Abstract
A large-scale field survey was conducted to screen major Saudi Arabian beekeeping locations for infection by Melissococcus plutonius. M. plutonius is one of the major bacterial pathogens of honeybee broods and is the causative agent of European Foulbrood disease (EFB). Larvae from samples suspected of infection were collected from different apiaries and homogenized in phosphate buffered saline (PBS). Bacteria were isolated on MYPGP agar medium. Two bacterial isolates, ksuMP7 and ksuMP9 (16S rRNA GenBank accession numbers, KX417565 and KX417566, respectively), were subjected to molecular identification using M. plutonius -specific primers, a BLAST sequence analysis revealed that the two isolates were M. plutonius with more than 98% sequence identity. The molecular detection of M. plutonius from honeybee is the first recorded incidence of this pathogen in Saudi Arabia. This study emphasizes the need for official authorities to take immediate steps toward treating and limiting the spread of this disease throughout the country.
Keywords: Honeybee, Molecular detection, Melissococcus plutonius, Saudi Arabia
1. Introduction
Beekeeping is one of the long-standing practice in rural Saudi Arabia and is one of the most important economic activities for the communities (Al-Ghamdi and Nuru, 2013a). Approximately 5000 beekeepers maintain more than one million honeybee colonies and produce approximately 9000 metric tons of honey annually (Al-Ghamdi, 2007). Apis mellifera jemenitica is the only race of A. mellifera naturally found in the country and traditional beekeeping is mostly practiced using this race, because it is well adapted to the semi-arid to semi-desert conditions of Saudi Arabia (Al-Ghamdi and Nuru, 2013a). Indigenous honeybee colonies are too scarce with low productivity per hive, and did not fulfill the increasing demand for honey in Saudi Arabia. Consequently, significant annual losses occur during the summer season, because of short flowering season and long hot summer (Al-Ghamdi et al., 2013, Alqarni et al., 2011). To compensate these annual losses, the country annually imports around 100,000 A. m. carnica and A. m. lingustica Bee colonies from Egypt and Australia (Al-Ghamdi and Nuru, 2013b). Most of the bee packages imported from Egypt lack quality control parameters and may include disease agents and parasites (Alattal et al., 2014). Despite the great potential and multiple opportunities for beekeeping in Saudi Arabia, the bee-keeping industry is steadily growing in the country with different opportunities and, of course, many challenges. The major challenge is occurrence and distribution of honeybee disease in the country (Al-Ghamdi, 1990, Al-Ghamdi, 2010, Alattal and Al-Ghamdi, 2015). These conditions greatly affect the health, performance and productivity of imported honeybee colonies. A decline in bee populations leads to a decline in pollination, crop yield, and food supply (Potts et al., 2010). Hence, researching these factors and diseases, including potential treatments and preventative measures, is beneficial to the agricultural industry and conservation strategies in general.
In the last decades, significant losses have been observed in imported bee colonies in Saudi Arabia (Alattal and Al-Ghamdi, 2015). A mysterious decline in honeybee colonies has gained worldwide attention, including in Saudi Arabia. Much attention has been given to Colony Collapse Disorder (CCD), which is a syndrome specifically defined as a dead colony with no adult bees and with no dead bee bodies but with a live queen, and usually honey and immature bees, still presents (Evans et al., 2009). Five major abiotic and biotic factors (parasites and pests, pathogens, poor nutrition, sublethal exposure to pesticides and harsh environmental conditions) threaten honey bee health on a global scale. In reality though, these factors tend to overlap and interact with one another, which complicates issues and synergistically result in the abrupt disappearance of worker bees from the colony. Abiotic factors include environmental stresses, such as high summer and low winter temperatures, a lack of foraging capacities and the use of insecticides in agriculture (Naug, 2009, Watanabe, 2008), whereas biotic factors include a range of disease causing organisms such as bacteria, viruses, protozoa, fungi and parasitic mites. Two of the most economically important diseases of honey bees are bacterial diseases affecting the brood. American foulbrood (AFB) and European foulbrood (EFB) are both widely distributed and potentially lethal to infected colonies (Forsgren, 2010).
European foulbrood (EFB) is an economically important disease of honey bee (Apis mellifera L.) larvae caused by the anaerobic Gram-positive lanceolate bacterium Melissococcus plutonius (ex White 1912) (Aleksandrova, 1949, Bailey and Collins, 1982). EFB is well distributed across every continent that honey bees inhabit (Matheson, 1993). EFB affects mainly unsealed larvae and kills them at the age of 4–5 days and in severe cases entire colonies can be lost. The dead larvae turn yellowish, then brown, decompose, and become watery. The larval remains often give off a foul or sour smell due to secondary invaders, such as Enterococcus faecalis and Paenibacillus sp. (Arai et al., 2012).
These findings have led to a demand for research that explores the disease-causing agents of A. m. jemenitica and imported Honeybees in relation to honeybee health under the local environmental conditions in the Kingdom of Saudi Arabia. However, even though some studies have reported on disease causing organisms of honeybee in Saudi Arabia (Nixon, 1982, El-Naga, 1987, Al-Ghamdi, 1990, Matheson, 1993, Ellis and Munn, 2005, Alattal and Al-Ghamdi, 2015, Abdel-Baki et al., 2016). El-Naga (1987) reported European Foulbrood infection in two out of 40 colonies of imported honeybees from Egypt. This was the first report of EFB infection in Saudi Arabia, Later on, Al-Ghamdi, 1990, Alattal and Al-Ghamdi, 2015 also confirmed, EFB infection in Saudi Arabia. There has been very little research reported on the molecular characterization honeybee pathogens in Saudi Arabia. Therefore, detailed studies of various honeybee pathogens, including their identification and characterization using various molecular and microbiological methods, are needed. The fundamental goal of the research described herein was to characterize pathogenic agents from different geographical locations in Saudi Arabia that infect honeybees, particularly bacterial pathogens, such as M. plutonius.
2. Materials and methodology
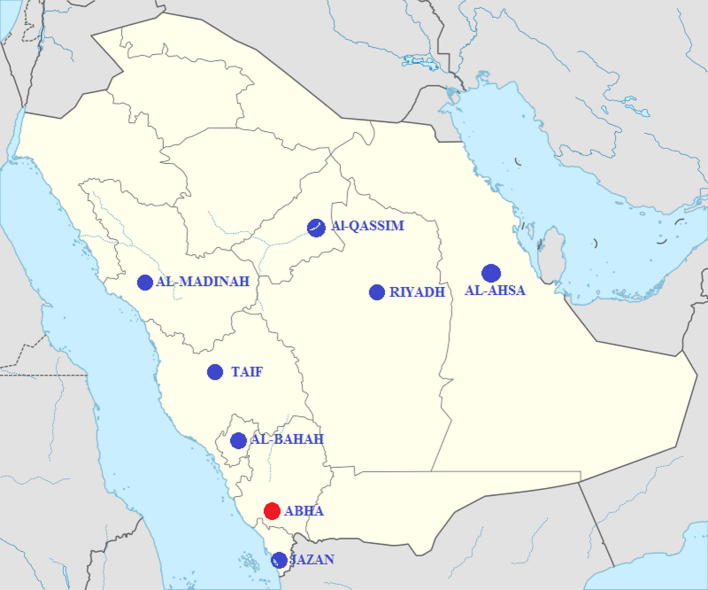
The presence of European Foulbrood (EFB) in honeybee colonies was investigated in different beekeeping locations during the spring season (March–April 2015), the active season for honeybees in Saudi Arabia. Eight different geographical localities where beekeeping is common were included in this survey (Fig. 1): Al-Ahsa (25°25′46″ N, 49°37′19″ E), Abha (18°13′24″ N, 42°30′26″ E), Jazan (16°53′21″ N, 42°33′40″ E), Taif (21°16′0″ N, 40°25′0″ E), Al-Madinah (24°28′0″ N, 39°36′0″ E), Al-Bahah (20°0′0″ N, 41°30′0″ E), Al-Qassim (25°49′19.72″ N, 42°50′6.85″ E) and Riyadh (24°43′19.2″ N 46°37′37.2″ E). At least 10 apiaries were visited in each area, and 10 colonies in each apiary were inspected.
Figure 1.
Map showing EFB inspection areas by region in Saudi Arabia. EFB has been detected only in the Abha region (red solid circle).
2.1. Sampling
A total of 800 hives in eight targeted localities (100 hives each) of A. m. jemenitica and imported bees (A. m. carnica and A. m. ligustica) were investigated in this study. Samples were collected from local (A. m. jemenitica) and imported bee races. Honeybee broods were visually inspected for any signs of abnormality and the clinical disease status. The clinical signs of AFB are very diverse and depend on the genotype involved, the stage of the disease and the strength of the bee colony (OIE, 2008). To preliminarily confirm EFB, suspect larvae were removed from the combs and tested using an EFB diagnostic field test kit (Vita, Europe) Limited, Basingstoke, UK according to the manufacturer’s instructions. EFB-suspected honeybee broods were collected for further lab examination. A piece of brood comb (10 × 10 cm) containing suspect larvae was excised, wrapped in paper towels, packaged in a plastic bag, labeled and transported to the laboratory of the Bee Research Unit (BRU) at the Department of Plant Protection of the Faculty of Food and Agriculture Sciences at King Saud University. The brood comb samples were subjected to microbiological examination and molecular analyses to test for the presence of the causative bacterium M. plutonius. Biosafety practices as recommended (De Graaf et al., 2013, OIE, 2008) were followed during the sampling period to make sure to not cause contamination between colonies during sampling.
2.2. Data collection
The data collected from the survey included the following information for each inspected apiary: the date of inspection, the apiary location (to facilitate repeat visits), the name of the owner, the hive type (local or modern), the honeybee race (indigenous or imported), the number of honeybee colonies and the number of colonies with positive visual clinical symptoms of EFB.
2.3. Bacterial strain isolation and identification
Pieces of comb approximately 10 cm2 in size were ground in phosphate-buffered saline (PBS), and different serial dilutions prepared. A total of 50 μL of each dilution was spread on KSBHI agar (15 g/L Agar (Difco), 10 g/L Soluble starch (Difco), 37 g/L Brain Heart Infusion broth (Difco) and 20.4 g/L KH2PO4) in Petri dishes. The plates were then incubated at 36 ± 0.5 °C under anaerobic conditions (10% CO2 condition) for 10 days, and the resulting bacterial colonies were subjected to microscopic and biochemical examination.
2.4. Microscopic examination of colonies
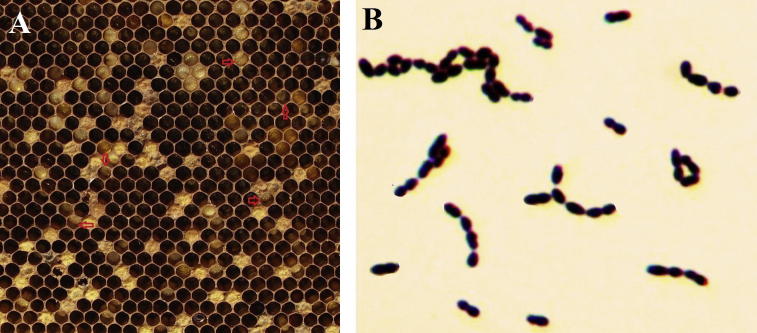
Colonies with a M. plutonius-like morphology (a small, whitish, shining and well defined appearance) were observed under microscope after gram staining. A smear of colonies were spread over the slide, pushing any excess off one end, to leave a thin smear. Smear was allowed to dry and heat fixed by flaming the slide and followed by gram staining (Holt et al., 1994), the slide was flooded with crystal violet for 60 s, washed and then flooded again with iodine solution for 1 min, decolorizing agent was used as ethanol for 5 s, the final steps involved applying safranin, after each step the slide was rinsed with water for 5 s. The prepared slide was then examined under microscope (Carl Zeiss, North Ryde, NSW, Australia) at 1000 times magnification (Fig. 2B), and photographed.
Figure 2.
(A) Irregular pattern of unsealed broods with sunken, darker cells and perforated cell caps with the foul odor (arrow) that typifies EFB disease. (B) Gram staining of Melissococcus plutonius. The coccoid-shaped bacteria forming pairs or even chains are clearly visible.
2.5. Biochemical assays
Bacterial isolates were further analyzed for gelatin and esculin hydrolysis, Glucose, Fructose, d-Mannose and l-Arabinose fermentation, β-glucosidase, β-galactosidase and catalase activity. All tests were made in triplicate.
Gelatin hydrolysis was tested on Brain heart infusion medium supplemented with potassium phosphate medium containing nutrient gelatin. A heavy inoculum of 18–24 h old bacteria was inoculated in the medium and kept at 25 °C up to one week and checking every day for gelatin liquefaction. After one week tubes kept in ice bath for 15–30 min. Hydrolysis of gelatin results in liquid medium even after the exposure of cold temperature, while the un-inoculated control remains solid (Levine and Carpenter, 1923). Esculin hydrolysis was determined by adding 0.1% (wt/vol) esculin (Merck, USA) and 0.05% (wt/vol) ferric citrate to KSBHI agar medium. The change of all or a significant portion of the medium to chocolate brown indicates breakdown of esculin to esculetin, a positive test. Negative results would be indicated by growth on the slant but no change in the color of the medium (Cowan and Steel, 1974). For salicin fermentation test, an inoculum from a pure culture is transferred aseptically to a sterile tube of phenol red salicin broth (Himedia, India), the inoculated tube is incubated at 35–37 °C for 24 h and the results are determined. A positive test consists of a color change from red to yellow, indicating a pH change to acidic (Schubert and Kexel, 1964). Strains were also tested for the fermentation of l-arabinose, d-glucose, d-mannose and d-fructose as described previously (Ventosa et al., 1982). β-galactosidase activity has been determined as per method given by Manafi et al. (1991). For the catalase activity test, part of the colony was transferred to a microscope slide and mixed with a drop of 30% H2O2. The production of bubbles indicates catalase activity, and the absence of bubbles indicates a lack of activity (Haynes, 1972).
2.6. Bacterial genomic DNA isolation
All the positively identified isolate (by cultural methods) samples were subjected to PCR analysis. Bacterial pellets were incubated in 200 μL enzyme solution (20 mg lysozyme, 20 mM Tris–HCl (pH 8.0), 2 mM EDTA, and 1.2% Triton) at 37 °C for 1 h. Then, 25 μL Proteinase K and 200 μL buffer AL (Qiagen) was added, and the lysates were incubated first at 56 °C for 30 min and then at 96 °C for 5 min. DNA was eluted with 200 μL of elution buffer and stored at −20 °C. Bacterial DNA was isolated using the QIAamp® genomic DNA isolation mini kit for gram-positive bacteria (Qiagen Inc., Valencia, CA) according to the manufacturer’s instructions. In addition, buffer controls were prepared in parallel during the DNA extraction to monitor for extraction contamination. Each DNA extract was tested for the presence of M. plutonius 16S rDNA.
2.7. PCR amplification using EFB-specific primers
One colony from each characterized bacterial isolate was subjected to molecular identification by PCR assay using the M. plutonius-specific primers (MP1, 5′ CTTTGAACGCCTTAGAGA 3′; MP2, 5′ ATCATCTGTCCCACCTTA 3′) described by Djordjevic et al. (1998). The PCR reactions were performed in a total volume of 50 μL containing 5 μL 10× PCR buffer (100 mM Tris–HCl, pH 8.3, 500 mM KCl, 4 mM MgCl2, 1% Triton X-100), 200 μM of each deoxynucleotide triphosphates, 2U Taq DNA polymerase enzyme (Promega, USA), 100 ng of each primer and 10 ng target DNA were added. The surface of the mixture was covered with 100 μL mineral oil. The following PCR conditions were used: one cycle at 95 °C (2 min), followed by 40 cycles of 95 °C (30 s), primer annealing (61 °C, 15 s), and primer extension (72 °C, 1 min) and a final extension cycle at 72 °C (1 min) ended the PCR. Samples of the amplicons were electrophoresed in 1.0% agarose gel. Approximate product size was determined using the 100-bp molecular size marker (Promega, USA). The PCR product was visualized and photographed using a Gel Doc EZ system (Bio-Rad, USA).
2.8. Hemi-nested PCR amplification
A third primer (MP3, 5′ TTAACCTCGCGGTCTTGCGTCTCTC 3′) was used in conjugation with MP1 primer to amplify a DNA fragments from 1 μL of the primary PCR product obtained in the previous reaction using primer MP1 and MP2. PCR conditions for the hemi-nested PCR are exactly as described above except that the MgCl2 concentration is lowered to 1.5 mM and the annealing temperature to 56 °C (Djordjevic et al., 1998).
2.9. 16S rRNA gene sequencing and phylogenetic analysis
The 16S rRNA gene sequencing was performed using a Genetic Analyzer DNA Sequencer (Applied Biosystems, USA) according to the manufacturer’s instructions. Sequence obtained were ‘cleaned up’ using MEGA4 software (Tamura et al., 2007). Partial 16S rRNA gene sequences of the isolates were compared with 16S rRNA gene sequences available by the BLAST search (Altschul et al., 1990), in the National Centre for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov/). Multiple sequence alignments were performed using CLUSTAL W version 1.8 (Thompson et al., 1994). The method of Jukes and Cantor (1969) was used to calculate evolutionary distances. Phylogenetic tree was constructed by the neighbor-joining method (Saitou and Nei, 1987), and the reliability of the tree topology was evaluated by bootstrap analysis using MEGA 4.1 software (Tamura et al., 2007).
3. Results
3.1. Field diagnosis tests
Infected honeybee combs were collected from the A. m. carnica colonies kept in the modern hives in the Abha region (Figure 1, Figure 2). No infection was reported in indigenous honeybee race, A. m. jemenitica. The collected infected combs had irregular capping of the brood with spotty brood pattern; capped and uncapped cells being found scattered irregularly over the brood frame. Most of the diseased larvae found to be yellow–brownish, decompose into a slimy mass and emit a sour odor before capping (Fig. 2A). Threads were slightly ropey with less than 1.5 cm long (Shimanuki, 1997). Dead and dying larvae appeared curled upwards, brown or yellow, melted, and/or dried out and rubbery. Larvae died before the cell was sealed (Bailey, 1961), but some larvae also died after the cell was sealed, resulting in sunken capping resembling the symptoms of American foulbrood (AFB). The whole comb emitted a sour smell. These field diagnostics all indicated an EFB infection in the colony.
3.2. Microscopic examination
The diagnosis in the field can be further verified by microscopic examination of brood smear preparations (Hornitzky and Smith, 1998, Hornitzky and Wilson, 1989) and gram staining of isolated colonies. M. plutonius were found to be short, lancet-shaped bacterial cells, which does not form spores. The cells occur singly, in pairs, or in chain (Fig. 2B).
3.3. Morphological, physiological, biochemical characterization
All the isolated colony of M. plutonius isolates was typical of M. plutonius: The colonies. Colonies grown on KSBHI agar. The plates were then incubated at 36 ± 0.5 °C under anaerobic conditions (10% CO2 condition) for 10 days. The colony observed were white, opaque and up to 1 mm in diameter (Arai et al., 2012). Cells were examined, and all isolates were Gram-positive cocci. Bacteria appeared as single cells or pairs and sometimes as short chains. Biochemical tests carried out on the plated bacterial isolates revealed that all were gram-positive and catalase- and β-galactosidase negative. When cultured on nutrient media, these isolates did not grow. Both isolates showed fermentation of salicin, Glucose and fructose. ksuMP7 was able to ferment d-Mannose, but ksuMP9 did not ferment d-mannose. The isolates failed to ferment l-Arabinose. Both isolates also failed to hydrolyze gelatin and esculin (Table 1). These results confirm and expand earlier reports of genotype-specific differences in the metabolism and biochemistry of M. plutonius isolates (Arai et al., 2012).
Table 1.
Phenotypic characteristics of isolated M. plutonius genotypes.
| Characteristics | Bacterial strain |
|
|---|---|---|
| ksuMP7 | ksuMP9 | |
| White colonies | + | + |
| Anaerobic | + | + |
| Gram staining | + | + |
| Motile | − | − |
| Sub culturing in nutrient broth | − | − |
| Hydrolysis of gelatin | − | − |
| Hydrolysis of esculin | − | − |
| Fermentation of salicin | − | − |
| Fermentation of glucose | + | + |
| Fermentation of fructose | + | + |
| Fermentation of d-mannose | + | − |
| Fermentation of l-arabinose | − | − |
| β-galactosidase activity | − | − |
| Catalase activity | − | − |
3.4. Molecular characterization of bacterial isolates
Amplification product from ksuMP7 and ksuMP9 using MP1 and MP2 primers was obtained, resulting in 485- and 486-bp DNA fragments, respectively corresponding to the expected size (Fig. 3), and All EFB suspected isolates were positive in PCR. The sequence of ksuMP7 (GenBank accession number: KX417565) and ksuMP9 (GenBank accession number: KX417566), resulting in 485- and 486-bp DNA fragments, respectively were deposited in NCBI GenBank (Fig. 5).
Figure 3.
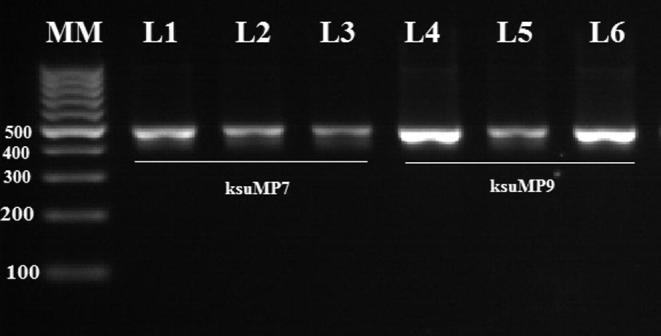
Visualization of the 16S rRNA gene PCR amplification products from the two selected bacterial isolates (ksuMP7 and ksuMP9). Lane MM: molecular size marker (100-bp ladder); Lanes 1–3: isolate ksuMP7; Lanes 4–5: isolate ksuMP9.
Figure 5.
Pairwise sequence alignment of representative 16S rRNA sequences belonging to the ksuMP7 and ksuMP9 bacterial isolates (NCBI accession no. KX417565 and KX417566, respectively). These sequences were aligned using the ClustalW pairwise alignment tool. The ranges of sequence identity and sequence similarity of the aligned sequences have been demonstrated and some mismatches are indicated by arrows.
Lowering of annealing temperature by 5 °C, MP1 and MP2 primers amplified the DNA of some related genera like Enterococcus faecalis (Djordjevic et al., 1998). In order to confirm M. plutonius amplification, a hemi-nested PCR was used with a combination of MP1 and a third primer MP3, using the DNA template from the amplified PCR product of MP1 and MP2 primers. Using hemi-nested PCR, all the isolates amplified a 276 bp M. plutonius specific product that was not amplified with E. faecalis DNA (Fig. 4). This confirmed that all the isolates belong to M. plutonius.
Figure 4.
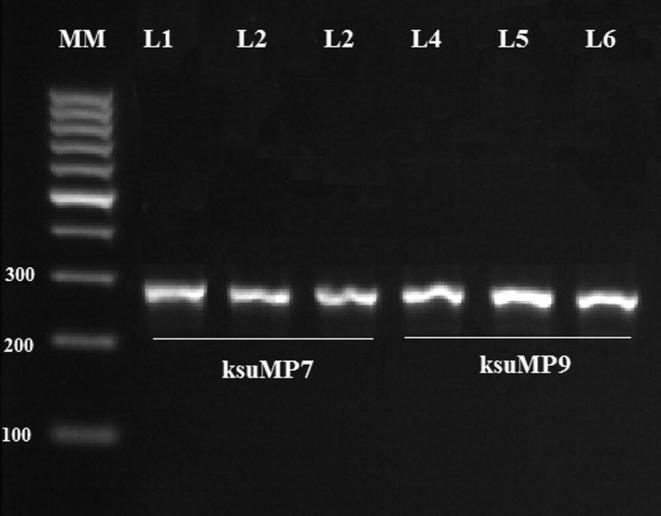
Hemi-nested PCR used with a combination MP1 and a third primer MP3, using the DNA template from the amplified PCR product of MP1 and MP2 primers. All the isolates amplified a 276 bp M. plutonius specific product that was not amplified with E. faecalis DNA.
3.5. BLAST and phylogenetic analysis
A similarity search using the Basic Local Alignment Search Tool (BLAST) (Altschul et al., 1990) showed that the ksuMP7 and ksuMP9 sequences exhibited more than 98% sequence identity, with some M. plutonius isolates (Fig. 6). The Nucleotide BLAST showed that the ksuMP7 isolate DNA sequence (485 bp) showed 98% sequence identity with the 16S rDNA of M. plutonius isolates (gb|AJ301842.1 and gb|AB614070.1). Similarly, the DNA ksuMP9 isolate sequence (486 bp) showed 99% sequence identity with the 16S rDNA of other M. plutonius isolates (gb|NR_043240.1).
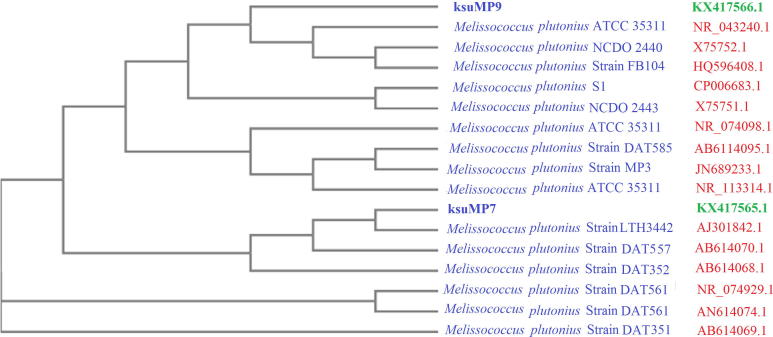
Figure 6.
Neighbor-joining phylogenetic tree of the two M. plutonius isolates (ksuMP7 and ksuMP9) based on 16S rRNA gene sequence comparisons and closest NCBI (BLASTn) strains based on the 16S rRNA gene sequences (neighbor-joining tree method). The scale bar indicates 0.0001 nucleotide substitutions per nucleotide position. The numbers at node show the bootstrap values obtained with 1000 resampling analyses.
The evolutionary relationship between the two isolates and previously reported isolates was constructed using MEGA4 software (Tamura et al., 2007). The results illustrate the degree of evolutionary relatedness between the two Saudi Arabian isolates and other previously reported isolates. From our study, the ksuMP7 and ksuMP9 formed a separate clade by itself. This indicates that the genotypes of the two isolates differ (Fig. 6).
4. Discussion
European foulbrood is the most dangerous and contagious of the infectious diseases in bees. The smell in the infected hives and the empty, shrunk, and uncapped comb cells observed in the combs with larvae have been reported to be among the specific symptoms of the disease (Forsgren et al., 2005). El-Naga (1987), reported EFB infection in Saudi Arabia for the first time in the imported bees from Egypt. Later on, Al-Ghamdi (1990) and Alattal and Al-Ghamdi (2015) also confirmed the presence of EFB infection in some apiaries in Saudi Arabia on the basis of morphological characterization. In this study, a large survey has been done and EFB caused by M. plutonius is reported in brood combs from Saudi Arabian hives. The honey comb of imported honeybee race, A. m. carnica kept in modern hives was collected from Abha regions of Saudi Arabia and found to be infected by EFB disease. Abha is a city in Aseer province of Saudi Arabia on the sloop of Sarawat Mountains, Abha has a mild summer and cold winter, and precipitation is low with more rain in spring and late autumn than in other months. The identification of this causative agent was based on symptomatology, morphological characteristics, biochemical reactions, microscopic analysis and molecular detection using PCR. Due to the low selectivity of M. plutonius on different growth media, various PCR methods have been developed to detect M. plutonius (Govan et al., 1998, Djordjevic et al., 1998). Adult bees, brood larvae and pupae are efficient testing materials because they are all susceptible to EFB (Budge et al., 2010).
PCR is a reliable, rapid and widely used method in microbiological diagnostics, and the testing of pathogen DNA is an alternative to classic cultivation tests on agar. Many types of samples can be used as a source of infectious material for EFB testing. Interpretation of positive and negative PCR results can be challenging. Similar to traditional pathogen detection techniques, PCR results must be strictly interpreted in conjunction with the history, clinical signs, and evidence of disease. A positive PCR result only indicates the detection of the target genetic sequence. It cannot differentiate between the incidental presence of an organism, colonization without disease, transient infection, or active infection with disease.
EFB already reported previously in some adjacent countries of Saudi Arabia, for instance, Iran (Ahmadi, 1984), Iraq (Bradbear, 1988), Jordan (Nixon, 1982, Bradbear, 1988), Syria (Bradbear, 1988, Matheson, 1993) and Egypt (Ali et al., 2010). We herein report for the first time the isolation and molecular characterization of M. plutonius, the causative agent of EFB in honeybees, in the kingdom of Saudi Arabia.
Fragments of the 16S rRNA gene of M. plutonius were amplified using PCR and hemi-nested PCR. Djordjevic et al. (1998) developed EFB-specific primers based on 16S rDNA that amplify a 486-bp fragment (MP1 and MP2 primers combination) and 276-bp fragment (MP1 and MP3 primers combination) of the target EFB-specific sequence. Borum et al. (2015) stated that the culture-dependent identification of M. plutonius is not a rapid confirmation method for identifying this pathogen. Therefore, the authors claim that a PCR-based method can be of greater utility to rapidly confirm the presence or absence of these bacteria.
Using the EFB primers developed by Djordjevic et al. (1998), we amplified 485- and 486-bp DNA fragments from the ksuMP7 and ksuMP9 isolates, respectively. McKee et al. (2003) succeeded in detecting M. plutonius strains by amplifying a 486-bp fragment from the 16S rRNA gene of M. plutonius, using MP1 and MP2 primers and 276-bp fragment amplified using primers MP1 and MP3 from M. plutonius crude DNA representing an important alternative for rapid EFB diagnosis. The results presented herein agree with those of McKee et al. (2003), who suggested that partial 16S rDNA PCR may be an easier method for rapidly confirming the presence of M. plutonius.
A Nucleotide BLAST search showed that the DNA sequence obtained from the ksuMP7 (485 bp) isolate showed a 98% sequence identity with the 16S rDNA of M. plutonius ATCC 35311 (gb|NR_043240.1) (Bailey and Collins, 1982). This is one of the confirmations that ksuMP7 is M. plutonius isolate and resembled M. plutonius ATCC 35311. Similarly, the DNA sequence obtained from the ksuMP9 isolate (486 bp) showed a 98% sequence identity with the 16S rDNA of M. plutonius isolate LTH 3442 (gb|AJ301842.1) and 99% sequence identity with 16S rDNA of M. plutonius strain DAT 557 (gb|AB614070.1). M. plutonius isolate LTH 3442 was first isolated in Germany (Behr et al., 2000) and M. plutonius strain DAT 557 was reported and isolated in Japan (Arai et al., 2012). This indicated that M. plutonius like isolates of Saudi Arabia are widely distributed all over the world and not restricted to this region only. Molecular diagnostic methods based on the comparative sequence analysis of the 16S rRNA gene are useful tools for the detection and identification of M. plutonius (Govan et al., 1998, Djordjevic et al., 1998). When the DNA nucleotide sequences of both the isolates in this study (ksuMP7 and ksuMP9) were compared, the resulting alignment score was approximately 98% (identities: 477/487; gaps: 3/487). For comparative analysis of the nucleotide sequences of M. plutonius genotypes of adjacent countries to Saudi Arabia, we searched the NCBI database for related sequences, but failed to find out any sequence of other isolates reported in Iran, Iraq, Syria, Jordan and Egypt.
Drought conditions, short nectar flow and long summers are major drawbacks of the Saudi Arabian beekeeping industry (Al-Ghamdi et al., 2013, Alqarni et al., 2011). As a result, Saudi Arabian beekeepers experience significant annual losses (Alattal and Al-Ghamdi, 2015). To compensate for these annual losses, beekeepers commonly import exotic honeybee packages, primarily from Egypt (Alattal et al., 2014). In 2012, approximately 200,000 package bees were introduced into the Kingdom (MoEP, 2012). These packages generally lack quality control and may be contaminated by disease-causing agents and parasites (Alattal et al., 2014). Recently EFB infection has been diagnosed in Egypt using morphological, cultural and biochemical methods (Ali et al., 2010). Egypt is the north-western border country of Saudi Arabia. Due to the fact that Saudi Arabia primarily imports bee packages from Egypt. These imported package bees may be one of the causes of EFB infection in imported bee colonies in Saudi Arabia.
Migratory beekeeping is a common practice in Saudi Arabia to avoid severe weather and food deficiency (Alqarni et al., 2011). Limited nectar sources spur many seasonal migratory beekeepers to move thousands of honeybee colonies for honey production during the flowering period of the target bee’s forage species (Adgaba et al., 2014). Moving infected or healthy colonies into close proximity to infected apiaries may also spread the disease in the Kingdom. This infection may originate from feeding on contaminated honey/or pollen and robbing diseased hives.
A phylogenetic tree was constructed based on the two obtained DNA nucleotide sequences of the 16S rRNA genes and the sequences of different Melissococcus isolates. The results presented in Fig. 6 reveal that ksuMP9 clusters into a clade with seven other bacterial isolates, whereas ksuMP7 is isolated in a separate clade with five other bacterial isolates (Fig. 6). The phylogenetic tree reflects the inferred evolutionary links between each bacterial isolate and the previously reported isolates. These results indicate that the two isolates and the other examined 12 isolates share a distant common origin. Based on all previous analyses, we assume that the two bacterial isolates are new isolates.
This is the first report of molecular detection of European foulbrood in Saudi Arabia. In this article we reported the infection in imported bee colonies from Abha region of Saudi Arabia. Further research and analysis of more colonies with and without apparent EFB symptoms are needed to determine the actual prevalence of this new agent in the country. Intensive survey and further research are thus necessary to determine the distribution and prevalence of M. plutonius in the Kingdom of Saudi Arabia and their Preventive measure. This report is an alarm for beekeeping industry of Saudi Arabia and protection from honeybee pathogens. Beekeepers must pay attention when moving their colonies in different season to void the pathogens including EFB infective agent.
5. Concluding remarks
Based on our results and the discussion presented above, the honeybee colonies infected with EFB is found only in one location (ABHA) out of eight locations surveyed for EFB infection. These test results emphasize the need to systematically monitor beekeeping locations in the Kingdom of Saudi Arabia for EFB. Such monitoring will lower the risk of the disease spreading without the need to destroy honeybee colonies in the Kingdom.
Acknowledgements
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award number (11-AGR2082-02). We are grateful to the beekeepers who allowed us to sample their hives.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Baki A.A.S., Mares M.M., Dkhil M.A., Al-Quraishy S. First detection of Nosema sp., microsporidian parasites of honeybees (Apis mellifera) in Riyadh city, Saudi Arabia. J. King Saud Univ. Sci. 2016 [Google Scholar]
- Adgaba N., Al-Ghamdi A., Shenkute A.G., Ismaiel S., Al-Kahtani S., Tadess Y., Ansari M.J., Abdulaziz M.Q.A. Socio-economic analysis of beekeeping and determinants of box hive technology adoption in the Kingdom of Saudi Arabia. J. Anim. Plant Sci. 2014;24(6):1876–1884. [Google Scholar]
- Ahmadi A.A. Incidence of honeybee (Apis mellifera) diseases and parasites in southern Iran. Bee World. 1984;65(3):134–136. [Google Scholar]
- Alattal Y., Al-Ghamdi A., Alsharhi M. Population structure of the Yemeni Honey Bee (Apis mellifera jemenitica) entails an urgent conservation strategy in Saudi Arabia. J. Entomol. 2014;11(3):163–169. [Google Scholar]
- Alattal Y., Al-Ghamdi A. Impact of temperature extremes on survival of indigenous and exotic honey bee subspecies, Apis mellifera, under desert and semiarid climates. Bull. Insectol. 2015;68(2):219–222. [Google Scholar]
- Aleksandrova L.V. Boleznipchel. Gosudarstvennue lzdatelístovo; Moscow: 1949. Growing the causative organism of European foulbrood (B. pluton) in pure culture. [Google Scholar]
- Al-Ghamdi A.A. University of Wales; Cardiff, United Kingdom: 1990. Survey of Honeybee Diseases, Pests and Predators in Saudi Arabia. MPhil Thesis. [xvii] + 127 pp. [Google Scholar]
- Al-Ghamdi, A.A., 2007. Saudi beekeeping industry. In: Fifth International Arab Apicultural Conference, November 25–28, Tripoli.
- Al-Ghamdi A.A. King Saud University, College of Agriculture, Bee Research Unit; Riyadh: 2010. Comprehensive Study for Current Beekeeping Industry of Imported and Native Honeybee in Kingdom of Saudi Arabia. [Google Scholar]
- Al-Ghamdi A., Nuru A. Beekeeping in the Kingdom of Saudi Arabia: past and present practices. Bee World. 2013;90(2):26–29. [Google Scholar]
- Al-Ghamdi A., Nuru A. Beekeeping in the Kingdom of Saudi Arabia: opportunities and challenges. Bee World. 2013;90(3):54–57. [Google Scholar]
- Al-Ghamdi A.A., Nuru A., Khanbash M.S., Smith D.R. Geographical distribution and population variation of Apis mellifera jemenitica Ruttner. J. Apicult. Res. 2013;52(3):124–133. [Google Scholar]
- Ali M.A., Olfat S.B., Al-Fattah M.A. A novel report on European foulbrood as the most recent disease in honeybee (Apis mellifera, L) colonies in Egypt; Instigating a control approach. Egypt. J. Microbiol. SI. 2010:195–209. [Google Scholar]
- Alqarni A.S., Hannan M.A., Owayss A.A., Engel M.S. The indigenous honey bees of Saudi Arabia (Hymenoptera, Apidae, Apis mellifera jemenitica Ruttner): their natural history and role in beekeeping. ZooKeys. 2011;134:83–98. doi: 10.3897/zookeys.134.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arai R., Tominaga K., Wu M., Okura M., Ito K., Okamura N., Onishi H., Osaki M., Sugimura Y., Yoshiyama M., Takamatsu D. Diversity of Melissococcus plutonius from honeybee larvae in Japan and experimental reproduction of European foulbrood with cultured atypical isolates. PLoS ONE. 2012;7(3):e33708. doi: 10.1371/journal.pone.0033708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey L. European foulbrood. Am. Bee J. 1961;101:89–92. [Google Scholar]
- Bailey L., Collins M.D. Reclassification of ‘Streptococcus pluton’ (White) in a new genus Melissococcus, as Melissococcus pluton nom. rev.; comb. nov. J. Appl. Bacteriol. 1982;53(2):215–217. [Google Scholar]
- Behr T., Koob C., Schedl M., Mehlen A., Meier H., Knopp D., Frahm E., Obst U., Schleifer K.H., Niessner R., Ludwig W. A nested array of rRNA targeted probes for the detection and identification of enterococci by reverse hybridization. Syst. Appl. Microbiol. 2000;23(4):563–572. doi: 10.1016/s0723-2020(00)80031-4. [DOI] [PubMed] [Google Scholar]
- Borum A.E., Özakin C., Güneş E., Aydin L., Ülgen M., Cakmak I. The Investigation by PCR and culture methods of foulbrood diseases in honey bees in South Marmara region. Kafkas Univ. Vet. Fak. Derg. 2015;21(1):95–99. [Google Scholar]
- Bradbear N. World distribution of major honeybee diseases and pests. Bee World. 1988;69(1):15–39. [Google Scholar]
- Budge G.E., Barrett B., Jones B., Pietravalle S., Marris G., Chantawannakul P., Thwaites R., Hall J., Cuthbertson A.G., Brown M.A. The occurrence of Melissococcus plutonius in healthy colonies of Apis mellifera and the efficacy of European foulbrood control measures. J. Invertebr. Pathol. 2010;105(2):164–170. doi: 10.1016/j.jip.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Cowan S.T., Steel K.J. Cambridge University Press; Cambridge: 1974. Manual for the Identification of Medical Bacteria. [Google Scholar]
- De Graaf D.C., Alippi A.M., Antúnez K., Aronstein K.A., Budge G., De Koker D., Genersch E. Standard methods for American foulbrood research. J. Apicult. Res. 2013;52(1):1–28. [Google Scholar]
- Djordjevic S.P., Noone K., Smith L., Hornitzky M.A. Development of a hemi-nested PCR assay for the specific detection of Melissococcus pluton. J. Apicult. Res. 1998;37(3):165–174. [Google Scholar]
- Ellis J.D., Munn P.A. The worldwide health status of honey bees. Bee World. 2005;86(4):88–101. [Google Scholar]
- El-Naga A.M.A. Diagnosis of European foulbrood (EFB) in Saudi Arabia. Arab Gulf J. Sci. Res. 1987;5(1):47–53. [Google Scholar]
- Evans J.D., Saegerman C., Mullin C., Haubruge E., Nguyen B.K., Frazier M., Pettis J.S. Colony collapse disorder: a descriptive study. PLoS ONE. 2009;4:e6481. doi: 10.1371/journal.pone.0006481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren E. European foulbrood in honey bees. J. Invertebr. Pathol. 2010;103:S5–S9. doi: 10.1016/j.jip.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Forsgren E., Lundhagen A.C., Imdorf A., Fries I. Distribution of Melissococcus plutonius in honeybee colonies with and without symptoms of European foulbrood. Microb. Ecol. 2005;50(3):369–374. doi: 10.1007/s00248-004-0188-2. [DOI] [PubMed] [Google Scholar]
- Govan V.A., Brözel V., Allsopp M.H., Davison S. A PCR detection method for rapid identification of Melissococcus pluton in honeybee larvae. Appl. Environ. Microbiol. 1998;64(5):1983–1985. doi: 10.1128/aem.64.5.1983-1985.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes W.C. Catalase test; an aid in the identification of Bacillus larvae. Am. Bee J. 1972;112:130–131. [Google Scholar]
- Holt J.G., Krieg N.R., Sneath P.H., Staley J.T., Williams S.T. ninth ed. Lippincott Williams & Wilkins; 1994. Bergey’s Manual of Determinate Bacteriology. p. 11. [Google Scholar]
- Hornitzky M.A.Z., Wilson S. A system for the diagnosis of the major bacterial brood diseases. J. Apicult. Res. 1989;28:191–195. [Google Scholar]
- Hornitzky M.A.Z., Smith L. Procedures for the culture of Melissococcus plutonfrom diseased brood and bulk honey samples. J. Apicult. Res. 1998;37:293–294. [Google Scholar]
- Jukes T.H., Cantor C.R. Evolution of protein molecules. In: Munro H.N., editor. vol. III. Academic Press; New York: 1969. pp. 21–132. (Mammalian Protein Metabolism). [Google Scholar]
- Levine M., Carpenter D.C. Gelatin liquefaction by bacteria. J. Bacteriol. 1923;8(4):297–306. doi: 10.1128/jb.8.4.297-306.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manafi M., Kneifel W., Bascomb S. Fluorogenic and chromogenic substrates used in bacterial diagnostics. Microbiol. Rev. 1991;55:335–348. doi: 10.1128/mr.55.3.335-348.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson A. World bee health report. Bee World. 1993;74(4):176–212. [Google Scholar]
- Mckee B., Djordjevic S., Goodman R., Hornitzky M. The detection of Melissococcus pluton in honey bees (Apis mellifera) and their products using a hemi-nested PCR. Apidologie. 2003;34(1):19–27. [Google Scholar]
- MoEP . Central Department of Statistics and Information, Ministry of Economy and Planning; Kingdom of Saudi Arabia: 2012. Import data of honeybees from different sources. [Google Scholar]
- Naug D. Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol. Conserv. 2009;142(10):2369–2372. [Google Scholar]
- Nixon M. Preliminary world maps of honeybee diseases and parasites. Bee World. 1982;63(1):23–41. [Google Scholar]
- OIE . vol. 1. OIE; Paris, France: 2008. European foulbrood of the honey bees; pp. 405–409. (OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Mammals, Birds and Bees), sixth ed.). (Chapter 9.3) [Google Scholar]
- Potts S.G., Biesmeijer J.C., Kremen C., Neumann P., Schweiger O., Kunin W.E. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 2010;25(6):345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schubert R.H.W., Kexel G. Der Ausfall der Butanedioldeshydrogenase-Reaktion bei einigen Pseudomonadacen und Vibrionen. Zbl. f. Bakt. 1 Orig. 1964;194:130–132. [PubMed] [Google Scholar]
- Shimanuki H. Bacteria. In: Morse R.A., Flottum K., editors. Honey Bee Pests, Predators, and Diseases. AI Root Co.; Medina, Ohio: 1997. pp. 35–54. [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24(8):1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventosa A.E., Quesada F., Rodriguez-Valera F., Ruiz-Berraquero, Ramos-Cormenzana A. Numerical taxonomy of moderately halophilic gram-negative rods. J. Gen. Microbiol. 1982;128:1959–1969. [Google Scholar]
- Watanabe M.E. Colony collapse disorder: many suspects, no smoking gun. Bioscience. 2008;58:384–388. [Google Scholar]