [DOI] [PMC free article] [PubMed] [Google Scholar]