Abstract
A fundamental goal of cognitive neuroscience is to explain how mental decisions originate from basic neural mechanisms. The goal of the present study was to investigate the neural correlates of perceptual decisions in the context of emotional perception. To probe this question, we investigated how fluctuations in functional MRI (fMRI) signals were correlated with behavioral choice during a near-threshold fear detection task. fMRI signals predicted behavioral choice independently of stimulus properties and task accuracy in a network of brain regions linked to emotional processing: posterior cingulate cortex, medial prefrontal cortex, right inferior frontal gyrus, and left insula. We quantified the link between fMRI signals and behavioral choice in a whole-brain analysis by determining choice probabilities by means of signal-detection theory methods. Our results demonstrate that voxel-wise fMRI signals can reliably predict behavioral choice in a quantitative fashion (choice probabilities ranged from 0.63 to 0.78) at levels comparable to neuronal data. We suggest that the conscious decision that a fearful face has been seen is represented across a network of interconnected brain regions that prepare the organism to appropriately handle emotionally challenging stimuli and that regulate the associated emotional response.
Keywords: decision making, emotion, functional MRI
The brain uses sensory information to make decisions that guide behavior. Currently, there is considerable knowledge, for example, about how contour, depth, and motion information is processed by early visual areas. In contrast, relatively little is known about how perceptual decisions are made. Even simple perceptual decisions, such as determining the presence or absence of a sensory stimulus, are not well understood. A fundamental goal of cognitive neuroscience is to explain how mental decisions originate from basic neural mechanisms.
In non-human primates, Newsome, Movshon, and colleagues (1, 2) have pioneered the investigation of the neural correlates of perceptual decisions. Their experiments have probed the mechanisms associated with deciding the direction of motion of an array of moving dots, only a fraction of which move coherently at a time. Newsome et al. (1, 2) showed that the activity of directionally selective neurons in middle temporal visual area (MT) could account for the psychophysical performance in a forced-choice direction discrimination task (1, 2). Related studies have quantified the link between performance and cell activity during depth perception (3) and the perception of structure from motion (4). Little is known, however, about the involvement of nonsensory regions in perceptual decisions. Previous neurophysiology studies typically have investigated one, and in some cases two [e.g., visual area 1 (V1) and MT], sensory processing regions (but see ref. 5).
Decision-making processes have been investigated with neuroimaging, too. Binder et al. (6) operationally defined decisions in an auditory object identification task in terms of reaction time (RT) and correlated RT with blood-oxygenation-level-dependent signals to reveal brain regions linked to decision processes. A more direct approach was taken by Heekeren et al. (7), who showed that activity in the dorsolateral prefrontal cortex covaried with the difference signal between face- and house-responsive regions in ventral temporal cortex in a face/house categorization task. Their results are important, because they suggest the existence of a general mechanism for perceptual decision making in the human brain based on the comparison of the outputs of different pools of selectively tuned neurons, as initially suggested in single-cell recording studies (8, 9).
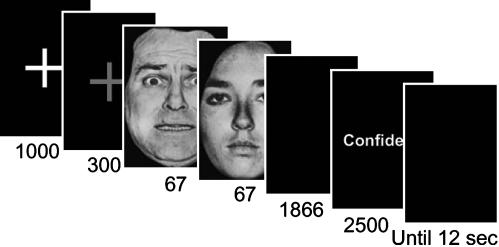
The goal of the present study was to investigate the neural correlates of decision making in the context of emotional perception. Although several brain regions have been implicated in the processing of emotion-laden visual stimuli (10), it is currently unknown whether they are also involved in perceptual decisions. To probe this question, we investigated how fluctuations in functional MRI (fMRI) signals were correlated with behavioral choice during a near-threshold fear detection task. Such correlation was quantified by adapting methods from single-cell physiology that allowed us to compute so-called choice probabilities (CPs) (8) in a voxel-wise manner. During our task, an initial neutral, happy, or fearful face was presented for 67 ms and immediately followed by a neutral face, which served as a mask (also shown for 67 ms; Fig. 1). In each trial, subjects indicated whether they had seen a fearful face. Like tasks studied in monkeys involving two-choice outcomes (e.g., left/right), the present study can also be viewed as a two-choice task (fear present/fear absent).
Fig. 1.
Experimental paradigm. For every trial, after the target-mask face pair, subjects indicated whether they saw a fearful face and then indicated their confidence in the response. The initial target face was a fearful, happy, or neutral face, and the mask was always a neutral face. Trials occurred every 12 s in a slow event-related design. The 300-ms fixation cross was actually green in the experiment.
Methods
For additional methods, please consult see Supporting Text, which is published as supporting information on the PNAS web site.
Stimuli and Procedure. Each trial began with a white fixation cross shown for 1,000 ms on a black background, followed by a green fixation shown for 300 ms on a black background, followed by the presentation of a fearful, happy, or neutral target face, immediately followed by a neutral face, which served as a mask (Fig. 1). The identities of the target and mask stimuli were always different. Faces subtended 4° of visual angle. The duration of the target face was 67 ms, and the mask face was 67 ms. Target presentation durations were confirmed by using a photodiode and an oscilloscope. Subjects were instructed that the stimulus would always comprise two faces and to respond “fear” if they perceived fear, however briefly. After the presentation of each face pair, subjects indicated fear or no fear with a button press. On each trial, subjects also rated the confidence in their response on a scale of 1 to 4 (low to high confidence). Happy faces were included to more closely match fearful faces in terms of low-level features, such as brightness around the mouth and eye regions, because both fearful and happy faces tend to be brighter than neutral ones in these regions. Thus, the inclusion of happy faces precluded subjects from using a strategy of detecting fearful faces by simply using low-level cues. The inclusion of happy faces also precluded subjects from adopting a strategy of indicating fear whenever facial features deviated from those of a neutral face. The total trial duration was ≈12 s (11,920 ms). Each subject performed 160 trials.
fMRI Data Analysis: CP Maps. Because we were interested in the neural correlates of the perceptual decision of fear, in choice-related analyses, we considered only high-confidence trials (values of 3 and 4); these comprised 75% of the total number of trials. In this manner, we largely eliminated guess trials.
To quantify the link between behavioral choice and fMRI signals, we computed individual CP maps. Thus, we sorted trials according to the two behavioral responses, namely, trials in which the subject reported seeing fear (hits plus false alarms) and trials during which the subject reported not seeing fear (correct rejects plus misses); these may be thought of as comprising the signal and noise distributions in signal detection theory. For high-confidence trials, on average, there were 51 reported fear trials and 60 reported no-fear trials per participant. Response strength was indexed by the average of the raw fMRI signal (after linear detrending) at times 3, 6, and 9 s relative to stimulus onset. Receiver operating characteristic (ROC) curves were constructed by using response strength and trial type in a manner analogous to that done in monkey physiology (8, 11). To create the ROC curve, we first determined the distribution of fMRI signal amplitudes as a function of trial type. The entire ROC curve was calculated by sliding a criterion threshold T value over the entire range of fMRI amplitudes, i.e., by determining P(a > T), where a is the fMRI signal amplitude, both for reported fear-present and -absent trials. Thus, to create the ROC curve, for a range of criterion response levels, we plotted on the x axis the proportion of reported fear-absent trials that exceeded the criterion, and on the y axis the proportion of reported fear-present trials that exceeded the same criterion.
The area under the ROC curve (A′) gave the CP value for the voxel. To determine the reliability of a CP, we used a permutation test (12) to compare it with the chance expectation of 0.5. The critical value of the CP was calculated after we randomly reassigned each fMRI response to either a reported fear or a reported no-fear behavioral choice, thus disrupting any correlation between fMRI signal and choices, while leaving the distributions of fMRI response and behavioral choice unchanged. In this manner, for each voxel, we generated the distribution of CPs expected in the absence of any association from 2,000 permutations (i.e., reassignments). Observed CP values were deemed statistically significant if they lay outside the central 95% of the distribution. In this manner, we created individual CP maps that displayed those voxels that reached significance based on the critical values computed above. Only choice values significantly >0.5 were considered, i.e., fear-present choices (see Supporting Text for a discussion of values significantly <0.5).
CPs were first computed by using only fear-neutral and neutral-neutral target-mask pairs. Given these trials types and behavioral responses, signal and noise distributions were determined as defined above and CP computed. Because different physical stimuli were used to compute CP, it is conceivable that stimulus differences could have contaminated the measure (see also Choice responses for constant physical stimuli in Supporting Text). Thus, we computed CPs in a separate manner by using happy targets rather than neutral ones, i.e., by using only fear- and happy-neutral target-mask pairs. To ensure that CPs reflected fear detection and not some low-level feature of the display, we determined the conjunction of the CP maps obtained in these two ways. By considering voxels that exhibited significant choice values in both contexts, we targeted voxels that were linked to deciding fear, irrespective of the type of nonfear target stimulus (neutral or happy). To assess the brain regions of greatest overlap in CP values and to display group results, we generated group CP maps. To create such maps, we smoothed each individual map with an 8-mm Gaussian filter (full width at half maximum) and averaged them. We then determined the conjunction of the choice maps obtained by separately considering neutral and happy-face trials as indicated above; the conjunction was obtained by determining the intersection of the two associated masks.
Because it was essential to formally assess the reliability of the individual CP maps in terms of inferences regarding the population (and not just in terms of the specific sample tested), we performed a standard two-stage random-effects analysis. The first stage corresponded to the individual CP maps, which were then submitted to a one-sample t test that evaluated whether the observed choice values significantly differed from 0.5 on a per-voxel basis. Because random-effects analysis may be fairly conservative (13), we used a threshold of P < 0.001 (uncorrected).
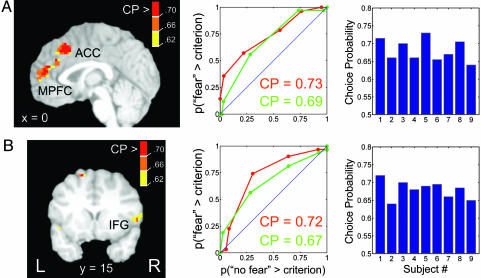
To illustrate the reliability of the individual participant's CP maps, for each brain region exhibiting strong fMRI signals predictive of behavioral choice independently of stimulus-driven signals, we selected the voxel coordinate corresponding to the peak CP value as the center of an 8-mm-radius spherical region. The values shown in Fig. 3 and Table 1 are the averaged-across-subject peak choice values obtained in the context of neutral and happy trials.
Fig. 3.
Individual CP data for the (A) MPFC and (B) right IFG. (Left) CP maps from two representative individuals. Maps were thresholded according to a per-voxel critical value for statistical significance (P < 0.05) obtained by a permutation test. The color bar indicates CP of individual voxels, which were the average value from the separate analyses with neutral and happy trials (see Methods). (Center) Choice-related ROC curve for the peak voxel of the activation (Left) when considering neutral trials only (red) and happy trials only (green). The x axis corresponds to the probability that fMRI magnitude in no-fear-response trials exceeds a criterion response magnitude; the y axis corresponds to the probability that fMRI amplitude in fear-response trials exceeds the same criterion. The ROC curve is determined by sliding the criterion value. The CP corresponds to the area under the ROC curve. The blue line (y = x) represents chance performance (area = 0.5), and departures from it indicate the possibility of distinguishing between the two trial types. In particular, ROC curves above the identity line indicate that responses during fear-response trials were consistently greater than responses during no-fear-response trials. Peak MPFC voxel coordinates: red and green curves, x = 0, y = 24, and y = 42; right IFG: red curve, x = 49, y = 17, and z = 3; green curve, x = 53, y = 15, and z = 3. (Right) Distribution of peak CP values for individual subjects for the (A) MPFC and (B) right IFG regions. Again, values were averaged from the analyses with neutral and happy trials.
Table 1. Brain regions exhibiting significant CP values at the group level.
| Montreal Neurological Institute coordinates
|
Peak CP*
|
Choice related†
|
|||
|---|---|---|---|---|---|
| Brain region | x | y | z | ||
| Right fusiform gyrus | 44 | -50 | -23 | No | |
| Right superior temporal sulcus | 56 | -45 | 23 | No | |
| PCC | 0 | -32 | 32 | 0.68 | Yes |
| ACC | 2 | 26 | 42 | No | |
| Right IFG | 44 | 19 | -10 | 0.67 | Yes |
| Left insula | -60 | 2 | 4 | 0.70 | Yes |
| MPFC | -2 | 50 | 12 | 0.69 | Yes |
| Right Anterior IFG | 44 | 46 | -4 | 0.68 | Yes |
CP values were the average of values obtained in separate analyses that considered neutral-neutral and happy-neutral trials as catch trials.
Only regions whose responses were not related to the physical characteristics of the stimulus (fear containing vs. neutral) or behavioral accuracy (correct vs. incorrect) were deemed to encode behavioral choice per se.
There are multiple ways to compare neuron- and fMRI-based CP values. In monkey studies, mean choice values are computed for all neurons that are carefully studied, which typically depends on meeting several criteria (e.g., exhibiting disparity tuning). In fMRI, because signal-to-noise ratios vary considerably across the brain, we did not average across all voxels; instead, we considered only those voxels with statistically significant CPs to determine the range of values.
The overlap between stimulus-driven, performance-related, and choice-related activation was formally assessed by computing their voxel-wise intersection by using masks. When any overlap with choice-related voxels was observed, they were marked with a “No” in Table 1. When no overlap was observed, the regions were deemed choice related (Table 1, “Yes”).
Results
For both the behavioral and fMRI results that follow, we considered only high-confidence trials. Analysis of the behavioral data showed that average accuracy across subjects for masked fear detection was 86% correct. Analysis of reaction times revealed no significant differences between reported fear-present [676 (mean) ± 148 (SD) ms] and fear-absent trials (726 ± 177 ms); P > 0.05, paired t test.
To investigate how fMRI signals were related to behavioral choice, we probed how the trial-by-trial variability in fMRI signal amplitude was correlated with the choices the subject made, independently of the effects of visual stimulation. In other words, in the fear-detection task used, could fMRI amplitude predict whether the subject responded fear or no fear on a trial-by-trial basis? We used a method based on signal detection theory, which is analogous to ROC analysis and which has been used in monkey physiology to link cell responses to behavioral choice (4, 11, 14). This method gives the probability that a so-called ideal observer, given access only to the fMRI amplitude in a trial, would be able to identify accurately which behavioral response was made in that particular trial (fear present or fear absent). Although in monkey physiology research, spike data are used as a measure of response, in the present case, fMRI amplitude was used as an index of response strength (see Methods). Because the task was to indicate whether fearful faces were shown, we discuss CPs linked to fear-present reports (see Supporting Text for further analyses). A value of 0.5 represents chance performance, and a value of 1.0 represents a perfect association between fMRI signals and behavioral response, i.e., fMRI amplitude would perfectly predict choice. Following previous work (8), we term this value CP.
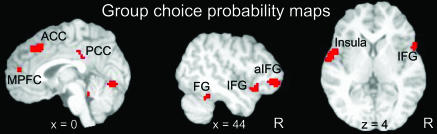
To probe regions involved in deciding whether fearful faces were perceived, we computed CPs in a voxel-wise manner. CP maps were computed for each individual and combined into a group map. To ensure that CPs truly reflected fear detection and not some low-level feature of the display, we determined ROCs in two separate contexts, by using only neutral-neutral trials as nontarget catch trials and separately only happy-neutral trials as catch trials. The conjunction of such maps was then determined and comprised our final results. In this manner, only voxels that predicted choice independently of the type of nontarget trial were considered to represent behavioral choice (see Methods). Moreover, we considered only high-confidence trials, which more directly reflect perceptual decision (thus largely eliminating guess trials). The resulting group CP maps revealed a set of brain regions that reliably predicted the subjects' responses (Fig. 2). The most strongly predictive regions included posterior cingulate cortex (PCC), medial prefrontal cortex (MPFC), right inferior frontal gyrus (IFG; this site encroached into the anterior insula), a more anterior site in the right IFG, and the left insula (see Table 1 for all regions and Supporting Text for discussion of motor-related activations). To formally assess the reliability of the group results, we performed a standard two-stage random-effects test of the CP values. The first stage corresponded to the individual CP values, which were then submitted to a one-sample t test that evaluated whether the observed values significantly differed from chance (0.5) on a per-voxel basis. With the exception of the right superior temporal sulcus, all regions were statistically significant.
Fig. 2.
Group CP maps illustrate areas that significantly predicted behavioral choices associated with reporting that fearful faces were seen according to trial-by-trial fluctuations in the fMRI signal. Note that the IFG site (on the right) abuts the insula. Group maps (n = 8) were obtained by averaging individual maps (see Methods) and were thresholded at a level to illustrate regions that predicted performance at the group level as indicated by random-effects analysis (Table 1). Slice coordinates are according to the Montreal Neurological Institute brain template. a, anterior; FG, fusiform gyrus.
To investigate the consistency of choice-related signals, we inspected individual choice maps (Table 1). To do so, individual maps were thresholded at a critical value obtained by a permutation test (12) to compare it with the chance expectation of 0.5. The averaged-across-subjects peak CPs in a set of regions (Table 1) ranged from 0.67 to 0.70. Fig. 3 displays CP maps and ROC curves for two representative individuals for the MPFC and the right IFG. The averaged-across-subjects peak CP was 0.69 for the MPFC (range, 0.64–0.78) and 0.67 for the right IFG (range, 0.63–0.73). Thus, the above results illustrate that results obtained at the group level were consistently observed across subjects.
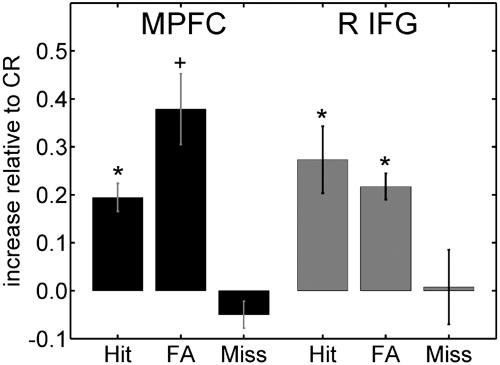
If fMRI signals in a brain region encode behavioral choice, then responses evoked during trials in which subjects reported seeing fear should be equivalent to one another. Thus, similar responses should be observed during hits (in which a target fearful face is correctly reported as fearful) and during false alarms (in which a nontarget stimulus is reported as fearful). Likewise, responses evoked for trials in which subjects reported not seeing fear should be equivalent to each other. Thus, similar responses should be observed during correct rejects (in which a nontarget stimulus is correctly reported as nonfearful) and during misses (in which a target fearful face is incorrectly reported as nonfearful). Deviations from this pattern would indicate that the region might be more closely tied to physical stimulus properties (containing or not fear) or performance (correct vs. incorrect trials); see below. Fig. 4 displays the responses to hits, false alarms, and misses relative to the responses to correct rejects for the MPFC and right IFG regions. Overall, the pattern is consistent with the encoding of behavioral choice (see Fig. 5, which is published as supporting information on the PNAS web site, and Supporting Text for further analyses).
Fig. 4.
Responses to hits, false alarms (FA), and misses, relative to correct reject (CR) responses. For both regions, hits and FA evoked similar responses that were stronger than responses evoked by misses and CR; the latter two trial types evoked similar responses. For both the MPFC and the right IFG, the pattern is thus consistent with the encoding of behavioral choice. An asterisk indicates P < 0.05 in a paired t test comparing that condition with miss trials; a plus sign indicates P = 0.06 in the paired t test comparing that condition with miss trials. Note that no other pairwise differences were observed (e.g., no significant differences were observed between hits and false alarms for the MPFC). Error bars are standard error of the mean pooled across subjects.
We also contrasted stimuli containing fearful targets (fear-neutral pairs) to those containing only neutral faces (neutral-neutral pairs). Several brain regions evoked greater responses during trials containing fearful targets than during trials containing only neutral faces (Table 2, which is published as supporting information on the PNAS web site). These regions included bilateral fusiform gyrus (at both more posterior and more anterior sites), bilateral superior temporal sulcus, and a site just dorsal to the right amygdala, which we interpret as ventral striatum. Such regions have been consistently reported during the perception of emotional faces in general, and fearful faces in particular (15). We also observed stronger activation during fear-containing trials in several other brain regions, including bilateral anterior insula, the anterior cingulate cortex (ACC), and the right middle frontal gyrus. The overlap between stimulus-driven and choice-related activation was formally assessed by computing their voxel-wise intersection (by using masks). Although there was some overlap (regions marked with a “No” in Table 1), the PCC, MPFC, right IFG (at both sites), and left insula were not driven by stimulus differences.
The next question we addressed was whether the areas that reliably predicted behavioral choice also predicted performance accuracy. To determine the regions that predicted task accuracy, we contrasted correct vs. incorrect trials. The resulting map revealed only two regions, bilateral anterior fusiform gyrus (at y = –45) and the ventral right amygdala/entorhinal cortex (z = –29; Fig. 6, which is published as supporting information on the PNAS web site). Thus, regions that predicted behavioral choice were distinct from those that predicted task accuracy.
Discussion
In the present paper, we computed whole-brain choice maps, which allowed us to characterize the distribution of CPs across regions and relate it to stimulus-evoked and performance-driven responses. Our results revealed a partial dissociation among regions that are sensitive to stimulus differences or performance and regions that are predictive of behavioral choice. We observed four regions that reliably predicted behavioral choice: PCC, MPFC, right IFG (at two sites), and left insula, which were neither sensitive to physical differences nor predictive of task accuracy. Thus, we suggest that these regions may have a role in perceptual decision making that is independent of visual stimulation and task accuracy (see Supporting Text and Figs. 7 and 8, which are published as supporting information on the PNAS web site, for further control analyses). The peak of the MPFC site was located in the most anterior aspect of the ACC and overlapped with a putative affective division of the ACC (16), which is connected to several emotional processing regions, including the amygdala, periaqueductal gray, nucleus accumbens, and hypothalamus, and has outflow to autonomic and visceromotor centers (17). Thus, the MPFC is well positioned to modulate brain responses according to the emotional significance of the incoming stimulus. The PCC is involved in emotion (18) and, in particular, the present finding that PCC signals are predictive of behavioral choice during the detection of fearful faces is consistent with a recent proposal that this region is involved in establishing an interface between attention and motivation (19). Interestingly, the PCC is also interconnected with the ACC, including its more anterior portion (20). The IFG has been proposed to be part of a network of regions that process emotionally arousing visual stimuli (21) and is connected to the amygdala and the more anterior portions of the ACC. Finally, the insula is a complex structure with important autonomic and other limbic functions, which is reciprocally connected with the amygdala (22). Overall, the PCC, MPFC, right IFG, and left insula may be part of a network of brain regions involved in emotional processing that participate in the decision that a fearful face has been seen.
Significant CP values were also observed in the left amygdala when these were computed based on fearful-neutral and neutral-neutral target-mask pairs. However, this site did not survive our stricter conjunction analysis. These findings suggest that amygdala responses may be predictive of behavioral choice when differences in arousal exist between the conditions but not when only valence differences exist (as in the context of happy faces). Moreover, a site in the inferior right amygdala predicted behavioral performance, suggesting that this region is important to the task, an interpretation consistent with a large body of data linking the amygdala to emotional perception (23).
CPs have been previously used in monkey neurophysiology to characterize the link between neuronal firing within one brain region and behavioral choice. In the original study by Britten et al. (8), a relatively modest but significant average CP of 0.55 was obtained for MT neurons tested in a direction discrimination task (8). Similar values have been obtained for MT neurons in depth perception (0.59) (3) and structure-from-motion tasks (0.57) (4). In one study (14), the mean CP value for MT neurons in a structure-from-motion task was 0.67. At the same time, single-cell unaveraged values in the 0.6–0.8 range have been reported (8). The present CP values were observed in a similar range (0.63–0.78). Our results thus demonstrate that fMRI signals can reliably predict behavioral choice in a rigorous and quantitative fashion at levels comparable with neuronal data.
In monkeys, activity that predicts behavioral choice or that appears to be linked to a perceptual decision has also been described in nonsensory regions, including the intraparietal area (24, 25), frontal eye fields (26), and medial (27) and dorsolateral (5, 28) prefrontal cortex. Although the involvement of some of these regions may have depended on the specific task used, it is noteworthy that we also observed choice-related activity in both medial and lateral prefrontal cortex (for some individuals, the latter site included responses in the inferior portions of the middle frontal gyrus).
Previous neuroimaging studies have also used signal detection theory to link behavior and fMRI signals. For example, Ress and Heeger (29) investigated a low-level visual detection task and showed that both hits and false alarms evoked greater responses than correct rejects and misses (see Fig. 3 as well as Fig. 6). Thus, in early visual areas V1, V2, and V3, activity corresponded to the subject's percept rather than the physically presented stimulus. A related approach was used by Grill-Spector et al. (30) to probe the neural correlates of detection and identification of faces in occipitotemporal cortex; their results revealed stronger responses in the fusiform gyrus when a face was detected vs. when it was missed.
In the present study, we showed that small fluctuations in fMRI responses were related to near-threshold fluctuations in the perceptual decision process. The present findings extend previous results from neurophysiology by showing that fMRI signals can quantitatively predict behavioral choice independent of stimulus properties and task accuracy. The present results show that certain perceptual decisions are not encoded by a single central brain region (see ref. 7). Instead, in the case of fear detection, decisions are represented over a network of nonsensory regions (see also ref. 5), which includes the PCC, MPFC, right IFG, and left insula. Our results do not imply, however, that the entire network is directly responsible for deciding that a fearful target is present. Choice-related signals in one region may reflect computations made at another site in the brain. Moreover, the complex decisions in our task are unlike simpler sensory decisions studied with monkeys. Thus, choice-related activations that predicted fear-present behavioral responses should be viewed to include a more general set of processes that are associated with fear detection. We suggest that the conscious decision that a fearful face is present is represented across a network of interconnected brain regions that prepare the organism to appropriately handle emotionally challenging stimuli and that regulate the associated emotional response. Our analysis demonstrates that we can quantify the link between the behavioral decision that fear is present and fMRI signals. Additional studies are needed to elucidate how and when these multiple regions are involved in perceptual decision making, and how their role might differ from other nonsensory regions (e.g., frontal eye fields, dorsolateral prefrontal cortex) shown to contribute to perceptual decisions (5, 7, 28).
Supplementary Material
Acknowledgments
We thank Mrim Boutla, Alexander Grunewald, and Shruti Japee, as well as the anonymous reviewers, for insightful discussions and feedback on the manuscript; Vishnu Murty for assistance in the analysis of the behavioral data; Alumit Ishai for making available a set of emotional faces; and Ziad Saad for the development of the Analyses of Functional Neuroimages (National Institute of Mental Health, National Institutes of Health)/matlab (Mathworks, Natick, MA) interface, which was instrumental in developing voxel-wise CP computations. This research was supported in part by Grant 1 R01 MH071589-01 from the National Institute of Mental Health, by the Brain Science Program (the Burroughs Wellcome Fund) of Brown University, and by the Ittleson Foundation.
Author contributions: L.P. designed research; S.P. performed research; L.P. contributed new reagents/analytic tools; S.P. analyzed data; and L.P. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: fMRI, functional MRI; ROC, receiver operating characteristic; PCC, posterior cingulate cortex; MPFC, medial prefrontal cortex; IFG, inferior frontal gyrus; ACC, anterior cingulate cortex; CP, choice probability; MT, middle temporal visual area.
References
- 1.Newsome, W. T., Britten, K. H. & Movshon, J. A. (1989) Nature 341, 52–54. [DOI] [PubMed] [Google Scholar]
- 2.Salzman, C. D. & Newsome, W. T. (1994) Science 264, 231–237. [DOI] [PubMed] [Google Scholar]
- 3.Uka, T. & DeAngelis, G. C. (2004) Neuron 42, 297–310. [DOI] [PubMed] [Google Scholar]
- 4.Grunewald, A., Bradley, D. C. & Andersen, R. A. (2002) J. Neurosci. 22, 6195–6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romo, R. & Salinas, E. (2003) Nat. Rev. Neurosci. 4, 203–218. [DOI] [PubMed] [Google Scholar]
- 6.Binder, J. R., Liebenthal, E., Possing, E. T., Medler, D. A. & Ward, B. D. (2004) Nat. Neurosci. 7, 295–301. [DOI] [PubMed] [Google Scholar]
- 7.Heekeren, H. R., Marrett, S., Bandettini, P. A. & Ungerleider, L. G. (2004) Nature 431, 859–862. [DOI] [PubMed] [Google Scholar]
- 8.Britten, K. H., Newsome, W. T., Shadlen, M. N., Celebrini, S. & Movshon, J. A. (1996) Visual Neurosci. 13, 87–100. [DOI] [PubMed] [Google Scholar]
- 9.Purushothaman, G. & Bradley, D. C. (2005) Nat. Neurosci. 8, 99–106. [DOI] [PubMed] [Google Scholar]
- 10.Adolphs, R. (2002) Current Opin. Neurobiol. 12, 169–177. [DOI] [PubMed] [Google Scholar]
- 11.Barlow, H. B., Levick, W. R. & Yoon, M. (1971) Vision Res. Suppl. 3, 87–101. [DOI] [PubMed] [Google Scholar]
- 12.Efron, B. & Tibshirani, R. J. (1993) An Introduction to the Bootstrap (Chapman & Hall, New York).
- 13.Worsley, K. J., Liao, C. H., Aston, J., Petre, V., Duncan, G. H., Morales, F. & Evans, A. C. (2002) NeuroImage 15, 1–15. [DOI] [PubMed] [Google Scholar]
- 14.Dodd, J. V., Krug, K., Cumming, B. G. & Parker, A. J. (2001) J. Neurosci. 21, 4809–4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haxby, J. V., Gobbini, M. I., Furey, M. L., Ishai, A., Shouten, J. L. & Pietrini, P. (2001) Science 293, 2425–2430. [DOI] [PubMed] [Google Scholar]
- 16.Bush, G., Luu, P. & Posner, M. I. (2000) Trends Cognit. Sci. 4, 215–222. [DOI] [PubMed] [Google Scholar]
- 17.Barbas, H. (2000) Brain Res. Bull. 52, 319–330. [DOI] [PubMed] [Google Scholar]
- 18.Maddock, R. J. (1999) Trends Neurosci. 22, 310–316. [DOI] [PubMed] [Google Scholar]
- 19.Small, D. M., Gitelman, D. R., Gregory, M. D., Nobre, A. C., Parrish, T. B. & Mesulam, M. M. (2003) NeuroImage 18, 633–641. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi, Y. & Amaral, D. G. (2003) J. Comp. Neurol. 466, 48–79. [DOI] [PubMed] [Google Scholar]
- 21.Yamasaki, H., LaBar, K. S. & McCarthy, G. (2002) Proc. Natl. Acad. Sci. USA 99, 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mesulam, M.-M. (2000) in Principles of Behavioral and Cognitive Neurology, ed. Mesulam, M. (Oxford Univ. Press, New York), pp. 1–120.
- 23.Adolphs, R. (2002) Curr. Opin. Neurobiol. 12, 169–177. [DOI] [PubMed] [Google Scholar]
- 24.Shadlen, M. N. & Newsome, W. T. (2001) J. Neurophysiol. 86, 1916–1936. [DOI] [PubMed] [Google Scholar]
- 25.Platt, M. L. & Glimcher, P. W. (1999) Nature 400, 233–238. [DOI] [PubMed] [Google Scholar]
- 26.Gold, J. I. & Shadlen, M. N. (2000) Nature 404, 390–394. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez, A., Zainos, A. & Romo, R. (2002) Neuron 33, 959–972. [DOI] [PubMed] [Google Scholar]
- 28.Kim, J. N. & Shadlen, M. N. (1999) Nat. Neurosci. 2, 176–185. [DOI] [PubMed] [Google Scholar]
- 29.Ress, D. & Heeger, D. J. (2003) Nat. Neurosci. 6, 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grill-Spector, K., Knouf, N. & Kanwisher, N. (2004) Nat. Neurosci. 7, 555–562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.