Abstract
BACKGROUND
Skeletal related events (SREs) are common complications of bone metastatic castration-resistant prostate cancer (mCRPC). To the authors’ knowledge, there are limited data regarding which factors predict SREs. The authors identified risk factors for SREs in men with bone mCRPC using characteristics commonly available in the medical record.
METHODS
Data from 454 patients with non-metastatic CRPC were identified from 2 Veteran Affairs Medical Centers from 2000 through 2013. Among these men, 233 (51%) developed bone metastases during follow-up and represent the cohort. First occurrence of an SRE was abstracted from the medical records. A stepwise multivariable Cox model was used to select the strongest predictors of time to SRE.
RESULTS
The median age of the patients at the time of diagnosis of bone mCRPC was 75 years (interquartile range, 68–81 years), and there were 153 non-black patients (66%). During follow-up (median, 7.8 months [intequartile range, 2.9–18.3 months]), 88 patients (38%) had an SRE. On univariable analysis, more recent year of metastasis (hazard ratio [HR], 0.91), prostate-specific antigen doubling time of ≥9 months versus <9 months (HR, 0.50), and bone pain (HR, 3.34) were all found to be associated with SRE risk. On multivariable analysis, year of metastasis (HR, 0.93), biopsy Gleason score 7 versus ≤6 (HR, 1.74), radiotherapy as the primary localized treatment versus none (HR, 2.33), and bone pain (HR, 3.64) were associated with SRE risk. The area under the curve for a multivariable model based upon these risk factors was 0.744.
CONCLUSIONS
The authors identified several significant predictors of SREs among men with mCRPC. In particular, men with bone pain are at high risk of an SRE. If confirmed, future trials should focus on prolonging life and reducing SRE risk in mCRPC patients with bone pain.
Keywords: bone metastases, bone pain, metastatic castration-resistant prostate cancer (mCRPC), prostate cancer, skeletal related event (SRE)
INTRODUCTION
Prostate cancer is the most common non-cutaneous malignancy and second most common cancer-related mortality among American men [1]. Patients who die of prostate cancer typically die of complications of metastatic castration-resistant prostate cancer (mCRPC). In particular, > 90% of patients with mCRPC have evidence of bone metastases [2] and mortality from prostate cancer is often secondary to complications from bone metastases [3,4]. Patients with bone mCRPC are at risk of skeletal-relative events (SREs), specifically pathologic fracture, spinal cord compression, radiotherapy (RT) to bone, or surgery to bone [5]. In addition to the morbidity and mortality from SREs, there is a substantial economic burden to the US health care system treating and managing patients with SREs [6,7].
Given the devastating outcomes of SREs, preventing such events is important. Several clinical trials involving bone-stabilizing agents, such as bisphosphonates and denosumab, have demonstrated pain relief and reduced SREs without an objective improvement in survival [5,8–10]. However, other systemic therapies for mCRPC (abiraterone, enzalutamide), as well as new bone-targeting therapies (radium-223) have demonstrated not only an improvement in time to first SRE but also an improvement in overall survival [11–13].
There currently is a lack of robust data identifying which factors are predictive of SRE for patients with bone mCRPC. To the best of our knowledge, previous studies regarding whether bone pain is predicts of subsequent SREs are few [5,14]. Data from a trial of zoledronic acid vs placebo in men with bone mCRPC demonstrated that pain and analgesic scores were increased in the placebo group but had no correlation to disease progression, performance status or quality of life [5]. A more recent analysis assessing the effect of zoledronic acid on SRE incidence as determined by the level of bone pain at the time of study entry found that zoledronic acid decreased SREs compared to placebo, irrespective of bone pain status [14]. It is interesting to note that this article demonstrated that bone pain was unrelated to the risk of developing an SRE over the next 24 months. However, because the time to SRE is highly variable, it is crucial to assess time to SREs, not just risk of SRE over a given time. As such, whether bone pain truly predicts risk of time to SRE is unclear.
Given the morbidity and mortality associated with SREs, improving the ability to predict these events in high-risk men is crucial. The objective of this study was to assess predictors of time to SRE using characteristics commonly available in the medical record at the time of bone mCRPC. Using the US Veterans Affairs Medical Center-based Shared Equal Access Regional Cancer Hospital (SEARCH) database, we tested the hypothesis that bone pain would be a strong predictor of time to SRE in patients with bone mCRPC.
MATERIALS AND METHODS
Study Population
After obtaining Institutional Review Board approval, data from patients with mCRPC were identified from 2 Veteran Affairs medical centers (San Diego, Calif and Durham, NC) in the SEARCH database, regardless of primary treatment modality. The methods used to create this specific cohort have been previously described [15]. In brief, from these 2 medical centers, we first identified 7,888 patients who received androgen deprivation therapy (ADT) between 1991 and 2013 and had prostate-specific antigen (PSA) levels ≥ 2 ng/mL after initiating ADT from a query of the electronic medical records. We then manually reviewed records to limit our cohort to those individuals who had documented CRPC as defined by the Prostate Cancer Working Group 2 definition (relative increase of 25% and absolute increase of >2ng/mL nadir while receiving continuous ADT, including gonadotropin-releasing hormone agonist, antagonist, or bilateral orchiectomy) [16]. All imaging reports were read by trained personnel to search for distant prostate cancer metastases. PSA doubling time (PSADT) was calculated by the natural log of 2 divided by the slope of the linear regression of the natural log of PSA over time in months [17]. Subjects with calculated PSADT <0 or >120 were assigned 120 months to facilitate analysis. All available PSA values before metastases but after CRPC diagnosis were used to calculate PSADT. To calculate PSADT, subjects had to have ≥2 PSAs levels over ≥3 months. Bone pain was determined from medical records within 2 months before or after diagnosis of bone metastasis that stated the patient was or was not experiencing bone pain [18]. Details were not consistently available in the charts to determine location or intensity of the pain. Patients who had metastatic disease at or before the time of CRPC diagnosis or had CRPC diagnosis before the year 2000 were excluded from the study. Figure 1 shows the process of how we arrived at our study cohort. We identified 454 patients with nonmetastatic CRPC who were followed to determine whether they developed metastases. Because we were interested in progression of bone mCRPC to SRE, we excluded 198 patients who did not develop metastases and 23 patients who only developed soft tissue metastases. Of the 454 with nonmetastatic CRPC, 233 (51%) developed bone metastases and were included in our final cohort. First occurrence of an SRE (pathologic fracture, spinal cord compression, RT to bone, or surgery to bone) [5] was collected from the Veterans Affairs medical records and any notes from outside the Veterans Affairs hospital that were available or any mention of care outside the Veterans Affairs hospital mentioned in the Veterans Affairs notes. Primary and secondary treatments for prostate cancer were at the discretion of the patient and treating physician.
Figure 1.
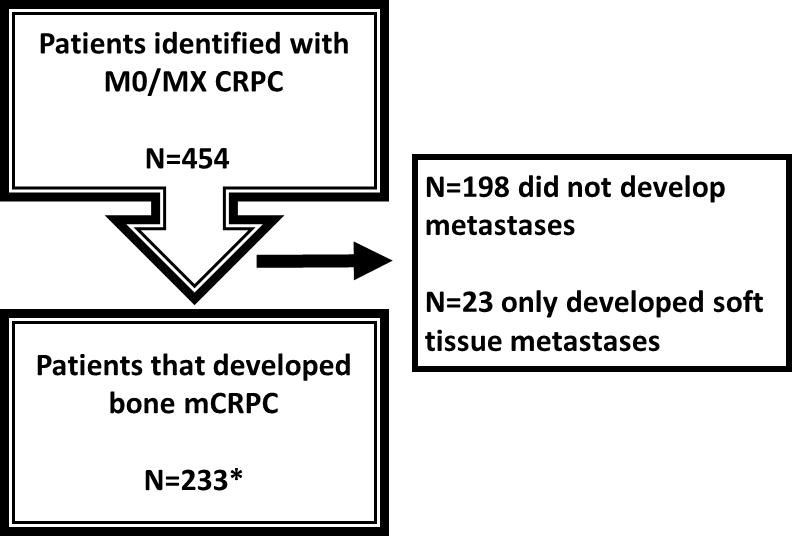
Trials (CONSORT) diagram. CRPC indicates castration-resistant prostate cancer; mCRPC, metastatic castration-resistant prostate cancer; MO/Mx, nonmetastatic.
MO/Mx=non-metastatic; CRPC=castration-resistant prostate cancer; mCRPC=metastatic castration-resistant prostate cancer
*Note: the final cohort is smaller than originally estimated in the protocol because of re-review of patients and fine-tuning of our definitions of positive and equivocal scans.
Statistical Analysis
Patient characteristics were summarized using median and 25th and 75th percentiles for continuous variables and count and percentages for categorical variables. We tested the association between various clinical factors and risk of SRE. Time zero was defined as the time of diagnosis of bone mCRPC and patients were followed to time of first SRE or date of last known contact with the Veterans Affairs health system or death. We estimated univariable hazard ratios (HRs) for each predictor using Cox proportional hazards models. We then used forward selection with α of .2 to select the strongest predictors of time to SRE. We chose a threshold value of α = .2 to not exclude variables that were important but did not reach statistical significance due to the small sample size and limited power. The predictors we assessed included age (continuous), year of metastasis diagnosis (continuous), race (black vs non-black), treatment center (center 1 vs center 2), biopsy Gleason score (2–6 vs 7 vs. 8–10 vs unknown/no biopsy), primary localized treatment (none vs radical prostatectomy with or without RT vs primary RT), PSA at metastasis (continuous, log-transformed), PSADT (<9 months vs ≥9 months vs missing), months from ADT to CRPC (continuous), months from CRPC to metastasis (continuous), number of bone metastases at the time of initial diagnosis of bone metastases (1 vs 2 vs 3–9 vs ≥10), and bone pain (yes vs no vs unknown). We calculated the area under the curve of the final multivariable model to assess the accuracy of the model to predict SREs.
Kaplan-Meier curves were used to graphically show time from mCRPC to SRE. Differences in time to SRE between groups were tested using the log-rank test.
RESULTS
Baseline Patient Characteristics
Table 1 shows the demographic and clinical characteristics of our study cohort of 233 men. Characteristics are reported at baseline, which is the time of initial bone metastases diagnosis. The median age at diagnosis of bone metastasis was 75 years (interquartile range [IQR], 68–81 years) and the median year of bone metastasis was 2007 (IQR, 2004–2010). There were 80 black patients (34%) and 153 non-black patients (66%). During follow-up (median 7.8 months [IQR, 2.9–18.3 months]), 88 patients (38%) had an SRE. Of these, 75 (85%) received RT to bone, 7 (8%) had a pathological fracture, 4 (4%) had spinal cord compression, and 2 (2%) had surgery to bone.
Table 1.
Baseline Patient and Disease Characteristics
| Variables | No. (%) or Median (25th Percentile, 75th Percentile) |
|---|---|
| Number of patients | 233 |
| Age at metastases (years) | 75 (68–81) |
| Year of metastases | 2007 (2004–2010) |
| Race | |
| Non-black | 153 (66%) |
| Black | 80 (34%) |
| Treatment center | |
| Center 1 | 119 (51%) |
| Center 2 | 114 (49%) |
| Biopsy Gleason score | |
| 2–6 | 34 (15%) |
| 7 | 63 (27%) |
| 8–10 | 61 (26%) |
| Unknown/No Biopsy | 75 (32%) |
| Primary localized treatment | |
| None | 79 (34%) |
| Radical Prostatectomy ± Radiation | 55 (24%) |
| Radiation Alone | 99 (42%) |
| PSA at metastases (ng/mL) | 44.1 (14.0–127.7) |
| PSADT at metastases (months) | |
| <9 | 113 (49%) |
| ≥9 | 73 (31%) |
| Missing | 47 (20%) |
| Months from ADT to CRPC | 40.9 (20.9–66.6) |
| Months from CRPC to metastases | 15.2 (5.1–31.0) |
| Soft tissue metastases | 35 (15%) |
| Number of bone metastases | |
| 1 | 43 (18%) |
| 2 | 27 (12%) |
| 3–9 | 93 (39%) |
| ≥10 | 70 (30%) |
| Bone pain | |
| No | 81 (35%) |
| Yes | 103 (44%) |
| Unknown | 49 (21%) |
| Had an SREa | 88 (38%) |
| Pathological fracture | 7 (8%) |
| Radiation to bone | 75 (85%) |
| Spinal cord compression | 4 (5%) |
| Surgery to bone | 2 (2%) |
| Total follow-up (months)b | 7.8 (3.9–18.3) |
Abbreviations: ADT, androgen deprivation therapy; CRPC, castration-resistant prostate cancer; IQR, interquartile range; PSA, prostate-specific antigen; PSADT: prostate-specific antigen doubling time; RT, radiotherapy; SRE: skeletal-related event
Indicates first occurrence of an SRE.
Among patients who did not have an SRE.
Predictors of SRE
On univariable analysis, year of metastasis (HR, 0.91, P= .005); PSADT of ≥9 versus <9 months (HR 0.50, p=0.011), and bone pain (HR, 3.34; P< .001) were all associated with risk of SRE (Table 2). There was a trend toward black patients to be at lower risk for SRE, although this did not reach statistical significance (HR, 0.65; P= .069). Similarly, there was a trend toward receiving primary treatment, particularly RT, and an increase risk of SRE compared with no primary treatment, although this was not statistically significant (P= .064).
Table 2.
Univariable and Multivariable Predictors of SRE Among Patients With mCRPC
| Variables | Univariable Results | Model Selectiona | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age at metastases, y | 0.99 | 0.97–1.01 | 0.318 | |||
| Year of metastases | 0.91 | 0.85–0.97 | 0.005 | 0.93 | 0.87–0.99 | 0.028 |
| Race | 0.069 | |||||
| Non-black | Referent | |||||
| Black | 0.65 | 0.41–1.03 | ||||
| Treatment center | 0.508 | |||||
| Center 1 | Referent | |||||
| Center 2 | 0.87 | 0.57–1.33 | ||||
| Biopsy Gleason score | 0.233 | Referent | ||||
| 2–6 | Referent | - | ||||
| 7 | 0.89 | 0.43–1.86 | 1.74 | 1.02–2.96 | 0.041 | |
| 8–10 | 1.58 | 0.79–3.17 | 1.54 | 0.90–2.62 | 0.112 | |
| Unknown/No Biopsy | 1.19 | 0.59–2.41 | ||||
| Primary localized treatment | 0.064 | |||||
| None | Referent | Referent | ||||
| Radical Prostatectomy ± Radiation | 1.38 | 0.75–2.53 | - | |||
| Radiation Alone | 1.82 | 1.09–3.03 | 2.33 | 1.50–3.62 | <0.001 | |
| Number of bone metastases | 0.107 | |||||
| 1 | Referent | Referent | ||||
| 2 | 0.42 | 0.17–1.06 | ||||
| 3–9 | 0.88 | 0.51–1.52 | ||||
| ≥10 | 1.26 | 0.70–2.27 | 1.60 | 1.00–2.57 | 0.052 | |
| PSA at metastases (ng/mL) | 1.13 | 0.98–1.30 | 0.102 | |||
| PSADT at metastases (months) | ||||||
| <9 | Referent | |||||
| ≥9 | 0.50 | 0.29–0.86 | 0.011 | |||
| Missing | 0.96 | 0.57–1.61 | 0.880 | |||
| Months from ADT to CRPC | 1.00 | 0.99–1.01 | 0.948 | |||
| Months from CRPC to metastases | 0.99 | 0.98–1.00 | 0.078 | |||
| Bone pain | ||||||
| No | Referent | Referent | ||||
| Yes | 3.34 | 2.04–5.48 | <0.001 | 3.64 | 2.30–5.75 | <0.001 |
| Unknown | 0.84 | 0.39–1.83 | 0.667 | - | ||
Abbreviations: 95% CI, 95% confidence interval; ADT, androgen deprivation therapy; HR, hazard ratio; mCRPC, metastatic castration-resistant prostate cancer; PSA, prostate-specific antigen; PSADT: prostate-specific antigen doubling time; RT, radiotherapy; SRE: skeletal-related event
Forward selection with α=0.2
On multivariable analysis, year of metastasis (HR, 0.93; P= .028), biopsy Gleason score of 7 versus ≤6 (HR, 1.74; P= .041), RT as primary localized treatment versus none (HR, 2.33; P< .001), and bone pain (HR, 3.64, P< .001) were found to be associated with risk of SRE. Although not significant, there was a trend toward an increased risk of SRE among patients with biopsy Gleason score 8 to 10 versus those with Gleason ≤6 (HR, 1.54; P= .112), as well as those individuals with ≥10 bone metastases versus 1 (HR, 1.60; P= .052). The area under the curve of the multivariable model was 0.744.
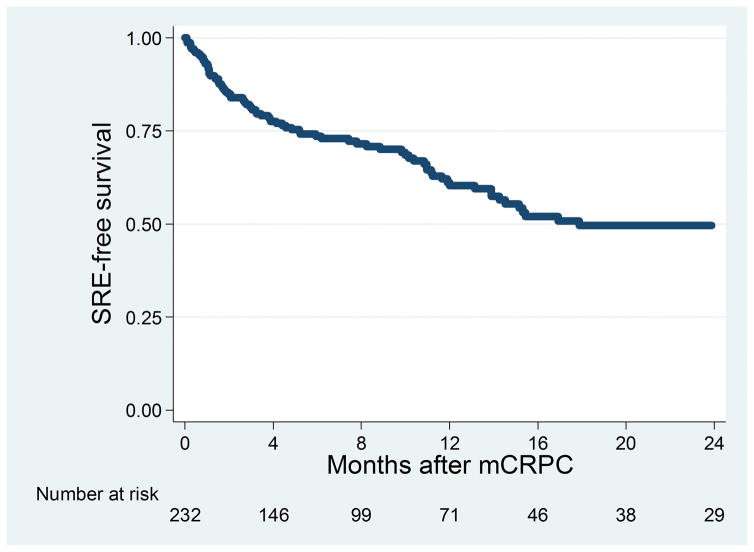
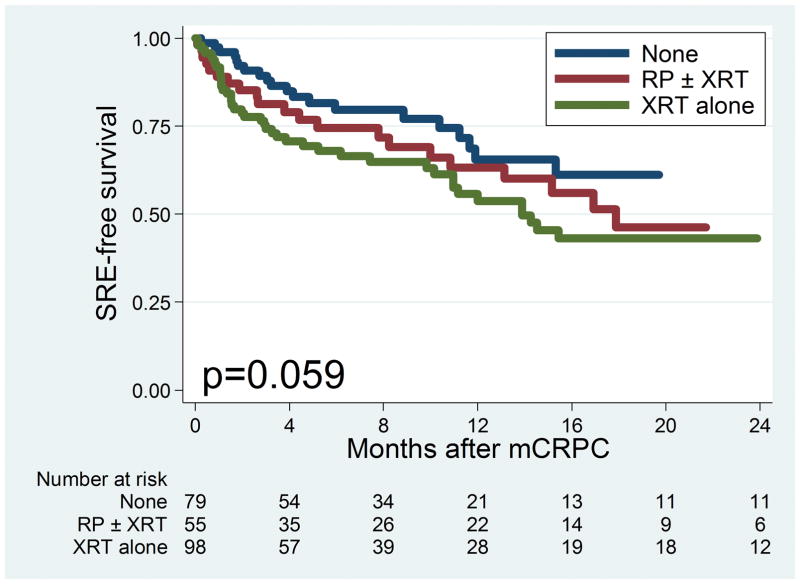
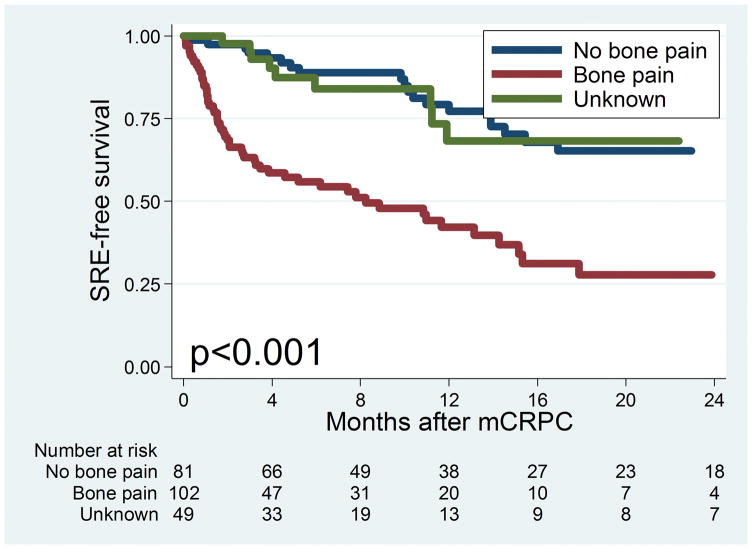
Figure 2 shows the Kaplan-Meier curve for time to SRE among all patients in the cohort. The median time to SRE was 17.9 months. Figure 3 Top shows the Kaplan-Meier curve for time to SRE stratified by primary localized treatment. There was no significant difference in time to SRE observed among patients who received different primary localized treatments (log-rank P= .059). Figure 3 Bottom shows the Kaplan-Meier curve for progression to SRE stratified by bone pain. There was a statistically significant difference in progression to SRE among patients with bone pain (log-rank P< .001), with patients with bone pain at time of metastasis having the greatest risk of SRE. The median time to SRE among men with bone pain was 8.2 months versus 48.5 months in those without bone pain.
Figure 2.
Kaplan-Meier curve for time to skeletal-related events (SREs) among all patients. mCRPC indicates castration-resistant prostate cancer.
Figure 3.
(Top) Kaplan-Meier curve for time to skeletal-related events (SREs) by primary localized treatment. (Bottom) Kaplan-Meier curve for time to SRE by bone pain at metastasis mCRPC, metastatic castration-resistant prostate cancer; RP, radical prostatectomy; XRT, radiotherapy.
DISCUSSION
Given the morbidity, economic burden, and pending mortality associated with SREs in patients with bone mCRPC, we sought to identify predictors of SRE using characteristics commonly available in the medical record at the time of bone mCRPC diagnosis. We found bone pain at the time of bone mCRPC diagnosis was the strongest predictor of subsequent SRE. Moreover, men treated with RT also were found to be at higher risk of SRE. Although there were trends for high-grade biopsy Gleason disease and high-volume skeletal metastatic burden to be associated with SRE risk, these did not reach statistical significance. To our knowledge, the current study is the first to unequivocally identify bone pain as the most predictive variable for SRE among the clinicopathologic, treatment and demographic variables commonly available to clinicians. These results highlight an opportunity to risk stratify these patients for SRE to identify high-risk men who can be offered aggressive therapies to reduce their risk of SREs.
Several studies have suggested that SREs are related to poorer survival, including an earlier study from our group using the same cohort as in the current study [6], [18–22]. In addition to poor clinical outcomes associated with SREs, the economic burden on the US health care system is substantial. In a Surveillance, Epidemiology, and End Results - Medicare study of 3,297 men aged >65 years with bone metastatic prostate cancer, McDougall et al. [6] found that men experiencing ≥1 SRE had a 2-fold increase in the number of emergency department visits, a 4-fold increase in number of hospitalizations, and $21,000 in additional health care costs compared to men without an SRE. Although the number of SREs and mortality secondary to SREs decreased from 1998 to 2010 in the United States, Roghmann et al. [7] reported in a Nationwide Inpatient Sample study that during this time period, SRE hospital charges rose by 94% to >$300 million in 2010. Beyond the potential clinical benefit of being able to predict and provide early treatment for patients at high risk for SRE, in today’s fiscally conscience health care system, the monetary benefits of preventing SREs should not be overstated.
The current study finding that bone pain predicted the risk of SRE at the time of bone mCRPC is consistent with predictors of SREs for other malignancies. In an analysis of 444 patients with bone metastatic breast cancer who were receiving zoledronic acid, Brown et al. [22] reported that pain score was a significant predictor of SREs on multivariable analysis. Indeed, in the current study, bone pain at the time of bone mCRPC (vs no bone pain; HR, 3.64 [95% confidence interval (95%CI), 2.30–5.75]) was found to be the strongest predictor of SRE, even more so than high-volume skeletal metastases (≥10 vs 1; HR, 1.60, [95%CI, 1.00–2.57]), which was included the same multivariable model. However, these findings are contrary to previous analysis that found bone pain was not significant linked to SREs within a phase 3 trial in patients with mCRPC [5,14]. In that trial, in which men with bone mCRPC were randomized to treatment with zoledronic acid versus placebo, bone pain was found to have no correlation with disease progression, performance status or quality of life [5]. A subsequent analysis of that study found that that although zoledronic acid decreased SREs in 422 men with CRPC compared to placebo, over 24 months of follow-up, bone pain was found to be unrelated to absolute risk of SRE (time to SRE was not examined) [14]. Given that the most common SRE in the current study was RT to the bone, which typically is administered for bone pain, it appears intuitive that bone pain should predict earlier development of an SRE. Coupled with the previously mentioned data from other cancers, the data from the current study provide strong support for the belief that bone pain is a predictor of SRE, though future larger studies are needed to confirm this.
Beyond bone pain, previous studies attempting to identify predictors of SREs in patients with bone mCRPC have primarily been limited to ad hoc analyses of phase 3 randomized controlled trials. In an analysis of 643 with bone mCRPC who were randomized to treatment with zoledronic acid versus placebo, Smith et al. [24] found that elevated bone-specific alkaline phosphatase (BSAP), a marker for osteoblastic activity, was significantly associated with a shorter time to first SRE on multivariable analyses. This finding also held true for subset analyses of the patients treated with placebo and zoledronic acid. In the current study, we did not examine BSAP as predictor of SRE because the data were only available for 62 of the 233 patients. Future studies are needed to better assess the value of BSAP as a predictor of SRE.
One factor we identified as being linked with SRE was receipt of primary curative RT. To our knowledge, this has not been reported previously and thus requires validation in other data sets. If confirmed, we speculate that RT to the prostate may lead to some damage to the surrounding pubic bone, which, at the time of mCRPC, increases the risk of SRE. It is interesting to note that no increased risk was seen for men treated with surgery and therefore it is unlikely a generalized effect of having received primary treatment. Again, however, these results require validation in future studies.
Several agents have been shown to reduce the risk of SRE. For example, a phase 3 study of 1904 men with bone mCRPC randomized to receive denosumab versus zoledronic acid demonstrated a median time to first SRE of 20.7 months for denosumab compared with 17.1 months for zoledronic acid (HR 0.82; 95% CI, 0.71–0.95) [9]. Among 921 men with bone mCRPC randomized to receive radium-223 or placebo, in addition to improved overall survival, men receiving radium-223 were found to have a decreased median time to first SRE compared with men receiving placebo (15.6 vs 9.8 months; HR, 0.66 [95% CI, 0.52–0.83]) [11]. Results from the phase 3 AFFIRM trial demonstrated that the median time to first SRE for patients being treated with enzalutamide was 16.7 months compared to 13.3 months for those receiving placebo (HR, 0.69; 95% CI, 0.57–0.84) [12]. Similarly, data from the phase 3 COU-AA-301 trial demonstrated that patients treated with abiraterone plus prednisone had a significantly longer time to first SRE compared with those receiving only prednisone (25.0 months vs 20.3 months; P= .001) [13]. It is interesting to note that the recently released guidelines from the American Urological Association for management of CRPC state that treatment for SRE prevention in men with bone mCRPC is considered optional [24]. Given the availability of multiple options that reduce the risk of SRE (i.e. denosumab, zoledronic acid, enzalutamide, abiraterone, radium-223) together with the findings of the current study, which identify men with bone pain at high risk men for SREs, we would suggest that if confirmed future studies, treatment of these men should not be optional, but rather standard of care. Ideally, enrollment of these patients onto clinical trials testing new and better treatments including combination treatments is needed.
The strengths of the current study include the fact that we had access to the complete medical records and thus could accurately assess SRE risk. Indeed, the rate of SRE in our study (38%), was comparable to that of previous studies from phase 3 trials [14], suggesting that we captured nearly all SREs in the current study cohort. Furthermore, we had sufficient sample size with enough end points to allow detailed multivariable analysis. However, the current study is not without limitations including its retrospective design. The current study cohort consisted of patients with mCRPC who were being treated at 2 Veterans Affairs hospitals, which limits the generalizability of the study findings. We acknowledge that 21% of the cohort was missing data regarding bone pain and only 27% of patients had information regarding BSAP levels, thereby preventing us from analyzing BSAP as a predictor of SRE. Furthermore, we did not have sufficient data regarding alkaline phosphatase, as well as the use of new mCRPC medications such as enzalutamide, abiraterone, radium-223, and denosumab. Certainly, future studies assessing outcomes among patients with mCRPC who are being treated with these medications in a Veterans Affairs health care system are warranted. In addition, we did not use a standardized questionnaire for assessing bone pain, and thus the patient responses may be subjective. Moreover, data were unavailable regarding the location (eg, rib vs pelvis) or intensity of the bone pain. Nonetheless, the fact that bone pain predicted SRE provides some level of validity to this measure. However, because inaccuracies in measurement tend to bias the results to the null, it is possible a prospectively collected bone pain assessment actually may be a stronger predictor of SRE than identified in our study. Clinically, it would seem reasonable to suggest that men with ≥10 bone metastatic lesions would be high risk of developing SRE. In the current study, this variable approached, but did not reach, statistical significance, although perhaps with a larger sample size and/or greater number of SRE events, the number of metastatic lesions would be predictive of SRE in multivariable analysis, although this requires further study. Although we found that primary RT increased the risk of SRE, the question of whether this reflects more aggressive disease at time of diagnosis, is a chance finding (type I error), or results from some other reason requires further testing in other data sets. Finally, because the Veterans Affairs health care system is an equal-access, equal payer government system, economic data were not readily available, thereby precluding such analyses to be performed.
CONCLUSIONS
In this study of US Veterans Affairs patients with prostate cancer, bone pain was found to be the strongest predictor of SRE among men with bone mCRPC. Given the morbidity, economic burden, and pending mortality associated with SREs in patients with bone mCRPC, these individuals should be considered for early and aggressive treatment in an attempt to prevent bone pain and pending SREs.
Acknowledgments
FUNDING SUPPORT
Funded by Bayer Pharmaceuticals, and National Institute of Health (grant K24CA160653 to Stephen J. Freedland and P50CA92131 to William J. Aronson).
Footnotes
CONFLICT OF INTEREST: Dr. Freedland is a consultant for Bayer Pharmaceuticals. The remaining authors made no disclosures.
CONFLICT OF INTEREST DISCLOSURES
Stephen J. Freedland has acted as a paid consultant for Bayer Pharmaceuticals.
AUTHOR CONTRIBUTIONS
Zachary Klaassen: Conceptualization, investigation, writing-original draft, writing-review and editing, and visualization.
Lauren E. Howard: Methodology, software, validation, formal analysis, writing-review and editing.
Amanda de Hoedt: Resources and writing-review and editing.
Christopher L. Amling: Resources and writing-review and editing.
William J. Aronson: Resources and writing-review and editing.
Matthew R. Cooperberg: Resources and writing-review and editing.
Christopher J. Kane: Resources and writing-review and editing.
Martha K. Terris: Resources and writing-review and editing.
Stephen J. Freedland: Conceptualization, methodology, investigation, resources, writing-original draft, writing-review and editing, visualization, supervision, project administration, and funding acquisition.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Bubendorf L, Schopfer A, Wagner U, et al. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 3.Lange PH, Vessella RL. Mechanisms, hypotheses and questions regarding prostate cancer micrometastases to bone. Cancer Metastasis Rev. 1998–1999;17:331–336. doi: 10.1023/a:1006106209527. [DOI] [PubMed] [Google Scholar]
- 4.Fizazi K, Massard C, Smith M, et al. Bone-related parameters are the main prognostic factors for overall survival in men with bone metastases from castration-resistant prostate cancer. Eur Urol. 2015;68:42–50. doi: 10.1016/j.eururo.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 6.McDougall JA, Bansal A, Goulart BH, et al. The clinical and economic impacts of skeletal-related events among Medicare Enrollees with prostate cancer metastatic to bone. Oncologist. 2016;21:320–326. doi: 10.1634/theoncologist.2015-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roghmann F, Antczak C, McKay RR, et al. The burden of skeletal-related events in patients with prostate cancer and bone metastasis. Urol Oncol. 2015;33:17e9–e18. doi: 10.1016/j.urolonc.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Fizazi K, Lipton A, Mariette X, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 9.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomized, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith MR, Coleman RE, Klotz L, et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol. 2015;26:368–374. doi: 10.1093/annonc/mdu519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 12.Fizazi K, Scher HI, Miller K, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomized, phase 3 AFFIRM trial. Lancet Oncol. 2014;15:1147–1156. doi: 10.1016/S1470-2045(14)70303-1. [DOI] [PubMed] [Google Scholar]
- 13.Logothetis CJ, Basch E, Molina A, et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 2012;13:1210–1217. doi: 10.1016/S1470-2045(12)70473-4. [DOI] [PubMed] [Google Scholar]
- 14.Saad F, Eastham J. Zoledronic acid improves clinical outcomes when administered before onset of bone pain in patients with prostate cancer. Urology. 2010;76:1175–1181. doi: 10.1016/j.urology.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Moreira DM, Howard LE, Sourbeer KN, et al. Predicting bone scan positivity in non-metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2015;18:333–337. doi: 10.1038/pcan.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton RJ, Aronson WJ, Terris MK, et al. Limitations of prostate specific antigen doubling time following biochemical recurrence after radical prostatectomy: results from the SEARCH database. J Urol. 2008;179:1785–1789. doi: 10.1016/j.juro.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard LE, De Hoedt AM, Aronson WJ, et al. Do skeletal-related events predict overall survival in men with metastatic castration-resistant prostate cancer? Prostate Cancer Prostatic Dis. 2016 Jul 5; doi: 10.1038/pcan.2016.26. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norgaard M, Jensen AO, Jacobsen JB, Cetin K, Fryzek JP, Sorensen HT. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007) J Urol. 2010;184:162–167. doi: 10.1016/j.juro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis. 2011;14:177–183. doi: 10.1038/pcan.2011.7. [DOI] [PubMed] [Google Scholar]
- 21.Onukwugha E, Yong C, Mullins CD, Seal B, McNally D, Hussain A. Skeletal-related events and mortality among older men with advanced prostate cancer. J Geriatr Oncol. 2014;5:281–289. doi: 10.1016/j.jgo.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Brown JE, Cook RJ, Lipton A, Costa L, Coleman RE. Prognostic factors for skeletal complication from metastatic bone disease in breast cancer. Breast Cancer Res Treat. 2010;123:767–779. doi: 10.1007/s10549-010-0981-1. [DOI] [PubMed] [Google Scholar]
- 23.Smith MR, Cook RJ, Coleman R, et al. Predictors of skeletal complications in men with hormone-refractory metastatic prostate cancer. Urology. 2007;70:315–319. doi: 10.1016/j.urology.2007.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cookson MS, Roth BJ, Dahm P, et al. Castration-resistant prostate cancer: AUA Guideline. J Urol. 2013;190:429–438. doi: 10.1016/j.juro.2013.05.005. [DOI] [PubMed] [Google Scholar]