Abstract
This study aimed to determine the bacterial species colonizing the nasal and oropharyngeal mucosa of fuel workers in Central Riyadh, Saudi Arabia on a microbiological and molecular level. Throat and nasal swab samples were obtained from 29 fuel station attendants in the period of time extending from March to May 2014 in Riyadh, Saudi Arabia. Microbiological identification techniques were utilized to identify the bacterial species isolated. Antibiotic sensitivity was assessed for each of the bacterial isolates. Molecular identification techniques based on PCR analysis of specific genomic sequences was conducted and was the basis on which phylogeny representation was done for 10 randomly selected samples of the isolates. Blood was drawn and a complete blood count was conducted to note the hematological indices for each of the study participants. Nineteen bacterial species were isolated from both the nasal cavity and the oropharynx including Streptococcus thoraltensis, alpha-hemolytic streptococci, Staphylococcus hominis, coagulase-negative staphylococci, Leuconostoc mesenteroides, Erysipelothrix rhusiopathiae and several others. We found 100% sensitivity of the isolates to ciprofloxacin, cefuroxime and gentamicin. Whereas cefotaxime and azithromycin posted sensitivities of 85.7% and 91.4%, respectively. Low sensitivities (<60% sensitivity) to the antibiotics ampicillin, erythromycin, clarithromycin and norfloxacin were observed. Ninety-seven percent similarity to the microbial bank species was noted when the isolates were compared to it. Most hematological indices recorded were within the normal range. In conclusion, exposure to toxic fumes and compounds within fuel products may be a contributing factor to bacterial colonization of the respiratory tract in fuel workers.
Keywords: Nasopharyngeal bacteria, Fuel workers, Fuel inhalation
1. Introduction
Air pollution has been a major health concern for decades. Motor exhaust emissions are a complex mixture of gases and particulate matter. Individuals working in stations that provide gasoline were found to be at greater risk of contracting respiratory diseases caused by the inhalation of toxic fumes of gasoline and petroleum products (Tunsaringkarn et al., 2010, De Oliveira et al., 2007). Combustion of vehicular exhaust was found to contain carbon aggregates consisting of tens to thousands of primary carbon particles and mineral particles. Of the particularly harmful substances within fuels are benzene and sulfur dioxide (SO2) both of which are known to modulate respiratory defenses leaving the respiratory tract susceptible to infections (Mohan et al., 2013).
Aromatic hydrocarbons (particularly benzene) are added to gasoline products as anti-knock agents (i.e. an agent that increases the octane rating of the fuel thus increasing the temperature and pressure necessary for that fuel to undergo auto-ignition). Previously, lead was used for this purpose but is nowadays considered hazardous to health and thus is not used as an anti-knock agent in most countries. Refined petroleum products usually include 2–3% benzene by volume, however in many countries, Saudi Arabia included, contents of benzene in gasoline may reach values ranging between 5% and 7% (Bahadar et al., 2014).
Benzene exposure has many deleterious and toxic effects in general on humans due to both acute and chronic exposure. Benzene is particularly toxic to both the respiratory and hematological systems. Acute exposure to benzene is so irritating to the respiratory system that large concentrations may cause lung edema, hemorrhage and even fatalities (Bahadar et al., 2014). Chronic exposure of the respiratory system to benzene within fuel fumes is also associated with toxic effects on the respiratory system. Apoptotic changes in the parenchymal components of the lungs were noted when rats were exposed to benzene for 7 days (Weaver et al., 2007). Such histological changes may promote the overgrowth of flora or make the system more susceptible to colonization or infection (Bahadar et al., 2014).
Benzene effects on the hematological system have been extensively studied due to its association with hematological malignancies and aplastic anemia. Chronic exposure to benzene is associated with a decrease in hemoglobin (HB), platelet count, and white blood cell (WBC) counts. Neutrophils and mean platelet volume (MPV) in the blood have been reported to be the most likely indices to be affected by benzene exposure in a study that was conducted on Chinese factory workers (Robert Schnatter et al., 2010).
Studies identifying the bacterial pathogens colonizing workers of fuel and gasoline stations are scarce. Most microbiological studies are dedicated to bacteria isolated from areas surrounding fuel stations. Bacterial species found included Pseudomonas sp., Flavobacterium sp., and Rhodococcus sp. (Lu et al., 2006). Other crude oil degrading bacteria such as Corynebacterium, Micrococcus sp. and Bacillus sp. were also isolated from soil samples collected from gasoline and diesel stations (Rahman et al., 2002). Thus far, no study has attempted to analyze and identify the molecular identity of respiratory bacteria isolated from a population exposed to fuel and fuel exhaust contaminants. Like human cells, bacteria have been shown to change in an environment containing toxins or pollutants (Wickham and Atlas, 1988) however there have been virtually no attempts to understand how those exposures would contribute to bacterial pathogenicity.
We conducted this cross-sectional study to determine, both microbiologically and molecularly, the bacterial species from the nasal and oropharynx of fuel workers in Central Saudi Arabia. We also aimed to study their sensitivity to many antibiotics and their possible effects on hematological parameters.
2. Materials and methods
2.1. Sample collection and isolation and testing for antibiotic resistance
This cross-sectional study investigates the effects of gasoline vapors and vehicular exhaust fumes on nasal and nasopharyngeal microbial flora in employees attending fuel stations. Participants were recruited from fuel stations in Riyadh, Saudi Arabia during the period of time between March and May 2014. All the participants were informed about the aim and objectives of the study and approval forms were obtained. The study protocol was reviewed by the Princess Noura Bint Abdul Rahman Research Ethics Committee.
Sterile swabs were used to sample the nares and posterior oropharynx of the participants. We have isolated, identified and assessed antibiotic resistance from 58 samples (where 29 were from the nasal cavity and 29 were from the oropharynx; 2 samples per participant) obtained from the 29 fuel station workers using Vitek® 2 compact system as described by Mezger et al. (2015). The swab samples where then inoculated onto special cards specific for the Vitek® 2 compact. The cards contained wells with reagents and antibiotics with which identification and antibiotic sensitivity could be easily assessed and documented via print out by the system. Alpha hemolysic activity of bacterial isolates was also identified via the Vitek® 2 compact system by pyrosequencing bacterial 16S rRNA gene. This method detects alpha hemolytic abilities and removed the need to inoculate stains onto blood agar plates and interpret their pattern of hemolysis thus leaving room for error in interpretation (Haanperä et al., 2007).
PCR molecular analysis and the phylogeny representation were performed on 10 randomly selected samples from the colonies isolated.
2.2. Blood sample collection and analysis
We also obtained blood samples from each of the participants after procuring their consent and performed complete blood counts (CBC) to assess whether bacterial colonization of the respiratory tract had elicited an immune response. In order to conduct the complete blood count, samples were collected and analyzed according to the standards proposed by the International Council for Standardization in Hematology (ICSH) (Barnes et al., 2005). Blood samples where drawn into EDTA tubes (lavender tops) under clean conditions. Samples were stored in a potable cool container and processed in the lab within 1 h of collection. The Sysmex XN-9000™ hematology analyzer system was utilized in the lab to obtain detailed information about the hematological makers of patient samples (Sysmex, 2015).
2.3. DNA extraction and sequence analyses
DNA was extracted from isolates using the CTAB (N-cetyl-N, N,N trimethyl ammonium bromide) method described by Murray (The Human Microbiome Jumpstart Reference Strains, 2010) and Thompson (Thompson et al., 1997). Small Subunit ribosomal RNA (mtSSU rRNA) and β-tubulin were then amplified by PCR using primer pairs under the conditions described by O’Donnell, White, Glass and Donaldson (O’donnell et al., 1998, White et al., 1990, Glass and Donaldson, 1995), respectively:
| GFP-F-272–293/GFP-R-356–332 | 5′ GCCATGCCAGAAGGTTATGTTC 5′ |
| 5′ CAAACTTGACTTCAGCTCTGGTCTT 3′ | |
| MM1133/MM1130 | 5′ TAGAGGACAGCCGTGATGTG 3′ |
| 5′ CAAACAGGGTTTCGGTCAGT 3′ | |
| Universal gene | 5′ TTCCGTGGTTCCGTCTCGC 3′ |
| 5′ CGGTCCAGACTCCTACGGG 3′ | |
| RST2/RST3 | 5′ AGGCCCTGGAAGGTGCCCTGGA 3′ |
| 5′ ATCGCACTGCGTACCGCGCGCGA 3′ |
PCR products were purified using the QIA quick PCR purification kit (QIAGEN, GmbH, Germany), and sequenced in both directions using the respective PCR primers. For this purpose, the Big Dye terminator sequencing kit (Version 3.1, Applied Biosystems) and an ABI PRISMTM 3100 DNA sequencer (Applied Biosystems) were used. All PCRs and sequencing reactions were performed on a GeneAmp PCR System 9700 (Applied Biosystems). Gene sequences were assembled using Sequence Navigator (Version 1.0.1, Applied Biosystems), and aligned using ClustalX (Version 1.8, Thompson et al., 1997), after which the alignments were manually corrected where needed. The predicted sequences were then compared with the corresponding sequences in GenBank to determine the possible positions of introns. All characters were weighted equally and alignment gaps were treated as missing data. Replication based bootstrap analysis was performed using the Hillis and Bull method (Hillis and Bull, 1993).
Data were analyzed using the Predictive Analysis Software PASW Statistics 19.0 statistical program (IBM – SPSS, Inc., 2009, Chicago, IL, USA). Results are presented as frequencies and as percentages.
3. Results
Twenty-nine male fuel station workers were included in the study. Their mean duration of years at work was 5.46 ± 3.0 years (range of 1–15 years). Nineteen bacterial species were isolated from both the nasal cavity and the oropharynx. Streptococcus thoraltensis was the most common isolate (n = 12, 20.7%), followed by alpha-hemolytic streptococci (n = 8, 13.8%), Staphylococcus hominis (n = 7, 12.1%), coagulase-negative staphylococci (n = 5, 8.6%), Leuconostoc mesenteroides (n = 5, 8.6%) and Erysipelothrix rhusiopathiae (n = 4, 6.9%). The complete list of the bacterial isolates is presented in Table 1.
Table 1.
Bacteria isolated from 58 samples from the nasal cavity and oropharynx in 29 fuel station workers.
| Bacterial isolates | n | % |
|---|---|---|
| Streptococcus thoraltensis | 12 | 20.7 |
| Alpha-hemolytic streptococci | 8 | 13.8 |
| Staphylococcus hominis | 7 | 12.1 |
| Coagulase-negative staphylococci | 5 | 8.6 |
| Leuconostoc mesenteroides | 5 | 8.6 |
| Erysipelothrix rhusiopathiae | 4 | 6.9 |
| Gardnerella vaginalis | 2 | 3.4 |
| Kocuria rosea | 2 | 3.4 |
| Pseudomonas aeruginosa | 2 | 3.4 |
| Staphylococcus epidermidis | 2 | 3.4 |
| Aerococcus viridians | 1 | 1.7 |
| Citrobacter koseri | 1 | 1.7 |
| Klebsiella pneumoniae | 1 | 1.7 |
| Keptococcus sedantaris | 1 | 1.7 |
| Micrococcus luteus | 1 | 1.7 |
| Pantoea spp | 1 | 1.7 |
| Staphylococcus aureus | 1 | 1.7 |
| Staphylococcus lentus | 1 | 1.7 |
| Staphylococcus vitulinus | 1 | 1.7 |
Table 2 details the results of the bacterial sensitivity and resistance testing of the bacterial isolates to commonly used antibiotics. A 100% sensitivity of the isolates to ciprofloxacin, cefuroxime, gentamicin and imipenem was found when the samples were tested against these antibiotics, whereas with cefotaxime, azithromycin and doxycycline, sensitivities of 94.3%, 91.4% and 90.5% respectively were recorded. Low sensitivities (less than 60% sensitivity) to the antibiotics ampicillin, erythromycin, clarithromycin, norfloxacin and cefaclor were observed. These antibiotics were tested only in less than 20% of the bacterial isolates, except for norfloxacin, which was tested in 66.1% of the isolates.
Table 2.
Bacterial sensitivity and resistance to antibiotics of all samples (n = 58).
| Antibiotic (antibiotic identification card Vitek®) | No. of samples tested N | Percentage of sample tested (%) | Sensitivity (%) | Resistance (%) |
|---|---|---|---|---|
| Amoxycillin | 51.0 | 87.9 | 84.3 | 15.7 |
| Ampicillin | 13.0 | 22.4 | 53.8 | 46.2 |
| Amoxycillin/Clavulanic acid | 46.0 | 79.3 | 82.6 | 17.4 |
| Aztreonam | 4.0 | 6.9 | 75.0 | 25.0 |
| Cefotaxime | 35.0 | 60.3 | 94.3 | 5.7 |
| Cefoxitin | 8.0 | 13.8 | 75.0 | 25.0 |
| Cefixime | 42.0 | 72.4 | 85.7 | 14.3 |
| Cephradine | 10.0 | 17.2 | 90.0 | 10.0 |
| Cefaclor | 4.0 | 6.9 | 50.0 | 50.0 |
| Chloramphenicol | 2.0 | 3.4 | 0.0 | 100.0 |
| Ciprofloxacin | 47.0 | 81.0 | 100.0 | 0.0 |
| Doxycycline | 42.0 | 72.4 | 90.5 | 9.5 |
| Erythromycin | 12.0 | 20.7 | 50.0 | 50.0 |
| Clindamycin | 47.0 | 81.0 | 70.2 | 29.8 |
| Gentamicin | 15.0 | 25.9 | 100.0 | 0.0 |
| Clarithromycin | 5.0 | 8.6 | 40.0 | 60.0 |
| Imipenem | 3.0 | 5.2 | 100.0 | 0.0 |
| Nitrofurantoin | 16.0 | 27.6 | 93.8 | 6.3 |
| Norfloxacin | 41.0 | 70.7 | 59.7 | 6.5 |
| Tetracycline | 10.0 | 17.2 | 70.0 | 30.0 |
| Trimethoprim-Sulfamethoxazole | 57.0 | 98.3 | 89.5 | 10.5 |
| Cefuroxime | 39.0 | 67.2 | 100.0 | 0.0 |
| Ofloxacin | 24.0 | 41.4 | 95.8 | 4.2 |
| Azithromycin | 35.0 | 60.3 | 91.4 | 8.6 |
| Vancomycin | 35.0 | 60.3 | 74.3 | 25.7 |
Ten bacterial isolates were randomly selected to undergo the process of gene sequencing and comparison. Table 3 shows an example of the results of the whole genome identification of single isolates of Klebsiella pneumoniae, Vibrio fluvialis and Xanthomonas campestris obtained from the participants. When the isolate was compared to the data within the GenBank sequence database, a 97% similarity in identity to the genome of the GenBank bacteria was found. In Fig. 1, the entire genome of a single isolate of Pseudomonas aeruginosa is displayed.
Table 3.
Results of the identification of Klebsiella pneumoniae sp., Xanthomonas campestris sp. and Vibrio fluvialis.
| Description | Max score | Total score | Query cover (%) | E value | Ident (%) |
|---|---|---|---|---|---|
| Klebsiella pneumoniae subsp. pneumoniae strain KPNIH31, complete genome | 518 | 4112 | 100 | 2e−143 | 97 |
| Klebsiella pneumoniae subsp. pneumoniae strain KPNIH30, complete genome | 518 | 4123 | 100 | 2e−143 | 97 |
| Klebsiella pneumoniae subsp. pneumoniae strain KPNIH29, complete genome | 518 | 4123 | 100 | 2e−143 | 97 |
| Klebsiella pneumoniae strain XH209, complete genome | 518 | 4112 | 100 | 2e−143 | 97 |
| Klebsiella pneumoniae subsp. pneumoniae strain KPNIH32, complete genome | 518 | 4123 | 100 | 2e−143 | 97 |
| Vibrio fluvialis strain XJ85003 CAI-1 autoinducer synthase (cqsA) gene, complete cds | 2117 | 2117 | 100 | 0.0 | 99 |
| Xanthomonas campestris pv. campestris str. ATCC 33913, complete genome | 2093 | 2093 | 99 | 0.0 | 98 |
| Xanthomonas campestris pv. campestris str. 8004, complete genome | 2093 | 2093 | 99 | 0.0 | 98 |
| Xanthomonas campestris pv. campestris complete genome, strain B100 | 2071 | 2071 | 99 | 0.0 | 98 |
| Xanthomonas campestris pv. raphani 756C, complete genome | 1988 | 1988 | 99 | 0.0 | 97 |
| Xanthomonas axonopodis pv. citrumelo F1, complete genome | 1328 | 1328 | 99 | 0.0 | 87 |
Figure 1.
Bacterial sequencing analysis results for Pseudomonas aeruginosa.
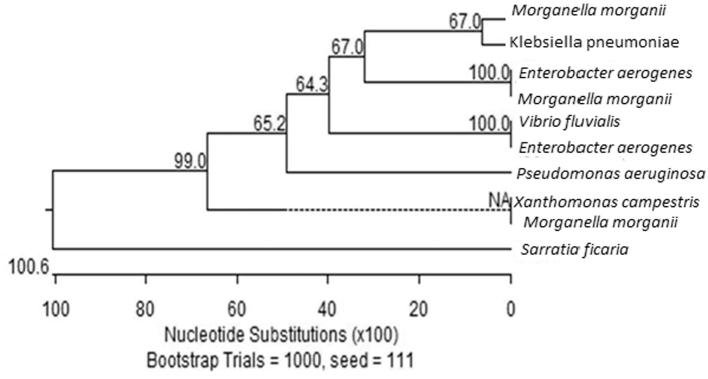
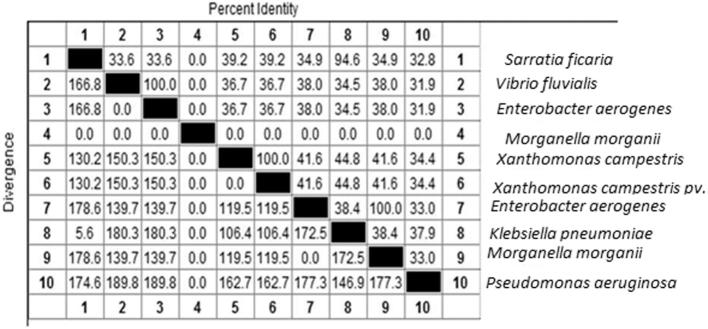
Figure 2, Figure 3 show the similarity in the gene sequences of the 10 isolates to those within the GenBank. Most of the isolates showed similarities that approached 100%.
Figure 2.
Bootstrap trial results in 10 random samples.
Figure 3.
Similarities in the 10 random isolates.
Finally in Table 4, the mean values of the 29 participants’ blood elements are displayed. All the CBC indices for the 29 workers were within the normal ranges except for two fuel workers who had low mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) possibly detonating anemia. None of the workers were symptomatic for respiratory bacterial infection or any other infection. A single participant displayed a low platelet count of 64 × 109/L cells but was asymptomatic.
Table 4.
Complete blood count results.
| Normal range | Mean | SD | |
|---|---|---|---|
| White cell count | 5.0–10.0 × 109/L | 7.8 | 1.4 |
| Red cell count | 4.5–5.5 × 1012/L | 5.3 | 0.2 |
| Hemoglobin | 14.0–17.0 g/dL | 15.9 | 1.0 |
| Hematocrit | 42–52% | 47.3 | 2.3 |
| Mean corpuscular volume | 84–96 fl. | 88.5 | 2.9 |
| Mean corpuscular hemoglobin | 28–34 pg | 28.9 | 1.0 |
| Platelets | 140–400 × 109/L | 265.6 | 66.1 |
Source: Laboratory Medicine: The Diagnosis of Disease in the Clinical Laboratory (Agrawal et al., 2010).
4. Discussion
The interplay between fuel inhalation and vehicular exhaust exposure and the normal flora of the nasopharyngeal tract is a field where virtually no studies have been conducted. The possibility that these exposures may lead to a higher likelihood of colonization by normal or abnormal flora is a hypothesis worth examining due to its possible deleterious effects on health. In this paper we had aimed to culture these organisms and test their susceptibility against common antibiotics. By sequencing the genomes of the isolated organisms and comparing them to the GenBank, we were able to get a perspective on how exposure to fuels and exhausts might affect the bacterial genome and the likelihood of mutability of the genome.
In this study, 19 bacterial species were isolated from the nasal cavities and oropharyngeal of 29 male fuel station workers. The most common bacterial species were S. thoraltensis, alpha hemolytic streptococci, S. hominis, coagulase-negative staphylococci, L. mesenteroides and E. rhusiopathiae. These species are of the normal flora that is known to colonize the upper respiratory tract (Yi et al., 2014). This is exemplified by the high degree of similarity in the identity of K. pneumoniae, X. campestris and V. fluvialis isolates with the genome of the bacteria within the GenBank as shown in Table 3.
Most of these bacteria, if immune defenses were lost or weakened, could possibly be the cause of respiratory and non-respiratory system infections. The antibiotics to which the bacteria showed complete sensitivity included very strong broad spectrum antibiotics and is in no way indicative that infections with these organisms when and if they happen will be easy to treat with first line agents. Alarmingly, 47% of our sample tested as resistant to ampicillin, a commonly used first-line agent for most infections of the upper respiratory tract. These bacteria also were 18% resistant to its stronger alternative, amoxycillin/clavulanic acid. This speaks of the significance of mounting antibiotic resistance in the area. Our results have confirmed that community-acquired microorganisms, whether specifically in relation to dangerous exposures such as fuels or simply due to their environment are harboring potentially resistant microbes that may not respond to conventional first line antibiotics.
With this study, we also made one of the first attempts in the region of studying the unique microbiome of these workers in relation to their exposures. Fuel additive control is not as strict in Saudi Arabia as it is in many western countries. With this small sample we hoped to introduce how these exposures play a role in diversifying the flora whenever the environment is altered. The fact that the bacterial species showed a high level of similarity to their counterparts within the GenBank is a positive finding, testifying to a high measure of gene content similarity between the strains compared which provides us with a sense of how ecologically related these species are (The Human Microbiome Jumpstart Reference Strains, 2010). When a bacterial genome is well understood and known, treating a possible infection and recognizing the emergence of resistance can be done more rapidly.
Another aspect of the study that needs to be credited is the high degree of resistance of these isolates to the more common antibiotics as shown in Table 2. An alarming finding was that some bacterial isolates showed very significant resistance (reaching over 50% in some) to the most commonly used antibiotics such as ampicillin, erythromycin, clarithromycin as well as some others. This is particularly worrying since ampicillin is one of the first agents that most primary care physicians prescribe for an upper respiratory tract infection. This study proves that a different antibiotic regime will soon have to be introduced to deal with such infections, as the bacteria are now resistant. The cause of this higher rate of resistance has been previously reported in the area and is unlikely to be due to the unique exposures that these workers face (Aly and Balkhy, 2012). Thus it will be vital that future studies not only attempt to understand the effect of fuels and exhaust on the microbiome on a larger scale but also will need to quantify and explain the emergent resistance patterns.
5. Conclusion
Potentially harmful microorganisms that compose the normal flora of the nasopharynx may be a possible cause of infection in workers who are exposed to exhaust fumes and fuels in their workplace. The resistance patterns and possible unique gene mutations in these organisms are areas of further research. There may be a need for close monitoring of fuel station workers health not only for environmental exposures but also for microbial pathogens that may find them susceptible because of their environmental exposures.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agrawal, Y.P., Aleryani, S.L., Alter, D.N., Apple, F.S., Dawling, S.P., Dufour, D.R., Finberg, K.E., Gronowski, A.M., Haas, J.J., Lakhan, V., 2010. Laboratory Medicine: The Diagnosis of Disease in the Clinical Laboratory.
- Aly M., Balkhy H.H. The prevalence of antimicrobial resistance in clinical isolates from Gulf Corporation Council Countries. Antimicrob. Resist. Infect. Control. 2012;1 doi: 10.1186/2047-2994-1-26. 26–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadar H., Mostafalou S., Abdollahi M. Current understandings and perspectives on non-cancer health effects of benzene: a global concern. Toxicol. Appl. Pharmacol. 2014;276:83–94. doi: 10.1016/j.taap.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Barnes P.W., Mcfadden S.L., Machin S.J., Simson E. The international consensus group for hematology review: suggested criteria for action following automated CBC and WBC differential analysis. Lab. Hematol. 2005;11:83–90. doi: 10.1532/LH96.05019. [DOI] [PubMed] [Google Scholar]
- De Oliveira K.M.P.G., Martins E.M., Arbilla G., Gatti L.V. Exposure to volatile organic compounds in an ethanol and gasoline service station. Bull. Environ. Contam. Toxicol. 2007;79:237–241. doi: 10.1007/s00128-007-9181-z. [DOI] [PubMed] [Google Scholar]
- Glass N.L., Donaldson G.C. Development of primer sets designed for use with the pcr to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haanperä M., Jalava J., Huovinen P., Meurman O., Rantakokko-Jalava K. Identification of alpha-hemolytic streptococci by pyrosequencing the 16s rRNA gene and by use of Vitek 2. J. Clin. Microbiol. 2007;45:762–770. doi: 10.1128/JCM.01342-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis D.M., Bull J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 1993;42:182–192. [Google Scholar]
- Lu S.J., Wang H.Q., Yao Z.H. Isolation and characterization of gasoline-degrading bacteria from gas station leaking-contaminated soils. J. Environ. Sci. (China) 2006;18:969–972. doi: 10.1016/s1001-0742(06)60023-5. [DOI] [PubMed] [Google Scholar]
- Mezger A., Gullberg E., Göransson J., Zorzet A., Herthnek D., Tano E., Nilsson M., Andersson D.I. A general method for rapid determination of antibiotic susceptibility and species in bacterial infections. J. Clin. Microbiol. 2015;53:425–432. doi: 10.1128/JCM.02434-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan D., Thiyagarajan D., Murthy P.B. Toxicity of exhaust nanoparticles. Afr. J. Pharm. Pharmacol. 2013;7:318–333. [Google Scholar]
- O’donnell K., Kistler H.C., Cigelnik E., Ploetz R.C. Multiple evolutionary origins of the fungus causing panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. 1998;95:2044–2049. doi: 10.1073/pnas.95.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman K.S., Rahman T., Lakshmanaperumalsamy P., Banat I.M. Occurrence of crude oil degrading bacteria in gasoline and diesel station soils. J. Basic Microbiol. 2002;42:284–291. doi: 10.1002/1521-4028(200208)42:4<284::AID-JOBM284>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Robert Schnatter A., Kerzic P.J., Zhou Y., Chen M., Nicolich M.J., Lavelle K., Armstrong T.W., Bird M.G., Lin L., Fu H., Irons R.D. Peripheral blood effects in benzene-exposed workers. Chem. Biol. Interact. 2010;184:174–181. doi: 10.1016/j.cbi.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Sysmex, 2015. Sysmex Lavender Top Management System® [Online]. Sysmex™ Available: “<http://www.sysmex.com/us/en/Products/Hematology/XNSeries/Pages/XN-9000-Hematology-Analyzer.aspx>” [Accessed Nov 20 2015].
- The Human Microbiome Jumpstart Reference Strains, C. A catalog of reference genomes from the human microbiome. Science. 2010;328:994–999. doi: 10.1126/science.1183605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The Clustal_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunsaringkarn T., Soogarun S., Rungsiyothin A., Zapuang K., Chapman S.R. Health status of gasoline station workers in Pathumwan Area, Bangkok, Thailand, in 2004 and 2009. J. Health Res. 2010;25:15–19. [Google Scholar]
- Weaver C.V., Liu S.P., Lu J.F., Lin B.S. The effects of benzene exposure on apoptosis in epithelial lung cells: localization by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (tunel) and the immunocytochemical localization of apoptosis-related gene products. Cell Biol. Toxicol. 2007;23:201–220. doi: 10.1007/s10565-006-0165-2. [DOI] [PubMed] [Google Scholar]
- White T., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M., Gelfand D., Shinsky J., White T., editors. PCR Protocols: A Guide to Methods and Applications. Academic Press; 1990. [Google Scholar]
- Wickham G.S., Atlas R.M. Plasmid frequency fluctuations in bacterial populations from chemically stressed soil communities. Appl. Environ. Microbiol. 1988;54:2192–2196. doi: 10.1128/aem.54.9.2192-2196.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H., Yong D., Lee K., Cho Y.J., Chun J. Profiling bacterial community in upper respiratory tracts. BMC Infect. Dis. 2014;14:583. doi: 10.1186/s12879-014-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]