Abstract
Background/Aims
The aim was to examine added benefits of a Comprehensive, Individualized, Person-Centered Management (CI-PCM) program, to memantine treatment.
Methods
This was a 28-week, clinician-blind, randomized, controlled, parallel-group study, with a similar study population, eligibility criteria, and design to the Reisberg, et al., 2003 memantine pivotal trial. Twenty eligible community-residing, Alzheimer’s disease (AD) subject-caregiver dyads were randomized to the CI-PCM program (n=10) or to Usual Community Care (n=10). Primary outcomes were the New York University Clinician’s Interview-Based Impression of Change-Plus Caregiver Input (NYU-CIBIC-Plus), assessed by one clinician set, and an Activities of Daily Living Inventory, assessed by a separate clinician set at baseline, and weeks 4, 12, and 28.
Results
Primary outcomes showed significant benefits of the CI-PCM program at all post-baseline evaluations. Improvement on the NYU-CIBIC-Plus in the management group at 28 weeks was 2.9 points over the comparator. The memantine 2003 trial showed a 0.3 point improvement on this global measure of memantine treated versus placebo randomized subjects at 28 weeks. Hence, globally, the management program intervention benefits were 967% > memantine treatment alone.
Conclusion
These results are ~10X those usually observed with both nonpharmacological and pharmacological treatments and indicate substantial benefits with the management program for advanced AD persons.
Keywords: Dementia, Activities of Daily Living, Behavioral Symptoms, Comprehensive Health Care, Caregivers, Community Health Care, Patient-Centered Care, Person-Centered Therapy, Delivery of Health Care, Community Health Education
Introduction
Present treatment of Alzheimer’s disease (AD) necessarily encompasses both pharmacological and nonpharmacological interventions. Pharmacologically, cholinesterase inhibitor treatments have been available for many years for mild-to-moderate AD. As a result of investigations conducted under our direction [1] and elsewhere [2, 3], memantine, was approved as the first treatment for moderate-to-severe AD, in the European Union (2002) [1, 2], and subsequently, in the United States [1–3]. Other studies have focused on affective and behavioral and psychological symptoms (BPSD) in AD [4, 5, 6]. Because these treatments are not curative, the advent of pharmacological treatments for persons with advanced AD accentuated the need for proper management of these persons.
Nonpharmacological AD treatment studies have traditionally focused on two approaches. One encompasses efforts to assist caregivers. These have helped e.g., by postponing institutionalization [7, 8]. Another approach is remediating deficits and disturbances. When we embarked on the present study modalities investigated included: reality orientation [9], music therapy [10], light therapy [11], environmental interventions [12], and validation therapy [13]. A combination of exercise with behavioral management had produced improvements in physical functioning and mood in persons with mild-to-moderate AD [14]. When we initiated the present investigation, comprehensive approaches had not been systematically investigated in the care of persons with AD. Also, in terms of AD person care, contemporaneous [15] and subsequent [16] reviews concluded that “for…outcomes (cognition, ADLs [activities of daily life], behavior, mood), the magnitude of the effect seemed to be similar to the effect obtained by drugs” [16].
We hypothesized that a Comprehensive, Individualized, Person (Patient)-Centered Management (CI-PCM) program, created and implemented by Sunnie Kenowsky (SK), incorporating elements we previously described [17], then current knowledge on AD [18, 19], as well as techniques and strategies SK created [20], would alleviate symptomatology and distress even in some of the most disturbed and impaired community-residing, AD persons. To facilitate comparisons with pharmacological treatment, we partly modeled our study on our previous memantine trial [1]. Based upon a prior, exercise-only, pilot investigation, we hypothesized that we would see robust results with a randomized sample of 20 subjects.
Methods
Design
This was a 28-week, clinician-blind, single-center, parallel-group study conducted in accordance with International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki [21, 22]. The Institutional Review Board of the New York University (NYU) School of Medicine approved the study protocol prior to study initiation, as well as subsequent modifications and continuing reviews. Also, prior to study initiation, the study was registered with ClinicalTrials.gov (identifier: NCT00120874 URL: https://clinicaltrials.gov). Subjects were assigned by simple randomization to either: (1) the CI-PCM intervention group, or (2) the Usual Community Care (UCC) Plus $50 Financial Compensation (FC) upon completion of baseline and week 28 study visits (total, $100), comparator group.
A statistician, blind to group assignment, computer-generated a sequential numerical list designating random study group assignments. Potential subjects were selected to be contacted by the coordinator if they were thought to have middle to late stage Alzheimer’s disease. The study coordinator assigned 54 potential participants, who agreed to be randomized, a sequential number and contacted them in numerical order. Then the coordinator consented all 54 potential participants to the corresponding treatments according to the procedures described below.
First, subject capacity to consent was determined by a qualified medical professional unassociated with the study. If the subject had capacity, they signed the consent form. If the subject did not have capacity, the subject’s legally authorized representative gave written consent on behalf of the subject, and the subject gave oral and written assent. The subject’s participating family and/or professional carers also gave written consent. Recruitment occurred from September, 2005 to November, 2010. All consented caregiver-subject dyads were screened for eligibility.
Eligible subjects were ≥ 50 years, community-residing at screening, and had a family and/or professional caregiver willing and able to participate in all aspects of the study. Eligible subjects also had a diagnosis of dementia of Alzheimer’s type by DSM-IV-TR criteria [23] and fulfilled NINCDS-ADRDA criteria for probable AD [24]. These diagnoses were confirmed by medical records, physical and neurological examinations, laboratory tests including corpuscular blood counts and differentials, comprehensive metabolic panels, and neuroimaging with computerized tomographic scans or, more commonly, brain magnetic resonance imaging. All subjects had moderate-to-severe AD on the Global Deterioration Scale (GDS), i.e., a GDS stage of 5 or 6 [25]. Also, all subjects had a deficit in performance of basic activities of daily life, including requiring some assistance with putting on clothing, signified by a Functional Assessment Staging (FAST) score of ≥ 6a [26]. Additionally, eligible subjects had Mini-Mental Status Examination (MMSE) [27] scores of 3–14.
Exclusion criteria included: non-AD dementias, including subjects with vascular dementia or a score > 4 on the modified Hachinski Ischemia Rating Scale [28], major depressive disorder, clinically significant laboratory abnormalities, and subjects receiving investigational medications.
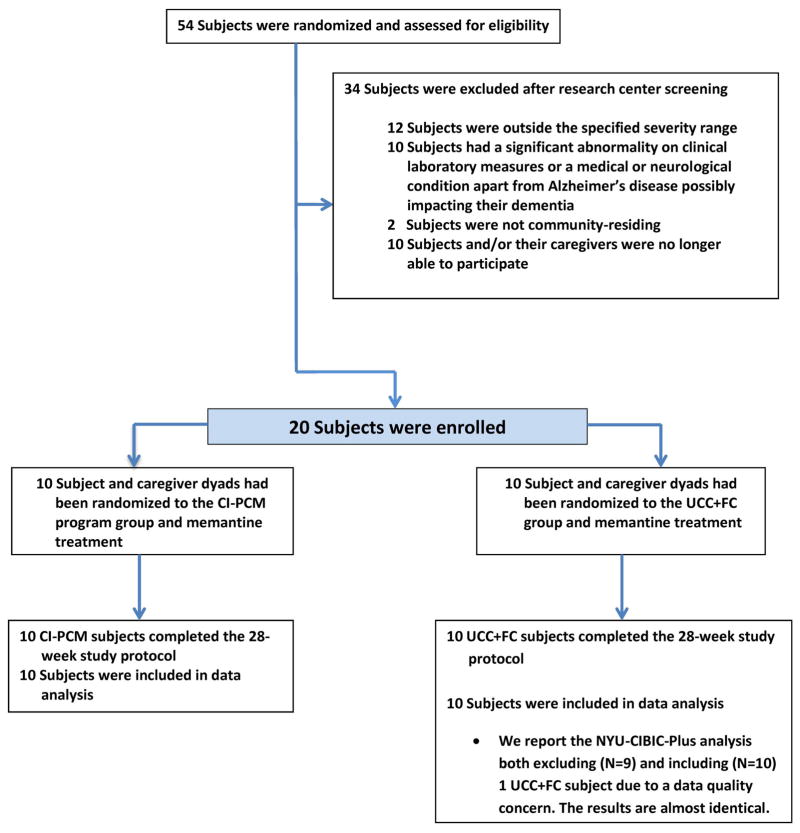
After screening, 20 subject-caregiver dyads were found fulfilling eligibility criteria. Ten dyads had been randomized to the CI-PCM group and 10 dyads had been randomized to the UCC+FC group. Enrollment occurred from 7/21/2006 through 11/5/2010. Twelve subjects were enrolled in the first 2 years; these comprised 7 intervention subjects and 5 comparators, whereas, 8 subjects were enrolled in the last two years; these comprised 3 intervention subjects and 5 comparators. Initial participants were delayed due to a medical injury in the sole trainer, SK. Subsequent enrollment was also delayed due to the personnel constraint of there being only one trainer. The last subject completed the 28-week study on 5/27/2011. Figure 1 diagrams the flow of participants through the study.
Figure 1. Flow of Participants Through Study.
CI-PCM, Comprehensive, Individualized, Person-Centered Management; UCC + FC, Usual Community Care Plus Financial Compensation; NYU-CIBIC-Plus, New York University Clinician's Interview-Based Impression of Change Plus Caregiver Input global score.
Subjects
Characteristics of eligible randomized subjects are shown in Table 1. There were 15 women and 5 men, age ranged from 54 to 92 years at study entry. Educational background ranged from 6 to 20 years.
Table 1.
Subject Characteristics
| CI-PCM* plus Memantine** N=10 |
UCC+FC*** plus Memantine** N=10 |
All Subjects N=20 |
|
|---|---|---|---|
| Gender (Females/Males) | 8/2 | 7/3 | 15/5 |
| Age (SD) (Years) | 77.7 (11.7) | 80.1 (7.1) | 78.9 (9.5) |
| Education (SD) (Years) | 14.9 (3.9) | 14.2 (2.8) | 14.5 (3.3) |
Note: Fisher’s exact test is used to compare proportions of females/males; Wilcoxon rank sum test is used to test age and education. There were no significant differences between the CI-PCM and the UCC+FC groups on any of the 3 variables.
CI-PCM, Comprehensive, Individualized, Person-Centered Management program.
Memantine, titrated to a maximum tolerated dose of 10 mg twice daily.
UCC+FC, Usual Community Care and Financial Compensation.
Intervention
All subjects received memantine (Namenda, Forest Pharmaceuticals) titrated to a maximum tolerated dose of 10mg twice daily.
Subject-carer dyads receiving UCC+FC had their questions addressed by the study Alzheimer’s care specialist, SK and/or NYU Alzheimer’s Disease Center (NYU-ADC) social workers and clinicians. When appropriate, they were referred to the Alzheimer’s Association and other community resources for caregiver training, care counseling, safe return/medic alert bracelets, day care center and support group programs.
The CI-PCM intervention was developed, conducted and implemented by SK. It included evidence based available knowledge and techniques, as well as, novel strategies and techniques developed by SK [20]. The intervention components were: caregiver training, management assessment, therapeutic home visits and caregiver support groups.
Caregiver training included: a course in Alzheimer’s care consisting of 8 education sessions, individualized, task specific training, and informal instruction, e.g., during home visits. Education sessions, created in advance by SK, consisted of ≥ 3 hours of didactic training weekly, at the NYU-ADC, for the first 8 study weeks. Sessions focused on : (1) The pathogenesis [29], course and personal impact of AD, retrogenesis, developmental age equivalence and management principles of AD care [17, 18, 30, 31]. (2) The impact of AD on communication and how to communicate effectively with persons with AD [32, 33]. (3) The nature, occurrence, identification, causes and management of behavioral and psychological symptoms in AD (BPSD), including how to determine meaning and possible responses [4, 5, 20, 34, 35, 36]. (4) Prevention and management strategies developed by SK, as well as appropriate carer responses to BPSD [20]. (5) Activities: which to do and how. (6) Exercises, cognitive and language stimulation: what to do and how. (7) How to remedy deficits and teach persons with AD, skills they have forgotten using memory coaching [20], activities and spaced retrieval [37]. (8) Nutrition, how to recognize and manage medical problems in AD persons, and caregiver stress. Some intervention principles, techniques and procedures are summarized in Table 2 [17, 19, 25–27, 30, 33, 34, 36, 38–41].
Table 2.
Intervention Principles, Techniques and Procedures
| Principles |
|
| Techniques and Procedures |
|
Subsequent carer questions were addressed in twice monthly support group meetings and residential visits. There was an initial residential visit. Subsequent visits were at the subject’s residence, SK’s office, or the NYU-ADC. There were ≥ 4 visits over the first 8 study weeks. Additional visits, at least monthly, were conducted as necessary. During these visits: (1) the environment was assessed and modifications to enhance safety and functioning were suggested, (2) recommendations were made for appropriate levels of care, supervision and caregiver assistance, (3) a plan of activities to remediate deficits, enhance functioning and maintain abilities was developed and instituted in accordance with the subject’s interests, stage, environment and capacity, (4) a subject specific exercise plan was initiated, (5) subjects were memory coached in skills and abilities which had been diminished or lost, such as continence of urine and feces, walking, eating with utensils, sewing, etc., (6) caregivers were taught how to continue the previously instituted management program.
After the education sessions concluded, caregiver support group meetings were held twice a month for 20 weeks. Caregiver stress identification and management was taught, as well as, how to take care of oneself “first,” without neglecting the AD person. New and incipient problems were recognized, possible solutions were discussed, successes were shared and celebrated. Participants were taught the stages of grief and actively grieved their losses and those of the AD person. Caregivers also learned teamwork and mutual support.
Outcome Measures
Outcomes were assessed at the NYU-ADC at baseline and at 4, 12, and 28 weeks. The primary efficacy variables were: (1) the Clinician’s Interview-Based Impression of Change-Plus Caregiver Input global score (NYU version) [42], (NYU-CIBIC-Plus), and (2) the Alzheimer’s Disease Cooperative Study Actitivies of Daily Living Inventory modified for more severe dementia [43], abbreviated first 12 questions version (ADCS-ADLsev-abv). Secondary outcomes included: cognitive measures, the Severe Impairment Battery (SIB) [44] and the MMSE [27]; a functional measure, the FAST-Disability Score (FAST-DS) [42]; a behavioral pathology assessment, the Behavioral Pathology in Alzheimer’s Disease Frequency-Weighted Severity Scale (BEHAVE-AD-FW) [45], a syncretic measure, assessing memory, emotional and other behavioral problems, the Revised Memory and Behavior Problems Checklist (RMBPC) [46]; and a global measure, the GDS [25]. Subjects and carers were assessed by 2 sets of clinicians; one group performed the NYU-CIBIC-Plus assessment and the second group assessed the other measures. All clinicians were unaware of treatment group assignment. In accord with standard NYU-CIBIC-Plus scale procedures, clinicians performing the NYU-CIBIC-Plus (primarily, IB, JG and MS), assessed subjects and caregivers without reference or access to, any other study data at the time of their assessment.
Statistical Analysis
To compare with the 2003 memantine pivotal trial, which was a model for the present investigation [1], efficacy outcomes were analyzed, using R version 3.2.5, by application of the Wilcoxon-Mann-Whitney test for independent samples to the change from baseline. The level of significance is 5% and hypothesis tests are two-sided. We report intention-to-treat (ITT) analyses. For the NYU-CIBIC-Plus, we report analyses both with the total, n=20, ITT analysis in the Supplement, see Supplement Figure, and the n=19 analysis, which excludes 1 subject due to a data quality concern. The results of the two analyses are almost identical.
In the 2003 study, we observed a mean difference of 0.3 between the NYU-CIBIC-Plus primary outcome of the two groups (memantine versus placebo) with a common SD=1.1. With a sample size of 10+10=20, assuming a similar SD, at the 5% alpha level, we have 93% power to detect a mean difference of 1.8 (6 times 0.3) using a two-sided, two-sample t-test.
Results
Outcomes
All 20 eligible subjects and their primary caregivers completed the 28-week study. Study outcomes are shown in Figures 2 to 9 and in the Supplement (see Supplement Figure).
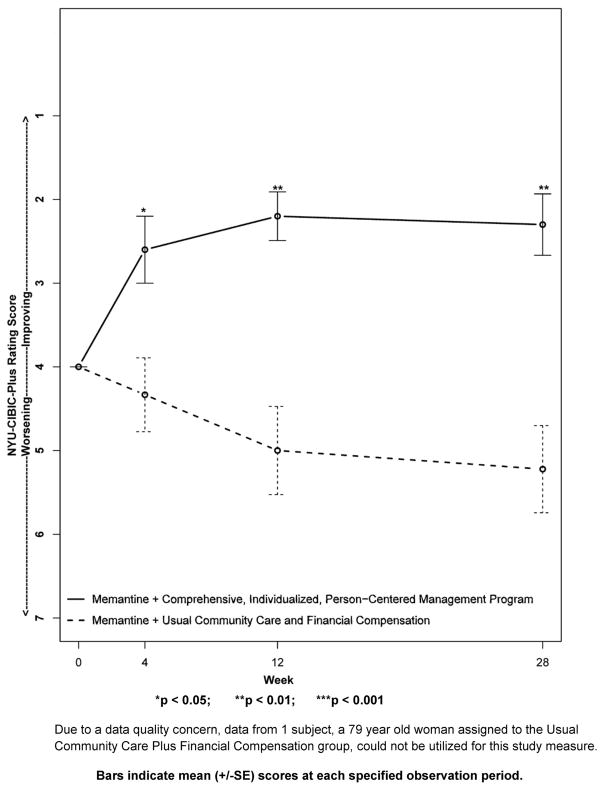
Figure 2. Primary Outcome Measure: The New York University Clinician’s Interview-Based Impression of Change-Plus Caregiver Input (NYU-CIBIC-Plus).
Change in the measure from baseline, set at 4 at week 0. Scores of 1, 2, and 3 correspond to “markedly,” “moderately,” and “minimally” improved, respectively, a score of 4 indicates, “unchanged,” and scores of 5, 6, and 7 correspond to “minimally,” “moderately,” and “markedly” worse, respectively.
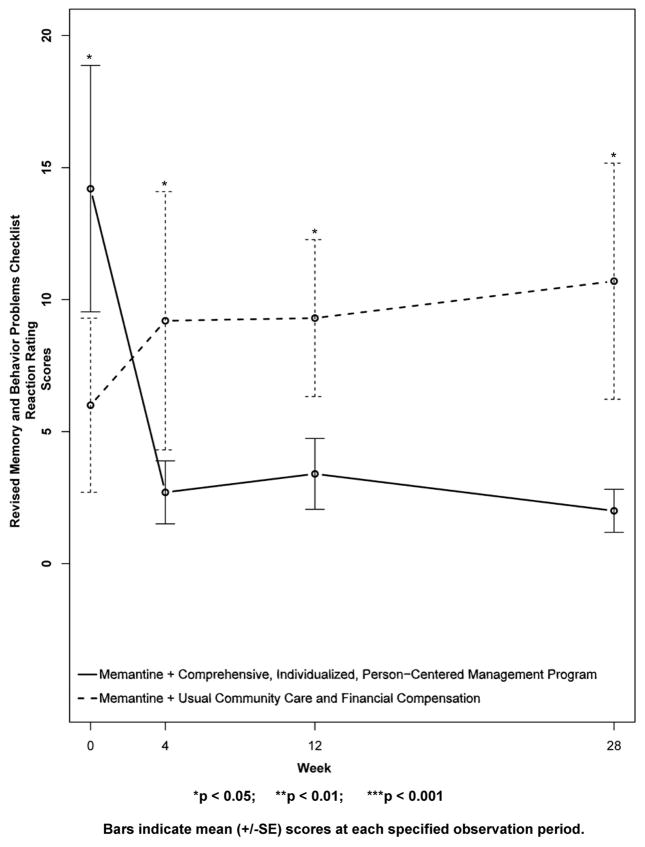
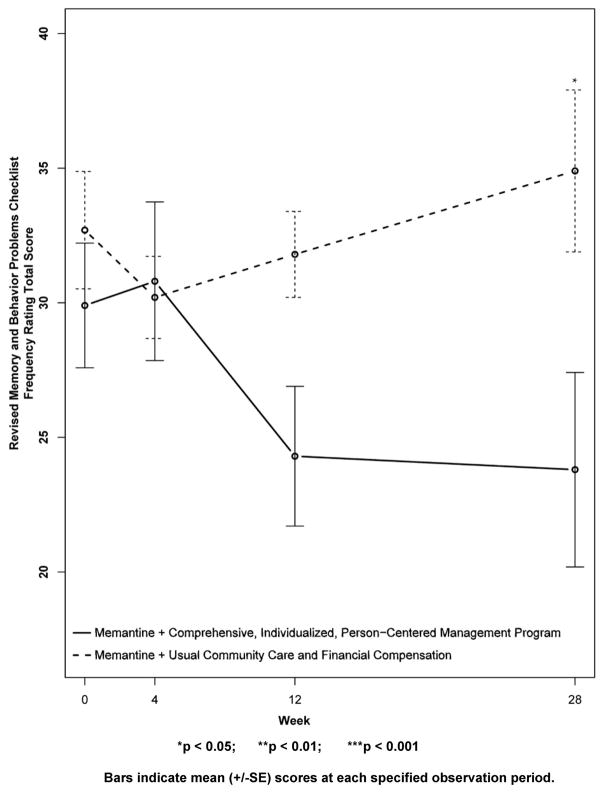
Figure 9. Secondary Outcome Measure: Revised Memory and Behavior Problems Checklist Reaction Ratings.
Higher scores indicate increased “bother or upset” in reaction to the memory and behavior problems of the subject, in the estimation of the caregiver. Note that the CI-PCM subject’s had significantly higher levels of “bother or upset” at baseline, and that the CI-PCM subjects had significantly lower levels of “bother or upset” at all subsequent visits.
For the NYU-CIBIC-Plus, due to a data quality concern, data from one subject, a 79-year old woman assigned to the UCC+FC group, was excluded. Figure 2, shows the result from the remaining 19 subjects. By convention, baseline for the NYU-CIBIC-Plus assessment is set at 4, which signifies “no change.” For post baseline assessments, lower values signify improvement, with 3 signifying “minimally improved,” a score of 2 signifying “moderately improved,” and a score of 1 signifying “markedly improved.” Higher scores on the NYU-CIBIC-Plus signify worsening. Specifically, scores of 5, 6, and 7 correspond to “minimally worse,” “moderately worse” and “markedly worse,” respectively. Beginning with the first post-baseline evaluation at week 4, the CI-PCM intervention group showed improvement on this primary outcome global measure. In contrast, subjects receiving UCC+FC comparator treatment showed worsening on the NYU-CIBIC-Plus at each post-baseline evaluation period. Between group differences were significant at week 4 (CI-PCM intervention 2.6±0.4[SE], UCC+FC comparator 4.3±0.4[SE], p < 0.05) and more robustly significant at week 12 (CI-PCM intervention 2.2±0.3[SE], UCC+FC comparator 5.0±0.5[SE], p < 0.01) and week 28 (CI-PCM intervention 2.3±0.4[SE], UCC+FC comparator 5.2±0.5[SE], p < 0.01), observation periods. Similarly robust outcomes were observed in the analysis with the entire, N=20, subject population (see Supplement Figure and Supplement Figure Legend).
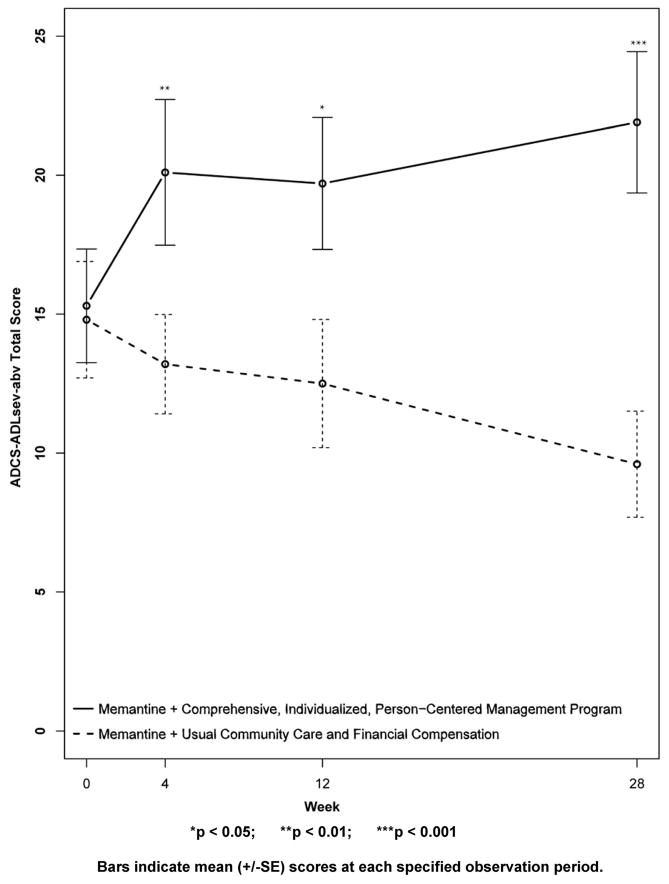
The other primary outcome, was the ADCS-ADLsev-abv on which a higher score indicates greater capacities. At baseline, the ADCS-ADLsev-abv mean score for the CI-PCM intervention subject group was 15.3±2.0[SE] and the mean score for the UCC+FC comparator subject group was 14.8±2.1[SE]. As shown in Figure 3, the CI-PCM intervention group improved in this assessment, in comparison with baseline, at all post-baseline assessments. In contrast, the UCC+FC comparator subjects worsened in ADCS-ADLsev-abv scores, in comparison with the baseline score, at all post-baseline assessments. Differences between the CI-PCM intervention and the UCC+FC comparator groups were robustly significant at week 4 (CI-PCM intervention 20.1±2.6[SE], UCC+FC comparator 13.2±1.8[SE], p < 0.01), significant at week 12 (CI-PCM intervention 19.7±2.4[SE], UCC+FC comparator 12.5±2.3[SE], p < 0.05), and very robustly significant (CI-PCM intervention 21.9±2.5[SE], UCC+FC comparator 9.6±1.9[SE], p < 0.001) at week 28.
Figure 3. Primary Outcome Measure: The Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory modified for more severe dementia, abbreviated first 12 questions version (ADCS-ADLsev-abv).
Higher scores indicate better functioning.
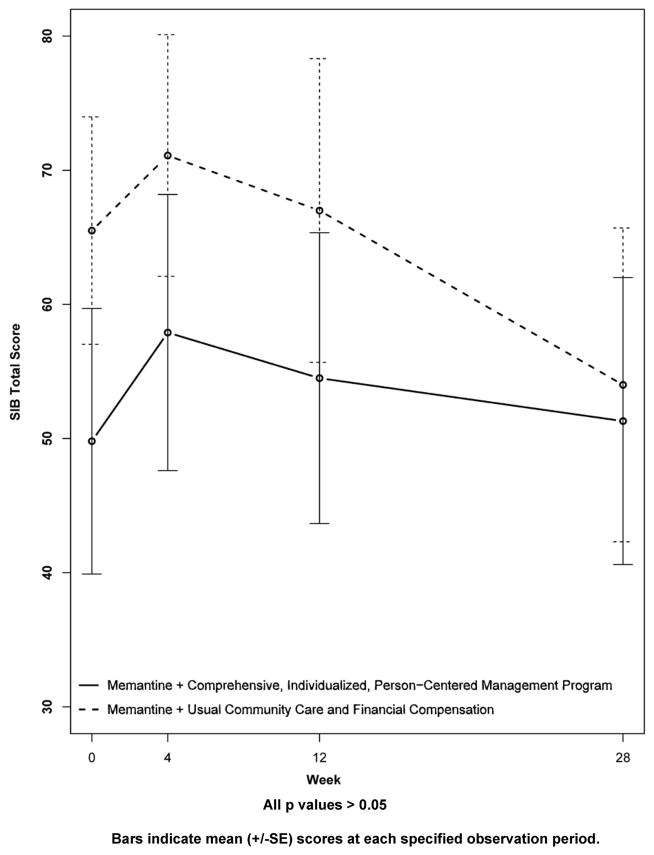
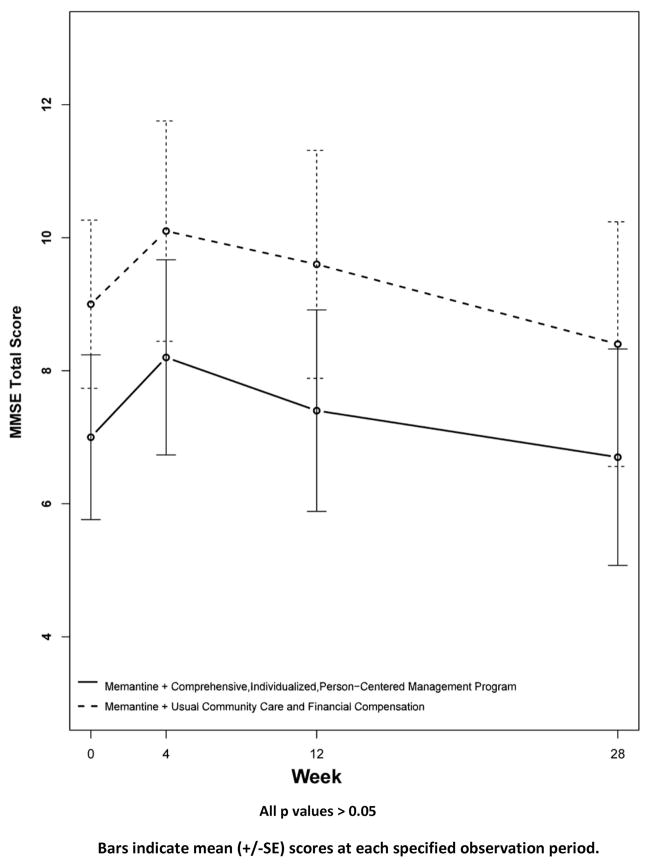
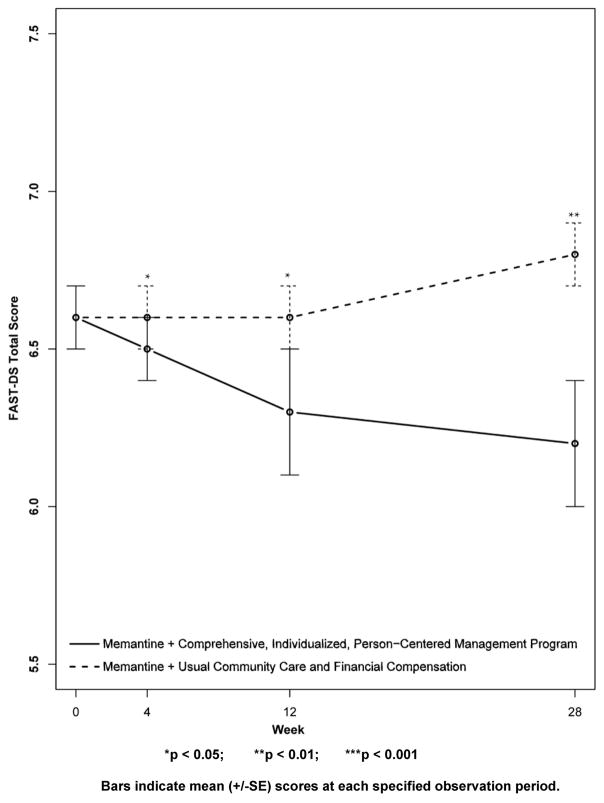
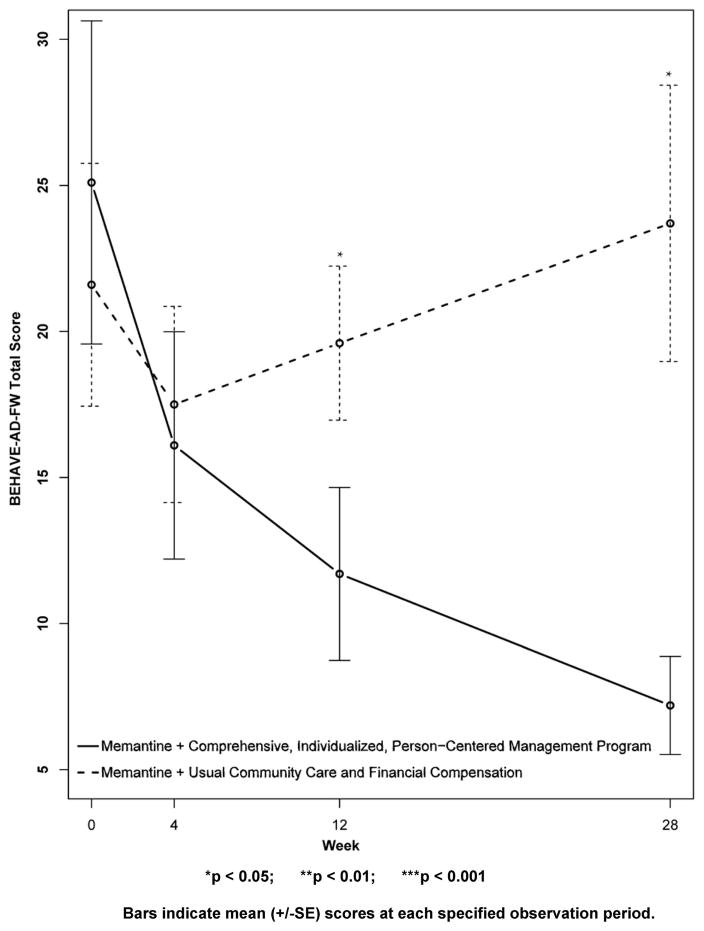
Results for secondary outcomes are shown in Figures 4 to 9. The cognitive secondary outcome assessments were the SIB and the MMSE. For both of these measures, a higher score is indicative of better performance. The baseline mean score for the CI-PCM intervention group on the SIB was 49.8±9.9[SE] and the mean score for the UCC+FC comparator group was 65.5±8.5[SE]. The baseline mean score for the CI-PCM intervention group on the MMSE was 7.0±1.2[SE] and the baseline mean MMSE score for the UCC+FC comparator group was 9.0±1.3[SE]. Neither objective cognitive assessment, the SIB or the MMSE, showed significant between group differences at any observation period (Figures 4 and 5). The functioning secondary outcome was the FAST-DS on which a higher score indicates greater impairment. At baseline, the mean FAST-DS score of the intervention group was 6.6±0.1[SE]. The baseline mean FAST-DS score of the comparator group was also 6.6±0.1[SE]. The FAST-DS, showed significant benefit in the CI-PCM group at weeks 4 (CI-PCM intervention 6.5±0.1[SE], UCC+FC comparator 6.6±0.1[SE], p < 0.05) and 12 (CI-PCM intervention 6.3±0.2[SE], UCC+FC comparator 6.6±0.1[SE], p < 0.05) and a robustly significant benefit at week 28 (CI-PCM intervention 6.2±0.2[SE], UCC+FC comparator 6.8±0.1[SE], p<0.01), (Figure 6). The behavioral disturbance evaluation, was the BEHAVE-AD-FW, in which a higher score indicates increased magnitude and frequency of behavioral disturbances. At baseline, the CI-PCM intervention subject group had a mean score of 25.1±5.5[SE] and the UCC+FC comparator subject group had a mean score of 21.6±4.2[SE]. Hence, at baseline, there was more behavioral disturbance in the future CI-PCM intervention subjects, than in the future UCC+FC comparator subjects. At week 4, this pattern reversed and there was more behavioral disturbance in the UCC+FC comparator subject group than in the CI-PCM intervention subject group. Beginning at week 12 the BEHAVE-AD-FW assessment showed significant benefit of the CI-PCM intervention (CI-PCM intervention 11.7±3.0[SE], UCC+FC comparator 19.6±2.6[SE], p<0.05) and this benefit was also observed at the 28 week observation period (CI-PCM intervention 7.2±1.7[SE], UCC+FC comparator 23.7±4.7[SE], p<0.05), with less disturbance in CI-PCM subjects (Figure 7). On the RMBPC frequency rating, a higher score indicates a higher frequency of occurrence of memory, emotional and behavioral problems. At baseline, the subjects assigned to the CI-PCM intervention group had a lower score (29.9±2.3[SE]) than the subjects assigned to the UCC+FC comparator group (32.7±2.2[SE]). As can be seen in figure 8, the pattern increased notably in week 12. Subsequently, at week 28, the RMBPC frequency rating showed significantly less frequent problems in the CI-PCM intervention group (CI-PCM intervention 23.8±3.6[SE], UCC+FC comparator 34.9±3.0[SE], p< 0.05), (Figure 8). For the reaction component of the RMBPC which assesses “how bothered or upset” the caregiver was by the subject’s memory and behavior, a significantly lower magnitude of reaction was present at baseline for the subjects assigned to the UCC+FC group (i.e., 6.0 ± 3.3[SE]) in comparison with the magnitude of “bother or upset” observed in the CI-PCM assigned subjects (i.e., a score of 14.2 ± 4.7[SE], p < 0.05, Figure 9). At all post baseline evaluations, the pattern completely reversed and significantly lower “bothered or upset” reactions were observed in the CI-PCM subjects at week 4 (CI-PCM intervention 2.7±1.2[SE], UCC+FC comparator 9.2±4.9[SE], p<0.05), week 12 (CI-PCM intervention 3.4±1.3[SE], UCC+FC comparator 9.3±3.0[SE], p<0.05), and week 28 (CI-PCM intervention 2.0±0.8[SE], UCC+FC comparator 10.7±4.5[SE], p<0.05) in comparison with the UCC+FC subjects (Figure 9).
Figure 4. Secondary Outcome Measure: The Severe Impairment Battery (SIB).
Higher scores indicate better cognition.
Figure 5. Secondary Outcome Measure: Mini-Mental Status Examination (MMSE).
Higher scores indicate better cognition.
Figure 6. Secondary Outcome Measure: Functional Assessment Staging Disability Score (FAST-DS).
Higher scores indicate increased functional disability.
Figure 7. Secondary Outcome measure: The Behavioral Disturbances in Alzheimer’s Disease Frequency Weighted Severity Scale (BEHAVE-AD-FW).
Higher scores indicate greater behavioral disturbance.
Figure 8. Secondary Outcome Measure: Revised Memory and Behavior Problems Checklist Frequency Ratings.
Higher scores indicate more frequent memory and behavioral problems.
All 20 eligible subjects were at GDS stage 6 at baseline and at the 4 week evaluation point. Seventeen were also in GDS stage 6 at all subsequent observation periods. Three subjects, all from the CI-PCM treatment group, improved to a GDS stage of 5 at the 12 week (n=1), or at the 28 week (n=2), post-baseline evaluations. There were no significant between group differences in the GDS stage at any study evaluation point.
The primary outcomes were consistent in demonstrating positive effects of the CI-PCM intervention in comparison with UCC+FC, at all post baseline evaluations.
Secondary outcomes were consistent in supporting positive effects of the CI-PCM intervention on functioning and behavioral disturbances with no significant effect on cognition. For the functioning outcomes and for two of the three behavioral disturbance outcome assessments, the significance level of improvement was greater at the final 28 week evaluations, than in the initial 4 week evaluations. For the remaining behavioral disturbance outcome assessment, the significance level of improvement was the same at all post baseline assessments.
Discussion
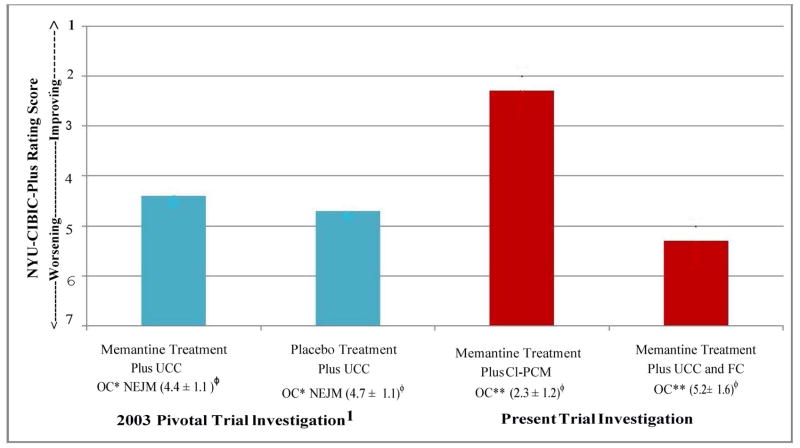
This study was modeled in part on the pivotal monotherapy trial associated with the EU and FDA’s approval of memantine [1]. Both studies had similar or identical inclusion criteria including age ≥ 50 years, DSM-IV [23] diagnoses of AD and McKhann, et al. criteria for probable AD [24], a FAST [26] score ≥ 6a, an MMSE [27] score of 3–14, and a Rosen-Hachinski rating [28] ≤ 4. The mean MMSE at baseline was 7.90 in the 2003 study and 7.85 in the present study and duration was 28-weeks in both studies. Both studies required reliable caregiver informants. A major difference between the studies was observed in outcomes which can be compared on the NYU-CIBIC-Plus primary outcome. By convention, baseline for the CIBIC-Plus assessment is set at “4.” Subsequent scores < 4 indicate progressive levels of improvement and > 4 indicate progressive levels of worsening. In the 2003 study, both treatment (memantine) and control (placebo) subjects declined on this global assessment (Figure 10) and the difference between the medication and placebo subject groups on the NYU-CIBIC-Plus, with the observed cases analysis, was 0.3 points.
Figure 10. Comparison Between Results in the 2003 Memantine Pivotal Trial and the Current Results with the Comprehensive, Individualized, Person-Centered Management (CI-PCM) Program or Usual Community Care Plus Financial Compensation (UCC+FC) on the Primary Global Outcome Measurement, the New York University Clinician’s Interview-Based Impression of Change Plus Caregiver Input (NYU-CIBIC-Plus). All results are at the 28 Week Study Endpoint.
NYU-CIBIC-Plus, New York University Clinician’s Interview-Based Impression of Change-Plus Caregiver Input; UCC, Usual Community Care; CI-PCM, Comprehensive, Individualized, Person-Centered Management program; FC, Financial Compensation, up to $100 per subject; *OC, Observed Cases, i.e., completers of the 28-week pivotal trial1 or** of the 28-week trial in the present investigation in which one subject in the UCC+FC group was excluded from this analysis because of data quality concerns; NEJM, New England Journal of Medicine, published pivotal trial in 2003.1 Mean NYU-CIBIC-Plus score ± standard deviation, at each 28-week study endpoint is shown. For scoring details, see Figure 2 Legend. The current UCC+FC group might appear to be different from the 2003 pivotal trial memantine treatment and placebo treatment groups on the NYU-CIBIC-Plus assessment. However, the UCC+FC treatment group has a much smaller sample size. Therefore the differences in the UCC+FC group results in the present study, are not significantly different from treatment or the placebo group in the 2003 controlled trial on the NYU-CIBIC-Plus assessment.
In the present study, in which both subject groups received memantine treatment, the subject group which received memantine and UCC+FC once again showed a decline on the NYU-CIBIC-Plus. The major difference between the 2003 pivotal trial and the present study is that in the 2003 trial the intervention subject group which received memantine treatment declined from baseline on the NYU-CIBIC-Plus by 0.4 points. In contrast, the CI-PCM plus memantine treatment group from the present study showed an improvement from baseline on the NYU-CIBIC-Plus of 1.7 points. Overall, the CI-PCM plus memantine treatment subjects improved 2.9 NYU-CIBIC-Plus points over the memantine treatment plus UCC+FC subject group. Of course, the 2003 pivotal trial participants in both study groups also received Usual Community Care and the $100 compensation in the present study comparator group is unlikely to have translated into meaningful clinical benefits. Therefore, the present results can be interpreted as improving the effects seen with medication treatment by a factor of 9.67 in comparison with the 0.3 point NYU-CIBIC-Plus improvement observed in the 2003 study of memantine versus placebo (i.e., a 967% improvement over medication treatment alone). This is ~ 10 times the traditional effect size previously observed with nonpharmacological interventions [15, 16]. We attribute the huge incremental effect in part to the severity range and corresponding needs of the subjects in both of these studies as well as to the therapeutic methodologies we employed in the CI-PCM program, which have been summarized herein.
A very brief case summary may be useful in further elucidating the procedures employed with the CI-PCM program participants.
Sole daughter and caregiver, C.G., was taught tools and techniques to help her mother, M.G. during the education sessions and home visits. C.G. was then able to better care for her mother relieving significant carer stress and anxiety regarding her ability to properly care for her mother. Using memory coaching, and various other strategies, C.G. was able to teach her mother to be urinary continent and sleep through the night. This alleviated M.G.’s disruptive behavior of rummaging through the refrigerator for snacks in the middle of the night and allowed both C.G. and M.G. to get some much needed rest. Being urinary continent meant there was much less laundry to do, further lightening the care burden. Additionally, M.G. was taught activities which she could do autonomously, with little to no supervision. For example, these activities included, crossword puzzles, folding laundry, etc. Participation in these activities served to mitigate M.G.’s purposeless repetitive behaviors, such as opening and closing a purse endlessly. As a result of these strategies, the subjects daughter, C.G., had the time to accomplish much needed tax paperwork, other important personal tasks, and the time to relax for a few moments of much needed respite.
Additionally, we have recently published a relatively detailed case history of a woman with Alzheimer’s disease, whom we (BR and SK) treated over a period of nearly 14 years [47]. Initially this woman, S.M., was in Global Deterioration Scale (GDS) stage and Functional Assessment Staging (FAST) stage 5, indicating moderate AD, and had an MMSE score of 19. We followed S.M. over 13 years and 9 months. Over this time, when she was in GDS stage 6, FAST stage 6c, and had an MMSE score of 2, she took up doing freestyle watercolor paintings for the first time. Subsequently, we show a picture of her carrying a cake and smiling sociably, 2 years after her MMSE score had reached zero. Over the nearly 14 year period, the subject progressed at less than half the rate typically observed in otherwise healthy AD persons.
In summary, from the evaluations of two independent groups of raters and observations with multiple rating instruments for each symptomatic domain, we conclude that the CI-PCM program in persons with moderate-to-severe AD, improved functional and behavioral disturbance symptomatology. No significant effects on cognition were observed. Globally, the magnitude of improvement in these community-residing persons living with AD appears to have been ~ 10X that conventionally observed in AD nonpharmacological and also, ostensibly, successful pivotal AD pharmacological trials. As a cure for AD remains elusive, these results, if replicated, might point the way toward less suffering for both carers and persons with advanced AD.
Supplementary Material
Acknowledgments
Supported by the Zachary and Elizabeth M. Fisher Center for Alzheimer’s Research Foundation, Forest Research Institute of Forest Laboratories, and United States Department of Health and Human Services (DHHS) grant P30 AG08051, R01 AG009127, R01 AG011505, and R01 AG003051, from the National Institute on Aging of the National Institutes of Health. Medication, Namenda, the brand formulation of memantine, was provided by Forest Research Institute of Forest Laboratories. Dr. Reisberg’s work is also supported by the Louis J. Kay and June E. Kay Foundation, the Hagedorn Fund, and Donations from Mrs. Miriam Glaubach and Dr. Felix Glaubach.
Footnotes
Additional Contributions: We thank all the persons living with AD and their care partners who consented to be screened and those who participated in the study. We especially thank the Usual Community Care and Financial Compensation participant dyads who received $100 each.
Disclosure Statement: Dr. Reisberg is the developer or codeveloper, and copyright holder of several of the assessment instruments used in this study. These instruments were selected because of their sensitivity in the severity range of the study subjects, their relevance for the respective domains of interest, and in several instances, also because of their use in the 2003 pivotal trial of memantine efficacy, with which the present study results are, to a large extent, being compared. Specifically, these copyrighted instruments are: the New York University Clinician’s Interview-Based Impression of Change-Plus Caregiver Input (NYU-CIBIC-Plus), (codeveloper and a copyright holder), the Functional Assessment Staging procedure and its associated disability scoring (developer and copyright holder), the Behavioral Pathology in Alzheimer’s Disease Frequency Weighted Severity Scale (developer and copyright holder), and the Global Deterioration Scale (developer and copyright holder). These instruments were provided to the study at no charge. None of the other authors of this study have any conflicts of interest to declare.
References
- 1.Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ for the Memantine Study Group. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348(14):1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 2.Winblad B, Poritis N. Memantine in severe dementia: results of the 9M-Best Study (Benefit and efficacy in severely demented patients during treatment with memantine) Int J Geriatr Psychiatry. 1999;14(2):135–146. doi: 10.1002/(sici)1099-1166(199902)14:2<135::aid-gps906>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I Memantine Study Group. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 4.Finkel SI, guest editor. Behavioral and Psychological Signs and Symptoms of Dementia: Implications for Research and Treatment. Proceedings of an international consensus conference. Lansdowne, Virginia, April 1996. Int Psychogeriatr. 1996;8(suppl 3):215–552. [PubMed] [Google Scholar]
- 5.Finkel SI, Burns A, guest editors. Behavioral and Psychological Symptoms of Dementia (BPSD): A Clinical and Research Update. Int Psychogeriatr. 2000;12(suppl 1):9–424. [Google Scholar]
- 6.Reisberg B, Monteiro I, Torossian C, Auer S, Shulman MB, Ghimire S, Boksay I, Guillo BenArous F, Osorio R, Vengassery A, Imran S, Shaker H, Noor S, Naqvi S, Kenowsky S, Xu J. The BEHAVE-AD assessment system: a perspective, a commentary on new findings, and a historical review. Dement Geriatr Cogn Disord. 2014;38(1–2):89–146. doi: 10.1159/000357839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodaty H, McGilchrist C, Harris L, Peters KE. Time until institutionalization and death in patients with dementia. Role of caregiver training and risk factors. Arch Neurol. 1993;50(6):643–650. doi: 10.1001/archneur.1993.00540060073021. [DOI] [PubMed] [Google Scholar]
- 8.Mittelman MS, Haley WE, Clay OJ, Roth DL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67(9):1592–1599. doi: 10.1212/01.wnl.0000242727.81172.91. [DOI] [PubMed] [Google Scholar]
- 9.Metitieri T, Zanetti O, Geroldi C, Frisoni GB, De Leo D, Dello Buono M, Bianchetti A, Trabucchi M. Reality orientation therapy to delay outcomes of progression in patients with dementia. A retrospective study. Clin Rehabil. 2001;15(5):471–478. doi: 10.1191/026921501680425199. [DOI] [PubMed] [Google Scholar]
- 10.Brotons M, Marti P. Music therapy with Alzheimer’s patients and their family caregivers: a pilot project. J Music Ther. 2003;40(2):138–150. doi: 10.1093/jmt/40.2.138. [DOI] [PubMed] [Google Scholar]
- 11.Ancoli-Israel S, Martin JL, Gehrman P, Shochat T, Corey-Bloom J, Marler M, Nolan S, Levi L. Effect of light on agitation in institutionalized patients with severe Alzheimer disease. Am J Geriatr Psychiatry. 2003;11(2):194–203. [PubMed] [Google Scholar]
- 12.Zeisel J, Silverstein NM, Hyde J, Levkoff S, Lawton MP, Holmes W. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697–711. doi: 10.1093/geront/43.5.697. [DOI] [PubMed] [Google Scholar]
- 13.Neal M, Briggs M. Validation therapy for dementia. Cochrane Database Syst Rev. 2003;(3):CD001394. doi: 10.1002/14651858.CD001394. [DOI] [PubMed] [Google Scholar]
- 14.Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, Kukull WA, LaCroix AZ, McCormick W, Larson EB. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA. 2003;290(15):2015–2022. doi: 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]
- 15.Luijpen MW, Scherder EJ, Van Someren EJ, Swaab DF, Sergeant JA. Non-pharmacological interventions in cognitively impaired and demented patients--a comparison with cholinesterase inhibitors. Rev Neurosci. 2003;14(4):343–368. doi: 10.1515/revneuro.2003.14.4.343. [DOI] [PubMed] [Google Scholar]
- 16.Olazarán J, Reisberg B, Clare L, Cruz I, Peña-Casanova J, Del Ser T, Woods B, Beck C, Auer S, Lai C, Spector A, Fazio S, Bond J, Kivipelto M, Brodaty H, Rojo JM, Collins H, Teri L, Mittelman M, Orrell M, Feldman HH, Muñiz R. Nonpharmacological therapies in Alzheimer’s disease: a systematic review of efficacy. Dement Geriatr Cogn Disord. 2010;30(2):161–178. doi: 10.1159/000316119. [DOI] [PubMed] [Google Scholar]
- 17.Reisberg B, Franssen EH, Souren LEM, Auer SR, Akram I, Kenowsky S. Evidence and mechanisms of retrogenesis in Alzheimer’s and other dementias: Management and treatment import. Am J Alz Disease. 2002;17(4):202–212. doi: 10.1177/153331750201700411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reisberg B, Javed A, Kenowsky S, Auer SR. Alzheimer’s disease. In: Zaretsky HH, Richter EF III, Eisenberg MG, editors. Medical Aspects of Disability. ed 3. New York: Springer; 2005. pp. 79–118. [Google Scholar]
- 19.Kitwood T, Bredin K. Towards a theory of dementia care: personhood and well-being. Ageing and Society. 1992;12(3):269–287. doi: 10.1017/s0144686x0000502x. [DOI] [PubMed] [Google Scholar]
- 20.Kenowsky S. What aspects of behavioral disturbances are important to caregivers? Perspective of a family caregiver. Int Psychogeriatr. 1996;8(suppl 3):449–453. doi: 10.1017/s1041610297003839. [DOI] [PubMed] [Google Scholar]
- 21.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonized tripartite guideline: guideline for good clinical practice. 1996 Jun 10;E6(R1) http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf.Published. [PubMed] [Google Scholar]
- 22.World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- 24.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 25.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139(9):1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 26.Sclan SG, Reisberg B. Functional assessment staging (FAST) in Alzheimer’s disease: Reliability, validity and ordinality. Int Psychogeriatr. 1992;4(1):55–69. doi: 10.1017/s1041610292001157. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of persons for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7(5):486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 29.Department of Health and Human Services National Institute of Health. Alzheimer’s Disease: Unraveling the Mystery. Dec, 2002. NIH Publication Number 02–3782. [Google Scholar]
- 30.Reisberg B, Kenowsky S, Franssen EH, Auer SR, Souren LEM. President’s Report: Towards a science of Alzheimer’s disease management: A model based upon current knowledge of retrogenesis. Int Psychogeriatr. 1999;11(1):7–23. doi: 10.1017/s1041610299005554. [DOI] [PubMed] [Google Scholar]
- 31.Reisberg B, Franssen EH. Clinical stages of Alzheimer’s disease. In: de Leon MJ, editor. An Atlas of Alzheimer’s Disease. Parthenon, Carnforth (U.K.): 1999. pp. 11–20. [Google Scholar]
- 32.Reisberg B, London E, Ferris SH, Borenstein J, Scheier L, de Leon MJ. The Brief Cognitive Rating Scale: language, motoric, and mood concomitants in primary degenerative dementia. Psychpharm Bull. 1983;19:702–708. [Google Scholar]
- 33.Reisberg B, Ferris SH, de Leon MJ, Sinaiko E, Franssen E, Kluger A, Mir P, Borenstein J, George AE, Shulman E, Steinberg G, Cohen J. Stage-specific behavioral, cognitive, and in vivo changes in community residing subjects with age-associated memory impairment and primary degenerative dementia of the Alzheimer type. Drug Develop Res. 1988;15(2–3):101–114. [Google Scholar]
- 34.Reisberg B, Auer SR, Monteiro I, Franssen E, Kenowsky S. A rational psychological approach to the treatment of behavioral disturbances and symptomatology in Alzheimer’s disease based upon recognition of the developmental age. International Academy for Biomedical and Drug Research. 1998;13:102–109. [Google Scholar]
- 35.Teri L, Logsdon RG, McCurry SM. Nonpharmacologic treatment of behavioral disturbance in dementia. Med Clin N Am. 2002;86(3):641–656. doi: 10.1016/s0025-7125(02)00006-8. [DOI] [PubMed] [Google Scholar]
- 36.Reisberg B, Franssen E, Sclan SG, Kluger A, Ferris SH. Stage specific incidence of potentially remediable behavioral symptoms in aging and Alzheimer’s disease: A study of 120 patients using the BEHAVE-AD. Bull Clin Neurosci. 1989;54:95–112. [Google Scholar]
- 37.McKitrick LA, Camp C, Black FW. Prospective memory intervention in Alzheimer’s disease. J Gerontol. 1992;47(5):337–343. doi: 10.1093/geronj/47.5.p337. [DOI] [PubMed] [Google Scholar]
- 38.Franssen EH, Souren LEM, Torossian CL, Reisberg B. Equilibrium and limb coordination in mild cognitive impairment and mild Alzheimer’s disease. J Am Geriatr Soc. 1999;47(4):463–469. doi: 10.1111/j.1532-5415.1999.tb07240.x. [DOI] [PubMed] [Google Scholar]
- 39.Franssen EH, Kluger A, Torossian CL, Reisberg B. The neurologic syndrome of severe Alzheimer’s disease: Relationship to functional decline. Arch Neurol. 1993;50(10):1029–1039. doi: 10.1001/archneur.1993.00540100024010. [DOI] [PubMed] [Google Scholar]
- 40.Souren LEM, Franssen EM, Reisberg B. Contractures and loss of function in patients with Alzheimer’s disease. J Am Geriatr Soc. 1995;43(6):650–655. doi: 10.1111/j.1532-5415.1995.tb07200.x. [DOI] [PubMed] [Google Scholar]
- 41.Reisberg B, Saeed MU. Alzheimer’s disease. In: Sadovoy J, Jarvik LF, Grossberg GT, Meyers BS, editors. Comprehensive Textbook of Geriatric Psychiatry - Third Edition. New York (NY): W.W. Norton; 2004. pp. 449–509. Sponsored by the American Association of Geriatric Psychiatry. [Google Scholar]
- 42.Reisberg B. Global measures: Utility in defining and measuring treatment response in dementia. Int Psychogeriatr. 2007;19(3):421–456. doi: 10.1017/S1041610207005261. [DOI] [PubMed] [Google Scholar]
- 43.Galasko DR, Schmitt FA, Jin S, Saxton J, Bennett D, Sano M, Ferris SH. Detailed assessment of cognition and activities of daily living in moderate to severe Alzheimer’s disease. Neurobiol Aging. 2000;21(suppl 1):168. [Google Scholar]
- 44.Panisset M, Roudier M, Saxton J, Boller F. Severe impairment battery. A neuropsychological test for severely demented patients. Arch Neurol. 1994;51(1):41–45. doi: 10.1001/archneur.1994.00540130067012. [DOI] [PubMed] [Google Scholar]
- 45.Monteiro IM, Boksay I, Auer SR, Torossian C, Ferris SH, Reisberg B. Addition of a frequency-weighted score to the Behavioral Pathology In Alzheimer’s Disease Rating Scale: the BEHAVE-AD-FW: methodology and reliability. Eur Psychiatry. 2001;16(suppl 1):5s–24s. doi: 10.1016/s0924-9338(00)00524-1. [DOI] [PubMed] [Google Scholar]
- 46.Teri L, Truax P, Logsdon R, Uomoto J, Zarit S, Vitaliano PP. Assessment of behavioral problems in dementia: the revised memory and behavior problems checklist (RMBPC) Psychol Aging. 1992;7(4):622–631. doi: 10.1037//0882-7974.7.4.622. [DOI] [PubMed] [Google Scholar]
- 47.Reisberg B, Franssen E, Souren L, Kenowsky S, Janjua K, Veigne S, Guillo Benarous F, Singh S, Khizar A, Shah U, Shah R, Bhandal A, Auer S. Alzheimer’s disease. In: Moroz A, Flanagan SR, Zaretsky H, editors. Medical Aspects of Disability. ed 5. Springer; New York: 2017. pp. 31–90. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.