Abstract
The antimicrobial activity of plant extract of Peganum harmala, a medicinal plant has been studied already. However, knowledge about bacterial diversity associated with different parts of host plant antagonistic to different human pathogenic bacteria is limited. In this study, bacteria were isolated from root, leaf and fruit of plant. Among 188 bacterial isolates isolated from different parts of the plant only 24 were found to be active against different pathogenic bacteria i.e. Escherichia coli, Methicillin-resistant Staphylococcus aureus (MRSA), Enterococcus faecium, Enterococcus faecalis and Pseudomonas aeruginosa. These active bacterial isolates were identified on the basis of 16S rRNA gene analysis. Total population of bacteria isolated from plant was high in root, following leaf and fruit. Antagonistic bacteria were also more abundant in root as compared to leaf and fruit. Two isolates (EA5 and EA18) exhibited antagonistic activity against most of the targeted pathogenic bacteria mentioned above. Some isolates showed strong inhibition for one targeted pathogenic bacterium while weak or no inhibition for others. Most of the antagonistic isolates were active against MRSA, following E. faecium, P. aeruginosa, E. coli and E. faecalis. Taken together, our results show that medicinal plants are good source of antagonistic bacteria having inhibitory effect against clinical bacterial pathogens.
Keywords: P. harmala, Antagonistic bacteria, Human pathogens, 16S rRNA, Phylogenetic analysis
1. Introduction
Medicinal plants are potential source of natural products that play an important role in preventing different human diseases. According to a survey of World Health Organization (WHO), 70–80% of the world population especially from developing countries rely on natural products of medicinal plants for their health care (Akerele, 1993). These natural products are either produced by plants or their associated microbes. Several previous studies have reported the beneficial effects of plant associated microbes. These microbes generally bacteria, are present in the phyllosphere, rhizosphere or reside inside the plant in a mutualistic relationship (Strobel et al., 2004). In this mutualism, bacteria play an important role in plant growth promotion by increasing nutrients uptake and mineral solubilization. Furthermore, this interaction helps in protecting host plant against different pathogens (El-Deeb et al., 2013).
In recent years, bioactive metabolites from medicinal plants have gained global attention. Bioactive metabolites are produced by medicinal plant or associated microbes. These bioactive metabolites are involved in symbiotic association with the host plant (Strobel, 2003). Bacteria produce bioactive metabolites exhibiting activities against phytopathogens as well as against bacteria, fungi, viruses, protozoans affecting humans and animals (Strobel et al., 2004).
The Kingdom of Saudi Arabia is so wide having more diverse flora as variation in climate and height of different area. Different medicinal plants and their extract have antibacterial, antifungal, anti-inflammatory activity and are used here by local people in kingdom in traditional medicine especially in remote area (Abulafatih, 1987, Al-Said, 1993, Ageel et al., 1986, Bokhari, 2009, Saadabi, 2006). Hermal (Peganum harmala L.) is a medicinal plant in the kingdom planta used in folk medicines due to insecticidal activity (Rharrabe et al., 2007), inhibition of reproduction (Nath et al., 1993, Adday, 1994), antimicrobial activity, and in cure of different diseases such as gastrointestinal, hypertension, cardiac, nervous system disorders, diabetes (Moloudizargari et al., 2013). The plant extract also shows in vivo and in vitro cytotoxicity in cancer cell line, rats and mouse model (Lamchouri et al., 2013).
In some previous studies in the kingdom planta many medicinal plants were used for antimicrobial studies (Alamri and Moustafa, 2012) but little is known about the distribution and isolation of bacteria from medicinal plants (El-Deeb et al., 2013). Therefore, the present study was designed to isolate and screen bacteria from P. harmala against human pathogenic bacteria. Furthermore, these potential bacteria were identified using 16S rRNA gene and phylogenetic analysis was performed.
2. Materials and methods
2.1. Plant collection and isolation of bacteria
P. harmala samples were collected in March 2014, from Taif region, Saudi Arabia. After collection, plant specimens were placed in a sterile bag and transferred to laboratory within 24 h for bacterial isolation. These plant samples were washed with sterile distilled water to remove soil and root, leaf and fruit of plant were separated. Bacteria were isolated from each part after cutting 1.0 g of tissue from each part mentioned above. These plant tissue samples were ground using sterile mortar and pestle. This homogenate was used to make serial dilutions using autoclaved distilled water and 0.1 ml aliquots were plated out on two different isolation media, 1/2 Tryptic soy agar and 1/2 R2A agar (HIMEDIA) supplemented with amphotericin B 25 μg/ml to inhibit fungal growth. These plates were then incubated at 28 °C for 1–2 weeks. Bacterial colonies were selected based on morphological features such as size, color and appearance. For pure culture, colonies were re-streaked and stocks were maintained in 15% (v/v) glycerol solution and stored at −80 °C for further use.
2.2. Screening of bacteria for antibacterial potential
Bacteria isolated from different parts of plants were screened for antibacterial activity using deferred antagonistic assay. In brief bacterial isolates were grown at 28 °C and 24 h grown culture of bacterial isolates was then overlaid with 0.1% soft agar mixed with test strains. All test strains were diluted to final concentration A600 = 0.1. Plates were again incubated at 28 °C for 24 h and the zone of inhibition was documented. The test strains of bacteria (Escherichia coli ATCC 8739, MRSA ATCC 43300, Enterococcus faecalis ATCC 29212, Enterococcus faecium ATCC 27270 and Pseudomonas aeruginosa ATCC 27853) were pregrown in LB broth at 37 °C.
2.3. DNA extraction and PCR analysis
The selected strains were subjected to extraction of genomic DNA for 16S rRNA gene analysis for identification of antagonistic bacterial strains. Genomic DNA of selected bacteria was extracted using commercial genomic DNA extraction kit (Thermo Scientific, Waltham, USA). The 16S rRNA gene was amplified from the extracted DNA using bacterial universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). Amplifications were performed with the following thermal cycle: one cycle of 95 °C for 5 min followed by 30 cycles of 94 °C for 1 min, an annealing of 58 °C for 50 s and extension at 72 °C for 1 min, with a final extension step at 72 °C for 10 min. The PCR products were separated by agarose gel electrophoresis and purified using PCR purification kit (Thermo Scientific, Waltham, USA) according to the manufacturer’s instructions and were sequenced by Macrogen (Seoul, Korea).
2.4. Phylogenetic analysis of antagonistic bacteria
For taxonomic identification of the antagonistic bacteria, the 16S rRNA gene sequences of all isolates were compared with sequences of matched type strains obtained from National Centre for Biotechnology Information (NCBI) and using the EzTaxon database (http://www.eztaxon.org/; Chun et al., 2007). The closest type species match was recorded along with the percent sequence similarity (Table 1). Multiple alignments were performed by using the CLUSTAL_X program (Thompson et al., 1997) and gaps were edited by using Bio Edit (Hall, 1999). The evolutionary distances were calculated using the Kimura two-parameter model (Kimura, 1983). The phylogenetic tree were constructed by using a neighbor-joining method (Saitou and Nei, 1987) in the MEGA4 Program (Tamura et al., 2007) with bootstrap values based on 1000 replications (Felsenstein, 1985).
Table 1.
Identification of antagonistic bacterial isolates on the basis of 16S rRNA and their antimicrobial activity against human pathogenic bacteria.
|
bAntibacterial activity against | ||||||||
|---|---|---|---|---|---|---|---|---|
| Strain lab no | Accession numbers | Closely related type strain | a% identity | E. coli | MRSA | P. faecium | P. faecalis | P. aeruginosa |
| Root | ||||||||
| EA 1 | KR812389 | Pseudomonas parafulva AJ 2129T | 98.60 | − | − | − | + | − |
| EA 2 | KR812390 | Leucobacter chromiiresistens JG 31T | 98.60 | − | − | + | + | + |
| EA 3 | KR812391 | Prolinoborus fasciculus CIP 103579T | 98.30 | − | − | − | − | + |
| EA 4 | KR812392 | Pantoea dispersa LMG 2603T | 97.90 | − | − | + | + | − |
| EA 5 | KR812393 | Erwinia toletana CECT 5263T | 98 | + | + | − | + | ++ |
| EA 6 | KR812394 | Erwinia piriflorinigrans CFBP 5888T | 98 | − | + | + | − | − |
| EA 7 | KR812395 | Carnobacterium viridans MPL-11T | 97.10 | − | − | + | − | − |
| EA 8 | KR812396 | Pseudomonas cremoricolorata NRIC 0181T | 99.30 | ++ | − | − | − | − |
| EA 9 | KR812397 | Bacillus subtilis subsp. inaquosorum KCTC 13429T | 98.90 | − | − | − | + | + |
| EA 10 | KR812398 | Bacillus endophyticus 2DTT | 98.70 | ++ | − | − | − | − |
| EA 11 | KR812399 | Bacillus methylotrophicus CBMB205T | 97.60 | − | + | − | − | − |
| EA 12 | KR812400 | Bacillus aryabhattai B8W22T | 99.70 | − | − | − | + | + |
| EA 13 | KR812401 | Staphylococcus sciuri subsp. sciuri DSM 20345T | 99.50 | − | + | − | − | − |
| EA 14 | KR812402 | Terribacillus aidingensis YI7–61T | 98.90 | − | − | + | − | − |
| EA 15 | KR812403 | Staphylococcus lentus ATCC 29070T | 99.40 | − | − | + | − | − |
| EA 16 | KR812404 | Leucobacter chromiiresistens JG 31T | 98.40 | + | ++ | − | − | ++ |
| EA 17 | KR812405 | Carnobacterium inhibens K1T | 98.60 | − | + | − | − | − |
| Leaf | ||||||||
| EA18 | KR812406 | Bacillus endophyticus 2DTT | 98.60 | ++ | + | + | + | + |
| EA 19 | KR812407 | Bacillus atrophaeus JCM 9070T | 99.80 | − | + | − | − | − |
| EA 20 | KR812408 | Pseudomonas chlororaphis subsp. piscium JF3835T | 97.80 | + | − | − | − | − |
| EA 21 | KR812409 | Bacillus aryabhattai B8W22T | 99.70 | − | − | + | − | − |
| EA 22 | KR812410 | Brevibacterium halotolerans DSM 8802T | 99.50 | − | + | + | − | − |
| Fruit | ||||||||
| EA 23 | KR812411 | Pseudomonas entomophila L48T | 99.60 | − | + | − | − | − |
| EA 24 | KR812412 | Leucobacter chromiiresistens JG 31T | 99.50 | − | ++ | − | − | − |
Based on the results of partial 16S rRNA gene sequence analysis of all strains.
The antibacterial activity was determined by in vitro Double agar overlay method. The activity was estimated after 24 h incubation at 28 °C by measuring the clear zone of bacterial inhibition: +, <3 mm; ++, between 4 and 5 mm; −, no activity.
2.5. Nucleotide sequence numbers
The nucleotide sequences obtained for 24 bacterial strains in this study have been deposited in the GenBank database under accession numbers KR812389 to KR812412.
3. Results
3.1. Isolation of antagonistic bacteria from plant
A total of 188 bacteria forming morphologically different colonies were isolated from roots, leaf and fruit of plant. All the isolated strains from different parts of the plant mentioned above were cultured on 1/2 TSA and 1/2 R2A. Bacterial count was high. In terms of plant tissue, the bacterial population was high from root of the plants (using both media) where 114 bacteria were recovered, following leaf (41) and fruit (33). Regarding antagonistic population of bacteria from these total isolates most of them were isolated from root (71%), following leaf (21%) and fruit (8%).
3.2. Screening for antibacterial activity
Double agar overlay method was used to test isolated strains against human pathogenic bacteria. Screening of 188 bacteria for their ability to inhibit human pathogenic bacterial growth, only 24 (12.7%) were found to be active. Of the 24 bacteria examined, only 1 isolate (KR812406) displayed inhibitory activity to all the pathogenic bacteria tested. Activities of rest of the isolates vary for different tested bacteria. Table 1 summarizes how each isolate inhibits the growth of bacterial pathogens tested. The antagonistic isolates EA5 and EA18 had inhibition activity against most of the target pathogens. These isolates strongly inhibited P. aeruginosa and E. coli respectively while weakly inhibited the others. From these active isolates, 11 isolates were active against MRSA, following 9 against E. faecium, 6 against E. coli, and 7 for P. aeruginosa and E. faecalis.
3.3. Identification of antagonistic bacteria on the basis of 16S rRNA gene sequence and their phylogenetic analysis
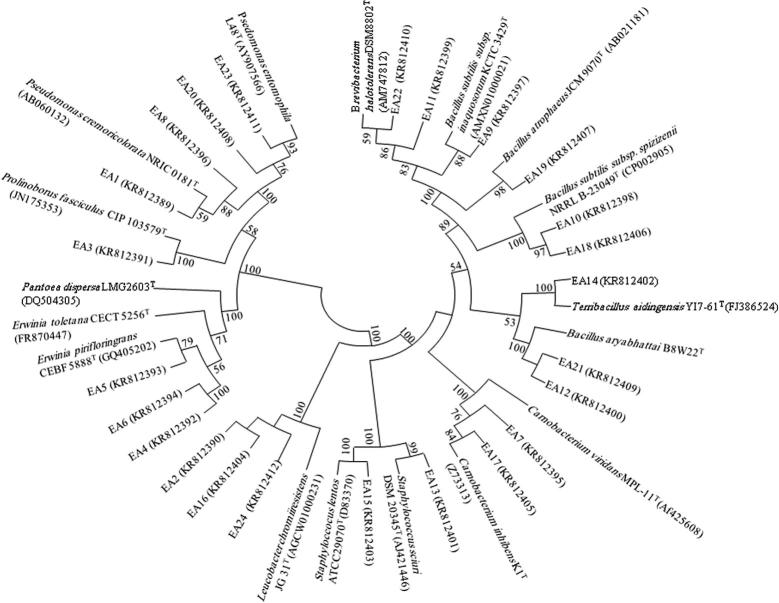
The 24 antagonistic bacteria isolated from the plant were further identified by partial 16S rRNA gene sequence analysis. Ten different genera were identified and in turn assigned to three major classes: Firmicutes (n = 13; 54%), γ-Proteobacteria (n = 8; 33%) and Actinobacteria (n = 3; 13%) (Fig. 1). A phylogenetic tree was generated from the distance data using the neighbor-joining method with the Jukes and Cantor model in a MEGA5 Program (Fig. 2). For the phylogenetic analysis on the basis of 16S rRNA gene, sequences of isolated strains along with sequences of type strains of closely related strains of different genera were included in the data set. Most of the sequenced strains have 16S rRNA gene similarities of 97–99%. Bacterial strains belonging to class Firmicutes were dominant and further related to 5 different genera i.e. Staphylococcus, Carnobacterium, Bacillus, Terribacillus and Brevibacterium. Strains EA51 and EA61 were almost similar to each other. Strains EA49 and EA58 were identical to each other with a high bootstrap value (96%) in phylogenetic analysis. The strains of class γ-Proteobacteria were placed in 4 different genera in the phylogenetic tree. The representatives of class γ-Proteobacteria were identified as Pseudomonas, Prolinoborus, Pantoea and Erwinia. All these strains of γ-Proteobacteria had high sequence similarity with already known strains (97–99%). Representatives of class Actinobacteria belonged to single genus i.e. Leucobacter.
Figure 1.
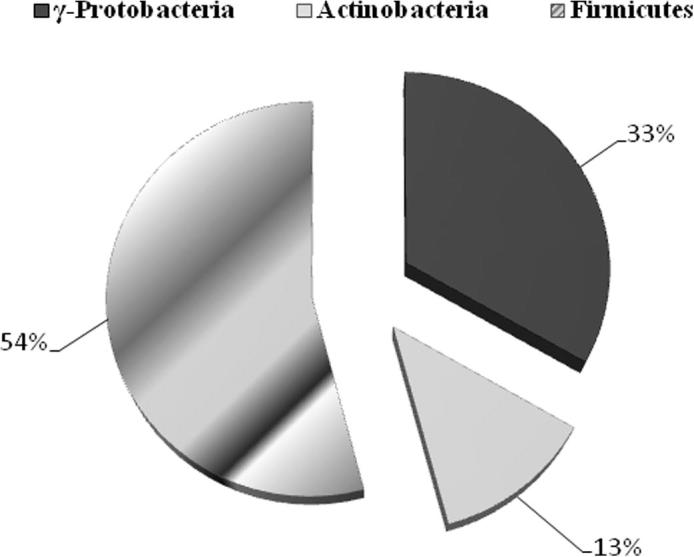
Percentage contribution of different phyla of antagonistic bacteria from total population on the basis of 16S rRNA gene sequence similarity.
Figure 2.
Phylogenetic analysis of antagonistic bacteria isolated from P. harmala on the basis of 16S rRNA gene sequences obtained from antagonistic bacteria and closely related sequences of the type strains representative of other species of genera. The phylogenetic relationships among taxa were inferred from the 16S rRNA gene by using the neighbor-joining method from distances computed with the Jukes–Cantor algorithm. Bootstrap values of >50% (1000 replicates) are shown next to the branches. GenBank accession numbers for each sequence are shown in parentheses. Bar, 0.01 accumulated changes per nucleotide.
4. Discussion
A culture-dependent method was used in the present study to isolate and investigate antagonistic bacteria from P. harmala against human pathogenic bacteria. The screening of 188 bacteria on the basis of their antagonistic potential against human pathogenic bacteria and subsequent identification on partial 16S rRNA gene sequence resulted in a diversity of different groups of bacteria comprising different genera. In the present study, it has been seen that medicinal plant harbored a large fraction of antagonistic bacteria similar as reported in previous studies (Aravind et al., 2009, Burch and Sarathchandra, 2006, Li et al., 2012, Preveena and Bhore, 2013). Our results show that bacterial population was high on TSA media as compared to R2A used in this study. To our knowledge, this is the first study in P. harmala to study bacterial diversity and their antagonistic potential against human pathogens.
P. harmala is a well-known medicinal plant used for its antifungal activities in Saudi Arabia (Saadabi, 2006). Several previous studies have shown antimicrobial activities of alkaloids derived from seeds of P. harmala (Moloudizargari et al., 2013). There is no study so far where antagonistic bacterial diversity has been analyzed from P. harmala. In this study bacterial colonization seems to be abundant in the rhizosphere of root tissues of plant as root is the primary site to gain entry into the plant tissue (Bloemberg and Lugtenberg, 2001, Lodewyckx et al., 2002). All parts of plant used in this study harbored a large fraction (n = 24) of potential bacteria active against different human pathogenic bacteria tested. Secondary metabolite production is the phenomenon used by bacteria to promote plant growth and defend against different soil pathogens (Dobbelaere et al., 2003, Compant et al., 2005, Bhore et al., 2010). In previous studies on medicinal plants it has been seen that each plant harbors a specific kind of bacterial population depending upon their alkaloids and secondary metabolites produced by associated bacteria (Qi et al., 2012).
Bacterial identification based on the 16S rRNA gene is a rapid and accurate method for identification of bacterial population from any source (Clarridge, 2004). Thus, we used this method for the identification of antagonistic bacteria isolated in our study. All 16S rRNA gene sequences obtained from antagonistic bacteria were further analyzed for their phylogenetic analysis. Using neighbor-joining phylogenetic method the tree topology grouped into three different groups: Firmicutes (Bacillus, Carnobacterium, Staphylococcus, Terribacillus, and Brevibacterium), γ-Proteobacteria (Pseudomonas, Prolinoborus, Pantoea and Erwinia) and Actinomycetes (Leucobacter) (Figure 1, Figure 2). Our results indicated that Firmicutes was the dominant group in this study based on 16S rRNA gene sequence analysis (Table 1). This is consistent with some previous studies (Tamilarasi et al., 2008) while contradictory results have been also found in some other studies, where the Actinobacteria was the dominant group in bacterial community analysis from different medicinal plants (Zhao et al., 2011). Members of Firmicutes, especially Bacillus are responsible for production of a wide range of antimicrobial metabolites, enzymes and surfactants which promote plant growth and induce systemic resistance in plants (Bibi et al., 2012). Nine isolates were identified as a member of γ-Proteobacteria including mainly Pseudomonas. The presence of Pseudomonas as a common soil inhabitant of plant, biocontrol agent and antimicrobial compound producer is already reported (Ó Sullivan and Ó Gara, 1992, Kobayashi et al., 1998). Only three actinobacterial isolates have been isolated in this study. Species of Actinobacteria have been isolated in previous studies from medicinal plants featuring antimicrobial and antitumor activities (Tamilarasi et al., 2008).
5. Conclusion
In conclusion, the present study demonstrates the phylogenetic diversity of antagonistic bacteria from medicinal plant. Our study of antagonistic bacteria of medicinal plants against human pathogens indicates that these bacteria produce some metabolites that inhibit different human pathogenic bacteria. Furthermore, it appears that medicinal plants can be an important source of antagonistic bacteria that confers many advantages to host plant. Further investigations are required to study the associated endophytic bacterial population of host plant that may be useful similar to rhizospheric bacteria.
Acknowledgements
This work was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No. (141-004-D1434). The author, therefore, acknowledges with thanks DSR technical and financial support. I am also thankful to Dr. Asif A. Jiman-Fatani from Department of Medical Microbiology and Parasitology, Faculty of Medicine, King Abdulaziz University, for providing all pathogenic bacterial type strains used in this study.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abulafatih H.A. Medicinal plants in Southwestern Saudi Arabia. Econ. Bot. 1987;41(3):354–360. [Google Scholar]
- Adday M.H. Some observations on the reproduction toxicity of the aqueous extract of Peganum harmala L. seeds. Fitoterapia. 1994;65(3):214–218. [Google Scholar]
- Ageel A.M., Parmar N.S., Mossa J.S., Al-Yahya M.A., Al-Said M.S., Tariq M. Anti-inflammatory activity of some Saudi Arabian medicinal plants. Agents Actions. 1986;17(3–4):383–384. doi: 10.1007/BF01982656. [DOI] [PubMed] [Google Scholar]
- Al-Said M.S. Traditional medicinal plants of Saudi Arabia. Am. J. Chin. Med. 1993;21(3–4):291–298. doi: 10.1142/S0192415X93000340. [DOI] [PubMed] [Google Scholar]
- Akerele O. Vol. 14. World Health Forum, WHO; Geneva: 1993. (Nature’s Medicinal Bounty: Don’t Throw it Away). pp. 390–395. [PubMed] [Google Scholar]
- Alamri S.A., Moustafa M.F. Antimicrobial property of 3 medicinal plants from Saudi Arabia against some clinical isolates of bacteria. Saudi Med. J. 2012;33(3):272–277. [PubMed] [Google Scholar]
- Aravind R., Kumar A., Eapen S.J., Ramana K.V. Endophytic bacterial flora in root and stem tissues of black pepper (Piper nigrum L.) genotype: Isolation, identification and evaluation against Phytophthora capsici. Lett. Appl. Microbiol. 2009;48(1):58–64. doi: 10.1111/j.1472-765X.2008.02486.x. [DOI] [PubMed] [Google Scholar]
- Bhore S.J., Ravichantar N., Loh C.Y. Screening of endophytic bacteria isolated from leaves of Sambung Nyawa [Gynura procumbens (Lour.) Merr.] for cytokinin-like compounds. Bioinformation. 2010;5(5):191–197. doi: 10.6026/97320630005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibi F., Yasir M., Song G., Lee S., Chung Y.R. Diversity and characterization of endophytic bacteria associated with tidal flat plants and their antagonistic effects on oomycetous plant pathogens. Plant Pathol. J. 2012;28(1):20–31. [Google Scholar]
- Bloemberg G.V., Lugtenberg B.J.J. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr. Opin. Plant Biol. 2001;4(4):343–350. doi: 10.1016/s1369-5266(00)00183-7. [DOI] [PubMed] [Google Scholar]
- Bokhari F.M. Antifungal activity of some medicinal plants used in Jeddah, Saudi Arabia. Mycopathologia. 2009;7(1):51–57. [Google Scholar]
- Burch G., Sarathchandra U. Activities and survival of endophytic bacteria in white clover (Trifolium repens L) Can. J. Microbiol. 2006;52(9):848–856. doi: 10.1139/w06-039. [DOI] [PubMed] [Google Scholar]
- Chun J., Lee J.H., Jung Y., Kim M., Kim S., Kim B.K., Lim Y.W. EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequence. Int. J. Syst. Evol. Microbial. 2007;57(10):2259–2261. doi: 10.1099/ijs.0.64915-0. [DOI] [PubMed] [Google Scholar]
- Clarridge J.E. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 2004;17(4):840–862. doi: 10.1128/CMR.17.4.840-862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compant S., Duffy B., Nowak J., Clément C., Barka E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005;71(9):4951–4959. doi: 10.1128/AEM.71.9.4951-4959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere S., Vanderleyden J., Okon Y. Plant growth-promoting effects of diazotrophs in the rhizosphere. Crit. Rev. Plant Sci. 2003;22(2):107–149. [Google Scholar]
- El-Deeb B., Khalaf F., Youssuf G. Isolation and characterization of endophytic bacteria from Plectranthus tenuiflorus medicinal plant in Saudi Arabia desert and their antimicrobial activities. J. Plant. Interact. 2013;8(1):56–64. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39(4):783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- Kimura M. Cambridge University Press; Cambridge: 1983. The Neutral Theory of Molecular Evolution. [Google Scholar]
- Kobayashi S., Hodaka S., Kawamura Y., Ozaki M., Hayase Y. Micacocidin A, B and C, novel antimycoplasma agents from Pseudomonas sp. J. Antibiotics. 1998;51(3):323–332. doi: 10.7164/antibiotics.51.323. [DOI] [PubMed] [Google Scholar]
- Lamchouri F., Zemzami M., Jossang A., Abdellatif A., Israili Z.H., Lyoussi B. Cytotoxicity of alkaloids isolated from Peganum harmala seeds. Pak. J. Pharm. Sci. 2013;26(4):699–706. [PubMed] [Google Scholar]
- Li J., Zhao G.Z., Huang H.Y., Qin S., Zhu W.Y., Zhao L.X., Xu L.H., Zhang S., Li W.J., Strobel G. Isolation and characterization of culturable endophytic Actinobacteria associated with Artemisia annua L. Antonie Van Leeuwenhoek. 2012;101(3):515–527. doi: 10.1007/s10482-011-9661-3. [DOI] [PubMed] [Google Scholar]
- Lodewyckx C., Vangronsveld J., Porteous F., Moore E.R.B., Taghavi S., Mezgeay M., van der L.D. Endophytic bacteria and their potential applications. Crit. Rev. Plant Sci. 2002;21(6):583–606. [Google Scholar]
- Moloudizargari M., Mikaili P., Aghajanshakeri S., Asghari M.H., Shayegh J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn. Rev. 2013;7(14):199–212. doi: 10.4103/0973-7847.120524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath D., Sethi N., Srivastava R., Jain A.K., Singh R.K. Studies on the teratogenic and antifertility of Peganum harmala L. Fitoterapia. 1993;64(4):312–324. [Google Scholar]
- Ó Sullivan D.G., Ó Gara F. Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol. Rev. 1992;56(4):662–676. doi: 10.1128/mr.56.4.662-676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preveena J., Bhore S.J. Identification of bacterial endophytes associated with traditional medicinal plant Tridax procumbens L. Anc. Sci. Life. 2013;32(3):173–177. doi: 10.4103/0257-7941.123002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X., Wang E., Xing M., Zhao W., Chen X. Rhizosphere and non-rhizosphere bacterial community composition of the wild medicinal plant Rumex patientia. World J. Microbiol. Biotechnol. 2012;28(5):2257–2265. doi: 10.1007/s11274-012-1033-2. [DOI] [PubMed] [Google Scholar]
- Rharrabe K., Bakrim A., Ghailani N., Sayah F. Bioinsecticidal effect of harmaline on Plodia interpunctella development (Lepidoptera Pyralidae) Pestic Biochem. Physiol. 2007;89(2):137–145. [Google Scholar]
- Saadabi A.M.A. Antifungal activity of some Saudi plants used in traditional medicine. Asian J. Plant Sci. 2006;5(5):907–909. [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Strobel G.A. Endophytes as source of bioactive products. Microb. Infect. 2003;5(6):535–544. doi: 10.1016/s1286-4579(03)00073-x. [DOI] [PubMed] [Google Scholar]
- Strobel G., Daisy B., Castillo U., Harper J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004;67(2):257–268. doi: 10.1021/np030397v. [DOI] [PubMed] [Google Scholar]
- Tamilarasi S., Nanthakumar K., Karthikeyan K., Lakshmanaperumalsamy P. Diversity of root associated microorganisms of selected medicinal plants and influence of rhizomicroorganisms on the antimicrobial property of Coriandrum sativum. J. Environ. Biol. 2008;29(1):127–134. [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24(8):1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K., Penttinen P., Guan T., Xiao J., Chen Q., Xu J., Kristina L., Lili Z., Xiaoping Z., Strobel G.A. The diversity and anti-microbial activity of endophytic actinomycetes isolated from medicinal plants in Panxi plateau, China. Curr. Microbiol. 2011;62(1):182–190. doi: 10.1007/s00284-010-9685-3. [DOI] [PubMed] [Google Scholar]