Abstract
Myxobacteria are known for complex social behaviors including outer membrane exchange (OME), in which cells exchange large amounts of outer membrane lipids and proteins upon contact. The TraA cell surface receptor selects OME partners based on a variable domain. However, traA polymorphism alone is not sufficient to precisely discriminate kin. Here, we report a novel family of OME-delivered toxins that promote kin discrimination of OME partners. These SitA lipoprotein toxins are polymorphic and widespread in myxobacteria. Each sitA is associated with a cognate sitI immunity gene, and in some cases a sitB accessory gene. Remarkably, we show that SitA is transferred serially between target cells, allowing the toxins to move cell-to-cell like an infectious agent. Consequently, SitA toxins define strong identity barriers between strains and likely contribute to population structure, maintenance of cooperation, and strain diversification. Moreover, these results highlight the diversity of systems evolved to deliver toxins between bacteria.
eLife digest
Most people do not think of bacteria as having a social life. However, some groups, such as myxobacteria, are highly cooperative. Although these microbes exist as individual cells, they can also move and hunt in coordinated packs and when nutrients are low, about a million cells come together to build spore-filled structures. To do so, myxobacteria need to recognize their sibling cells among the vast number of different species of microbes found in soil.
One way that the bacteria recognise their kin is by displaying a variable cell surface protein, called TraA, that identifies other individuals that display the same protein on their surface. Upon recognition, cells exchange resources by briefly fusing their outer membranes. This allows bacteria to help to rejuvenate damaged sibling cells by delivering healthy cell components to them. Now, using a genetic approach, Vassallo et al. present evidence that bacteria can also exchange toxins.
The newly identified toxin-exchange system works alongside the TraA kin recognition system to allow myxobacteria to recognize and verify their true sibling cells in diverse environments. The cells involved in the exchange must contain matching immunity proteins to survive the interaction – thus the exchange does not harm sibling cells. Strikingly, once the toxic proteins are delivered, they can be passed on to other cells by a series of transfers, much like an infection spreads throughout a population.
The study performed by Vassallo et al. provides a new framework for understanding how microbes recognize their kin to build a community. These insights will help investigators to explore other microbial ecosystems, including those found inside the human body. Additionally, the results also suggest ways in which cells can be engineered to specifically recognize other cells to transfer materials between them. This system could be adapted to program different cell types so that they interact with specific partners and perform complex tasks.
Introduction
Cooperative, social organisms benefit by resource sharing and division of labor between individuals in a population. These behaviors entail directing beneficial action toward kin, often at a fitness cost to the actor. Relatedness between individuals must be high for such cooperative action to remain evolutionarily viable (Hamilton, 1964a, 1964b). This requires that social organisms recognize their kin, and direct preferential action toward them (kin discrimination). The mechanisms by which social microbes recognize and direct benefits toward kin cells are not well understood. However, insights in this area will help us to understand the organization of microbes into social groups and the behaviors that maintain cooperation despite seemingly opposing evolutionary pressures to be selfish.
The soil bacterium Myxococcus xanthus is a model organism for the study of social behavior and cooperation (Cao et al., 2015). Myxobacterial populations divide labor and share resources during coordinated behaviors such as swarming, predation, and starvation-induced fruiting body development. Their social lifestyle, which includes multicellular development by an aggregation strategy, requires that they direct cooperative behavior towards their clonemates or close kin. One such cooperative behavior is outer membrane exchange (OME). During OME, swarming cells in a population simultaneously donate and receive prodigious amounts of outer membrane (OM) material between one another during cell contact. Exchanged material includes membrane lipids, lipoproteins and lipopolysaccharide (Nudleman et al., 2005; Wei et al., 2011; Vassallo et al., 2015; Pathak et al., 2012). The mechanism for exchange is thought to involve transient OM fusion catalyzed by the OM receptor TraA and an associated protein, TraB (Cao et al., 2015; Pathak et al., 2012). Our model predicts that transient OM fusion between two cells enables the lateral diffusion of OM lipids and proteins between OMs until cells move apart and the membranes are again separated (Cao et al., 2015). This process occurs constitutively on surfaces and facilitates efficient OM homogenization of populations with heterogeneous OMs (Wei et al., 2011). Exchange of fluorescent OM lipoprotein reporters, as well as endogenous OM lipoproteins, demonstrates that nearly all recipient cells receive substantial amounts of cargo protein within two hours of co-culture (Nudleman et al., 2005; Wei et al., 2011). Furthermore, cells with lethal defects in lipopolysaccharide biosynthesis can be sustained in a population by OME with wild-type (WT) donors (Vassallo et al., 2015). Based on this observation, OME is hypothesized to help physiologically heterogeneous populations move toward homeostasis and buffer cell damage to support synchronized and cohesive group behaviors (Vassallo et al., 2015; Vassallo and Wall, 2016). This robust system for sharing cellular goods must be discriminately targeted to closely related cells – that is, clonemates. Otherwise, this organism risks donating private goods to competing, non-kin genotypes. In this regard, we previously showed that TraA has a variable domain that specifies recognition between cells through homotypic interactions (Pathak et al., 2013; Cao and Wall, 2017). Thus, myxobacteria with divergent, incompatible TraA receptors do not engage in OME. traA is therefore a greenbeard gene in that it allows myxobacteria to identify cells with identical alleles and to direct beneficial treatment toward those cells (Dawkins, 1976). Greenbeard alleles do not exclusively recognize kin genotypes, but instead recognize any genotype that possesses the same allele (kind discrimination) (Queller, 2011; Strassmann et al., 2011). Indeed, although TraA sequence diversity in the variable domain is high, some non-kin genotypes share compatible traA alleles (Pathak et al., 2013). In fact, some Myxococcus isolates that antagonize one another in co-culture possess the same traA alleles (Pathak et al., 2013). Based on this observation, we hypothesized that there are additional genetic determinants that more precisely discriminate kin during social interactions.
Bacterial kin discrimination is often mediated by antagonism toward non-kin. One mechanism that bacteria use to this end is the delivery of polymorphic toxins between cells in close contact (Zhang et al., 2012; Ruhe et al., 2013; Cardarelli et al., 2015; Wenren et al., 2013). These toxins usually share homology in species-specific amino-terminal domains required for presentation and/or delivery, but vary in their carboxy-terminal toxin domains (Zhang et al., 2012). Each toxin is associated with a cognate immunity protein, typically encoded together in an operon, which specifically neutralizes toxicity in the producing cell and in clonemates or close kin that share the locus. The presence of a polymorphic toxin/immunity pair in one strain leads to antagonism toward related strains that do not possess immunity (Riley and Wertz, 2002). Examples include contact-dependent growth inhibition (CDI; a type Vb secretion system) (Aoki et al., 2005, Aoki et al., 2010); modular type IV secretion system (T4SS) (Souza et al., 2015), type VI secretion system (T6SS) (Russell et al., 2011; MacIntyre et al., 2010; Schwarz et al., 2010; Hood et al., 2010), and type VII secretion system (T7SS) (Cao et al., 2016) effectors; as well as the MafB toxins of Neisseria (Jamet and Nassif, 2015a). The competitive advantages offered by these toxins likely drives positive selection for novel toxin/immunity pairs, which in turn helps to define kin groups through inter-strain antagonism. Mining of prokaryotic genomes revealed that polymorphic toxins are indeed quite prevalent and diverse (Zhang et al., 2012). Additionally, many homologous C-terminal toxin domains are shared between distantly related toxin-delivery systems from diverse organisms, suggesting that these toxins have evolved from a common pool of domains (Zhang et al., 2012). Currently there is a knowledge gap between the number of toxin domains discovered through bioinformatic analysis and the experimental characterization of their delivery mechanisms (Jamet and Nassif, 2015b; Benz and Meinhart, 2014). It seems likely that additional, uncharacterized modes of polymorphic toxin delivery remain to be discovered, with each mechanism adapted to the host’s particular lifestyle. As mechanisms that promote inter-strain and inter-species conflict, polymorphic toxins appear to play a strong role in the evolution of microbes. For instance, kin discrimination by polymorphic toxins may help maintain cooperation in social organisms such as myxobacteria by promoting local relatedness (Hamilton, 1964a; Vos and Velicer, 2009). In addition, they likely play an important role in symbiosis (Hillman and Goodrich-Blair, 2016) and in population structure within ecological niches such as the soil (Varivarn et al., 2013), rhizosphere (Ma et al., 2014), and human gut (Zheng et al., 2015; Russell et al., 2014).
We previously showed that the widely used DK1622 reference strain of M. xanthus is killed by ancestral strains when co-cultured on surfaces (Dey et al., 2016). A traA mutation in either strain abolishes this behavior, indicating that OME is required for antagonism. Further, this antagonism requires a hyper-variable region of the chromosome called Mx-alpha, which is composed of roughly 100 kb of prophage and mobile genetic elements and can be found in multiple copies of imperfect repeats in M. xanthus genomes (Dey et al., 2016). In ancestral strains that antagonize DK1622, there are three homologous Mx-alpha units apparently arranged in tandem. However, two of these units (~200 kb) were lost by spontaneous deletion during the construction of DK1622 (Dey et al., 2016). From these observations, we hypothesized that OME-delivered toxins encoded within the Mx-alpha repeat elements are responsible for antagonism.
Here, we identify the genetic determinant of this antagonism as one of several related, polymorphic, OM lipoprotein toxins that are encoded on Mx-alpha and transferred to target cells by OME. OME between strains that contain different toxins leads to mutual cell death, which establishes territorial barriers between populations. These toxins belong to a large and diverse family found in myxobacteria and display features that make them unique among polymorphic toxin systems. Strikingly, we show that these toxins are serially transferred from cell-to-cell by OME, which results in a potent killing system. Finally, we provide evidence that OME-mediated antagonism contributes to the ecology and evolution of these social microbes.
Results
SitA1 is the swarm inhibition toxin
M. xanthus inter-strain antagonism related to the presence of Mx-alpha was originally observed as ‘swarm inhibition’, during which a nonmotile ancestor strain (Mx-alpha+) inhibited the outward swarming of a motile strain (missing two of three Mx-alpha units) during co-culture on agar. This phenomenon is traA-dependent and therefore is likely an outcome of OME (Pathak et al., 2013) (see Figure 1). Swarm inhibition was further demonstrated to be caused by cell death of the motile strain (Dey et al., 2016). We first sought to identify the specific genetic determinant on Mx-alpha that was required for antagonism and cell death of the susceptible motile strain. Sequence analysis of the two Mx-alpha units retained in the ancestral DK101 strain (a.k.a. DZF1) (Müller et al., 2013) but lost in DK1622 revealed a candidate toxin gene (MXF1DRAFT_07513), which we have designated sitA1 for swarm inhibition toxin. This gene encodes a predicted lipoprotein that contains a C-terminal nuclease domain with a WHH motif (Zhang et al., 2011) (Figure 1A). The absence of lysine at the +2 position or alanine at the +7 position relative to the +1 cysteine in the N-terminal lipobox suggests that this protein is localized to the OM (Bhat et al., 2011) (see Supplementary file 1). Given that OME efficiently transfers OM lipoproteins between cells (Wei et al., 2011), this open reading frame (ORF) represents a promising candidate for the antagonistic determinant. Immediately downstream of sitA1 is a gene (sitI1) that shows homology to the SUKH-family of immunity proteins commonly found in polymorphic toxin systems (Zhang et al., 2011) (Figure 1A). Upstream of sitA1 is a hypothetical gene (sitB1) of unknown function. The sitB1 ORF overlaps with sitA1 by 11 base pairs, suggesting that the genes form an operon and function together (Figure 1A).
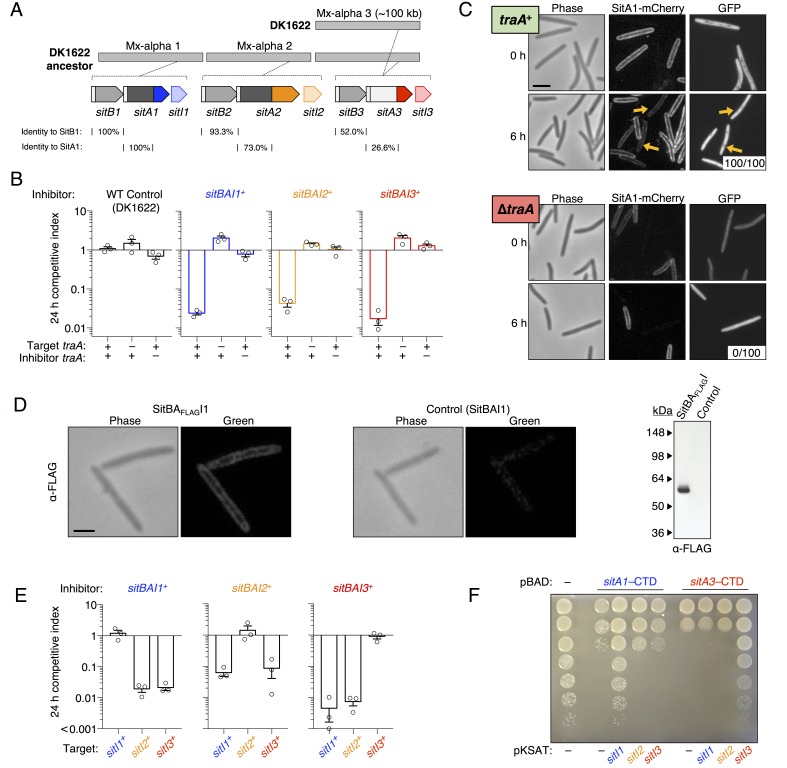
Figure 1. SitA1 is the swarm inhibition determinant.
(A) sitBAI1 operon found on one of the Mx-alpha elements that was lost from DK1622. SS, signal sequence. (B) Swarm inhibition assays with indicated motile and nonmotile strains. White arrow illustrates swarm inhibition with control strains. NA, not applicable. Bar, 1 mm. (C) Expression of sitBAI2 in a non-antagonistic nonmotile background results in modest swarm inhibition (indicated by *) compared to sitBAI1+ (shown in B). Expression of sitBAI3 in the non-antagonistic nonmotile background results in complete swarm inhibition of ∆sitBAI3. Here and elsewhere see Supplementary file 2A for strain details.
To test if sitA1 is the swarm inhibition toxin, we used the swarm inhibition assay as a readout for the contribution of sitA or sitI toward cell death of the susceptible motile strain during co-culture with the antagonistic, nonmotile ancestral strain. In this assay, cell death of the susceptible strain results in no cells visibly escaping the mixed culture spot. In contrast, abrogation of cell death results in the appearance of the motile strain moving outward from the colony co-culture. As shown previously (Pathak et al., 2013; Dey et al., 2016; Dey and Wall, 2014), nonmotile ancestral cells inhibited the motility of DK1622, but not the DK1622 ∆traA strain (Figure 1B, rows 1–4). Importantly, nonmotile ancestors carrying a sitA1 mutation did not inhibit DK1622 (Figure 1B, row 5). Further, expression of sitI1 in motile DK1622 cells also prevented antagonism (Figure 1B, row 6), consistent with the prediction that sitI encodes an immunity protein that neutralizes SitA1. To test whether sitBAI1 is sufficient to convert non-antagonistic cells into killers, we expressed the gene cassette in a DK1622-derived nonmotile strain, which lacks two Mx-alpha units (see Figure 2A), and does not cause swarm inhibition. As predicted, ectopic sitBAI1 expression allowed the nonmotile ∆Mx-alpha cells to inhibit DK1622, thus recapitulating the antagonistic phenotype exhibited by the nonmotile ancestor strain (Figure 1B, rows 7–8). These combined results suggest that sitBAI1 may function as a toxin/immunity system responsible for the antagonistic behaviors previously described (Pathak et al., 2013; Dey et al., 2016; Dey and Wall, 2014). Therefore, the loss of two Mx-alpha units, and thus the sitBAI1 operon (Figure 2A), during the construction of DK1622 from an ancestral DK101 strain explains why the latter strain antagonizes the former.
Figure 2. SitA polymorphic toxins found on Mx-alpha units are delivered by OME.
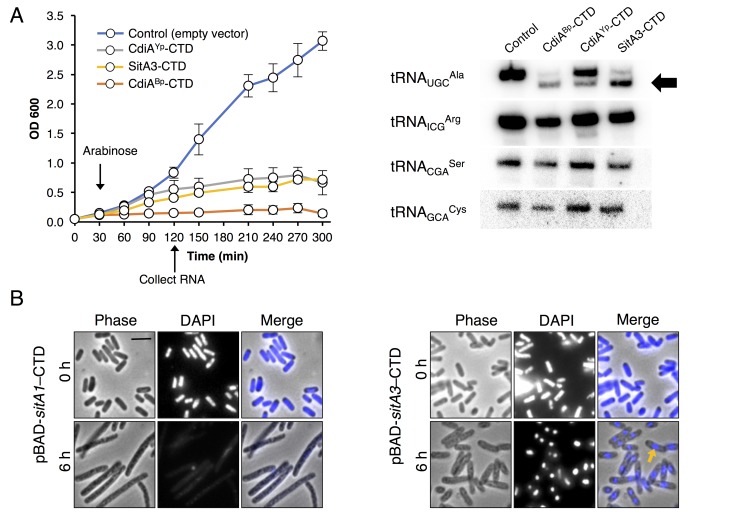
(A) Strain DK101 (the ancestor of DK1622) carries three Mx-alpha repeats, whereas DK1622 retains only one copy. Each Mx-alpha unit contains a unique sitBAI cassette. SitB proteins contain type I signal sequences (white boxes) whereas SitA proteins contain type II signal sequences (white boxes) with a lipobox and C-terminal toxin domains. The relative sequence identities are shown. (B) Competition outcomes when inhibitor strains each expressing one of three sitBAI cassettes were competed against susceptible target strains that lack the corresponding sitBAI cassette. Mock-inhibitor control is shown at left (WT vs. WT). See text for the calculation of competitive index. Strain genotypes (‘–’, traA deletion) are shown below histograms and further strain details provided in Supplementary file 2A. (C) Cells harvested from an agar co-culture of a strain expressing a SitA1-mCherry fusion with a GFP-labeled target at 0 and 6 hr. GFP targets are traA+ in the top panel and ∆traA in the bottom panel. Yellow arrows indicate two examples of GFP cells that have acquired the mCherry reporter. Boxes represent the number of mCherry positive GFP cells out of 100. Bar, 5 μm. (D) Fixed-cell immunofluorescence of C-terminal FLAG-tagged SitA1 and untagged control. Bar, 2.5 μm. Immunoblot of protein isolated from the same strains (right). SitAFLAG predicted size is 62.6 kDa. (E) Competition outcomes when inhibitor expresses one of the three sitBAI cassettes and the target strains express one of the three sitI genes. Data points at <0.001 indicate that no target cells remained. (F) E. coli MG1655 plating efficacy when equal number of cells were 10-fold serially diluted, spotted onto arabinose-supplemented agar and incubated overnight. Strains express either SitA1 or SitA3 C-terminal toxin domain (CTD) from a pBAD plasmid either in the absence (‘–’, empty vector) or presence of the indicated sitI genes expressed constitutively from a separate plasmid (pKSAT). This image is representative of three biological replicates. In this figure and the figures below, error bars represent standard error of the mean from at least three independent experiments.
Figure 2—figure supplement 1. Morphology of SitA-poisoned target cells.
Figure 2—figure supplement 2. SitA-CTD expression in M. xanthus is toxic.
Figure 2—figure supplement 3. Heterologous sitAI cassettes from M. fulvus HW-1 are active in DK1622.
DK1622 ancestors contain three functional sitBAI toxin/immunity cassettes
The three tandem Mx-alpha units in the ancestor strain are related and contain different alleles of >80 genes. This region therefore represents a rare bacterial polyploid element – that is, it contains three Mx-alpha prophage genomes with divergent gene allele sets (Dey et al., 2016). Inspection of the other two Mx-alpha units revealed additional putative sitBAI operons. The second Mx-alpha unit (absent from DK1622) carries sitA2 (MXF1DRAFT_07313), which encodes a putative lipoprotein with clear homology to the N-terminal region of SitA1, though the C-terminal domains are unrelated (Figure 2A). The sitA2 gene is flanked by a sitB1 homolog, sitB2, and a downstream candidate immunity gene, sitI2 (Figure 2A). The third Mx-alpha unit, which is shared between the ancestral strain and DK1622, also appears to contain a sitBAI operon. Although the sitA3 gene (MXF1DRAFT_05864 or MXAN_1899) has low sequence homology with sitA1 and sitA2, the three genes nevertheless share several key features: (1) sitA3 is preceded by sitB3, which is homologous to sitB1 and sitB2, (2) sitA3 occupies a similar position within its Mx-alpha unit as the other sitA genes, (3) sitA3 encodes an OM lipoprotein signal sequence, (4) sitA3 encodes a predicted C-terminal tRNase toxin domain, and (5) sitA3 is adjacent to a downstream putative immunity gene, sitI3 (Figure 2A). This analysis suggests that the three Mx-alpha units each contain distinct sitBAI toxin/immunity operons.
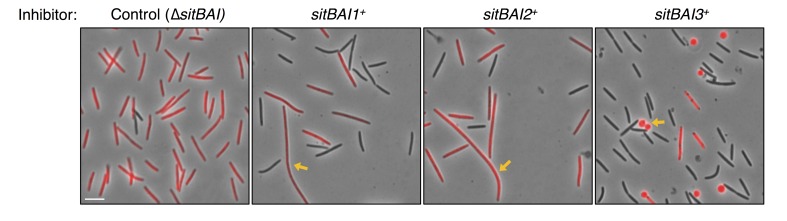
To determine whether SitA lipoproteins function as toxins, we expressed each sitBAI cassette in DK1622 and tested the competitive fitness of the resulting inhibitor strains against parental DK1622 target cells that lack the corresponding sitI gene. Target strains were labeled with fluorescent markers and co-cultured with inhibitor strains on agar for 24 hr. Competition outcomes were assessed by competitive index, which is the ratio of target cells to toxin-producing inhibitor cells at 24 hr relative to the starting ratio. For example, a competitive index of 0.01 indicates that the ratio of target cells to inhibitor cells decreased 100-fold. In all instances, sitBAI-expressing inhibitor cells significantly outcompeted target cells, whereas the mock-inhibitor did not (Figure 2B). Delivery of SitA1 and SitA2 over a 24 hr period induced filamentation and lysis of target cells, whereas SitA3 induced rounding and lysis of target cells (Figure 2—figure supplement 1). Furthermore, a ∆traA mutation in either the target or inhibitor strain abolished the inhibitor’s competitive advantage (Figure 2B). We note that assessing competitive index by microscopy gives a quick and reproducible metric of one strain’s ability to outcompete another, but does not capture the full dynamic range of competition because many enumerated target cells have severe morphological abnormalities and are likely not viable at the 24 hr time point. However, microscopy allows competitive indices to be determined for these otherwise WT strains, which are not easily amenable to enumeration as colony forming units (CFU) because they form extraordinarily cohesive biofilms in isogenic co-cultures (dependent on type IV pili). These results show that SitA lipoproteins provide a competitive advantage, conferring the ability to kill and/or inhibit the growth of competitors in a TraA-dependent manner.
To examine SitA localization, we generated an mCherry reporter that carries the N-terminal lipobox from SitA1. Cells expressing this fusion have membrane-localized fluorescence as expected for a lipoprotein (Figure 2C). The TraA-dependent function of SitA shown in Figure 2B suggests that the protein is delivered by OME. Therefore, we tested whether the reporter fusion is transferred between cells. We co-cultured the reporter strain with a target strain expressing cytoplasmic GFP (which is not exchanged [Wei et al., 2011]) and microscopically assayed for transfer of the reporter. At 6 hr of co-culture, we observed the mCherry signal present in the cell envelope of the GFP target strain, indicating cell-to-cell transfer of the SitA1-mCherry reporter (Figure 2C, upper panel). Deletion of traA in the GFP target strain prevented the acquisition of mCherry signal (Figure 2C, lower panel), recapitulating our earlier findings that OM-localized reporters are exchanged between cells in a TraA/B-dependent manner (Nudleman et al., 2005; Wei et al., 2011; Pathak et al., 2012). Because inner membrane lipoproteins are not transferred during OME (Wei et al., 2011), these data also suggest that the lipobox directs SitA1 to the OM. We confirmed that full-length SitA1 localizes to the cell envelope using immunofluorescence microscopy to detect FLAG epitope-tagged SitA1 in formaldehyde-fixed cells (Figure 2D). Taken together, these results demonstrate that SitA1 resides in the OM and is transferred cell-to-cell by OME.
The fact that sitI1 expression protects WT DK1622 cells from swarm inhibition suggests that this gene encodes an immunity protein that neutralizes SitA1 toxicity. To determine whether SitI proteins block SitA-mediated growth inhibition, we expressed each sitI allele individually in DK1622 ∆sitBAI3 cells and co-cultured the resulting strains with strains that express each of the three sitBAI cassettes. For each competition, only strains that express the cognate sitI were protected from growth inhibition (Figure 2E), consistent with immunity function.
Immunity proteins typically interact with the C-terminal domain of polymorphic toxins (Zhang et al., 2012; Poole et al., 2011). To test whether this was true for SitA, we expressed the predicted C-terminal toxin domains (CTD) of each SitA toxin in E. coli MG1655 under the inducible PBAD promoter. Expression of SitA1-CTD and SitA3-CTD blocked growth, but co-expression of cognate sitI from a second plasmid restored cell growth (Figure 2F). These results confirm that SitI proteins specifically neutralize cognate SitA toxins. In addition, because the SitA-CTD constructs lack secretion signal sequences, these data show that the domains exert their toxic effects in the cytoplasm. We also tested SitA2-CTD expression constructs, but none inhibited E. coli MG1655 growth. Because SitA2-mediated inhibition is obvious in M. xanthus competition co-cultures (Figure 2B and E), we tested the SitA-CTD expression constructs in M. xanthus and found that each inhibited cell growth (Figure 2—figure supplement 2A). Therefore, SitA2-CTD is indeed toxic when expressed in the cytoplasm of M. xanthus.
Given that sitBAI2 expression confers a significant advantage in competition co-culture (see Figure 2B), it is unclear why swarm inhibition was not observed with the sitA1– nonmotile ancestral strain (see Figure 1B, row 5), considering these cells should still deploy SitA2 and that DK1622 lacks the SitI2 immunity protein. Therefore, we tested whether nonmotile ∆Mx-alpha cells that ectopically express sitBAI2 are able to inhibit DK1622 swarming. In agreement with the prior result, we found that DK1622 motility was only partially inhibited by the sitBAI2-expressing strain (Figure 1C, row 1). This result confirms that SitA2 contributes to the swarm inhibition phenotype, but is not sufficiently potent by itself to block outward swarming of DK1622. Together, these results indicate that SitA1 is the major swarm-inhibition toxin. We note that SitA3 does not contribute to the originally observed swarm inhibition phenotype because both ancestor and DK1622 strains contain the sitBAI3 operon (see Figure 2A). However, we found that a nonmotile strain expressing sitBAI3 fully inhibits the motility of a DK1622 ∆sitBAI3 strain that lacks the sitI3 immunity gene (Figure 1C, row 2).
SitA C-terminal domains are nuclease toxins
Homologous CTDs are often associated with different toxin delivery systems from phylogenetically distant bacteria (Zhang et al., 2012). SitA3-CTD is homologous to a previously characterized tRNase domain found at the C-terminus of CdiA from Burkholderia pseudomallei 1026b (Morse et al., 2012; Nikolakakis et al., 2012) and an orphan CdiA-CTD encoded by Yersinia pseudotuberculosis YPIII (Figure 3—figure supplement 1). To determine whether SitA3-CTD also has tRNase activity, we expressed the toxin in E. coli and compared its activity to the CDI toxins. Induction of SitA3-CTD expression inhibited cell growth in the same manner as the CdiA-CTD toxins (Figure 3A). Examination of tRNA from SitA3-CTD intoxicated cells revealed cleavage of tRNAUGCAla, similar to the specific tRNase activity of the B. pseudomallei toxin (Figure 3A).
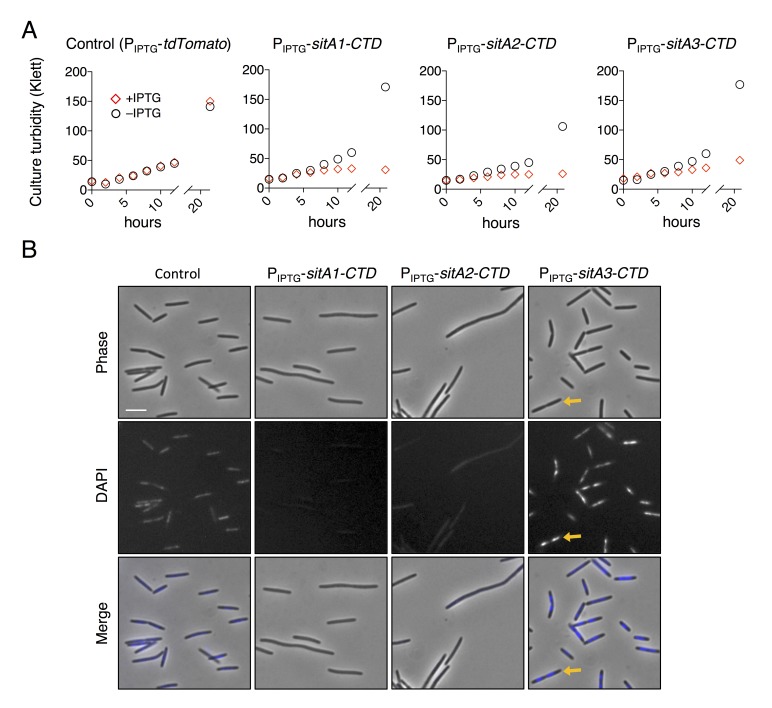
Figure 3. Toxic function of SitA1 and SitA3 CTDs.
(A) SitA3-CTD is a toxic tRNase. Expression of the indicated CTDs was induced with arabinose in E. coli, and cell growth monitored by measuring the optical density of the cultures at 600 nm (OD600). OD600 values are reported as the average ± standard error for three independent experiments (left). RNA was isolated after 90 min of toxin expression and analyzed by Northern blot hybridization using probes to the indicated tRNAs (right). The arrow indicates cleaved tRNAUGCAla. (B) SitA1-CTD has DNase activity. E. coli cells were stained with DAPI at 0 hr and after 6 hr of toxin expression. Cells expressing sitA1-CTD became filamentous and lost DAPI staining at 6 hr (left). In contrast, sitA3-CTD expressing cells retained DAPI staining (right), though their nucleoids became compacted (yellow arrow). Bar, 5 μm.
Figure 3—figure supplement 1. Alignment of SitA3-CTD with CdiA-CTD tRNase toxins.
Next, we investigated the toxic activities of SitA1-CTD and SitA2-CTD. We had previously observed that SitA1 induces cell filamentation and loss of DAPI staining in M. xanthus target cells, which is consistent with DNase activity mediated by the predicted Colicin-DNase domain (Pfam 12639, E = 6.7 e-21) containing the WHH motif (Zhang et al., 2011). To test this, we induced SitA1-CTD expression in E. coli and found that cells became filamentous and had reduced DAPI stain signal (Figure 3B). By contrast, E. coli cells that were intoxicated by SitA3-CTD retained DAPI staining, though their nucleoids became more compact (Figure 3B). Together, these results suggest that SitA1-CTD has DNase activity. HMM-HMM comparison (HHpred [Söding et al., 2005]) of C-terminal residues 699–783 of SitA2 revealed distant homology to another CdiA-CTD from Y. pseudotuberculosis YPIII (locus tag, Ga0077885_11586), which was previously characterized as a DNase (Morse et al., 2015). To examine this possibility, we expressed each SitA-CTD in M. xanthus under the control of an IPTG-inducible promoter. Expression of SitA2-CTD in M. xanthus resulted in cell filamentation and reduced DAPI staining (Figure 2—figure supplement 2B), suggesting that SitA2-CTD degrades DNA. Expression of SitA1-CTD and SitA3-CTD in the cytoplasm of M. xanthus cells yielded similar results as when they were expressed in E. coli (Figure 2—figure supplement 2B), although the DAPI signal from SitA3-CTD expressing cells was brighter than the control and many cells contained two distinct nucleoids (Figure 2—figure supplement 2B), suggesting a block in cell division.
Polymorphic SitA toxins are conserved in myxobacteria
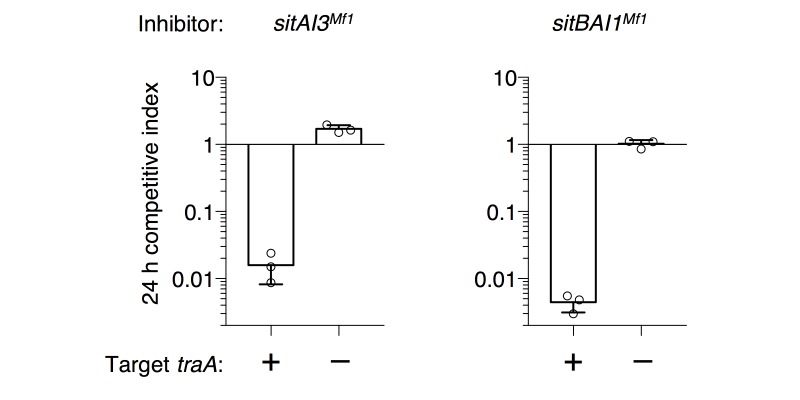
To determine the phylogenetic distribution of SitA toxins, we conducted a BLAST search using the N-terminal domains of SitA1/2 (which are homologous) and SitA3 as query sequences. This search recovered >100 sitA orthologs that are common in the Myxococcales (Supplementary file 1). More sensitive search algorithms such as HMMER (Finn et al., 2011) failed to identify significant homologs outside of the Myxococcales. Consistent with the finding that SitA is delivered through OME, all orthologs contain lipoboxes and are only found in species that contain traAB. Moreover, SitA C-terminal domains are variable and typically show homology to nuclease domains when subjected to HMM-HMM comparison using HHpred (Supplementary file 3). Interestingly, many sitA genes are not linked to upstream sitB orthologs, particularly in species that are distantly related to M. xanthus (Supplementary file 1). This suggests that SitB may not be required for SitA function, or perhaps that SitB proteins, encoded at unlinked loci, function promiscuously between multiple SitA proteins. Notably, some sitA loci are found outside of Mx-alpha-like elements, however, these genes are typically adjacent to other mobile genetic elements. To test cross-genotype compatibility of SitA orthologs, we cloned two sitA gene cassettes from Myxococcus fulvus HW1 for heterologous expression in M. xanthus. One of these operons (sitAI3Mf1) does not contain a sitB gene. As predicted, M. xanthus cells that express heterologous sitAI3Mf1 or sitBAI1Mf1 outcompeted the parental strain in a traA-dependent manner (Figure 2—figure supplement 3). These results indicate that SitA toxin delivery is not limited by its specific species/strain of origin, and that the systems are functional after horizontal gene transfer (HGT) of a minimal set of components (sitAI).
SitA toxins define barriers to social compatibility
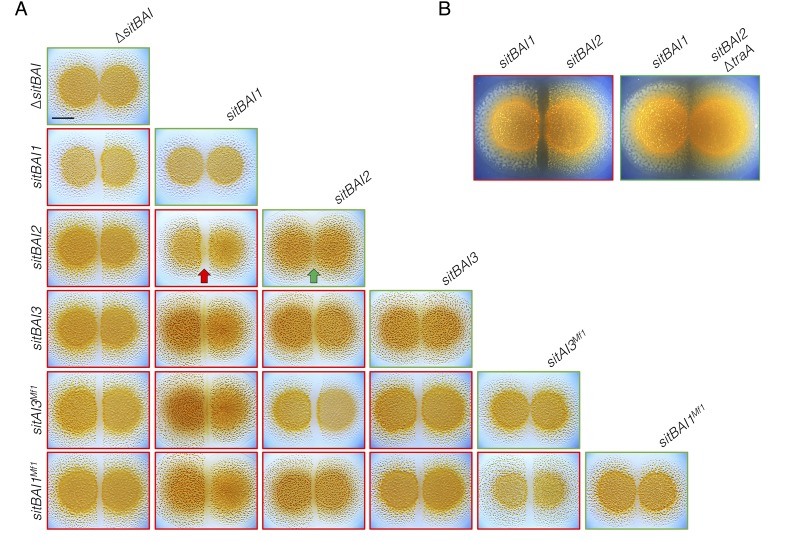
Our results show that sitBAI-expressing cells inhibit OME-compatible strains that lack a cognate SitI immunity protein. We hypothesized that this antagonism should be sufficient to mediate territorial exclusion. Territorial exclusion promotes physical segregation of nonself organisms (Gibbs and Greenberg, 2011), which in turn drives both diversification and maintenance of cooperation (Papke and Ward, 2004; Velicer and Vos, 2009). Territorial exclusion between wild M. xanthus genotypes, including those that are closely related and that are isolated in close proximity to one another has been well studied, but the specific determinants underlying this behavior are unknown (Vos and Velicer, 2009; Rendueles et al., 2015; Vos and Velicer, 2006; Wielgoss et al., 2016). We tested whether SitA is sufficient for territorial exclusion by conducting colony merger assays with every combination of DK1622 strains expressing one of the five described sitAI alleles. In these assays, two liquid cultures are spotted next to one another on agar, and the colonies are allowed to swarm toward each other. If the converging swarms merge, then the strains are considered compatible. For each combination, the expression of different sitAI cassettes resulted in dramatic lines of demarcation between the two strains (Figure 4A), and the formation of these demarcation zones was traA-dependent (Figure 4B). These results show that otherwise isogenic strains are rendered socially incompatible and geographically isolated by the acquisition of a single sitAI cassette.
Figure 4. sitAI alleles determine the social compatibility of M. xanthus swarms.
(A) M. xanthus colonies expressing identical sitAI cassettes merge (as illustrated by the green arrow) when spotted adjacent to one another (top of each column). Strains that express different sitAI cassettes form demarcation zones between colonies (illustrated by the red arrow). Labels on the left indicate toxin expressed by left colony, while top labels indicate toxin expressed by colony on the right. Green borders indicate colony merging and red indicates demarcation. (B) Demarcation zone formation is traA-dependent. Bar, 5 mm.
SitA toxins are infectious
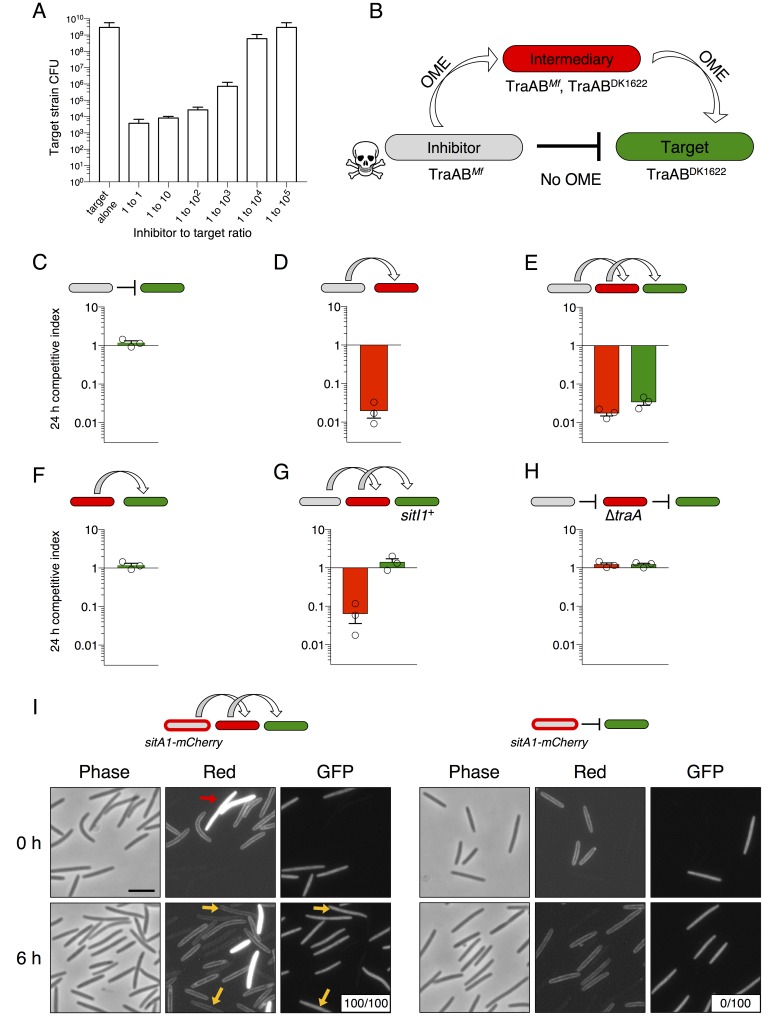
We previously found that swarm inhibition occurs efficiently even when motile cells outnumber the antagonistic nonmotile strain 40 to 1 (Dey and Wall, 2014). This observation suggests that an individual SitA-expressing cell can inhibit many targets. To further explore this phenomenon, we quantified viable target cells in a series of competition co-cultures in which the ratio of SitA producing cells to targets was progressively decreased by factors of 10. To facilitate CFU enumeration in these experiments, we used ∆pilA cells, which are unable form type IV pili-dependent biofilms. We found that target cell CFU were reduced approximately 106-fold when the strains were mixed at a 1:1 ratio (Figure 5A). The higher degree of killing reported here (compared to competitive index in Figure 2B) provides a clearer understanding of the killing efficiency because the CFU assay measures a broader dynamic range of viable cell number. Remarkably, the SitA-producing strain still reduced target cell viability >104-fold in co-cultures seeded at a 1:1000 ratio of inhibitors to target cells (Figure 5A). These observations imply that each inhibitor cell intoxicates several thousand target cells during co-culture. In one explanation we hypothesized that OME delivery allows a series of SitA transfer events from one target cell to other cells. We consider this serial transfer mechanism plausible if translocation of all toxin molecules from the target cell OM to the cytoplasm is not completed before subsequent OME events occur. This model predicts that SitA toxins could spread through the population like an infectious agent, intoxicating target cells that never made direct contact with the producer. To test this hypothesis, we conducted three-strain competitions with (1) a sitBAI1 inhibitor strain that contains M. fulvus traAB (traABMf) as its only traAB alleles, (2) a susceptible target strain that contains M. xanthus traABDK1622 alleles and thus is incompatible for OME with the inhibitor, and (3) a susceptible intermediary strain that carries both traABMf and traABDK1622 (Figure 5B). If serial toxin transfer occurs, the traAB merodiploid strain should act as an intermediary carrier/conduit to deliver toxin to traABDK1622 targets (Figure 5B). As a control, we first showed that traABMf inhibitors do not inhibit traABDK1622 targets (Figure 5C), consistent with the incompatibility of their traAB alleles. Importantly, inclusion of intermediary cells, which are inhibited (Figure 5D), also resulted in the inhibition of traABDK1622 target cells (Figure 5E). As expected, the intermediary strain (which lacks sitBAI1) did not inhibit traABDK1622 targets in co-cultures containing only those two strains (Figure 5F). To exclude a SitA-independent mechanism of target cell inhibition, we conducted the same three-strain competition, but provided traABDK1622 targets with the sitI1 immunity gene. In this latter co-culture, the intermediary strain was inhibited, but traABDK1622 targets were not (Figure 5G). Finally, we tested an intermediary strain that lacks traA and found that neither intermediary nor target cells were inhibited during co-culture (Figure 5H).
Figure 5. SitA toxins are serially transferred by OME.
(A) Viable cells (CFU) of a target population as a function of inhibitor to target cell ratio quantifies the efficiency of SitA1 and OME delivery. Strains were co-cultured on agar for 48 hr at indicated ratios before determining CFU of the marked (Kmr) target strain. (B) Experimental design to test serial transmissibility of SitA1. The grey cell produces the SitA1 toxin and contains traABMf alleles. The target cells (green) are susceptible, but carry incompatible traABDK1622 alleles that preclude OME with inhibitors. Intermediary cells (red) express both traAB alleles. (C–F) Competitive indices of intermediary (red) and target (green) strains from two- and three-strain co-cultures. (G) Three-strain competition when the target strain expresses SitI1. (H) Three strain competition when the intermediary strain is ∆traA. Competition outcomes were determined at 24 hr by fluorescent microscopy. Competitive index was calculated relative to the inhibitor (C–E, G–H) or relative to intermediary strain (F). Starting ratio was 1:5:5 inhibitor to intermediary to target. (I) Serial transfer of the SitA1-mCherry fusion. The left panel shows a 10:1:1 mixture of sitA1-mCherry cells to intermediary to target visualized at 0 and 6 hr. Red arrow indicates a representative example of an intermediary cell that expresses cytoplasmic tdTomato (which does not transfer). Yellow arrows indicate GFP-labeled target cells that have acquired an OM-localized mCherry signal at 6 hr. Boxes represent the number of mCherry positive GFP cells out of 100. Right panel: otherwise identical experiment omitting the intermediary strain. Bar, 5 μm.
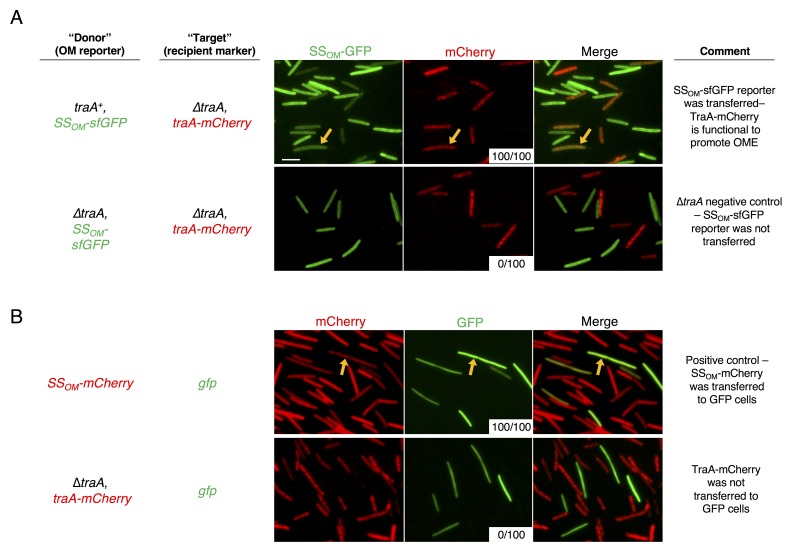
Figure 5—figure supplement 1. TraA is not transferred during OME.
To directly visualize serial transfer, we co-cultured traABMf cells that express the SitA1-mCherry fusion (described in Figure 2) with traAB merodiploid intermediary cells that express cytoplasmic tdTomato, and GFP-labeled, traABDK1622 target cells. Microscopic examination of target cells at 6 hr revealed that all had acquired mCherry fluorescence, with the signal localized to the cell envelope (Figure 5I, left panels). By contrast, no SitA1-mCherry transfer was detected when the intermediary was absent from the co-culture (Figure 5I, right panels). Although these results are consistent with serial transfer of SitA, it is also possible that target cells acquire TraAMf and/or inhibitor cells acquire TraADK1622 by OME-dependent exchange of TraA with the intermediary strain, which would then allow direct transfer of SitA between inhibitor and target cells. To test if TraA is transferred during OME, we used a TraA-mCherry fusion to monitor transfer of TraA. The TraA fusion promoted efficient transfer of an OM sfGFP reporter (Figure 5—figure supplement 1A), demonstrating that it is functional to catalyze OME. However, TraA-mCherry itself was not transferred (Figure 5—figure supplement 1B). One explanation for why TraA does not transfer is that it interacts with TraB, which contains an OmpA domain known to bind the cell wall. For this reason, we suspect TraA is anchored to the cell envelope and is unable to transfer (Cao and Wall, 2017). Taken together, these results indicate that SitA1 can be transferred from the initial target to secondary recipients, supporting a model in which SitA acts like an infectious agent that disseminates through a population by OME.
SitB is an accessory protein that contributes to SitA function
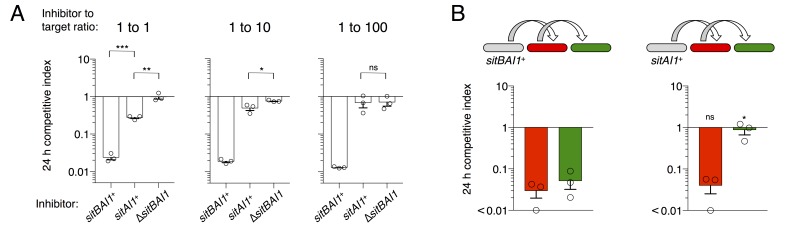
In M. xanthus and its close relatives, sitA is typically accompanied by an overlapping sitB cistron. In the case of DK101 the genes overlap by 11 bp in all three sitBAI cassettes. SitB shows no significant homology to other proteins or domains using HMMER or HHpred, though it does contain a type I signal sequence (SignalP 4.1 [Petersen et al., 2011]). I-TASSER (Zhang, 2008) predicts that SitB adopts a transmembrane β-barrel structure characteristic of OM proteins. To examine the role of SitB1, we tested the activity of inhibitor cells that express either sitBAI1 or sitAI1 (cells lack sitB1) against a susceptible target strain. The inhibitors in these experiments also carried a ∆sitB3 mutation to eliminate the possibility of promiscuous interactions between SitB3 and SitA1. At a 1:1 (inhibitor to target) ratio, sitAI inhibitors had less of an advantage against targets than sitBAI inhibitors, but still retained activity compared to the mock inhibitor control (Figure 6A, left). The sitAI inhibitors were less effective at a 1:10 ratio, and at 1:100 were indistinguishable from mock inhibitors (Figure 6A). In contrast, sitBAI1 inhibitors were equally effective at outcompeting the target strain at each of the three ratios (Figure 6A). Thus, SitB1 contributes significantly to SitA1-mediated inhibition.
Figure 6. SitB contributes to SitA function and serial transfer.
(A) The indicated SitA1 inhibitor strains were co-cultured with target cells at three different inhibitor to target ratios. Competitive index was measured at 24 hr by counting the ratios of fluorescently marked cells. Asterisks indicate level of statistical significance, ns = not significant. P-values of indicated comparisons from left to right: 0.0002, 0.006, 0.0257, 0.9359. (B) Serial transfer was monitored as in Figure 5 using sitBAI or sitAI inhibitors that express traABMf. Co-cultures were seeded at a 10:1:1 ratio of inhibitor to intermediary to target strains. Significance indicators refer to comparisons between the inhibitor strains. P-values from left to right: 0.6341, 0.0193.
Progressive loss of function at increasing target to inhibitor ratios with the sitAI inhibitors could indicate defects in serial toxin transfer compared to sitBAI inhibitors. Therefore, we tested serial transfer using three-strain co-cultures as described in Figure 5B. To improve the sensitivity of this assay, we increased the inhibitor to intermediary to target strain ratio to 10:1:1 to compensate for the inhibition defect of sitAI1 cells. As observed in Figure 5, sitBAI1 cells outcompeted both the intermediary and target strains (Figure 6B). Because of the high inhibitor cell ratio, the sitAI1 cells outcompeted the intermediary strain to a similar extent as sitBAI cells, but importantly, sitAI cells had little to no effect on target cells (Figure 6B). We extended the experiment to 48 hr and also performed experiments with SitA-resistant intermediary cells, to increase the number of conduit cells, but again we did not observe SitAI-mediated antagonism of target cells. These results support the hypothesis that SitB promotes SitA transfer, including the serial transfer from primary to secondary target cells. Although SitB1 clearly contributes to SitA1-mediated inhibition, it is not strictly required, which may explain why many myxobacterial sitA genes are not linked to sitB. Finally, these results are congruent with our above conclusion that TraA is not transferred, because if it was, then direct transfer of SitA1 would occur between sitAI inhibitors and the target strain. However, this did not occur because the target strain was not inhibited.
OME and SitA are critical for competitive fitness within TraA recognition groups
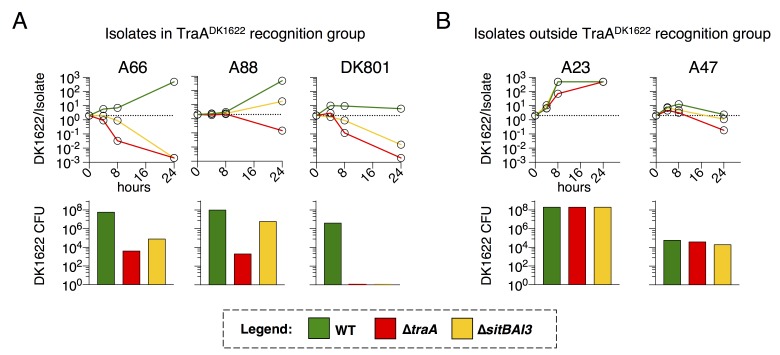
M. xanthus uses multiple inhibitory mechanisms to antagonize non-kin. However, because SitA toxins are serially transferred between cells, we hypothesized that they should be powerful determinants of competitive outcomes during inter-genotype conflict. To test this hypothesis, we quantified the contribution of TraA and SitA to competitive outcomes in co-cultures of DK1622 with wild M. xanthus soil isolates. Isolates A66 and A88 (from Tübingen, Germany) (Vos and Velicer, 2006) and DK801 (from California, USA) (Martin et al., 1978) contain traA alleles in the same recognition group as DK1622 (originally isolated from Iowa, USA) (Pathak et al., 2013; Dey et al., 2016). We compared the fitness outcomes of WT, ∆traA, and ∆sitBAI3 genotypes when co-cultured with these environmental isolates by monitoring the ratio of fluorescently labeled DK1622-derived cells to isolate cells at 4, 8 and 24 hr. As a second metric, we enumerated CFU of the DK1622-derived strains at the 24 hr time point. In every case, mutant strains that cannot deploy SitA3 had dramatically decreased competitive fitness outcomes and viability compared to WT (Figure 7A). Remarkably, against all three isolates, the presence of traA was the determining factor in which strain prevailed, demonstrating up to a 106-fold swing in strain ratio (vs. A66) and a near 107-fold difference in CFU (vs. DK801) between WT and ∆traA strains (Figure 7A). These results indicate that the ability to deliver SitA3 is a dominant determinant of competitive fitness under these conditions. Not surprisingly, the finding that environmental isolates outcompeted and killed ∆traA strains (Figure 7A), confirms the existence of OME-independent killing mechanisms at play. Interestingly, these experiments revealed that ∆traA cells had less competitive fitness than ∆sitBAI3, indicating a competitive fitness defect in ∆traA cells beyond just the inability to deploy SitA3. As a control, we competed the DK1622 genotypes against isolates A23 and A47 (from Tübingen [Vos and Velicer, 2006]), which are outside the DK1622 TraA recognition group (Pathak et al., 2013). ∆traA and ∆sitBAI3 genotypes resulted in similar competitive outcomes as WT as would be expected when inter-strain OME does not occur and SitA3 cannot be deployed (Figure 7B). These results show that SitA contributes significantly to fitness during competition with TraA-compatible non-kin genotypes, and likely plays a key role in competition and survival in nature.
Figure 7. TraA and SitA are dominant determinants of competitive outcome within TraA recognition groups.
(A) Line graphs represent strain ratio over time when the three indicated, DK1622-derived strains, which were fluorescently labelled, were competed with wild isolates (A66, A88, DK801). These isolates belong to the same TraA recognition group as DK1622. Histograms indicate viable cells (CFU) of the DK1622-derived strains (Tcr) after the 24 hr competition. (B) Identical experiments as in A, except the lab strains were competed with wild isolates that belong to different TraA recognition groups.
Discussion
SitA promotes kin discrimination of OME partner cells
Here, we describe a novel family of proteins that carry polymorphic toxin domains and are delivered between myxobacteria by OME. SitA proteins are similar to other polymorphic toxins in that they carry diverse C-terminal domains, are neutralized by cognate immunity proteins, and are delivered in a cell contact-dependent manner. However, they are unique in their N-terminal domains, and in that they are lipoproteins transferred with other cargo during OME. To our knowledge, this is the first example of a polymorphic toxin system in which the toxin itself is a lipoprotein. Unlike CDI, T4SS, T6SS and T7SS toxins, there appears to be no requirement for a specialized apparatus to export the toxins. Instead, toxins are exchanged bi-directionally and simultaneously during OME. Therefore, SitA delivery likely only requires OM localization of the toxin and compatible TraA receptors. This discovery highlights the diversity of mechanisms used by bacteria to deliver polymorphic toxins.
Importantly, the SitA toxin family constitutes a second identity constraint upon OME with partner cells. For two cells to engage in OME, not only must they present compatible TraA receptors, but they must also contain immunity proteins to each other’s toxins. In this ‘recognize and verify’ system (Wall, 2016), if the latter constraint is not met, then the recipient of the toxin is poisoned. TraA homotypic interactions alone are considered kind or greenbeard recognition, in which social interactions are based on a single gene locus. The finding that myxobacteria verify relatedness with sitAI confirms the notion that myxobacteria apply a bona fide kin discrimination mechanism during OME by requiring identity verification at multiple polymorphic loci. Interestingly, this system allows TraA interactions to promote contrasting behaviors – cooperative or antagonistic – depending on relatedness. Either outcome makes OME potentially beneficial regardless of the partner by conferring the ability to both share goods with clonemates and poison non-kin.
SitA delivery range is restricted to within a single TraA recognition group. Considering traA allele diversity is high (Pathak et al., 2013), this significantly limits the use of SitA to related but nonself individuals. This suggests that one of the primary functions of SitA is the discrimination of exchange partners, consistent with the notion that sharing large amounts of goods with non-kin is costly. Within TraA recognition groups, OM material is a shared good; a resource to be guarded from exploitation by OME compatible, yet nonself populations. Myxobacteria achieve this safeguard by inextricably linking the delivery of these goods with the delivery of SitA toxins. Another example is the Burkholderia thailandensis CDI system, which couples a communication signal with polymorphic toxin delivery during biofilm formation (Anderson et al., 2014). Similarly, the CDI system of E. coli mediates both antagonism and cooperative intercellular adhesion to related cells (Ruhe et al., 2013, 2015). In Proteus mirabilis, IdsD/IdsE interactions communicate identity and may promote cooperative behaviors (Cardarelli et al., 2015). This intercellular communication is also coupled to toxic T6SS effector delivery (Wenren et al., 2013). In these examples, organisms link goods, signals, and/or cooperative behaviors to polymorphic toxin delivery, which ensures that potential cooperators are related.
SitA diversity and myxobacterial ecology
Differential acquisition of antagonistic systems can affect cooperation compatibility between originally identical genotypes. We demonstrated that when two otherwise isogenic colonies express different SitA toxins, they are no longer able to merge swarms. The acquisition of a single sitAI operon would therefore alter strain identity and population structure between previously clonal cells. However, the impact of SitA on strain identity and population structure in natural soil habitats depends on two factors: (1) strains that belong to the same traA recognition group must exist in proximity, and (2) sitA loci must be sufficiently diverse to ensure different toxin/immunity types are represented at fine geographic scales. Velicer and colleagues have examined the compatibility of natural M. xanthus isolates obtained from a centimeter-scale plot of soil (Vos and Velicer, 2009; Wielgoss et al., 2016). Colony merger assays between these geographically proximal strains reveal compatibilities among the most closely related isolates, but also strong incompatibilities between strains that differ by only several dozens of mutations outside of the Mx-alpha region (Wielgoss et al., 2016). Indeed, these incompatibilities correlate with gene variation at hyper-variable Mx-alpha loci, where sitBAI genes commonly reside. Our analysis of their published sequences reveals 69 total and 15 unique sitA alleles distributed over 22 isolates (see Materials and methods for search criteria). Between these strains there are two traA alleles that are known to be incompatible (Pathak et al., 2013). Within each TraA recognition group we have observed an apparently high degree of correlation between the published colony merger compatibility of the strains (Wielgoss et al., 2016) and the sitA genes they possess. This suggests that sitBAI polymorphisms contribute to swarm incompatibility and inter-strain competition among natural soil isolates. We are currently investigating this possibility. Moreover, because Mx-alpha produces defective phage particles that promote specialized transduction (Starich and Zissler, 1989; Starich et al., 1985), these elements are apparent hotspots for HGT, perhaps explaining the high degree of Mx-alpha variation discovered between otherwise related isolates (Wielgoss et al., 2016). These variations likely contribute to the emergence of new compatibility types which underlie complex population structures, and explain the observation of rapidly evolving social antagonisms in M. xanthus (Velicer and Vos, 2009). Thus, TraA and SitA may act as powerful evolutionary drivers of myxobacteria diversification.
sitAI genes are often associated with prophage-like elements or other mobile elements and thus are likely acquired by HGT. By conferring a fitness advantage to their host, they may play an important role in transmission and retention of mobile DNA. For example, a HGT event into one cell in a population endowing it with a novel sitBAI operon allows that cell to infect its clonemates with toxins, thus ensuring the propagation of that element within the population. Similarly, the loss of the element would be lethal because the surrounding cells harbor this toxin-immunity pair would kill susceptible cells. Importantly, this model explains why many lab strains have stably maintained three large tandem repeats of Mx-alpha, which is expected to be genetically unstable (Starich and Zissler, 1989; Roth et al., 1996). In cases in which strains have spontaneously lost Mx-alpha units (Dey et al., 2016), those events likely occurred during propagation in liquid media, where OME cannot occur. Mx-alpha has the attributes of a selfish or addictive element that exploits the social nature of myxobacteria and OME. However, the origin of sitBAI loci is unclear because these genes also reside outside of selfish elements in myxobacterial genomes.
An infectious polymorphic toxin system
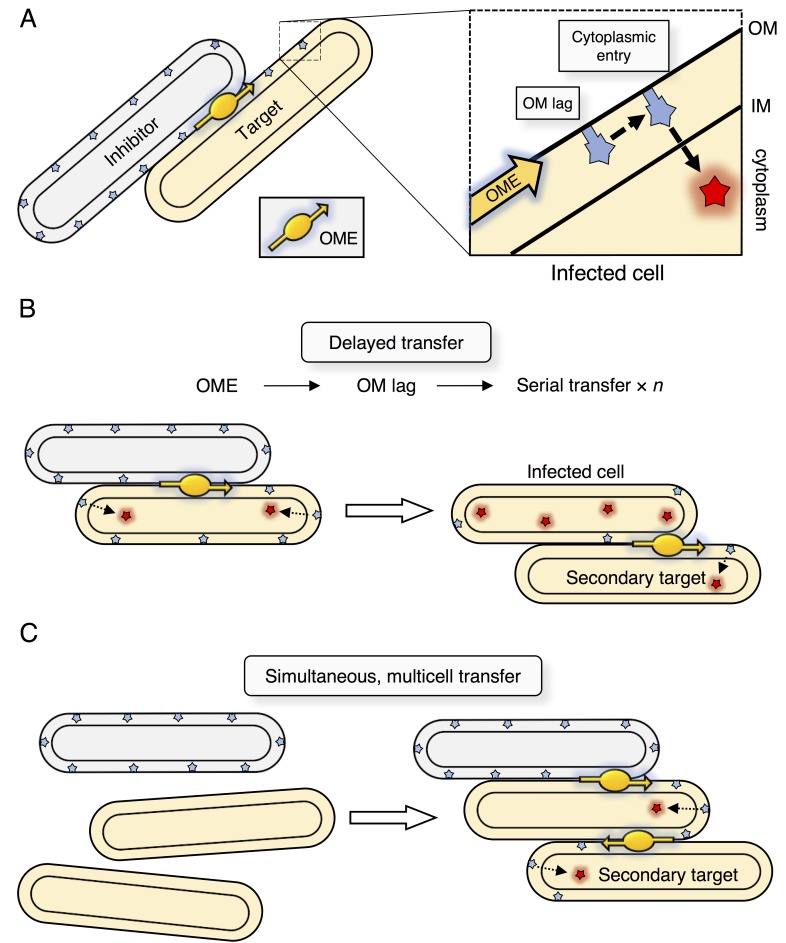
SitA toxins are uniquely powerful determinants of identity, likely because they are transmitted as infectious agents between recipient cells. The infectious model is consistent with the observations that individual M. xanthus cells typically make contact with multiple cells simultaneously within a swarm, OME is constitutively active, and that prodigious amounts of material are transferred during OME (Nudleman et al., 2005). Furthermore, SitA entry into the cytoplasm occurs by a secondary and uncoupled pathway to OME (Dey et al., 2016; Dey and Wall, 2014). Thus, it is possible that SitA lingers in the OM of the primary target long enough to allow transfer to secondary target cells through subsequent OME events (Figure 8A). Perhaps a cellular protein is required for SitA cell entry (Dey et al., 2016; Dey and Wall, 2014), but this protein is outnumbered by SitA molecules in the OM, making cytoplasmic entry a rate-limiting step. Based on these inferences, we propose two non-exclusive models that explain serial transfer: (1) Following transfer of SitA to a primary infected cell, OME with a secondary cell occurs before the full complement of SitA enters the cytoplasm of the initial recipient (Figure 8B); or (2) that three or more cells are engaged in OME simultaneously (Figure 8C). Our results suggest that SitB functions to promote serial transfer. Given that ∆sitB inhibitors have defects in direct transfer, serial transfer could be blocked simply by a decrease in number of SitA molecules delivered or a decrease in rate of delivery. Alternatively, SitB could stabilize SitA in the OM of the inhibitor and/or target cell, thereby increasing OM dwell times to promote serial transfer. The finding that SitB is an accessory protein is consistent with bioinformatics analysis in which many of the sitA genes that reside outside of the M. xanthus species are not linked with a sitB gene. In the case of M. xanthus isolates (Wielgoss et al., 2016), we found only 3 of 69 sitA genes were not associated with sitB. The mechanism of serial transfer, the function of SitB, and the ability of SitA to traverse the cell envelope are topics for future study. With respect to the latter point, in prior work we found that a mutation that disrupts the inner membrane protein, OmrA, renders target cells resistant to SitA1 (Dey et al., 2016; Dey and Wall, 2014). Thus, as proposed for CDI toxins, one possibility is that SitA toxins exploit inner-membrane proteins to gain access to the cytoplasm (Willett et al., 2015).
Figure 8. Model for serial transfer of SitA.
(A) SitA is delivered cell-to-cell by OME. After OME, SitA may enter the cytoplasm or linger (lag) in the OM. (B) In the delayed entry model, the infected cell can undergo OME of SitA to another naïve cell before all SitA molecules enter the cytoplasm and before cell death. n = number of target cells poisoned by infected cell. (C) Alternative, but non-exclusive model in which OME and SitA transfer occurs between three or more cells simultaneously. Here SitA is delivered to a tertiary cell via an intermediary cell.
Remarkably, SitA appears to determine the competitive outcome of two-strain co-cultures between DK1622 and wild-isolates, despite the T6SS and a host of other antagonistic machinery at play (Konovalova et al., 2010; Smith and Dworkin, 1994). We hypothesize that the rapid spread of SitA by serial transfer may disrupt the antagonistic capabilities of competitors. When deployed between converging swarms, serial transfer provides a mechanism to inhibit cells behind the front-lines. Thus, SitA mediated antagonism results in the formation of distinct territorial boundaries (see Figure 4), which in turn minimize OME, resource sharing, and social interactions between non-clonal swarms.
A solution to Crozier’s Paradox
The selective pressure from SitA antagonism within a TraA recognition group may help drive the generation and fixation of traA polymorphisms that determine recognition specificity. For instance, we recently showed that simply substituting a single amino acid residue in TraA can alter recognition specificity while retaining OME function (Cao and Wall, 2017). More broadly, it is a puzzle how organisms select and maintain genetic variation in social genes such as traA. Diversification of beneficial greenbeard genes is theorized to be selected against because social groups with more common alleles receive benefit more often than those with less common alleles. This pressure to possess the allele that gains the most benefit is thought to ultimately erode allele diversity that originally allowed discrimination. This problem is known as Crozier’s paradox (Strassmann et al., 2011; Crozier, 1986). Our results provide one solution to this paradox in that variation at a second locus (sitA, a ‘harming greenbeard' [Gardner and West, 2010]) exerts selective pressure to diversify TraA, a helping greenbeard (Wall, 2016). For example, if one strain is killed by another via SitA, a TraA mutation that alters specificity within the losing population would be immune and retain OME function, and would thus be selected for. We suggest that other greenbeard systems could involve a similar balance between antagonism and cooperation that promotes maintenance of diversity for beneficial greenbeard genes.
Concluding remarks
Our results provide a description of a novel polymorphic toxin system that helps direct cellular goods to clonemates to promote multicellular cooperation. Polymorphic toxin systems are widely prevalent in bacteria and their role in population structure, ecology and evolution of microbes is only beginning to be understood. Importantly, this study highlights the diversity of delivery mechanisms for these domains and how they have adapted to the lifestyle of their host genomes.
Materials and methods
Growth conditions
All strains are listed in Supplementary file 2A. M. xanthus was routinely grown in CTT medium [1% casitone; 10 mM Tris⋅HCl (pH 7.6); 8 mM MgSO4; 1 mM KH2PO4] in the dark at 33°C. E. coli TOP10 and MG1655 were grown in LB media at 37°C. As needed for selection or induction, media were supplemented with kanamycin (50 µg/mL), oxytetracycline (10 µg/mL), ampicillin (100 µg/mL), streptomycin (50 µg/mL), arabinose (0.2%), or IPTG (1 mM). TPM buffer (CTT without casitone) or PBS was used to wash cells. CTT or LB agar was used as a solid growth medium for routine strain maintenance. For all assays, strains were grown to logarithmic growth phase, washed, and re-suspended to the appropriate density.
Cloning and strain construction
All plasmids and primers are listed in Supplementary file 2B,C. Plasmids were constructed and maintained in E. coli and subsequently electroporated into M. xanthus. In the case of cloning IPTG-dependent sitA-CTDs, plasmids were maintained in XL1-Blue, which overexpresses LacI and reduces clone toxicity. Insertion mutations were created by amplifying an approximately 500 bp fragment of the gene of interest by PCR and cloning the fragment into the pCR-TOPO XL or pCR-TOPO 2.1 vectors (Invitrogen, Carlsbad, CA). For gene expression in M. xanthus, we cloned the appropriate gene(s) into pMR3487 using XbaI and NdeI restriction sites and T4 DNA ligase. If the gene(s) of interest contained these restriction sites, we used Gibson Assembly (Gibson et al., 2009) (New England Biolabs, Ipswich, MA) for plasmid construction. pMR3487 recombines at a specific site in the M. xanthus chromosome and expression is induced with IPTG (Iniesta et al., 2012). Traditional restriction endonuclease cloning was used to create pBAD30, pCH450 and pKSAT derived plasmids in which the sitA-CTD fragments had an ATG start codon engineered into the insert. Expression of sitI genes in pKSAT is constitutive, driven by the Kmr promoter. pCV10 for GFP expression was created by ligating tandem rRNA promoters, used for expressing lacI from the pMR3487 plasmid, with EGFP and the pSWU19 plasmid backbone. This plasmid recombines into the M. xanthus chromosome at the Mx8 phage attachment site. The deletion of sitBAI3 was constructed by cloning in-frame regions flanking and partially overlapping the start and stop codons of sitB3 and sitI3, respectively, into pBJ114 using Gibson Assembly. After recombination in M. xanthus, mutants were grown in CTT for 24 hr and plated on CTT containing 2% galactose to select for spontaneous loss of the galK marker. Deletion mutants were distinguished from WT by PCR with primers that flanked the deletion site.
M. xanthus competition experiments
Competition experiments, unless otherwise noted, were done using 1:1 strain mixtures of 3 × 108 cells per mL spotted (20 µL) on agar plates containing 0.5× CTT with 2 mM CaCl2 and 1 mM IPTG (competition media). Culture spots were harvested at 24 hr and observed on glass slides by microscopy to quantify strain ratios based on fluorescent labels. Typically, between 200 and 800 cells were counted. Competitive index in all assays was quantified by calculating the change in ratio of target to toxin-producing inhibitor cells over 24 hr. For example, if the 0 hr ratio was 1 to 1 and the 24 hr ratio was 1 to 100, the competitive index was. 01, indicating that the target strain was outcompeted. Swarm inhibition experiments were done identically but cells were not collected and instead were imaged after 72 hr. To determine the potency of killing, competition assays were conducted where the target cell volume and density were held constant (50 μL, 3 × 108 cells per mL) while the number of toxin producing cells were titrated 1 to 10 for each sample. Cells were harvested at 48 hr, serially diluted and plated on CTT containing Km to enumerate viable target cells.
For the SitA1 serial transfer assay, competition was done at 1:5 or 1:5:5 mixtures of inhibitors to target(s) using a culture density of 3 × 109 cells per mL. For competition with environmental isolates, liquid cultures of the DK1622-derived strain and the environmental isolate were adjusted to 3 × 109 cells per mL liquid culture, mixed (100 µL DK1622 to 50 µL isolate), and spread onto competition media to ensure there were enough non-lysed cells to count by fluorescent microscopy. At the indicated time points, 2 mL of TPM was added to the agar plate, agitated with a plate spreader, and collected by pipette. Cells were centrifuged at low speed to help prevent clumping and either cell ratio was quantified by microscopy (as above) or the cells were resuspended in 1 mL TPM for CFU determination. Clumping was not an issue due to a non-isogenic mix of strains and massive cell lysis interfering with cell-cell adhesion. CFU of DK1622-derived strains were enumerated by 1 to 10 serial dilution and plating on CTT with oxytetracycline. All figures that contain error bars indicate the experiments were done in triplicate on different days. All statistical tests comparing two results are unpaired, two-tailed t-tests.
E. coli experiments
To test for growth inhibition by toxin expression, overnight cultures of each strain grown in LB with appropriate antibiotics were adjusted to OD600 = 1.0 and back diluted 1 to 10 into fresh media containing 0.2% arabinose (to induce expression from pBAD), ampicillin 100 μg/mL and streptomycin 50 μg/mL. Cultures were then incubated in a shaker at 37°C for 5 hr. 1 to 10 serial dilutions of each culture were plated on LB with 0.2% arabinose and 100 μg/mL ampicillin and imaged after overnight growth at 37°C.
For DAPI staining, cells were grown as described above. At 0 hr and 6 hr post arabinose induction cells were adjusted to OD600 = 0.4 and 1 mL was collected by centrifugation. Cells were fixed in freshly prepared 4% (vol/vol) formaldehyde in PBS for 15 min, rotating at room temperature (RT). The reaction was quenched by addition of an equal volume of 250 mM glycine (pH 7.5) in PBS. Cells were collected and washed 3x and resuspended to 100 μL. Cells were then spotted on poly-L-lysine coated slides and incubated for 10 min. Excess liquid was removed and cells were rinsed with water. 5 μL of fluorogel-II with DAPI (Electron Microscopy Sciences, Hatfield, PA) was placed on cells, a coverslip was applied and cells were imaged at 100× magnification with a Nikon E800 microscope coupled to a digital imaging system (Wei et al., 2011).
To assess tRNA processing, total RNA was isolated from E. coli clones using guanidine isothiocyanate-phenol extraction as described (Garza-Sánchez et al., 2006). Here, cultures were grown to mid-log phase and then diluted to OD600 = 0.05 and grown for 30 min before 0.4% arabinose was added to induce toxin expression. tRNAs were analyzed by Northern blot hybridization using the following 5´-radiolabeled oligonucleotide probes: tRNAUGCAla (5´ - TCC TGC GTG CAA AGC AG), tRNAICGArg (5´ - CCT CCG ACC GCT CGG TTC G), tRNACGASer (5´ - GTA GAG TTG CCC CTA CTC CGG), and tRNAGCACys (5´ - GGA CTA GAC GGA TTT GCA A).
Swarm merging assay
These assays used a modified competition media (replacing 1.5% agar with 1.0% agarose for imaging and addition of 5 μg/mL oxytetracycline). A multichannel pipette was used to simultaneously pipette competing strain suspensions (1.5 × 109 cells per mL) until culture spots were nearly touching. Aliquots were air dried and plates were then incubated in a humid chamber at 33°C for 3 days. Spots were viewed on an Olympus SZX10 stereomicroscope coupled to a digital imaging system.
Sequence analysis
To discover SitA homologs, we performed BLAST (Altschul et al., 1990) analysis against the IMG database (Markowitz et al., 2012), using the conserved N-terminal sequence (first 507 amino acids of SitA1 and the first 441 amino acids of SitA3) as queries. HMMER (Finn et al., 2011), HHPred (Söding et al., 2005) and I-TASSER (Zhang, 2008) analysis were performed as described above using default parameters.
To detect any correlation between colony merger and sitA genes from the referenced study (Wielgoss et al., 2016), we first identified SitA homologs by performing local BLAST analysis of the sequences published at http://www.odose.nl/u/michiel/h/22-myxo-genomes-w-annotation (Wielgoss et al., 2016) using SitB1 as the query. Any gene located downstream of a SitB homolog that contained the described features of SitA was considered a SitA homolog. We also used SitA homolog sequences as queries to find any sitA genes without an accompanying sitB. Redundant sequences were removed using NCBI FASTA Tools Unique Sequences webpage. The sequences were then clustered according to >96% pairwise amino acid identity and each cluster was considered a unique polymorphic toxin group. The two closest toxin groups were 90% identical. This analysis was done blind with respect to the published colony merger compatibility types (Wielgoss et al., 2016).
Fluorescent transfer experiments
Log-phase reporter and target cell liquid cultures were adjusted to 1.5–3 × 109 cells per mL, mixed at the indicated ratios, and plated on ½ CTT agar with 2 mM CaCl2, +/- IPTG, as needed. Strains were incubated at 33°C for the indicated time-period, collected, and visualized by fluorescent microscopy as described (Wei et al., 2011).
Immunofluorescence and blotting
Log phase cultures were resuspended to 6 × 108 cells per mL in reduced osmolarity PBS solution (“mPBS” = 7.2 mM NaCl, 5.4 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4). 100 μL of 10% paraformaldehyde solution (in mPBS) and 1 μL of 5% glutaraldehyde solution (in H2O) were added to 400 μL of cell suspension. Each mixture was spotted on a poly-lysine coated slide. Fixation proceeded for 30 min at RT. After rinsing with mPBS, cells were permeabilized with 0.025% Triton X-100 for 10 min and washed. Cells were blocked for 1 hr at RT with 4% BSA in mPBS, then probed with a 1:1000 final concentration of anti-FLAG antibody (in 4% BSA, Sigma, St. Louis, MO) for 1 hr at RT and subsequently washed 2× for 5 min and 1× for 10 min with mPBS. Cells were then probed with a 1:2000 final concentration of secondary antibody (in 4% BSA, Alexa Fluor 488-conjugated donkey anti-rabbit IgG; Jackson ImmunoResearch, Westgrove, PA) for 30 min at RT and subsequently washed as before. SlowFade Gold antifade reagent (Invitrogen) was added to the slide and the cells were visualized with a 100× objective lens. Western blot was performed according to standard protocols, using anti-FLAG antibody described above, and horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Thermo Scientific, Waltham, MA).
Expression of SitA-CTDs in M. xanthus
Each SitA-CTD was expressed from an IPTG-inducible promoter. For liquid growth-inhibition, cells were grown to log-phase and diluted to 5 × 107 cells per mL in two separate flasks containing fresh media (CTT, 2.5 μg/mL oxytetracycline). To one of two flasks, IPTG was added to 1.0 mM. Cells were grown in the dark with shaking at room temperature. Culture growth was monitored at the indicated time points by measuring turbidity using a Klett meter. For DAPI staining, cells were grown as above for 30 hr, washed and resuspended in mPBS to a concentration of 1.5 × 109 cells per mL. 1 μL of a 50 μg/mL DAPI solution (Life Technologies, Carlsbad, CA) was added for each mL of culture. Cells were incubated for 20 min in the dark with rotation, washed, concentrated, and visualized by fluorescent microscopy.
Acknowledgements
We would like to thank Dr. Greg Velicer and Dr. Sébastien Wielgoss for constructive comments.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Funding Information
This paper was supported by the following grants:
National Institute of General Medical Sciences GM101449 to Daniel Wall.
National Institute of General Medical Sciences Wyoming INBRE to Daniel Wall.
Additional information
Competing interests
The authors declare that no competing interests exist.
Author contributions
CNV, Conceptualization, Data curation, Formal analysis, Validation, Investigation, Methodology, Writing—original draft, Writing—review and editing.
PC, Conceptualization, Data curation, Formal analysis, Methodology.
AC, Data curation, Validation, Methodology, and Data generation.
HF, Data curation, Validation, Methodology, and Data generation.
CSH, Conceptualization, Formal analysis, Supervision, Writing—review and editing.
DW, Conceptualization, Resources, Data curation, Formal analysis, Supervision, Funding acquisition, Validation, Writing—original draft, Project administration, Writing—review and editing.
Additional files
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Garcia EC, Cotter PA. Kind discrimination and competitive exclusion mediated by contact-dependent growth inhibition systems shape biofilm community structure. PLoS Pathogens. 2014;10:e1004076. doi: 10.1371/journal.ppat.1004076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki SK, Pamma R, Hernday AD, Bickham JE, Braaten BA, Low DA. Contact-dependent inhibition of growth in Escherichia coli. Science. 2005;309:1245–1248. doi: 10.1126/science.1115109. [DOI] [PubMed] [Google Scholar]
- Aoki SK, Diner EJ, de Roodenbeke CT, Burgess BR, Poole SJ, Braaten BA, Jones AM, Webb JS, Hayes CS, Cotter PA, Low DA. A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature. 2010;468:439–442. doi: 10.1038/nature09490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz J, Meinhart A. Antibacterial effector/immunity systems: it's just the tip of the iceberg. Current Opinion in Microbiology. 2014;17:1–10. doi: 10.1016/j.mib.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Bhat S, Zhu X, Patel RP, Orlando R, Shimkets LJ. Identification and localization of Myxococcus xanthus porins and lipoproteins. PLoS One. 2011;6:e27475. doi: 10.1371/journal.pone.0027475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Dey A, Vassallo CN, Wall D. How myxobacteria cooperate. Journal of Molecular Biology. 2015;427:3709–3721. doi: 10.1016/j.jmb.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Casabona MG, Kneuper H, Chalmers JD, Palmer T. The type VII secretion system of Staphylococcus aureus secretes a nuclease toxin that targets competitor bacteria. Nature Microbiology. 2016;2:16183. doi: 10.1038/nmicrobiol.2016.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Wall D. Self-identity reprogrammed by a single residue switch in a cell surface receptor of a social bacterium. PNAS. 2017;114:3732–3737. doi: 10.1073/pnas.1700315114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardarelli L, Saak C, Gibbs KA. Two proteins form a heteromeric bacterial self-recognition complex in which variable subdomains determine allele-restricted binding. mBio. 2015;6:e00251. doi: 10.1128/mBio.00251-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier RH. Genetic clonal recognition abilities in marine invertebrates must be maintained by selection for something else. Evolution. 1986;40:1100–1101. doi: 10.1111/j.1558-5646.1986.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Dawkins R. The Selfish Gene. Oxford University Press; 1976. [Google Scholar]
- Dey A, Wall D. A genetic screen in Myxococcus xanthus identifies mutants that uncouple outer membrane exchange from a downstream cellular response. Journal of Bacteriology. 2014;196:4324–4332. doi: 10.1128/JB.02217-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A, Vassallo CN, Conklin AC, Pathak DT, Troselj V, Wall D. Sibling rivalry in Myxococcus xanthus is mediated by kin recognition and a polyploid prophage. Journal of Bacteriology. 2016;198:994–1004. doi: 10.1128/JB.00964-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Research. 2011;39:W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A, West SA. Greenbeards. Evolution. 2010;64:25–38. doi: 10.1111/j.1558-5646.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- Garza-Sánchez F, Janssen BD, Hayes CS. Prolyl-tRNA(Pro) in the A-site of SecM-arrested ribosomes inhibits the recruitment of transfer-messenger RNA. Journal of Biological Chemistry. 2006;281:34258–34268. doi: 10.1074/jbc.M608052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs KA, Greenberg EP. Territoriality in Proteus: advertisement and aggression. Chemical Reviews. 2011;111:188–194. doi: 10.1021/cr100051v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour. I. Journal of Theoretical Biology. 1964a;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour. II. Journal of Theoretical Biology. 1964b;7:17–52. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- Hillman K, Goodrich-Blair H. Are you my symbiont? Microbial polymorphic toxins and antimicrobial compounds as honest signals of beneficial symbiotic defensive traits. Current Opinion in Microbiology. 2016;31:184–190. doi: 10.1016/j.mib.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Hood RD, Singh P, Hsu F, Güvener T, Carl MA, Trinidad RR, Silverman JM, Ohlson BB, Hicks KG, Plemel RL, Li M, Schwarz S, Wang WY, Merz AJ, Goodlett DR, Mougous JD. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host & Microbe. 2010;7:25–37. doi: 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniesta AA, García-Heras F, Abellón-Ruiz J, Gallego-García A, Elías-Arnanz M. Two systems for conditional gene expression in Myxococcus xanthus inducible by isopropyl-β-D-thiogalactopyranoside or vanillate. Journal of Bacteriology. 2012;194:5875–5885. doi: 10.1128/JB.01110-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamet A, Nassif X. Characterization of the maf family of polymorphic toxins in pathogenic Neisseria species. Microbial Cell. 2015a;2:88–90. doi: 10.15698/mic2015.03.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamet A, Nassif X. New players in the toxin field: polymorphic toxin systems in bacteria. mBio. 2015b;6:e00285–00285215. doi: 10.1128/mBio.00285-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konovalova A, Petters T, Søgaard-Andersen L. Extracellular biology of Myxococcus xanthus. FEMS Microbiology Reviews. 2010;34:89–106. doi: 10.1111/j.1574-6976.2009.00194.x. [DOI] [PubMed] [Google Scholar]
- Ma LS, Hachani A, Lin JS, Filloux A, Lai EM. Agrobacterium tumefaciens deploys a superfamily of type VI secretion DNase effectors as weapons for interbacterial competition in planta. Cell Host & Microbe. 2014;16:94–104. doi: 10.1016/j.chom.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre DL, Miyata ST, Kitaoka M, Pukatzki S. The Vibrio cholerae type VI secretion system displays antimicrobial properties. PNAS. 2010;107:19520–19524. doi: 10.1073/pnas.1012931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz VM, Chen IM, Palaniappan K, Chu K, Szeto E, Grechkin Y, Ratner A, Jacob B, Huang J, Williams P, Huntemann M, Anderson I, Mavromatis K, Ivanova NN, Kyrpides NC. IMG: the integrated microbial genomes database and comparative analysis system. Nucleic Acids Research. 2012;40:D115–D122. doi: 10.1093/nar/gkr1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Sodergren E, Masuda T, Kaiser D. Systematic isolation of transducing phages for Myxococcus xanthus. Virology. 1978;88:44–53. doi: 10.1016/0042-6822(78)90108-3. [DOI] [PubMed] [Google Scholar]
- Morse RP, Nikolakakis KC, Willett JL, Gerrick E, Low DA, Hayes CS, Goulding CW. Structural basis of toxicity and immunity in contact-dependent growth inhibition (CDI) systems. PNAS. 2012;109:21480–21485. doi: 10.1073/pnas.1216238110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse RP, Willett JL, Johnson PM, Zheng J, Credali A, Iniguez A, Nowick JS, Hayes CS, Goulding CW. Diversification of β-Augmentation interactions between CDI toxin/immunity proteins. Journal of Molecular Biology. 2015;427:3766–3784. doi: 10.1016/j.jmb.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S, Willett JW, Bahr SM, Scott JC, Wilson JM, Darnell CL, Vlamakis HC, Kirby JR. Draft genome of a type 4 pilus defective Myxococcus xanthus strain, DZF1. Genome Announcements. 2013;1:e00392-13. doi: 10.1128/genomeA.00392-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolakakis K, Amber S, Wilbur JS, Diner EJ, Aoki SK, Poole SJ, Tuanyok A, Keim PS, Peacock S, Hayes CS, Low DA. The toxin/immunity network of Burkholderia pseudomallei contact-dependent growth inhibition (CDI) systems. Molecular Microbiology. 2012;84:516–529. doi: 10.1111/j.1365-2958.2012.08039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudleman E, Wall D, Kaiser D. Cell-to-cell transfer of bacterial outer membrane lipoproteins. Science. 2005;309:125–127. doi: 10.1126/science.1112440. [DOI] [PubMed] [Google Scholar]
- Papke RT, Ward DM. The importance of physical isolation to microbial diversification. FEMS Microbiology Ecology. 2004;48:293–303. doi: 10.1016/j.femsec.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Pathak DT, Wei X, Bucuvalas A, Haft DH, Gerloff DL, Wall D. Cell contact-dependent outer membrane exchange in myxobacteria: genetic determinants and mechanism. PLoS Genetics. 2012;8:e1002626. doi: 10.1371/journal.pgen.1002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak DT, Wei X, Dey A, Wall D. Molecular recognition by a polymorphic cell surface receptor governs cooperative behaviors in bacteria. PLoS Genetics. 2013;9:e1003891. doi: 10.1371/journal.pgen.1003891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Poole SJ, Diner EJ, Aoki SK, Braaten BA, t'Kint de Roodenbeke C, Low DA, Hayes CS. Identification of functional toxin/immunity genes linked to contact-dependent growth inhibition (CDI) and rearrangement hotspot (Rhs) systems. PLoS Genetics. 2011;7:e1002217. doi: 10.1371/journal.pgen.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queller DC. Expanded social fitness and Hamilton's rule for kin, kith, and kind. PNAS. 2011;108:10792–10799. doi: 10.1073/pnas.1100298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendueles O, Zee PC, Dinkelacker I, Amherd M, Wielgoss S, Velicer GJ. Rapid and widespread de novo evolution of kin discrimination. PNAS. 2015;112:9076–9081. doi: 10.1073/pnas.1502251112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley MA, Wertz JE. Bacteriocins: evolution, ecology, and application. Annual Review of Microbiology. 2002;56:117–137. doi: 10.1146/annurev.micro.56.012302.161024. [DOI] [PubMed] [Google Scholar]
- Roth JR, Benson N, Galitski T, Haack K, Lawrence J. G, Miesel L. Rearrangements of the bacterial chromosome: formation and applications. In: Neidhardt F. C, Curtis R, Ingraham J. L, Lin E. C. C, Low K. B, Magasanik B, Reznikoff W. S, Riley M, Schaechter M, Umbarger H. E, editors. Escherichia coli and Salmonella: Cellular and Molecular Biology. Vol. 120. ASM Press; 1996. pp. 2256–2276. [Google Scholar]
- Ruhe ZC, Wallace AB, Low DA, Hayes CS. Receptor polymorphism restricts contact-dependent growth inhibition to members of the same species. mBio. 2013;4:e00480-13. doi: 10.1128/mBio.00480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhe ZC, Townsley L, Wallace AB, King A, Van der Woude MW, Low DA, Yildiz FH, Hayes CS. CdiA promotes receptor-independent intercellular adhesion. Molecular Microbiology. 2015;98:175–192. doi: 10.1111/mmi.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AB, Hood RD, Bui NK, LeRoux M, Vollmer W, Mougous JD. Type VI secretion delivers bacteriolytic effectors to target cells. Nature. 2011;475:343–347. doi: 10.1038/nature10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AB, Wexler AG, Harding BN, Whitney JC, Bohn AJ, Goo YA, Tran BQ, Barry NA, Zheng H, Peterson SB, Chou S, Gonen T, Goodlett DR, Goodman AL, Mougous JD. A type VI secretion-related pathway in Bacteroidetes mediates interbacterial antagonism. Cell Host and Microbe. 2014;16:227–236. doi: 10.1016/j.chom.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S, West TE, Boyer F, Chiang WC, Carl MA, Hood RD, Rohmer L, Tolker-Nielsen T, Skerrett SJ, Mougous JD. Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathogens. 2010;6:e1001068. doi: 10.1371/journal.ppat.1001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Dworkin M. Territorial interactions between two Myxococcus species. Journal of Bacteriology. 1994;176:1201–1205. doi: 10.1128/jb.176.4.1201-1205.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza DP, Oka GU, Alvarez-Martinez CE, Bisson-Filho AW, Dunger G, Hobeika L, Cavalcante NS, Alegria MC, Barbosa LR, Salinas RK, Guzzo CR, Farah CS. Bacterial killing via a type IV secretion system. Nature Communications. 2015;6:6453. doi: 10.1038/ncomms7453. [DOI] [PubMed] [Google Scholar]
- Starich T, Cordes P, Zissler J. Transposon tagging to detect a latent virus in Myxococcus xanthus. Science. 1985;230:541–543. doi: 10.1126/science.2996138. [DOI] [PubMed] [Google Scholar]
- Starich T, Zissler J. Movement of multiple DNA units between Myxococcus xanthus cells. Journal of Bacteriology. 1989;171:2323–2336. doi: 10.1128/jb.171.5.2323-2336.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassmann JE, Gilbert OM, Queller DC. Kin discrimination and cooperation in microbes. Annual Review of Microbiology. 2011;65:349–367. doi: 10.1146/annurev.micro.112408.134109. [DOI] [PubMed] [Google Scholar]
- Söding J, Biegert A, Lupas AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Research. 2005;33:W244–W248. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varivarn K, Champa LA, Silby MW, Robleto EA. Colonization strategies of Pseudomonas fluorescens Pf0-1: activation of soil-specific genes important for diverse and specific environments. BMC Microbiology. 2013;13:92. doi: 10.1186/1471-2180-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassallo C, Pathak DT, Cao P, Zuckerman DM, Hoiczyk E, Wall D. Cell rejuvenation and social behaviors promoted by LPS exchange in myxobacteria. PNAS. 2015;112:E2939–E2946. doi: 10.1073/pnas.1503553112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassallo CN, Wall D. Tissue repair in Myxobacteria: a cooperative strategy to heal cellular damage. BioEssays. 2016;38:306–315. doi: 10.1002/bies.201500132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer GJ, Vos M. Sociobiology of the myxobacteria. Annual Review of Microbiology. 2009;63:599–623. doi: 10.1146/annurev.micro.091208.073158. [DOI] [PubMed] [Google Scholar]
- Vos M, Velicer GJ. Genetic population structure of the soil bacterium Myxococcus xanthus at the centimeter scale. Applied and Environmental Microbiology. 2006;72:3615–3625. doi: 10.1128/AEM.72.5.3615-3625.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos M, Velicer GJ. Social conflict in centimeter-and global-scale populations of the bacterium Myxococcus xanthus. Current Biology. 2009;19:1763–1767. doi: 10.1016/j.cub.2009.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall D. Kin recognition in bacteria. Annual Review of Microbiology. 2016;70:143–160. doi: 10.1146/annurev-micro-102215-095325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Pathak DT, Wall D. Heterologous protein transfer within structured myxobacteria biofilms. Molecular Microbiology. 2011;81:315–326. doi: 10.1111/j.1365-2958.2011.07710.x. [DOI] [PubMed] [Google Scholar]
- Wenren LM, Sullivan NL, Cardarelli L, Septer AN, Gibbs KA. Two independent pathways for self-recognition in Proteus mirabilis are linked by type VI-dependent export. mBio. 2013;4:e00374-13. doi: 10.1128/mBio.00374-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielgoss S, Didelot X, Chaudhuri RR, Liu X, Weedall GD, Velicer GJ, Vos M. A barrier to homologous recombination between sympatric strains of the cooperative soil bacterium Myxococcus xanthus. The ISME Journal. 2016;10:2468–2477. doi: 10.1038/ismej.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett JL, Gucinski GC, Fatherree JP, Low DA, Hayes CS. Contact-dependent growth inhibition toxins exploit multiple independent cell-entry pathways. PNAS. 2015;112:11341–11346. doi: 10.1073/pnas.1512124112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Iyer LM, Aravind L. A novel immunity system for bacterial nucleic acid degrading toxins and its recruitment in various eukaryotic and DNA viral systems. Nucleic Acids Research. 2011;39:4532–4552. doi: 10.1093/nar/gkr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, de Souza RF, Anantharaman V, Iyer LM, Aravind L. Polymorphic toxin systems: comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biology Direct. 2012;7:18. doi: 10.1186/1745-6150-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Gänzle MG, Lin XB, Ruan L, Sun M. Diversity and dynamics of bacteriocins from human microbiome. Environmental Microbiology. 2015;17:2133–2143. doi: 10.1111/1462-2920.12662. [DOI] [PubMed] [Google Scholar]