Abstract
The aim of this study was to quantify the contents of individual quercetin glycosides in red, yellow and chartreuse onion by High Performance Liquid Chromatography (HPLC) analysis. Acid hydrolysis of individual quercetin glycosides using 6 M hydrochloric acid guided to identify and separate quercetin 7,4′-diglucoside, quercetin 3-glucoside, quercetin 4′-glucoside, and quercetin. The contents of total quercetin glycosides varied extensively among three varieties (ranged from 16.10 to 103.93 mg/g DW). Quercetin was the predominant compound that accounted mean 32.21 mg/g DW in red onion (43.6% of the total) and 127.92 mg/g DW in chartreuse onion (78.3% of the total) followed by quercetin 3-glucoside (28.83 and 24.16 mg/g DW) respectively. Quercetin 3-glucoside levels were much higher in yellow onion (43.85 mg/g DW) followed by quercetin 30.08 mg/g DW. Quercetin 4′-glucoside documented the lowest amount that documented mean 2.4% of the total glycosides. The varied contents of glycosides present in the different onion varieties were significant.
Keywords: Allium cepa L., HPLC, Colorful onions, Quercetin glycosides
1. Introduction
Onion (Allium cepa L.) is a biennial plant belonging to the Liliaceae family, one of the most important vegetable crops with a world production of about 55 million tons (Shokoohinia et al., 2016, Griffiths et al., 2002, Teena et al., 2016, Raja et al., 2016). It is known to contain many phytochemicals such as carotenoids, copaenes, flavonoids, minerals, phenolics, phytoestrogens, terpenoids, vitamins, anthocyanins, and amino acids. Approximately 80% of the onion bulbs consist of nonstructural carbohydrates such as glucose, fructose, sucrose, and low molecular weight fructooligosaccharides (Benkeblia et al., 2007, Galdon et al., 2008). Onion is a versatile vegetable and typically used as an ingredient in many dishes besides a variety of uses in the fresh form, such as salads and in sandwiches. The bulbs are the main edible part, with a distinctive strong flavor and pungent odor. They are classified based on their color into yellow, red and white and based on their taste as sweet and non-sweet (Miean and Mohamed, 2001). White, yellow and red types of onions are reported to be rich in flavonols including mainly quercetin, kaempferol, tannins and organosulfur compounds like allyl propyl disulfide and diallyl disulfide (Miean and Mohamed, 2001). Among the flavonols, quercetin was connected with a hydroxyl and carbonyl at the carbon position of benzene ring and a double bond between 2 and 3 carbons. Quercetin aglycone, quercetin-3,4′-O-diglucoside and quercetin-4′-O-glucoside are the predominant forms of quercetin in onions (Price and Rhodes, 1996). A research conducted by Hertog et al. (1992) reported that onions ranked the highest in quercetin content of 28 vegetables and 9 fruits. Gorinstein et al., 2008 claimed that the levels of quercetin in red onions were 14-fold higher than that of garlic, whereas the levels were twofold higher than white onions. In addition, quercetin amounts in the onion peel were 48-fold more than the flesh.
In the recent years, many researchers involved in comparing the antioxidant activity of Allium species but a limited number of reports are available on the identification and quantification of some beneficial compounds, such as quercetin aglycone, quercetin-3,4′-O-diglucoside and quercetin-4′-O-glucoside (Stratil et al., 2006, Yoo et al., 2010, Ko et al., 2015). Therefore, the present paper reports the identification and quantification of quercetin glycosides from three kinds of onions (red, yellow, and chartreuse, commercial chartreuse) cultivated in Korea using HPLC technique.
2. Materials and methods
2.1. Reagents and chemicals
The HPLC-grade methanol was obtained from J. T. Baker Chemical Co. (Phillipsburg, NJ, USA). tert-Butylhydroquinone (TBHQ) was obtained from Alfa Aesar (Heysham, Lancashire, UK) and hydrochloric acid was purchased from Wako Pure Chemical Co., Industries, Ltd. (Osaka, Japan). Quercetin glycosides were provided from Extrasynthèse (Genay, France).
2.2. Plant materials
Onions of different colors (red, yellow, and chartreuse) were provided by the Biotechnology Research Institute of Chonnam National University (Gwangju, Korea). The onions were divided into two parts (outer and inner). Each part of onion samples was carefully collected and packed using aluminum foil and frozen dried at 0 °C for 6 h followed by −20 °C for 6 h and finally at −70 °C for 48 h. The frozen dried samples were powdered using pestle and mortar and used for the analysis of quercetin glycoside compounds.
2.3. Extraction of quercetin glycosides
Extraction of quercetin glycosides was performed following the method of Pérez-Gregorio et al. (2010) with slight modifications. Freeze-dried onion powder (10 mg) was mixed with 0.5 mL of methanol: formic acid: water (MFW; 50:5:45; v/v/v) stabilized with 2 g/L of tert-butylhydroquinone (TBHQ) and vortexed for 15 min. The extract solution was sonicated for 20 min at room temperature and subsequently centrifuged at 4000 rpm at 4 °C for 15 min. Additionally, the extractions were repeated four times and each extract solution was combined (approximately 2 mL). The solutions were filtered through PTFE hydrophilic syringe filter, 13 mm diameter, 0.45 μm pore size prior to injection.
2.4. Acid hydrolysis
Acid hydrolysis was carried out for the transformation of glycosides into aglycone Pérez-Gregorio et al. (2010). Commercial chartreuse onion extract (100 μL) was mixed with 100 μL 6 M hydrochloric acid (1:1; v/v) and determined whether the formation of the aglycone. Briefly, 6 M HCl (0.2 mL) was added to a TBHQ containing extracts (0.8 mL) and incubated at 70 °C for 30 min with vortexing. After that the extracts were sonicated for 5 min, and then centrifuged at 12,000 rpm for 15 min at 4 °C. The extracts passed through a 0.45 μm PTFE hydrophilic syringe filter before HPLC analysis.
2.5. Quantification of individual quercetin glycosides by HPLC analysis
The analyses of the onion extracts were performed using an Agilent 1200 HPLC system (Agilent Technologies, CA, USA). Samples were separated on a Capcell PAK C18 column (250 × 4.6 mm i.d., 5 μm particle size) maintained at 40 °C. The flow rate was 1.0 mL/min, and the injection volume was 10 μL. The mobile phase consisted of (A) water: formic acid (95:5, v/v) and (B) 100% methanol. The binary gradient used to be as follows: 0–25 min, 20–60% B; 25–25.1 min, 60–100% B; 25.1–30 min, 100–60% B; 30–30.1 min, 60–20% B; 30.1–35 min, 20% B. The absorbance was measured at 360 nm. Identification of the quercetin glucosides was based on spectra, standards, and literature data. External standards were used for identification and quantification, and results are presented as milligram per gram of dry weight (Kim et al., 2006).
3. Results and discussion
3.1. Extraction and HPLC separation of quercetin glycosides
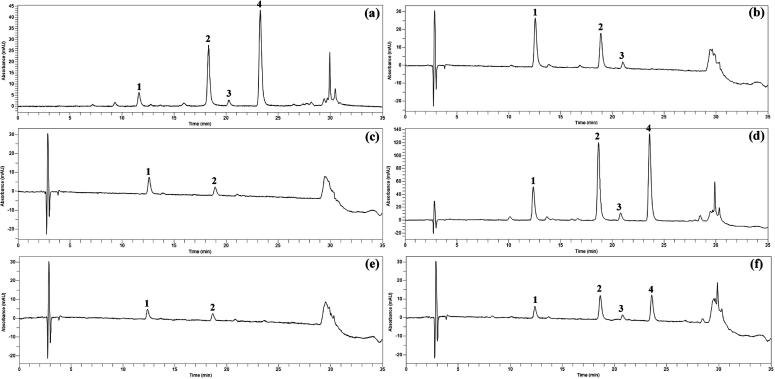
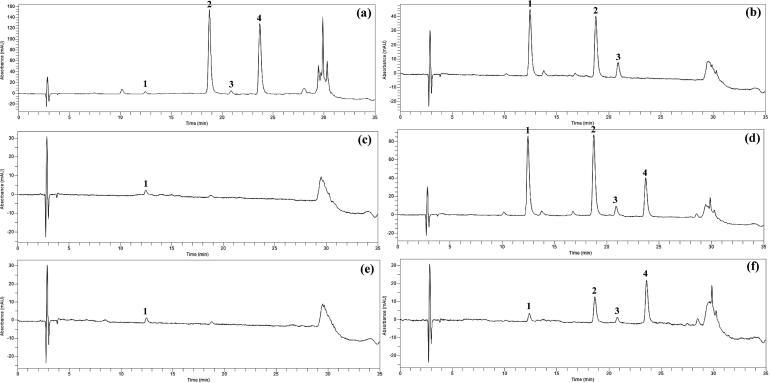
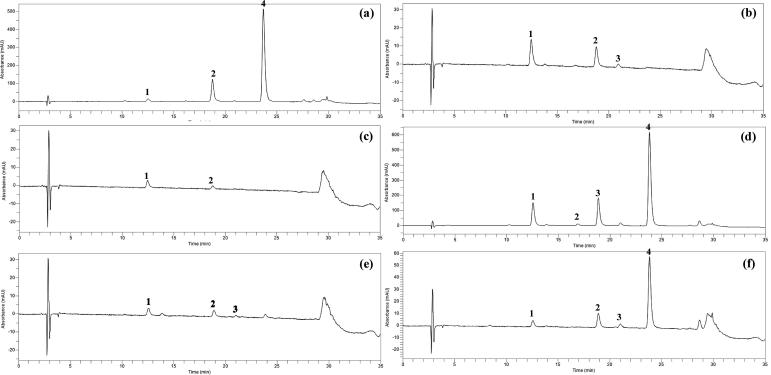
Quercetin and quercetin glycoside derivatives present in different varieties of onions were extracted using the mixture of MeOH: formic acid: water (MFW; 50:5:45; v/v/v) containing the antioxidant TBHQ (tert-butylhydroquinone, 2 g/L) (Pérez-Gregorio et al., 2010). To improve the extraction efficiency of quercetin glycosides in onions, the samples were treated with a higher concentration of hydrochloric acid (6 M). The total extraction process was repeated four times which increases the efficiency of releasing the quercetin from the lyophilized powder. HPLC analyses of quercetin glycosides were conducted by slightly modified method in buckwheat flavonoids (chlorogenic acid, orientin, isoorientin, vitexin, isovitexin, rutin, and quercetin) (Kim et al., 2006). Each quercetin glycosides were characterized based on the chromatographic retention time and UV–Vis wavelength data by comparing the previously identified quercetin glycosides (Sellappan and Akoh, 2002). The quercetin glycoside derivatives found in onions were similar which coincided with previously published data (Sellappan and Akoh, 2002). HPLC analysis guided to exhibit four peaks with different retention time. The HPLC chromatogram of red onion, yellow onion and chartreuse onion extracts recorded in 360 nm is shown in Figure 1, Figure 2, Figure 3.
Figure 1.
HPLC chromatograms of red onion detected at 360 nm. (a), skin; (b), first SL (scaly leaf); (c), third SL; (d), top; (e), bottom; (f), root. Peak: 1, quercetin 7,4′-diglucoside; 2, quercetin 3-glucoside; 3, quercetin 4′-glucoside; 4, quercetin.
Figure 2.
HPLC chromatograms of yellow onion detected at 360 nm. (a), skin; (b), first SL (scaly leaf); (c), forth SL; (d), top; (e), bottom; (f), root. Peak: 1, quercetin 7,4′-diglucoside; 2, quercetin 3-glucoside; 3, quercetin 4′-glucoside; 4, quercetin.
Figure 3.
HPLC chromatograms of chartreuse onion detected at 360 nm. (a), skin; (b), first SL (scaly leaf); (c), third SL; (d), top; (e), bottom; (f), root. Peak: 1, quercetin 7,4′-diglucoside; 2, quercetin 3-glucoside; 3, quercetin 4’-glucoside; 4, quercetin.
3.2. Quantification of quercetin glycosides
The amounts of total quercetin glycosides in outer layer of onions ranged from 16.1 mg/g dry weight (DW) (red onion) to 103.93 mg/g DW (chartreuse onion), whereas in the inner layer the highest levels of total quercetin glycosides were detected in SL 1 layer of yellow onion (1.22 mg/g DW) and the lowest in chartreuse onion (0.99 mg/g DW) (Table 1). The root of chartreuse onion documented the highest levels of total quercetin glycosides (163.3 mg/g DW) followed by the yellow onion (94.95 mg/g DW) and red onion (73.83 mg/g DW), respectively. It was clear that yellow onions contained more total quercetin than red onions, whereas, Kiviranta et al. (1988) claimed that red onions contained more total quercetin. In chartreuse onion, quercetin documented average 78.3% of total quercetin glycosides, whereas in yellow and red onion 43.6 and 31.7%. Chartreuse onion contained the highest level of quercetin (163.3 mg/g DW) while it had low contents of other onions. Quercetin 3-glucoside and quercetin were documented in an average 93%, 82.6% and 77.8% of the quercetin glycosides in chartreuse, red and yellow onions. This is consistent with the previous reports (Leighton et al., 1992). It was observed that among quercetin 3-glucoside was the major glycoside (43.85 mg/g DW) followed by quercetin (30.08 mg/g DW) and quercetin 7,4′-diglucosidein (18.28 mg/g DW) in yellow onion, whereas; in red onion, quercetin documented predominant (32.21 mg/g DW) followed by quercetin 3-glucoside (28.83 mg/g DW) and quercetin 7,4′-diglucosidein (11.1 mg/g DW), respectively. Among the glucosides, quercetin 4′-glucoside detected comparatively lower amounts in all the onions. Many reports suggest that aglycone form of onions has greater pharmacological activity than its glycosides. Therefore, onions bred for high aglycone content may have more health benefits (Patil and Pike, 1995, Patil et al., 1995).
Table 1.
Quercetin contents (mg·g−1 dry wt.) in four types of onions (n = 2).
| Cultivars | Parts | Quercetin 7,4′-diglucoside | Quercetin 3-glucoside | Quercetin 4′-glucoside | Quercetin | Total | |
|---|---|---|---|---|---|---|---|
| Red onion | Outer | First SLa | 17.31 | 7.04 | 41.39 | 0.59 | 66.33 |
| Second SL | 4.52 ± 0.48 | 1.10 ± 0.14 | 5.70 ± 0.61 | 0.03 ± 0.00 | 11.33 ± 1.20 | ||
| Third SL | 0.54 ± 0.02 | 0.10 ± 0.01 | 0.56 ± 0.04 | NDb | 1.20 ± 0.06 | ||
| Inner | 0.04 ± 0.01 | 0.01 ± 0.00 | 0.07 ± 0.01 | ND | 0.12 ± 0.03 | ||
| Yellow onion | First SL | 6.92 ± 0.95 | 0.69 ± 0.10 | 9.04 ± 1.20 | 0.07 ± 0.02 | 16.71 ± 2.27 | |
| Second SL | 0.72 ± 0.14 | 0.11 ± 0.02 | 0.75 ± 0.14 | ND | 1.58 ± 0.30 | ||
| Third SL | ND | ND | ND | ND | ND | ||
| Inner | ND | ND | ND | ND | ND | ||
| Chartreuse onion | First SL | 11.30 ± 0.75 | 5.66 ± 0.45 | 21.72 ± 1.63 | 0.35 ± 0.26 | 39.02 ± 2.57 | |
| Second SL | 1.42 ± 0.01 | 0.44 ± 0.02 | 1.73 ± 0.12 | ND | 3.60 ± 0.15 | ||
| Third SL | 0.38 ± 0.04 | 0.18 ± 0.01 | 0.69 ± 0.06 | ND | 1.25 ± 0.11 | ||
| Inner | ND | ND | ND | ND | ND | ||
| Commercial chartreuse onion | First SL | 9.29 ± 0.85 | 1.35 ± 0.12 | 20.27 ± 1.92 | 32.81 ± 1.63 | 63.71 ± 4.52 | |
| Second SL | 5.24 ± 1.58 | 0.70 ± 0.22 | 8.26 ± 2.42 | 9.88 ± 2.54 | 24.09 ± 6.75 | ||
| Third SL | 4.09 ± 0.29 | 0.76 ± 0.07 | 4.83 ± 0.34 | 0.50 ± 0.12 | 10.18 ± 0.59 | ||
| Inner | 0.44 ± 0.11 | 0.09 ± 0.03 | 0.48 ± 0.14 | 0.03 ± 0.02 | 1.04 ± 0.30 | ||
SL, scaly leaf; the number of SL 1 is the outermost part of onion skin.
ND, not detected.
4. Conclusion
Four quercetin glycosides were extracted and separated in varieties of onions using HPLC method. The variations in the composition and contents of quercetin glycosides among four different varieties were analyzed. A distinct gradient in total and individual quercetin glycoside contents were observed between the inner and outer parts of the skin. Quercetin was identified as one of the major compounds in all the varieties in chartreuse onion (127.92 mg/g DW) and red onion (32.21 mg/g DW) whereas only quercetin 3-glucoside content was higher (43.85 mg/g DW) in yellow onion. Onions contained rich antioxidants, and is an excellent choice for human daily consumption. The differences in quercetin glycoside content among different onion varieties could be useful for future breeding initiatives.
Acknowledgements
This research was supported by grants 110046-3 and PJ010269 from MAFRA (Ministry for Food, Agriculture, Forestry) and RDA (Rural Development Administration), Korea.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mariadhas Valan Arasu, Email: mvalanarasu@gmail.com.
Moo Kyoung Yoon, Email: yoonmk@rda.go.kr.
Sun-Ju Kim, Email: kimsunju@cnu.ac.kr.
References
- Benkeblia N., Shiomi N., Osaki M. Kinetics and hydrolysis parameters of total Fructo-oligosaccharides of onion bulbs: effect of temperature regimes and cultivars. J. Food Biochem. 2007;31:14–27. [Google Scholar]
- Galdon B.R., Rodriguez E.M., Romero C.D. Flavonoids in onion cultivars (Allium cepa L.) J. Food Sci. 2008;73:C599–C605. doi: 10.1111/j.1750-3841.2008.00903.x. [DOI] [PubMed] [Google Scholar]
- Gorinstein S., Leontowicz H., Leontowicz M., Namiesnik J., Najman K., Drzewiecki J., Cvikrová M., Martincová O., Katrich E., Trakhtenberg S. Comparison of the main compounds and antioxidant activities in garlic and white and red onion after treatment protocols. J. Agric. Food Chem. 2008;56(12):4418–4426. doi: 10.1021/jf800038h. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Trueman L., Crowther T., Thomas B., Smith B. Onions – a global benefit to health. Phytother. Res. 2002;16:603–615. doi: 10.1002/ptr.1222. [DOI] [PubMed] [Google Scholar]
- Hertog M.G.L., Hollman P.C.H., Venema D.P. Optimization of a quantitative HPLC determination of potentially anticarcinogenic flavonoids in vegetables and fruits. J. Agric. Food Chem. 1992;40:1591–1598. [Google Scholar]
- Kim S.J., Kawaharada C., Suzuki T., Saito K., Hashimoto N., Takigawa S. Effect of natural light periods on rutin, free amino acid and vitamin C contents in the sprouts of common (Fagopyrum esculentum M) and Tartary (F. tataricum G) buckwheats. J. Food Sci. Technol. 2006;12:199–205. [Google Scholar]
- Kiviranta J., Huovinen K., Hiltunen P. Variation of phenolic substances in onion. Acta Pharm. Fenn. 1988;91:67–72. [Google Scholar]
- Ko E.Y., Nile S.H., Sharma K., Li G.H., Park S.W. Effect of different exposed lights on quercetin and quercetin glucoside content in onion (Allium cepa L.) Saudi J. Biol. Sci. 2015;22(4):398–403. doi: 10.1016/j.sjbs.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton, T., Glinther, C., Fluss, L., Harte, W.K., Cansado, J., Notario, V., 1992. Molecular characterization of quercetin and quercetin glycosides in Allium vegetables: their effects on cell transformation. In: Huang, M.T., Lee, C.Y., Ho C.T. (eds.). p. 221–238.
- Miean K.H., Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001;49:3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- Patil B.S., Pike L.M. Distribution of potentially anticarcinogenic quercetin content in different rings of various colored onion (Allium cepa L.) cultivars. J. Am. Soc. Hortic. Sci. 1995;70(4):643–650. [Google Scholar]
- Patil B.S., Pike M.L., Kil S.Y. Variation in the quercetin content in different colored onions (Allium cepa L.) J. Am. Soc. Hortic. Sci. 1995;120(6):909–913. [Google Scholar]
- Pérez-Gregorio M., García-Falcon M.S., Simal-Gándara J. Flavonoids changes in fresh-cut onions during storage in different packaging systems. Food Chem. 2010;124:652–658. [Google Scholar]
- Price K.R., Rhodes M.J.C. Analytical problems in the study of flavonoid compounds in onions. Food Chem. 1996;57:113–117. [Google Scholar]
- Raja I., Rajendran K., Kumariah M., Rajasekaran S. Isolation and characterization of mannose-binding lectin gene from leaves of Allium ascalonicum (Shallot) and its putative role in insect resistance. South Ind. J. Biol. Sci. 2016;2(2):245–255. [Google Scholar]
- Sellappan S., Akoh C.C. Flavonoids and antioxidant capacity of Georgia grown vidalia onions. J. Agric. Food Chem. 2002;50:5338–5342. doi: 10.1021/jf020333a. [DOI] [PubMed] [Google Scholar]
- Shokoohinia Y., Rashidi M., Hosseinzadeh L., Jelodarian Z. Quercetin-3-O-β-d-glucopyranoside, a dietary flavonoid, protects PC12 cells from H2O2-induced cytotoxicity through inhibition of reactive oxygen species. Food Chem. 2016;167:162–167. doi: 10.1016/j.foodchem.2014.06.079. [DOI] [PubMed] [Google Scholar]
- Stratil P., Klejdus B., Kuban V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables – evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006;54:607–616. doi: 10.1021/jf052334j. [DOI] [PubMed] [Google Scholar]
- Teena M.T., Soumya K.R., Sudha K.S. Cytotoxic effect of sewage effluent on root tip cells of Allium cepa L. South Ind. J. Biol. Sci. 2016;2(1):1. 8–23. [Google Scholar]
- Yoo K.S., Lee E.J., Patil B.S. Quantification of quercetin glycosides in 6 onion cultivars and comparisons of hydrolysis-HPLC and spectrophotometric methods in measuring total quercetin concentrations. J. Food Sci. 2010;75(2):C160–C165. doi: 10.1111/j.1750-3841.2009.01469.x. [DOI] [PubMed] [Google Scholar]