Abstract
Purpose
Expression of programmed death-ligand 1 (PD-L1) is a potential predictive marker for response and outcome after treatment with anti–programmed death 1 (PD-1). This study explored the relationship between anti–PD-1 activity and PD-L1 expression in patients with advanced melanoma who were treated with pembrolizumab in the phase Ib KEYNOTE-001 study (clinical trial information: NCT01295827).
Patients and Methods
Six hundred fifty-five patients received pembrolizumab10 mg/kg once every 2 weeks or once every 3 weeks, or 2 mg/kg once every 3 weeks. Tumor response was assessed every 12 weeks per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 by independent central review. Primary outcome was objective response rate. Secondary outcomes included progression-free survival (PFS) and overall survival (OS). Membranous PD-L1 expression in tumor and tumor-associated immune cells was assessed by a clinical trial immunohistochemistry assay (22C3 antibody) and scored on a unique melanoma (MEL) scale of 0 to 5 by one of three pathologists who were blinded to clinical outcome; a score ≥ 2 (membranous staining in ≥ 1% of cells) was considered positive.
Results
Of 451 patients with evaluable PD-L1 expression, 344 (76%) had PD-L1–positive tumors. Demographic and staging variables were equally distributed among PD-L1–positive and –negative patients. An association between higher MEL score and higher response rate and longer PFS (hazard ratio, 0.76; 95% CI, 0.71 to 0.82) and OS (hazard ratio, 0.76; 95% CI, 0.69 to 0.83) was observed (P < .001 for each). Objective response rate was 8%, 12%, 22%, 43%, 57%, and 53% for MEL 0, 1, 2, 3, 4, and 5, respectively.
Conclusion
PD-L1 expression in pretreatment tumor biopsy samples was correlated with response rate, PFS, and OS; however, patients with PD-L1–negative tumors may also achieve durable responses.
INTRODUCTION
Cancer cells can exploit many pathways to evade the immune system.1 One such mechanism involves the exploitation of endogenous inhibitory checkpoints that serve to terminate the immune response after antigen activation.2-4 Antibodies against cytotoxic T-lymphocyte–associated protein 4, such as ipilimumab, release one such negative regulatory pathway,5 which results in a survival benefit in patients with metastatic melanoma.6,7 Programmed death 1 (PD-1) is another key immune inhibitory checkpoint. The PD-1 pathway is a powerful regulator of peripheral tolerance. Initially described in 1992,8 PD-1 is preferentially expressed by activated T cells, B cells, and myeloid cells in the setting of chronic antigen exposure.9 Two ligands for PD-1 have been identified to date: programmed death-ligand 1 (PD-L1) and programmed death-ligand 2. PD-L1 was first described in 200010 and is widely expressed by myeloid and lymphoid tissues as well as in nonlymphoid tissues, such as the lung, heart, pancreas, and placenta.11 In the tumor microenvironment, PD-L1 is known to be induced by types I and II interferon. Both tumor cells and tumor-infiltrating cells of multiple cancers have been shown to express PD-L1.12-15
Recent clinical trials that assessed monoclonal antibodies against PD-1 and PD-L1 have shown that these therapies provide antitumor activity against diverse tumor histologies. Pembrolizumab, a humanized immunoglobulin G4 monoclonal antibody against PD-1, has been assessed in multiple clinical trials and has demonstrated antitumor activity and a manageable safety profile in patients with solid tumors and hematologic malignancies.16-27 Although these data highlight the effectiveness of PD-1 pathway inhibition, it is clear that most patients, even those with prototypically immunogenic tumors, such as melanoma, will not achieve objective responses. Currently, the best marker of response to anti–PD-1 and anti–PD-L1 antibodies is the presence of PD-L1 in tumor cells or tumor-associated stromal cells.14,15,28 Despite the availability of preliminary evidence that supports the use of PD-L1 as a biomarker, published correlative data remain scarce. Here, we report on the relationship between anti–PD-1 activity and PD-L1 expression in patients with advanced melanoma who were treated with pembrolizumab in the KEYNOTE-001 clinical trial.
PATIENTS AND METHODS
Study Design and Conduct
KEYNOTE-001, an international, multicohort, open-label, phase I study that assessed the efficacy and safety of pembrolizumab in patients with advanced melanoma, was sponsored by Merck & Co (Kenilworth, NJ). The study was conducted in accordance with the protocol, Good Clinical Practice standards, and the Declaration of Helsinki. The protocol and its amendments were approved by the relevant institutional review boards or ethics committees of the participating institutions. All patients provided written informed consent to participate. The study was registered with ClinicalTrials.gov.
As described previously,24 patients with ipilimumab-naive, ipilimumab-treated, or ipilimumab-refractory melanoma were enrolled in non–randomly assigned and randomly assigned cohorts and were treated with pembrolizumab 2 mg/kg once every 3 weeks, 10 mg/kg once every 3 weeks, or 10 mg/kg once every 2 weeks. Regardless of dose or schedule, pembrolizumab was administered intravenously over a 30-minute period on day one of each cycle and continued until disease progression, intolerable toxicity, withdrawal of consent, or investigator decision.
Patient Eligibility
Detailed eligibility criteria have been published previously.16,17 In brief, enrolled patients were ≥ 18 years of age with advanced unresectable melanoma, had measurable disease per investigator assessment, an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate organ function. Previous systemic therapy was limited to two or fewer regimens for patients with ipilimumab-naive disease and was unlimited for patients who were previously treated with ipilimumab. A new tumor biopsy sample collected within 60 days of the first pembrolizumab dose was required. Key exclusion criteria included active autoimmune disease, systemic corticosteroid therapy (except for managing ipilimumab-related adverse events in patients with ipilimumab-refractory disease), and previous treatment with a PD-1 or PD-L1 inhibitor. There was no protocol requirement for baseline magnetic resonance imaging of the brain, but patients with previously treated brain metastases could enroll if they were clinically stable for ≥ 8 weeks. A later protocol amendment permitted patients with brain metastases who were stable for ≥ 4 weeks to enroll.
Assessments
Tumor response was assessed every 12 weeks per RECIST v1.129 by independent central review and per immune-related response criteria30 by investigator review. Pembrolizumab treatment decisions were made on the basis of immune-related response criteria. The primary end point was the objective response rate (ORR) assessed per RECIST v1.1 by central review. Secondary end points included response duration (time from first documentation of response to first documentation of disease progression), progression-free survival (PFS; time from start of treatment to documentation of disease progression or death as a result of any cause), and overall survival (OS; time from start of treatment to death as a result of any cause).
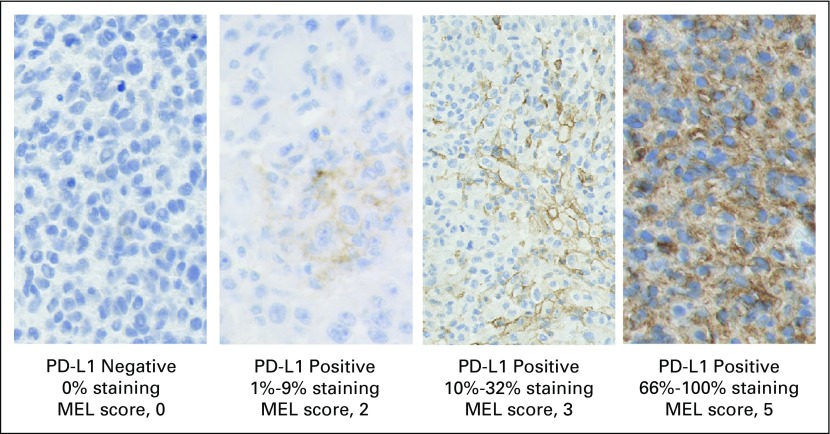
PD-L1 expression was assessed in pretreatment tumor biopsy samples by using an investigational version of an immunohistochemistry assay, which is now commercially available (PD-L1 IHC 22C3 pharmDx; Dako North America, Carpinteria, CA) and approved by the US Food and Drug Administration for use in non–small-cell lung cancer. All samples were obtained within 60 days of the first pembrolizumab dose and were analyzed either internally at Dako North America (n = 195; 30%) or at Labcorp Clinical Trials (Los Angeles, CA; n = 460; 70%). Each sample was scored by one of three pathologists who were blinded to clinical outcome. Examples of stained samples are shown in Fig 1.
Fig 1.
Photomicrographs showing immunohistochemical staining of programmed death-ligand 1 (PD-L1; 22C3 antibody) in melanoma (MEL) samples. MEL score ≥ 2 is considered PD-L1 positive. PD-L1 staining is evidenced by the presence of the brown chromogen, whereas blue is hematoxylin counterstain.
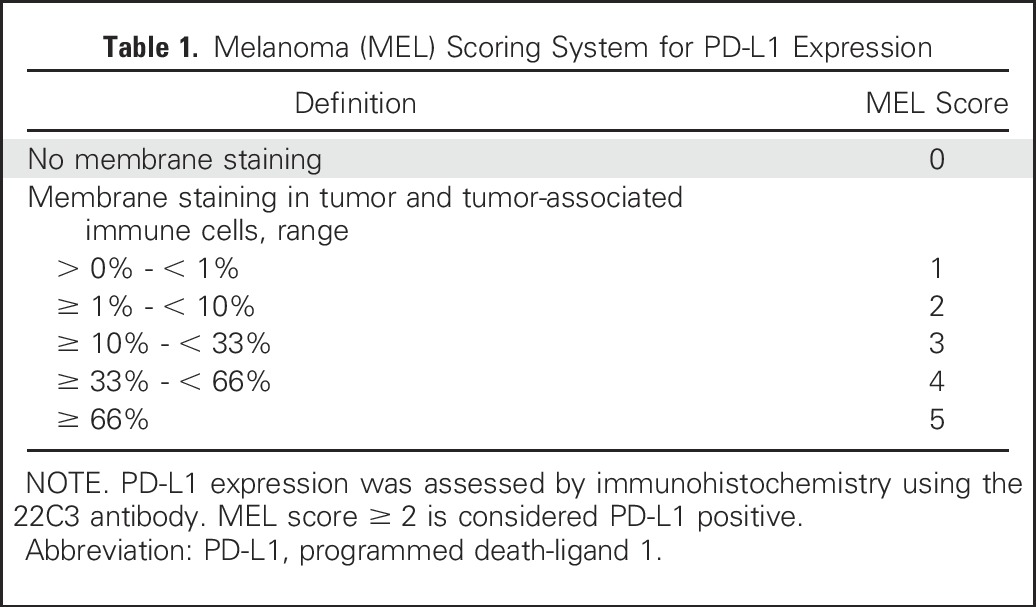
Melanoma samples were scored on the MEL 0 to 5 scale, which was derived from a scheme commonly used for hormone receptors in breast cancer.31 The MEL score was described using the hormone receptor terminology of the Allred proportion score in the European Union pembrolizumab label. However, we abandoned that terminology herein to avoid confusion, because, other than the 0 to 5 scale, PD-L1 scoring for melanoma differs considerably from scoring hormone receptors. Of note, PD-L1 scoring is distinct from that used for non–small-cell lung cancer. Table 1 lists the correspondence between the MEL 0 to 5 scores and the percentage of stained cells. Cells scored include tumor cells themselves as well as mononuclear inflammatory cells, which are intercalated within or are contiguous with tumor nests. Inflammatory cells within the stroma, distinct from the tumor nests, are excluded from scoring. MEL scores of 0 or 1, corresponding to < 1% staining, are considered to be negative, whereas scores of 2 to 5, corresponding to ≥ 1% staining, are considered positive. The positive and negative interpretation is based on receiver operating characteristic analysis, which favored the true-positive rate—and, thus, negative predictive value—at the expense of the false-positive rate.
Table 1.
Melanoma (MEL) Scoring System for PD-L1 Expression
Statistical Analyses
ORR was assessed in all patients with evaluable PD-L1 expression who received one or more doses of study treatment and had measurable disease per RECIST v1.1 by central review. ORR and its associated 95% CI was estimated by using the exact binomial method. The Kaplan-Meier method was used to estimate time-to-event outcomes, including PFS, duration of response, and OS, in all patients with evaluable PD-L1 expression who received one or more doses of study treatment. The relationship between tumor PD-L1 positivity and ORR was explored by using the Miettinen and Nurminen method.32 The relationship between ORR and PD-L1 expression via the MEL score was explored by using logistic regression analysis. The relationships between tumor PD-L1 expression and PFS and OS were explored by Cox proportional hazards regression analysis. All analyses were performed using a data cutoff date of October 18, 2014.
RESULTS
Study Design and Patient Characteristics
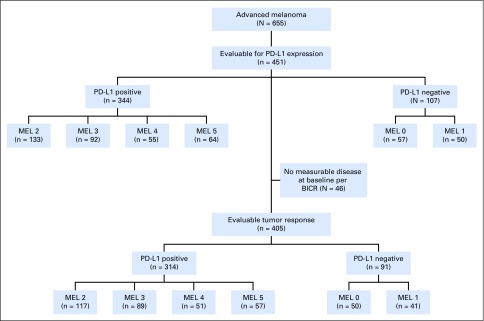
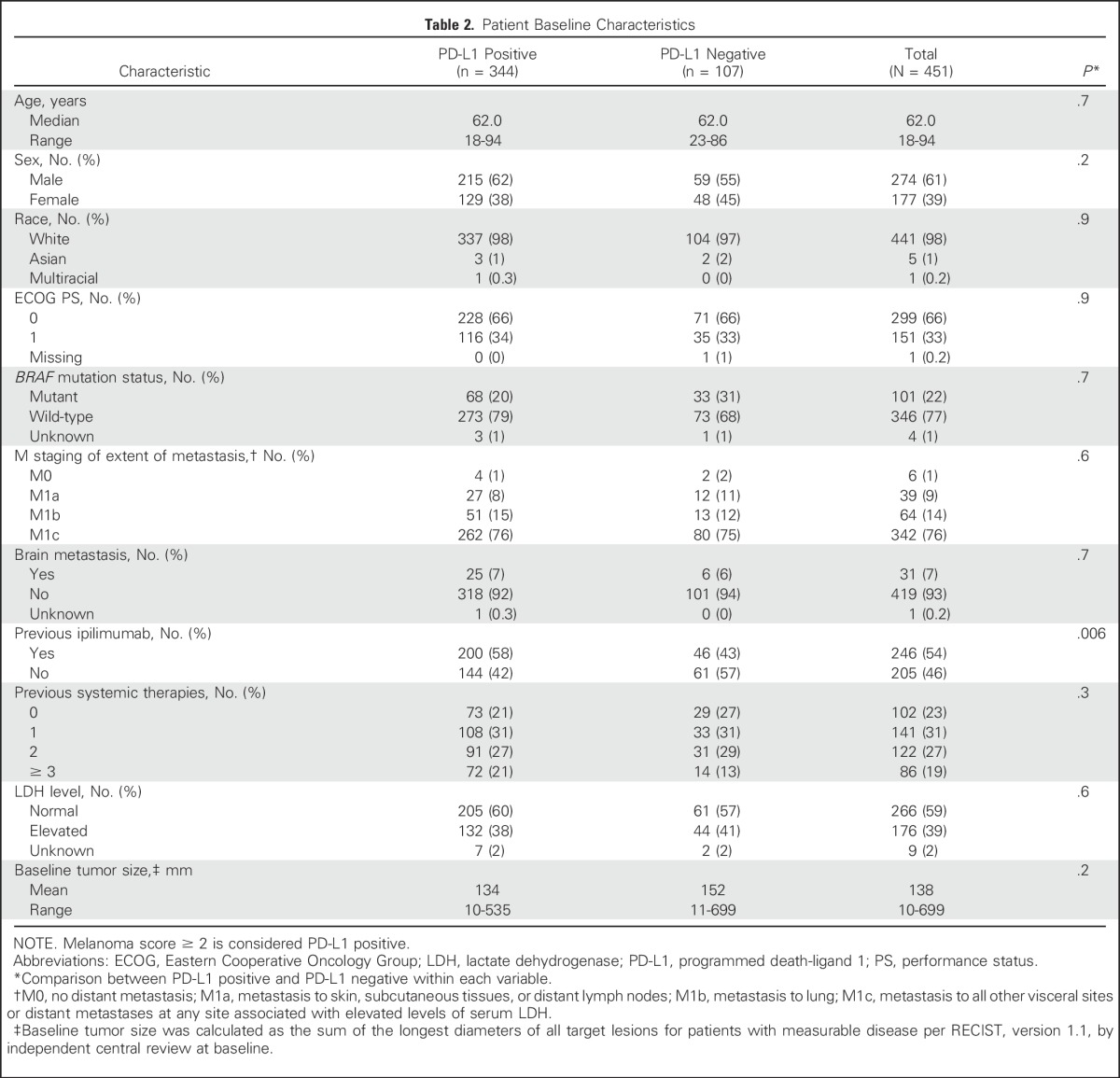
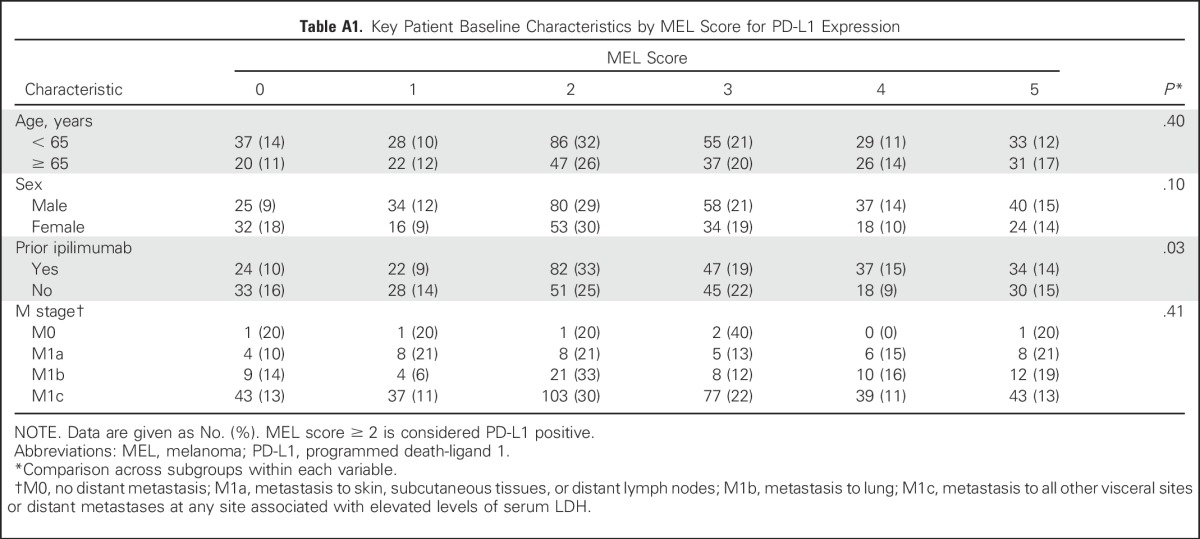
Between December 2011 and September 2013, 655 patients with advanced melanoma were enrolled in KEYNOTE-001 (Appendix Fig A1, online only). Among these 655 patients, biopsy samples from 451 patients that were collected in the 60 days before the first dose of pembrolizumab was administered were evaluable for PD-L1 expression. Of 451 evaluable patients, 344 (76%) had PD-L1–positive tumors and 107 (24%) had PD-L1–negative tumors. Patient baseline demographics and disease characteristics were mostly similar between patients with PD-L1–positive and those with PD-L1–negative tumors, with the exception of higher percentages of BRAF wild-type tumors and ipilimumab treatment in patients with PD-L1–positive tumors (Table 2). A breakdown of key baseline characteristics by MEL score is presented in Appendix Table A1 (online only).
Table 2.
Patient Baseline Characteristics
Relationship Between PD-L1 Expression and Efficacy
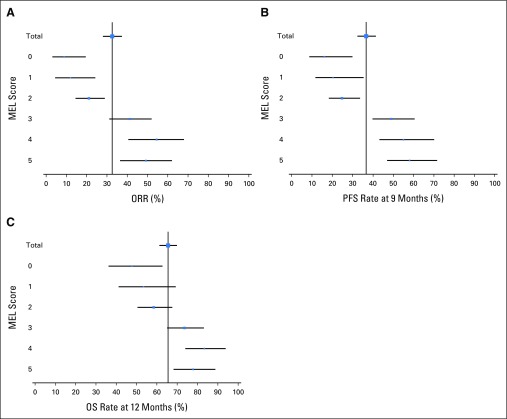
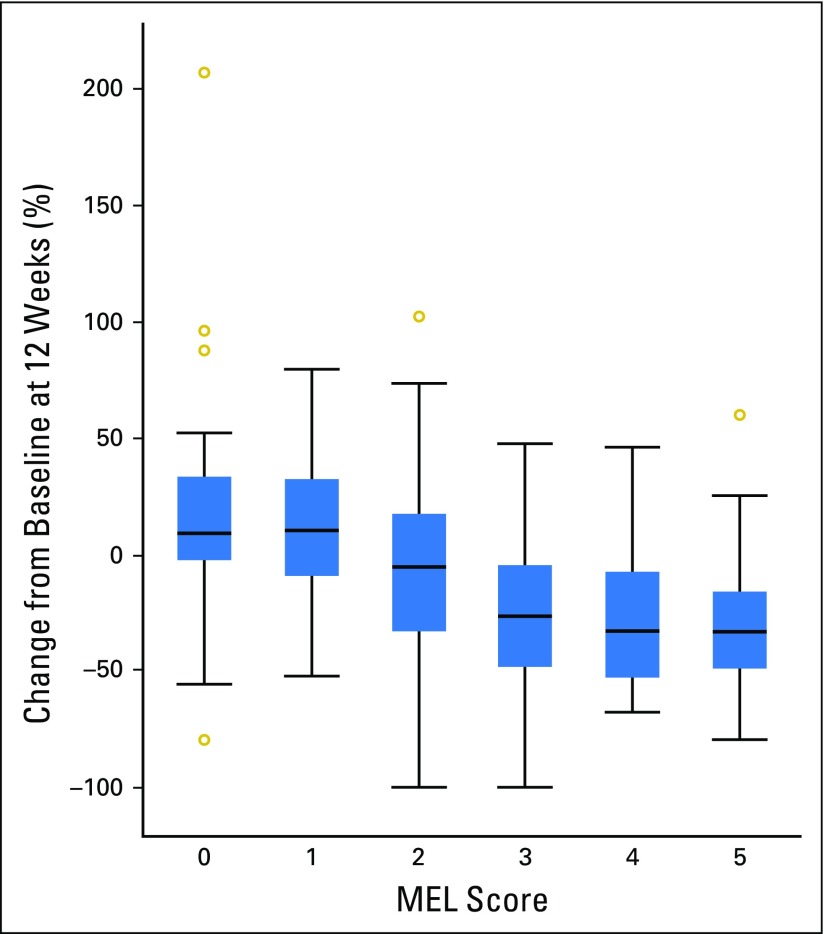
The confirmed ORR per RECIST v1.1 by independent central review was 33% (95% CI, 28% to 37%) in 405 patients who were evaluable for both PD-L1 expression and tumor response. An association between a higher MEL score for PD-L1 expression and ORR was observed (P < .001; Fig 2; panel A). The highest and lowest ORRs were observed in patients with tumors with a MEL score of 4 (ORR, 57%) and 0 (ORR, 8%). The pattern of change in tumor size compared with baseline seemed to vary by MEL score (Fig 3). Among evaluable patients at week 12, 35%, 38%, 57%, 78%, 84%, and 86% showed reductions from baseline tumor measurements for MEL scores 0 to 5, respectively. Of note, patients with MEL scores of 4 or 5, 33% and 22%, respectively, showed a reduction of ≥ 50% at week 12. Qualitatively, patients with MEL scores of 0 or 1 were less likely to have a decrease from baseline tumor size overall, whereas more patients with MEL scores of 2 to 5 had a 100% decrease from baseline.
Fig 2.
Efficacy according to melanoma (MEL) score for programmed death-ligand 1 (PD-L1) expression. (A) Objective response rate (ORR) and associated 95% CIs. (B) Kapan-Meier estimates of progression-free survival (PFS) and associated 95% CIs at 9 months. (C) Kaplan-Meier estimates of overall survival (OS) and associated 95% CIs at 12 months. Response was assessed per RECIST, version 1.1, by independent central review. MEL score ≥ 2 is considered PD-L1 positive.
Fig 3.
Box plots of observed percentage change from baseline in tumor size at week 12 by melanoma (MEL) score. The analysis population was patients with evaluable programmed death-ligand 1 (PD-L1) expression who had tumor size data at week 12 (n = 292). MEL score, 0 (0% staining); MEL score, 1 (> 0% to < 1% staining); MEL score, 2 (≥ 1% to < 10% staining); MEL score, 3 (≥ 10% to < 33% staining); MEL score, 4 (≥ 33% to < 66% staining); and MEL score, 5 (≥ 66% to 100% staining). MEL score ≥ 2 is considered PD-L1 positive. Outer boundaries of boxes represent the 1st and 3rd quartiles, and lines in between represent the median.
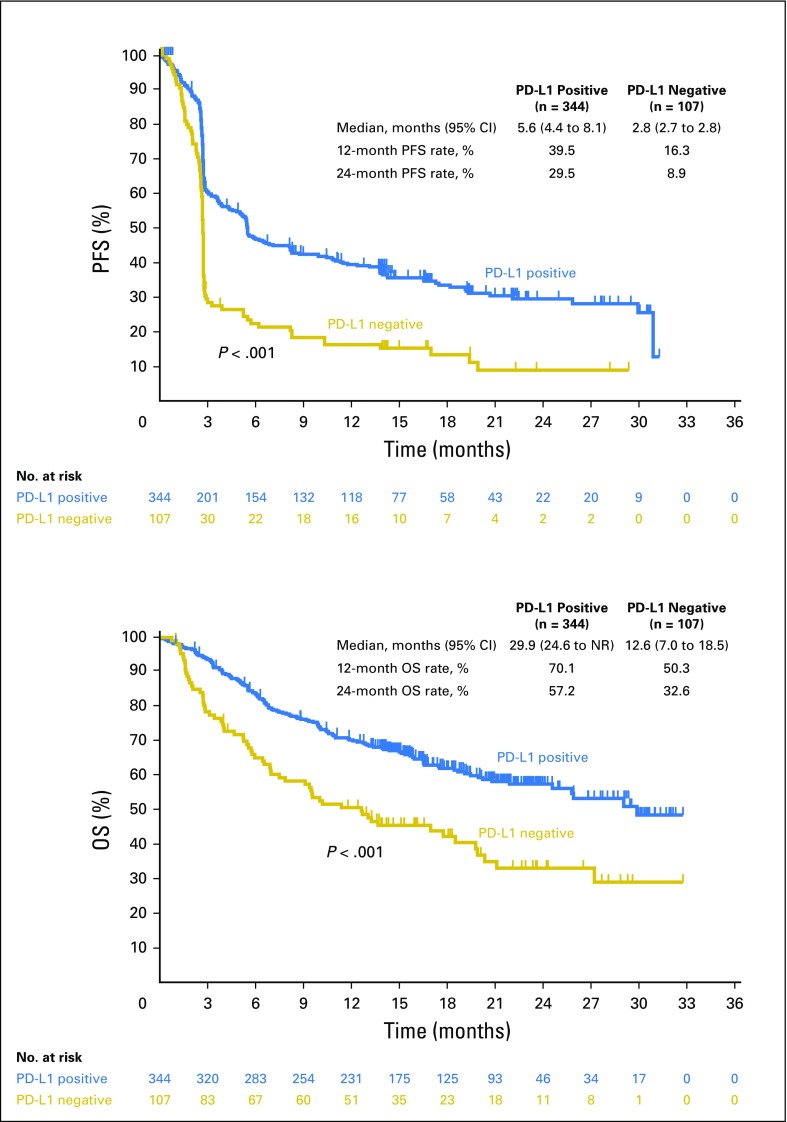
In 451 patients who were evaluable for PD-L1 expression, associations between a higher MEL score and the 9-month PFS and 12-month OS rates were observed (Fig 2; panels B and C). Similar to ORR, patients with tumors with a MEL score of 4 had the best outcomes in terms of survival, whereas a similar proportion of patients with tumors with MEL scores of 4 and 5 were alive and free of progression at 9 months. Associations between higher PD-L1 MEL score and PFS (hazard ratio [HR], 0.76; 95% CI, 0.71 to 0.82; P < .001) and OS (HR, 0.76; 95% CI, 0.69 to 0.83; P < .001) were observed. Similarly, significant associations were also observed when PD-L1 expression was categorized as positive (ie, MEL score 2 to 5) or negative (ie, MEL score 0 or 1; Fig 4), with a HR of 0.51 (95% CI, 0.40 to 0.65) and 0.50 (95% CI, 0.37 to 0.67) for PFS and OS, respectively.
Fig 4.
Efficacy by PD-L1 positivity. (A) Kaplan-Meier estimate of progression-free survival (PFS) assessed per RECIST, version 1.1, by independent central review. (B) Kaplan-Meier estimate of overall survival (OS). Melanoma score ≥ 2 is considered PD-L1 positive. NR, not reached; PD-L1, programmed death-ligand 1.
DISCUSSION
PD-1 and PD-L1 inhibitors are rapidly emerging as a central therapeutic modality for patients with advanced melanoma. Results of recent clinical trials have shown the superiority of pembrolizumab over chemotherapy for patients with ipilimumab-refractory melanoma and, if BRAFV600 mutant, a BRAF inhibitor;25 of pembrolizumab over ipilimumab for patients with ipilimumab-naive melanoma;26 of nivolumab over chemotherapy as first-line therapy;33 and of nivolumab alone or combined with ipilimumab over ipilimumab as first-line therapy.34 Anti–PD-1 and anti–PD-L1 antibodies are also widely active across diverse nonmelanoma cancers.16-27 Given the targeted nature of these antibodies, it seems logical that PD-L1 expression should be correlated with outcome; however, the data that have emerged so far are complex and ambiguous. Whereas PD-L1 expression seems to correlate with response, the thresholds required for response vary widely by both tumor histology and immunohistochemistry method used.14,15 PD-L1 assays that incorporate different cut points to define PD-L1 positivity also pose a challenge for interpretation.35 The variability of tumor PD-L1 status according to assay and cut point used is illustrated by the PD-L1 positivity rates observed in similar advanced melanoma populations in the current study (76.3%) and in a recent randomized trial of ipilimumab plus nivolumab versus each agent alone in patients with treatment-naive melanoma (23.6%).34 Along with differences in assays and antibodies, use of newly collected versus archival tumor samples may impact the rate of PD-L1 positivity.31 Furthermore, PD-L1 expression is dynamic and is affected by many factors, including prior therapy and presence of tumor-infiltrating immune cells,34 which may limit its ability to discriminate between patients who will and will not respond to therapy. Physicians should be made aware of the various antibodies and assays in use, including their differences and their limitations. Of note, the clinical use of the assay described here may be less uniform in practice than readings done by more experienced pathologists. Nonetheless, tumor PD-L1 expression remains of predictive value.36,37 One of the critical unanswered questions is whether PD-L1 expression is correlated with survival, which remains the gold standard for therapeutic intervention. Other correlative markers, such as CD8+ T-cell infiltration of the tumor or of the tumor margin, have also been studied.36 Whereas these are promising biomarker candidates, there are currently few prospective data that assess their potential use.
To our knowledge, the data described here represent one of the most comprehensive analyses of the relationship between PD-L1 expression and treatment outcome presented to date for any tumor. KEYNOTE-001 was a large phase I trial, with 655 patients enrolled in the melanoma arms. Of these 655 patients, 451 patients had tumor samples that were evaluable for PD-L1 expression by using the 22C3 antibody. Clinical outcomes in KEYNOTE-001 were measured rigorously, with radiologic response to therapy evaluated per RECIST v1.1 by independent central review. Using this robust dataset, a relationship was observed between PD-L1 expression assessed by using a unique MEL scoring system and ORR. The highest ORR was observed among patients with an MEL score of 4. Of most importance, survival was also correlated with PD-L1 expression. Reflective of the significance of PD-L1 expression, Kaplan-Meier curves for PFS and OS diverged when PD-L1 expression was categorized as positive (ie, MEL score 2 to 5) and negative (ie, MEL score 0 or 1).
Given the data described here, PD-L1 expression is correlated with clinical outcome in patients with advanced melanoma. Whereas there is some uncertainty regarding the optimal level of PD-L1 expression, a level that corresponds to MEL scores of 3 to 5 seems to best capture the population that is most highly responsive to PD-1 blockade. Conversely, MEL scores of 0 or 1 represents the least responsive population, and a MEL score of 2, which represents the largest proportion of patients (29%), seems to be intermediate, with many patients experiencing a response to therapy. Furthermore, tumors with the highest levels of PD-L1 staining exhibited deeper responses (33% and 22% of patients showed reductions of ≥ 50% at week 12 with MEL scores of 4 and 5, respectively). Although there was no clear evidence of differences between MEL score and age or sex, there did seem to be some evidence of increased PD-L1 positivity with prior ipilimumab treatment; however, it is not clear that this relationship is reproducible in other studies, as PD-L1 expression—using the same assay and scoring guidelines—was observed in 81% of ipiliumab-naive patients in KEYNOTE-006.26
Randomized controlled studies are needed to better understand the clinical benefits of pembrolizumab in patients with PD-L1–positive and PD-L1–negative melanoma, particularly given that a proportion of patients who have PD-L1–negative tumors may experience a durable response with pembrolizumab. Of note, both intertumoral and intratumoral heterogeneity of PD-L1 expression have been observed,37,38 as have changes in PD-L1 expression over time.13,39 Therefore, analysis of a single tissue sample, as in this study, may result in the classification of a tumor with some PD-L1–positive areas as PD-L1 negative. Further limitations of this analysis are that the study included non–randomly assigned cohorts, and a sizeable fraction (31%) of the overall study population was not evaluable for PD-L1 expression.
Important questions remain regarding the relationship between PD-L1 expression and the therapeutic effects of anti–PD-1 and anti–PD-L1 antibodies. It is clear that even with a MEL score of 0 in the assay used in this study, durable responses were still observed; response rates were even higher in the other MEL score groups. The high prevalence of PD-L1 positivity observed in this study, along with the durable responses observed in PD-L1–negative tumors, suggest that pembrolizumab treatment should not be limited to patients with PD-L1–positive tumors. Ongoing clinical trials with correlative studies will further delineate the role of PD-L1 expression in melanoma.
ACKNOWLEDGMENT
We thank the patients and their families and caregivers, as well as all investigators and site personnel. We also thank Roger Dansey (Merck) for critical review of the manuscript and supervision of the research group, Kevin Gergich (Merck) for study support, and Scott Diede (Merck) for critical review of the manuscript. Medical writing and editorial assistance was provided by Tricia Brown and Melanie Leiby (The ApotheCom oncology team, Yardley, PA), which was funded by Merck & Co.
Appendix
Fig A1.
CONSORT diagram. BICR, blinded independent central review; MEL, melanoma; PD-L1, programmed death-ligand 1.
Table A1.
Key Patient Baseline Characteristics by MEL Score for PD-L1 Expression
Footnotes
Supported by Merck & Co.
Presented in part at the 105th Annual Meeting of the American Association for Cancer Research, Philadelphia, PA, April 5-9, 2014; the 2014 Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014; the 11th International Congress of the Society for Melanoma Research, Zurich, Switzerland, November 13-17, 2014; the 51st Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 29-June 2, 2015; and the Medical Oncology Group of Australia Incorporated Annual Scientific Meeting 2015, Hobart, Tasmania, August 5-7, 2015.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01295827.
AUTHOR CONTRIBUTIONS
Conception and design: Adil I. Daud, Caroline Robert, Wen-Jen Hwu, Antoni Ribas, Grant Toland, Xiaoyun Nicole Li, Kenneth Emancipator, S. Peter Kang, Scot Ebbinghaus
Provision of study materials or patients: Adil I. Daud, Caroline Robert, Wen-Jen Hwu, Jeffrey S. Weber, Antoni Ribas, Anthony M. Joshua, Richard Kefford, Roxana Dronca, Amita Patnaik
Collection and assembly of data: Wen-Jen Hwu, Jeffrey S. Weber, Antoni Ribas, F. Stephen Hodi, Anthony M. Joshua, Peter Hersey, Roxana Dronca, Amita Patnaik, Hassane Zarour, Charlotte Roach, Grant Toland, Kenneth Emancipator, Marisa Dolled-Filhart, Omid Hamid
Data analysis and interpretation: Adil I. Daud, Jedd D. Wolchok, Caroline Robert, Wen-Jen Hwu, Jeffrey S. Weber, Antoni Ribas, F. Stephen Hodi, Anthony M. Joshua, Richard Kefford, Richard Joseph, Roxana Dronca, Amita Patnaik, Charlotte Roach, Jared K. Lunceford, Xiaoyun Nicole Li, Kenneth Emancipator, Marisa Dolled-Filhart, S. Peter Kang, Scot Ebbinghaus, Omid Hamid
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Programmed Death-Ligand 1 Expression and Response to the Anti–Programmed Death 1 Antibody Pembrolizumab in Melanoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Adil I. Daud
Stock or Other Ownership: OncoSec
Consulting or Advisory Role: Novartis, Genentech, Merck, Bristol-Myers Squibb, Array BioPharma
Jedd D. Wolchok
Stock or Other Ownership: Potenza Therapeutics, Vesuvius Pharmaceuticals
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, MedImmune, ZIOPHARM Oncology, Polynoma, Polaris, Jounce Therapeutics, Genentech
Research Funding: Bristol-Myers Squibb (Inst), MedImmune (Inst), GlaxoSmithKline (Inst), Merck (Inst)
Patents, Royalties, Other Intellectual Property: Co-inventor on an issued patent for DNA vaccines for treatment of cancer in companion animals
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Caroline Robert
Consulting or Advisory Role: Amgen, Bristol-Myers Squibb, Merck, Novartis, GlaxoSmithKline
Wen-Jen Hwu
Consulting or Advisory Role: Merck
Research Funding: Merck, Bristol-Myers Squibb, GlaxoSmithKline, MedImmune
Jeffrey S. Weber
Stock or Other Ownership: Altor BioScience, Celldex, cCam Biotherapeutics
Honoraria: Bristol-Myers Squibb, Merck, Genentech, AbbVie, AstraZeneca, Daiichi Sankyo, GlaxoSmithKline, Eisai, Altor BioScience, Lion Biotechnologies, Amgen, Roche, Ichor Medical Systems, Celldex, cCam Biotherapeutics, Pieris
Consulting or Advisory Role: Celldex, Ichor Medical Systems, cCam Biotherapeutics, Lion Biotechnologies, Pieris, Altor, BioScience, Bristol-Myers Squibb, Merck, Genentech, Roche, Amgen, AstraZeneca, GlaxoSmithKline, Daiichi Sankyo, AbbVie, Eisai
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst), GlaxoSmithKline (Inst), Genentech (Inst), Astellas Pharma (Inst), Incyte (Inst), Roche (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, GlaxoSmithKline, Daiichi Sankyo, Pieris, cCam Biotherapeutics, Lion Biotechnologies, Roche, Celldex, Amgen, Merck, AstraZeneca, Genentech
Antoni Ribas
Stock or Other Ownership: Kite Pharma, Compugen, FLX Bio, CytomX Therapeutics, Five Prime Therapeutics, Arcus, Advaxis
Consulting or Advisory Role: Merck, Genentech, Novartis
F. Stephen Hodi
Consulting or Advisory Role: Merck Sharp & Dohme, Novartis, Genentech, Amgen, EMD Serono
Research Funding: Bristol-Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Genentech (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Patent pending as per institutional policy, patent pending royalties received on MICA related disorders application to institution per institutional IP policy
Travel, Accommodations, Expenses: Novartis, Bristol-Myers Squibb
Other Relationship: Bristol-Myers Squibb, Genentech
Anthony M. Joshua
No relationship to disclose
Richard Kefford
Honoraria: Merck, Bristol-Myers Squibb, Novartis
Consulting or Advisory Role: Roche (Inst), Amgen (Inst), Bristol-Myers Squibb (Inst), Merck (Inst), Novartis (Inst), TEVA Pharmaceuticals (Inst)
Speakers' Bureau: GlaxoSmithKline, Merck
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck
Peter Hersey
No relationship to disclose
Richard Joseph
Consulting or Advisory Role: Bristol-Myers Squibb, Nektar, Merck, Genoptix, Cerulean Pharma
Research Funding: Merck (Inst), Bristol-Myers Squibb (Inst), Genentech (Inst), Amgen (Inst)
Tara C. Gangadhar
Honoraria: Merck Sharp & Dohme
Research Funding: Incyte (Inst), Bristol-Myers Squibb (Inst), Merck (Inst), Roche (Inst), Cerulean Pharma (Inst)
Roxana Dronca
Research Funding: Merck Sharp & Dohme (Inst)
Amita Patnaik
Research Funding: Merck (Inst), Pfizer (Inst), AVEO (Inst), Eli Lilly (Inst), Plexxikon (Inst), Jiangsu Hengrui Medicine (Inst), Symphogen (Inst), Corvus Pharmaceuticals (Inst), Tesaro (Inst), Bayer (Inst)
Hassane Zarour
Research Funding: Merck, Bristol-Myers Squibb
Charlotte Roach
Employment: DAKO
Stock or Other Ownership: DAKO
Grant Toland
Employment: DAKO
Stock or Other Ownership: DAKO
Jared K. Lunceford
Employment: Merck
Stock or Other Ownership: Merck
Xiaoyun Nicole Li
Employment: Merck & Co
Stock or Other Ownership: Merck & Co
Kenneth Emancipator
Employment: Merck, Celgene (I)
Stock or Other Ownership: Merck, Johnson & Johnson, Bayer, Celgene (I)
Marisa Dolled-Filhart
Employment: Merck & Co
Stock or Other Ownership: Merck, Merck (I)
S. Peter Kang
Employment: Merck
Stock or Other Ownership: Merck
Patents, Royalties, Other Intellectual Property: MK-3475 related
Scot Ebbinghaus
Employment: Merck
Stock or Other Ownership: Merck
Omid Hamid
Consulting or Advisory Role: Amgen, Novartis, Roche, Bristol-Myers Squibb
Speakers' Bureau: Bristol-Myers Squibb, Genentech, Novartis, Amgen
Research Funding: Astra Zeneca (Inst), Bristol-Myers Squibb (Inst), Celldex (Inst), Genentech (Inst), Immunocore (Inst), Incyte (Inst), Merck (Inst), Merck Serono (Inst), MedImmune (Inst), Novartis (Inst), Pfizer (Inst), Rinat (Inst), Roche (Inst)
REFERENCES
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Page DB, Postow MA, Callahan MK, et al. Immune modulation in cancer with antibodies. Annu Rev Med. 2014;65:185–202. doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- 5.Krummel MF, Allison JP. Pillars article: CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. The Journal of Experimental Medicine. 1995. 182: 459-465. J Immunol. 2011;187:3459–3465. [PubMed] [Google Scholar]
- 6.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 8.Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 10.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang SC, Latchman YE, Buhlmann JE, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 12.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 18.Robert C, Joshua AM, Weber JS, et al. Pembrolizumab (pembro; MK-3475) for advanced melanoma (MEL): Randomized comparison of two dosing schedules. Ann Oncol. 2014;25 (suppl 4; abstr LBA34) [Google Scholar]
- 19.Plimack ER, Gupta S, Bellmunt J, et al. A phase 1b study of pembrolizumab (pembro; MK-3475) in patients with advanced urothelial cancer. Ann Oncol. 2014;25 (suppl 4; abstr LBA23) [Google Scholar]
- 20.Muro K, Chung HC, Shankaran V, et al. doi: 10.1016/S1470-2045(16)00175-3. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial. Lancet Oncol 17:717-726, 2016. [DOI] [PubMed] [Google Scholar]
- 21.Chow LQM, Haddad R, Gupta S, et al. doi: 10.1200/JCO.2016.68.1478. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: Results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol 34:3838-3845, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 study. J Clin Oncol. 2015;34:2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armand P, Shipp MA, Ribrag V, et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol. 2016;34:3733–3739. doi: 10.1200/JCO.2016.67.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 25.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 27.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 28.Daud AI, Hamid O, Antoni-Ribas F, et al. Antitumor activity of the anti-PD-1 monoclonal antibody MK-3475 in melanoma (MEL): Correlation of tumor PD-L1 expression with outcome. Cancer Res. 2014;74((suppl 19; abstr CT104)) [Google Scholar]
- 29.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 31.Allred DC, Harvey JM, Berardo M, et al. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 32.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–226. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]
- 33.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 34.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerr KM, Tsao MS, Nicholson AG, et al. Programmed death-ligand 1 immunohistochemistry in lung cancer: In what state is this art? J Thorac Oncol. 2015;10:985–989. doi: 10.1097/JTO.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 36.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jilaveanu LB, Shuch B, Zito CR, et al. PD-L1 expression in clear cell renal cell carcinoma: An analysis of nephrectomy and sites of metastases. J Cancer. 2014;5:166–172. doi: 10.7150/jca.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madore J, Vilain RE, Menzies AM, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: Implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res. 2015;28:245–253. doi: 10.1111/pcmr.12340. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Hamrouni A, Wolowiec D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-γ and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]