Abstract
Diabetic retinopathy (DR) is a severe complication of diabetes and the leading cause of blindness among working adults worldwide. DR is being widely recognized as a neurodegenerative disease of the retina, since, retinal neurons are damaged soon after diabetes onset. Diabetes-induced oxidative stress is considered as central factor that dysregulates neurotrophic factors and activates apoptosis, thereby damages neurons in the diabetic retina. Flavonoids being a powerful antioxidant have been considered to protect neurons in diabetic retina. The purpose of this study was to analyze the beneficial effects of flavonoid, quercetin to protect neurons in the diabetic rat retina. We quantitated the expression levels of BDNF, NGF, TrkB, synaptophysin, Akt, Bcl-2, cytochrome c and caspase-3 using Western blotting techniques in the diabetic retina with and without quercetin treatments and compared with non-diabetic rats. In addition, we employed ELISA techniques to determine the level of BDNF. Caspase-3 activity and the level of glutathione were analyzed by biochemical methods. Our results indicate that quercetin treatment to diabetic rats caused a significant increase in the level of neurotrophic factors and inhibited the level of cytochrome c and caspase-3 activity in the diabetic retina. Furthermore, the level of an anti-apoptotic protein Bcl-2 was augmented in quercetin treated diabetic retina. Thus, quercetin, may protect the neuronal damage in diabetic retina by ameliorating the levels of neurotrophic factors and also by inhibiting the apoptosis of neurons. Therefore, this study suggests that quercetin can be a suitable therapeutic agent to prevent neurodegeneration in diabetic retinopathy.
Keywords: Diabetic retinopathy, Retina, Neurodegeneration, Oxidative stress, Apoptosis
1. Introduction
Diabetic retinopathy (DR) is one of the severe complications of diabetes and the leading cause of blindness. It is estimated that about 75% people who have diabetes for more than 20 years develop some form of retinopathy (Prevention of Blindness from Diabetes Mellitus, WHO 2005). Clinically, DR has been mainly considered as a microvascular disease; however, recently a neurodegenerative view of the disease has emerged. Numerous cellular and molecular studies of diabetic retinas suggest that neurons are vulnerable to be damaged shortly after the onset of diabetes, before vascular damage (Han et al., 2004, Bearse et al., 2004, Fletcher et al., 2007, Barber et al., 1998). In addition, retinal functional tests such as multifocal electroretinography (ERG), flash ERG, contrast sensitivity and color vision indicate early neuronal dysfunction in diabetic retina (Han et al., 2004, Bearse et al., 2004, Fletcher et al., 2007). We and others have reported that diabetes causes a chronic loss of retinal neurons by increasing the frequency of apoptosis, dysregulated levels of neurotrophic factors and increased oxidative stress which are key features of retinal neurodegeneration (Barber et al., 1998, Ola and Alhomida, 2014, Ola et al., 2013a, Ola et al., 2014).
Apoptosis is one of the major pathways that lead to cell death in diabetic retina. Diabetes induced oxidative stress may damage mitochondrial membrane that results in translocation of BAX from the cytosol to mitochondria and release the cytochrome c. This process is controlled by Bcl-2 proteins, which either inhibit or promote cell death (Kuwana et al., 2002). For example, Bcl-2 inhibits apoptosis while BAX is proapoptotic whose levels were found to be dysregulated in diabetic retina (Podestà et al., 2000). Cytochrome c released into the cytosol activates procaspase-3 and the active caspase-3 fragments the DNA (Li et al., 1997). Among caspases, caspase-3 serves as executioner caspase, which activates other caspases in diabetic retina (Barber et al., 2005, Busik et al., 2008). Thus, the ideal preventive or therapeutic approach would indeed be to target apoptosis. Recent studies suggest that flavonoids such as hesperetin, rutin and total flavonoids from Flos Puerariae inhibited the level of caspases and ameliorated apoptotic regulatory proteins in the retina of rodent model of diabetes (Kumar et al., 2013, Li et al., 2013, Ola et al., 2015). In this study, we sought to analyze expression levels of caspase-3, Bcl-2 and cytochrome c proteins in the diabetic retina after quercetin treatments.
Another key factor for neurodegeneration is the imbalance of neurotrophic factors in the diabetic retina which correlate with the pathogenesis of diabetic retinopathy (Carmeliet and Tessier-Lavigne, 2005, Suchting et al., 2006). Neurotrophic factors play important roles in the interactions between neuronal and vascular cells, and thereby regulate survival, growth, and functional maintenance of neuronal cells (Park et al., 2008). Retinal brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF) are produced by neurons and glial cells which are known for maintenance and survival of neuronal and vascular cells (von Bartheld, 1998, Seki et al., 2005, Hackam, 2008, Cohen-Cory et al., 2010, Nagahara and Tuszynski, 2011). BDNF activates several intracellular signaling pathways, including the activation of Akt through high affinity tropomyosin-related kinase B (TrkB) receptor (Patapoutian and Reichardt, 2001). Activation of protein kinase B/Akt has been demonstrated to result in inhibition of apoptotic signals and promotion of cell survival signals (Nuñez and del Peso, 1998). Downregulation in the levels of neurotrophic factors or altered signaling through BDNF-TrkB-Akt might cause serious alterations in retinal and brain function (Rohrer et al., 1999, Yoshii and Constantine-Paton, 2010, Yao et al., 2012). We and others have reported decreased levels of BDNF and NGF in the retina of diabetic animals (Ola et al., 2013b, Ola et al., 2015, Krabbe et al., 2007, Yamanaka et al., 2008). Furthermore, we also showed the increased expression of BDNF and NGF in the diabetic retina after rutin treatments (Ola et al., 2015). However, it is not clear whether quercetin (a plant based flavonoid) supplementation regulates their levels and signaling through them to protect neurons in the diabetic retina. Therefore, we investigated the effects of quercetin on neuroprotective pathway through BDNF–TrkB–Akt-synaptophysin in the diabetic rat retina.
Oxidative stress is considered as the major factor that causes neurodegeneration by dysregulating neurotrophic factors and activating apoptosis in the diabetic retina. Therapeutic approaches have shown that supplementation with antioxidants that reduce oxidative stress may play important roles in the treatment of diabetic neurological complications (Ola and Alhomida, 2014). Flavonoids are known for their strong antioxidant activities and thereby ameliorate neurodegeneration in diabetic retina (Ola et al., 2014, Sasaki et al., 2010, Gupta et al., 2011, Silva et al., 2013). However, the beneficial effects of quercetin towards oxidative stress, apoptosis and neurotrophic factors in the diabetic retina are not well characterized. Quercetin is the main representative of the flavonol subclass of the flavonoid family found in vegetables and fruits, with beneficial pharmacological effects on biological systems. In this study, we used streptozotocin-induced rats and measured the level of glutathione, neurotrophic factors and apoptotic markers in the retina with and without oral treatments of the flavonoid, quercetin.
2. Materials & methods
2.1. Animals and experimental model
Three months aged male Wistar albino rats, weighing 250–280 g were used for experiments. Single dose of streptozotocin 65 mg/kg body weight made in citrate buffer injected intraperitoneally to make rats diabetic. Diabetes was confirmed after 3 days by measuring fasting blood glucose level more than 250 mg/dl. For drug treatments, animals were divided into three groups (n = 7) as follows; (1) control (C), (2) diabetic (D), (3) diabetic treated with quercetin at a dose 50 mg/kg/day (D + Q). Quercetin dissolved in normal saline was administered orally by gavage to those rats. Vehicle and quercetin treatments started once a day, after one week of diabetes induction and continued for five consecutive weeks. All procedures including euthanasia were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, and the Ethical Guidelines of the Experimental Animal Care Center, King Saud University, Saudi Arabia.
2.2. Retina harvesting
At the end of the treatments, animals were fasted overnight and blood samples were collected though cardiac puncture under deep anesthesia. Retinas were quickly dissected, rinsed in ice-cold phosphate buffer saline to remove any blood/serum from the retina. Retinas were transferred into eppendorf tubes, flash frozen in liquid nitrogen and stored at −70 °C until assay. Blood samples were kept on ice for 30 min, centrifuged, and serum glucose levels were assayed using a commercially available kit (RANDOX Laboratories Ltd., UK).
2.3. Quantification of BDNF by enzyme-linked immunosorbent assay
Retinal homogenate was prepared by lysing each retinal tissue in the 150 μl of 10 mM HEPES lysis buffer, pH 7.4, containing 100 mM NaCl, 1% triton X-100, 0.2% SDS, and a protease inhibitor cocktail by applying short burst of ultrasonication. After homogenization, retinal lysates were centrifuged at 15,000g for 10 min and supernatants decanted and the protein concentrations estimated using the Bio-Rad protein assay kit (Bio-Rad, USA). Equal volumes of supernatant from non-diabetic, diabetic and quercetin rat retinas were used for quantitative determination of BDNF using enzyme-linked immunosorbent assay (ELISA) kits (Quantikine Human Brain Derived Neurotrophic Factor, R&D Systems, Minneapolis, MN) according to the manufacturer’s instruction. The level of BDNF was measured in 50 μl retinal homogenate. Each assays were performed in duplicate. Using the 4-parameter fit logistic (4-PL) curve equation, the actual concentration of BDNF in each sample was calculated. The detection limit of BDNF ELISA kit was 20 picograms/mL (pg/mL). The ELISA plate readings were done using Auto Bio Labtech Instruments, Co, Ltd, China.
2.4. Western blotting
To determine the BDNF protein levels in the retinas of non-diabetic and diabetic rats, retinal tissues were lysed by ultrasonication in the 10 mM HEPES lysis buffer, pH 7.4, containing 100 mM NaCl, 1 mM Na3VO4, 10 mM sodium pyrophosphate, 10 mM NaF, 2 mM EDTA, 1 mM PMSF, 1 mM benzamidine, 1% triton X-100, 0.2% SDS, and a protease inhibitor cocktail. Samples were centrifuged at 15,000g for 10 min and supernatants decanted and the protein concentrations estimated using the Bio-Rad protein assay kit (Bio-Rad, USA). Protein samples were boiled in Laemmli’s sample buffer for 5 min, and 50 μg proteins in each lane were separated on 10–12% SDS–polyacrylamide gels and transferred onto nitrocellulose membranes. After transferring proteins, the membranes were blocked for 1.5 h at room temperature with 5% non-fat milk made in Tris-buffered saline containing 0.1% Tween-20 (TBS-T). The membranes were incubated overnight with anti-BDNF and anti-TrkB (1 μg/ml, R&D system, Minneapolis, MN); anti-synaptophysin, anti-cytochrome c and anti-NGF (1 μg/ml; Abcam, Cambridge, MA); anti-Bcl-2 and anti-caspase-3 (1 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA); anti-phospho Akt (ser473) and anti-Akt (1 μg/ml; Cell Signaling, Beverly, MA) antibodies. After overnight incubation with primary antibodies, membranes were washed three times with TBS-T (5 min each) and then incubated with their respective secondary horseradish peroxidase-conjugated antibodies (1:2000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at room temperature for 1.5 h. Membranes were then washed four times with TBS-T, 5 min each, and the immunoreactivity of bands were visualized on a LI-COR C-digit blot scanner from Biosciences, Lincoln, USA, using enhanced chemiluminescence (Western blotting luminol reagents (1:1). For internal control, membranes were washed and incubated with a mouse monoclonal β-actin antibody (1:2000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and all remaining steps were followed as detailed above.
2.5. Glutathione (GSH) assay
The total GSH levels were measured in the retina of quercetin treated and non-treated diabetic and non-diabetic rats using Cayman’s GSH assay kit (Cayman Chemical Company, Ann Arbor, MI). Retinal homogenate was prepared as described above. Retinal homogenate was deproteinized by adding an equal volume of metaphosphoric acid (2.5% w/v). After 5 min, the mixture was centrifuged at 10,000 rpm and supernatant collected. In the supernatant, 5 μl of 4 M triethanolamine per 100 μl was added and assay was performed using 50 μl supernatant from the retina. A standard curve of GSH was prepared from 0 to 10 μM, and unknown concentration of GSH in the samples was calculated by using linear regression program. The level of total GSH was measured as nM/μg retinal protein.
2.6. Caspase-3 assay
The caspase-3 colorimetric assay kit (R&D Systems, Minneapolis, MN, USA) measured the increased enzymatic activity of the caspase-3 class of proteases in retinal tissue as per manufacturer’s instructions. Briefly, the enzymatic reaction for caspase activity was carried out by the addition of 250 μg protein/50 μl of rat retinal homogenate in a 96 well microplate. The cleavage of caspase-3 colorimetric substrate (DEVD-pNA) by the caspase, releases the chromophore pNA, which was quantitated spectrophotometrically at a wavelength of 405 nm using microplate reader (Auto Bio Labtech Instruments, Co, Ltd, China). The results are expressed as fold increase in caspase activity as represented by an increase in optical density in diabetic retina over control retina.
2.7. Statistical analysis
All values are expressed as the means ± standard error of mean (SEM). The Mann–Whitney test was used to compare means from two independent groups in the case of quercetin treated and untreated control and diabetic rats. Statistical Package for the Social Sciences Version 12 (SPSS 12.0) was used for the statistical analyses. P value <0.05 was considered as significant.
3. Results
3.1. Blood glucose
Blood glucose levels in the diabetic rats (428.50 ± 23.46 mg/dl) was significantly higher than in the normal rats (92.85 ± 6.5 mg/dl) (p < 0.001) at the end of 5 weeks period. However, the level was significantly lower (325.52 ± 29.82 mg/dl) in quercetin-treated rats (50 mg/kg body weight) compared to untreated diabetic rats (p < 0.05).
3.2. Effects of quercetin on retinal BDNF levels
The levels of BDNF in the retinal homogenate of quercetin treated and untreated control and diabetic rats were measured using ELISA method. The level of BDNF in the retina of diabetic rats was significantly lower as compared to corresponding non-diabetic control group (37.3 ± 3.5 vs. 21.42 ± 2.6 pg/μg protein; p < 0.01). However, quercetin treatment significantly increased the level of BDNF in the retina of diabetic rats as compared to untreated diabetic rats (31.2 ± 2.7 vs. 21.42 ± 2.6 pg/μg protein), p < 0.05) (Fig. 1).
Figure 1.
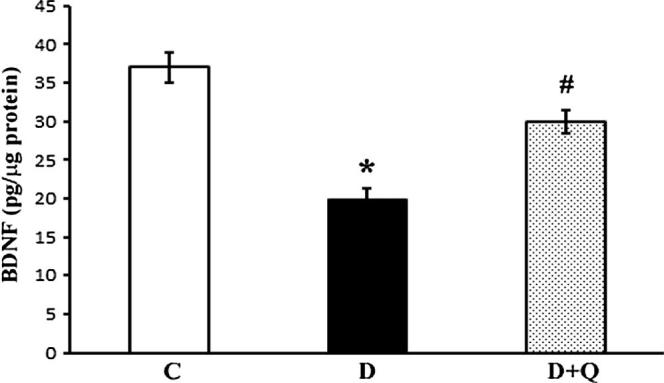
Quantification of BDNF level in the retina of quercetin treated and untreated control and diabetic rats. BDNF levels were measured by ELISA kit. Reduced levels of BDNF in diabetic groups were compared to the level from control and diabetic rats treated with quercetin. Values are means ± SEM (standard error of mean) for six determinations, ∗,#P < 0.05 compared to control and diabetic rats respectively. Experiments were repeated twice. C represents control, D as diabetic, and D + Q as quercetin treated diabetic rats.
3.3. Effects of quercetin on BDNF, NGF, synaptophysin and TrkB protein expression levels in the diabetic rat retinas
Brain derived neurotrophic factor (BDNF), NGF and TrkB and synaptophysin protein expression levels were quantified in retinas of non-diabetic control, diabetic and quercetin treated diabetic rats by Western blotting techniques (Figure 2, Figure 3). Densitometric analysis of the protein bands showed a significant reduction in the expression level of retinal BDNF in diabetic rats as compared to controls (100 ± 6.2 vs. 40 ± 4.9%; p < 0.01) (Fig. 2). However, quercetin treatment to diabetic rats caused a significant increase in the level of BDNF in the retina compared to the untreated diabetic rats (40 ± 4.9 vs. 65 ± 6.1%; p < 0.05). Similarly, the level of NGF significantly decreased in the diabetic retina as compared to the control rats (100 ± 5.0 vs. 35 ± 4.5%; p < 0.01), but the quercetin treatment improved the NGF level in the retina of diabetic rats (35 ± 4.5 vs. 60 ± 6.2%; p < 0.03). The level of TrkB, the specific receptor of BDNF was significantly low in diabetic retina compared to the non-diabetic rats (100 ± 6.4 vs. 35 ± 4.5; p < 0.01) (Fig. 2). In the quercetin treated diabetic rats retina, the level of TrkB was significantly increased as compared to the diabetic rats (35 ± 4.5 vs. 80 ± 7.2%; p < 0.05). In addition, the level of synaptophysin observed was also low compared to control (100 ± 7.4 vs. 60 ± 6.3%; p < 0.05). However, in the quercetin treated diabetic groups, the level was only slightly increased (Fig. 3).
Figure 2.
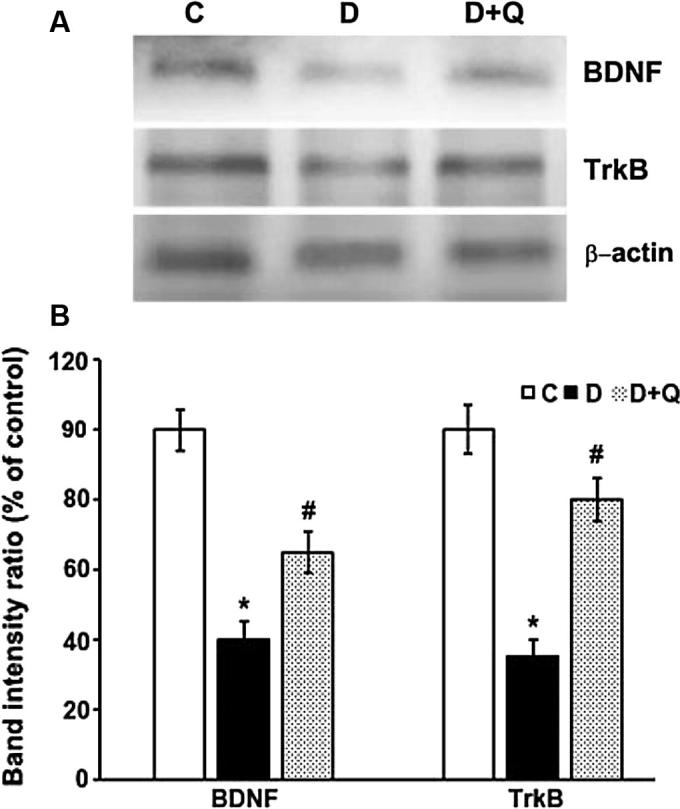
Western blot analysis of the expression of BDNF and TrkB in retinas from control, diabetic, and quercetin-treated diabetic rats. The intensities of the bands were quantified by densitometry. Panel A; Representative immunoblots of BDNF, TrkB, and β-actin bands. Panel B; Data presented as percent of control of band intensities ratios of those protein bands to β-actin. Values are means ± SEM for six determinations. ∗P < 0.01, significantly different from their controls; #P < 0.05, significantly different from diabetic. Immunoblotting experiments were repeated twice. Brain derived neurotrophic factor (BDNF).
Figure 3.
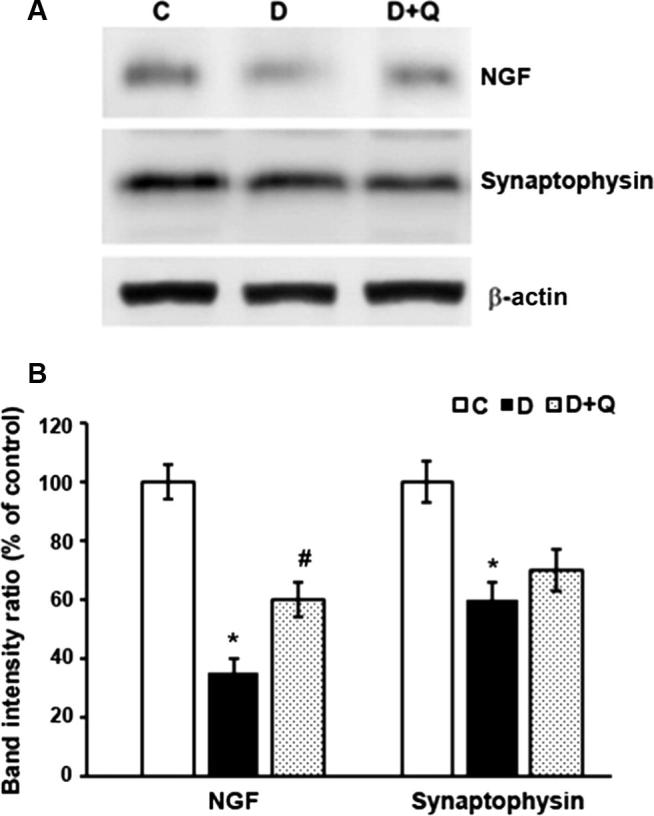
Western blot analysis of the expression of NGF and synaptophysin in retinas from control, diabetic, and quercetin-treated diabetic rats. The intensities of the bands were quantified by densitometry. Panel A; Representative immunoblots of NGF, synaptophysin and β-actin bands. Panel B; Data presented as percent of control of band intensities ratios of those protein bands to β-actin. Values are means ± SEM for six determinations. ∗,#P < 0.05, significantly different from their controls; #P < 0.03, significantly different from diabetic. Experiments were repeated twice.
3.4. Effects of quercetin on phospho-Akt protein levels in the diabetic rat retinas
Amelioration of BDNF pathway by quercetin was reflected by the activation of the Akt survival pathway. Akt protein phosphorylation was decreased to almost 62% in the diabetic retina, while quercetin augmented its level to almost normal level relative to the control group (Fig. 4).
Figure 4.
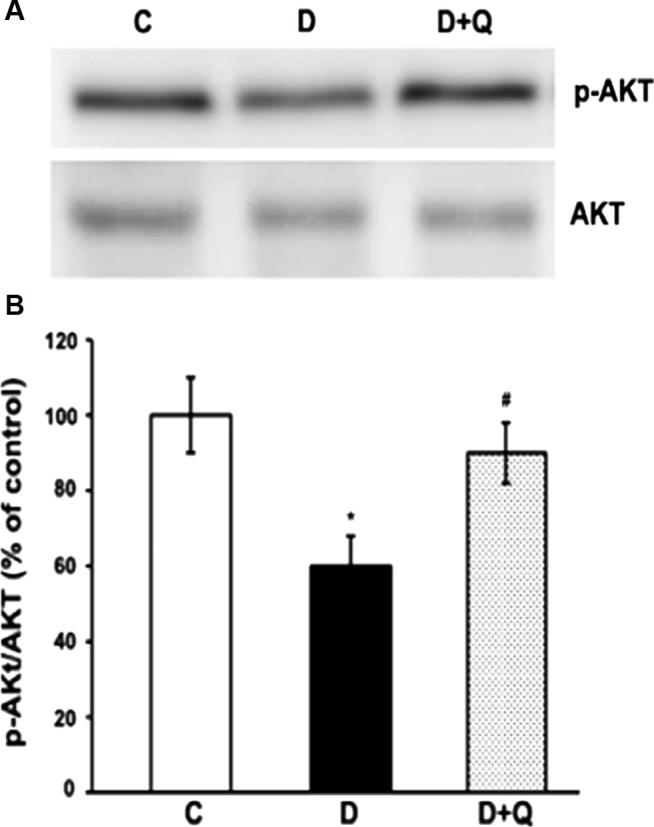
Western blot analysis of the expression of p-Akt and Akt in retinas from control, diabetic, and quercetin-treated diabetic rats. The intensities of the bands were quantified by densitometry. Panel A; Representative immunoblots of p-Akt and Akt, bands. Panel B; Data presented as percent of control of band intensities ratios of those protein bands to Akt. Values are means ± SEM for six determinations. ∗P < 0.01, significantly different from their controls; #P < 0.05, significantly different from diabetic. Experiments were repeated twice.
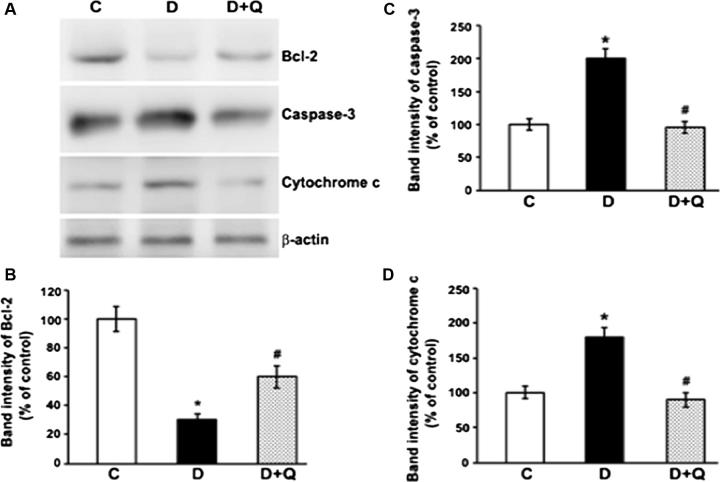
3.5. Effects of quercetin on the protein expression levels of Bcl-2, caspase-3 and cytochrome c in the diabetic rat retinas
The expression of proapoptotic proteins (cleaved caspase-3 and cytochrome c), and anti-apoptotic protein Bcl-2, were examined in the retinas of control, diabetic, and quercetin-treated diabetic rats by Western blot analysis (Fig. 5). Densitometric analyses of the bands show that expression levels of anti-apoptotic Bcl-2 reduced significantly in the diabetic retina compared to controls (100 ± 8.2 vs. 30 ± 4.3%; P < 0.01). However, the decreased level of Bcl-2 in diabetic retinas was significantly augmented when treated with quercetin (30 ± 4.3 vs. 60 ± 7.2; P < 0.05). Expression levels of proapoptotic, caspase-3 and cytochrome c increased significantly in the diabetic retinas as compared to controls (P < 0.01). However, quercetin administration to diabetic rats lowered the levels of both caspase-3 and cytochrome c in the diabetic retina to their control levels (P < 0.05) (Fig. 5).
Figure 5.
Western blot analysis of Bcl-2, caspase-3 and cytochrome c proteins in the retinas from control (C), diabetic (D), and quercetin-treated diabetic rats (D + Q). Western blot analysis was performed using antibodies against and Bcl-2 caspase-3 and cytochrome c followed by antibody against β-actin. The intensities of the bands were quantified by densitometry. Panel A; Representative immunoblots of Bcl-2, caspase-3, cytochrome c and β-actin bands. Panel B, C & D; Data presented as percent of control of band intensities ratios of those protein bands to β-actin. Values are means ± SEM for six determinations. ∗P < 0.01, significantly different from their controls (C) and #P < 0.05, significantly different from diabetic rats (D). Experiments were at-least repeated twice.
3.6. Capase-3 activity
We measured the caspase-3 activity in the retina of quercetin treated diabetic rats and compared with controls. Capsase-3 activity in the diabetic rat retinas was increased significantly in the retina of diabetic rats as compared to the control groups (p < 0.01) (Fig. 6). However, quercetin treatment to diabetic rats significantly decreased the caspase-3 activity in the retina nearly to the control level (p < 0.05).
Figure 6.
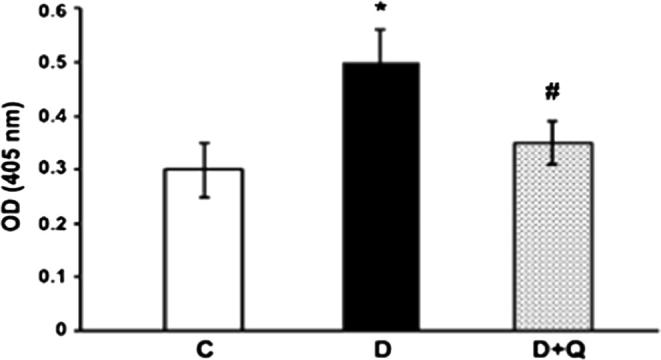
Effect of quercetin on caspase-3 activity in diabetic rat retinas. The enzymatic activity of caspase-3 in retinal tissue of control, diabetic, and quercetin-treated diabetic rats was measured by caspase-3 colorimetric assay. The results are expressed as fold increase in the caspase activity as represented by an increase in optical density. The diabetic retina had significantly higher caspase activity compared to that of controls (∗P < 0.01). Quercetin treatments to diabetic rats reduced the increased caspase activity significantly (#P < 0.05). Values are means ± SEM (n = 7). Experiments were repeated three times.
3.7. Effect of quercetin on glutathione level
Glutathione (GSH) is the endogenous antioxidant in the retina. The level of GSH was measured with and without quercetin treatment in the retina of diabetic rats as a measure of oxidative stress. The level of GSH significantly decreased (P < 0.05) in diabetic retinas as compared to controls. Quercetin treatments significantly increased the GSH level in the retina of diabetic rats as compared to non-treated diabetic rats (P < 0.05) (Fig. 7).
Figure 7.
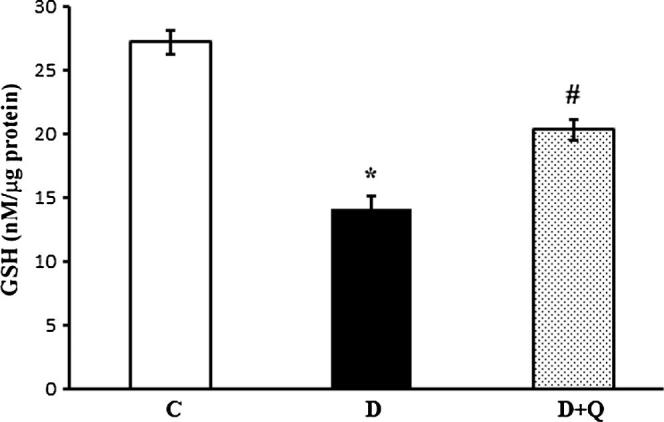
Effect of quercetin on the levels of total glutathione in the control and diabetic rats. Reduced levels of GSH in diabetic groups were compared with controls and diabetic rats treated with quercetin. The level of GSH was significantly decreased in diabetic retina compared to control rats. Quercetin treatments to diabetic rats increased GSH level significantly in the retina compared to non-treated diabetic retina. Values are means ± SEM for six determinations (∗,#P < 0.05). C represents control, D as diabetic, and D + R as diabetic rats treated with quercetin. Experiments were repeated twice.
4. Discussion
The purpose of the present study was to analyze the neuroprotective effects of the oral treatment of quercetin in the retina of diabetic rats, specifically at the level of key neurotrophic factors and apoptosis. We found ameliorative effects of quercetin by alleviating the expression levels of BDNF, NGF, TrkB receptor, synaptophysin and phosphorylation of Akt (p-Akt) in the diabetic rat retinas. In addition, quercetin treatments attenuated the increased expression levels of proapoptotic caspase-3 and cytochrome c, and increased the expression of anti-apoptotic Bcl-2 in the diabetic rat retinas. Furthermore, the decreased level of antioxidant GSH in diabetic retina was enhanced by the quercetin treatment.
Neurotrophic factors play important roles in neuronal survival and maintenance. Reduced levels of BDNF and NGF have been shown to affect systemically by impairing insulin function, and dysregulating glucose and lipid metabolism and locally causing neurodegeneration in diabetic rodents retina (Krabbe et al., 2007, Fujinami et al., 2008, Arentoft et al., 2009, Navaratna et al., 2011). Previous studies by us and others reported a significant reduction in the level of BDNF in the retina of diabetic rats compared to that of non-diabetic controls (Ola et al., 2013b, Sasaki et al., 2010). Consistent with previous studies, in this study, we found a decreased level of both BDNF and NGF in the retina of diabetic rats, which may cause neurodegeneration in the retina. Interestingly, treatments with quercetin could markedly increase their levels in diabetic retinas. Our results are in agreement with other studies that quercetin may induce synthesis and secretion of neurotrophic factors as reported in glial cells and brain (Xu et al., 2013, De Nicoló et al., 2013). More recently, we found the potential of another flavonoid rutin supplementation to enhance both the levels of BDNF and NGF in the diabetic retinas (Ola et al., 2015). Thus, increased levels of neurotrophic factors induced by quercetin may be neuroprotective in the diabetic retina.
Furthermore, our analysis of the expression of TrkB, the specific receptor of BDNF indicated a lowered expression of the receptor downstream of BDNF, which may implicate in neuronal dysfunction. Reduced levels of both BDNF and its receptor might exacerbate glutamate excitotoxicity of postsynaptic neurons by overstimulating glutamate receptor as suggested previously by others (Kohara et al., 2001, Dai et al., 2012). Consequently, excitotoxicity may decrease the level of synaptic protein (synaptophysin) as observed in the diabetic retina. Notably, quercetin treatment caused an increase in the expression level of both TrkB and synaptophysin in the diabetic retina. Previously, Al-Gayyar et al. (2011) reported the beneficial effects of another flavonoid, epicatechin towards improving expression and signaling through NGF and its receptor in protecting neurons in diabetic retina (Al-Gayyar et al., 2011). In addition, the level of p-Akt in diabetic retinas was decreased however; quercetin markedly enhanced its level. Activation of Akt has been demonstrated to result in inhibition of apoptotic signals and promotion of cell survival signals (Nuñez and del Peso, 1998). Wang et al. (2010) reported that stimulation of Akt reduced oxidative stress in diabetic retinopathy (Wang et al., 2010). The enhanced expression of p-Akt in diabetic retinas by quercetin treatment may contribute to the anti-apoptotic effects. Thus, the flavonoid, quercetin possesses neuroprotective effects in the diabetic retina by ameliorating neurotrophic factors and their downstream signaling molecules.
Apoptosis is the hallmark of neurodegeneration in diabetic retina, which is regulated by both proapoptotic and antiapoptotic molecules in diabetic retinas. Pro-apoptotic molecules including caspase-3 and cytochrome c activate other caspases in the diabetic retina to cause apoptosis while Bcl-2 protein serves as an inhibitor of apoptosis (Podestà et al., 2000, Barber et al., 2005, Busik et al., 2008). In agreement with few previous studies, we also found relatively increased expression of those pro-apoptotic proteins and lowered expression of anti-apoptotic Bcl-2 protein in the diabetic rat retina. Remarkably, quercetin ameliorated the dysregulated levels of those apoptotic regulatory proteins. Our results are well supported by other studies that flavonoids inhibited the level of caspases and ameliorated apoptotic proteins in the retina of diabetic rodents (Kumar et al., 2013, Li et al., 2013, Ola et al., 2015, Sasaki et al., 2010). More recently, Kumar et al. (2014), showed lower expression of caspase-3 in the quercetin treated diabetic rat retinas (Kumar et al., 2014). Thus, quercetin exerts potential anti-apoptotic effects via BDNF–TrkB/Akt-synaptophysin signaling pathway in the diabetic retina which may protect neuronal damage.
Flavonoids are known for their strong antioxidant activities and thereby to ameliorate neurodegeneration in diabetic retina (Ola et al., 2014, Sasaki et al., 2010, Gupta et al., 2011, Silva et al., 2013). Indeed, quercetin supplementation to diabetic rats showed significant effects towards amelioration of oxidative stress by increasing glutathione level in the diabetic retina. The antioxidant activities of quercetin is consistent with many previous studies, showing other flavonoids such as rutin, lutein, epigallocatechin gallate, and curcumin supplementation lowered oxidative stress in the diabetic rodents’ retina (Ola et al., 2015, Sasaki et al., 2010, Gupta et al., 2011, Silva et al., 2013, Muriach et al., 2006, Kowluru and Kanwar, 2007). Neuronal cell culture studies also suggest that quercetin prevented neuronal death when subjected to oxidative insult by increasing the level of glutathione (Arredondo et al., 2010). Since, oxidative stress is known to activate pro-caspases and also lower survival factor in diabetic retina (Barber et al., 1998, Mohr et al., 2002, Kowluru et al., 2004, Lopes et al., 2012). Thus, our results indicate that supplementation of quercetin may protect neurons in the diabetic retina by inhibiting oxidative stress, proapoptotic caspases and activating neurotrophic support in the diabetic retina. Therefore, quercetin can prove to be a suitable drug in neuroprotection and thereby in prevention of diabetic retinopathy early in diabetes.
Acknowledgments
The authors extend their appreciation to the funding support from King Abdulaziz City for Science and Technology; Grant number (ARP-30-23) and King Saud University for the help. Authors have no conflict of interest or commercial interest in this study.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Gayyar M.M., Matragoon S., Pillai B.A., Ali T.K., Abdelsaid M.A., El-Remessy A.B. Epicatechin blocks pro-nerve growth factor (proNGF)-mediated retinal neurodegeneration via inhibition of p75 neurotrophin receptor expression in a rat model of diabetes. Diabetologia. 2011;54:669–680. doi: 10.1007/s00125-010-1994-3. [DOI] [PubMed] [Google Scholar]
- Arentoft A., Sweat V., Starr V., Oliver S., Hassenstab J., Bruehl H., Tirsi A., Javier E., McHugh P.F., Convit A. Plasma BDNF is reduced among middle-aged and elderly women with impaired insulin function: evidence of a compensatory mechanism. Brain Cognit. 2009;71:147–152. doi: 10.1016/j.bandc.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo F., Echeverry C., Abin-Carriquirym J.A., Blasina F., Antúnez K., Jones D.P., Go Y.M., Liang Y.L., Dajas F. After cellular internalization, quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Radical Biol. Med. 2010;49:738–747. doi: 10.1016/j.freeradbiomed.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Barber A.J., Lieth E., Khin S.A., Antonetti D.A., Buchanan A.G., Gardner T.W. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J. Clin. Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A.J., Antonetti D.A., Kern T.S., Reiter C.E., Soans R.S., Kradym J.K., Levison S.W., Gardner T.W., Bronson S.K. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest. Ophthalmol. Vis. Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- Bearse M.A., Jr., Ying H., Marilyn E.S., Barez S., Carl J., Anthony J.A. Local multifocal oscillatory potential abnormalities in diabetes and early diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2004;45:3259–3265. doi: 10.1167/iovs.04-0308. [DOI] [PubMed] [Google Scholar]
- Busik J.V., Mohr S., Grant M.B. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008;57:1952–1965. doi: 10.2337/db07-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P., Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S., Kidane A.H., Shirkey N.J., Marshak S. Brain derived neurotrophic factor and the development of structural neuronal connectivity. Dev. Neurobiol. 2010;70:271–288. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M., Xia X.B., Xiong S.Q. BDNF regulates GLAST and glutamine synthetase in mouse retinal Müller cells. J. Cell. Physiol. 2012;227:596–603. doi: 10.1002/jcp.22762. [DOI] [PubMed] [Google Scholar]
- De Nicoló S., Tarani L., Ceccanti M. Effects of olive polyphenols administration on nerve growth factor and brain derived neurotrophic factor in the mouse brain. Nutrition. 2013;29:681–687. doi: 10.1016/j.nut.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Fletcher E.L., Phipps J.A., Ward M.M., Puthussery T., Wilkinson-Berka J.L. Neuronal and glial cell abnormality as predictors of progression of diabetic retinopathy. Curr. Pharm. Des. 2007;13:2699–2712. doi: 10.2174/138161207781662920. [DOI] [PubMed] [Google Scholar]
- Fujinami A., Ohta K., Obayashi H., Fukui M., Hasegawa G., Nakamura N., Kozai H., Imai S., Ohta M. Serum brain derived neurotrophic factor in patients with type 2 diabetes mellitus: relationship to glucose metabolism and biomarkers of insulin resistance. Clin. Biochem. 2008;41:812–817. doi: 10.1016/j.clinbiochem.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Gupta S.K., Kumar B., Nag T.C., Agrawal S.S., Agrawal R., Agrawa P., Saxena R., Srivastava S. Prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J. Ocul. Pharmacol. Ther. 2011;27:123–130. doi: 10.1089/jop.2010.0123. [DOI] [PubMed] [Google Scholar]
- Hackam A.S. Regulation of neurotrophin expression and activity in the retina. Adv. Exp. Med. Biol. 2008;613:343–349. doi: 10.1007/978-0-387-74904-4_40. [DOI] [PubMed] [Google Scholar]
- Han Y., Adams A.J., Bearse M.A., Jr., Schneck M.E. Multifocal electroretinogram and short-wavelength automated perimetry measures in diabetic eyes with little or no retinopathy. Arch. Ophthalmol. 2004;122:1809–1815. doi: 10.1001/archopht.122.12.1809. [DOI] [PubMed] [Google Scholar]
- Kohara K., Kitamura A., Morishima M., Tsumoto T. Activity-dependent transfer of brain derived neurotrophic factor to postsynaptic neurons. Science. 2001;291:2419–2423. doi: 10.1126/science.1057415. [DOI] [PubMed] [Google Scholar]
- Kowluru R.A., Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr. Metab. (Lond) 2007;16:4–8. doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru R.A., Chakrabarti S., Chen S. Re-institution of good metabolic control in diabetic rats and activation of caspase-3 and nuclear transcriptional factor (NF-kappaB) in the retina. Acta Diabetol. 2004;41:194–199. doi: 10.1007/s00592-004-0165-8. [DOI] [PubMed] [Google Scholar]
- Krabbe K.S., Nielsen A.R., Krogh-Madsen R., Plomgaard P., Rasmussen P., Erikstrup C., Fischer C.P., Lindegaard B., Petersen A.M., Taudorf S., Secher N.H., Pilegaard H., Bruunsgaard H., Pedersen B.K. Brain derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50:431–438. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- Kumar B., Gupta S.K., Srinivasan B.P., Nag T.C., Srivastava S., Saxena R., Jha K.A. Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats. Microvasc. Res. 2013;87:65–74. doi: 10.1016/j.mvr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Kumar B., Gupta S.K., Nag T.C., Srivastava S., Saxena R., Jha K.A., Srinivasan B.P. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp. Eye Res. 2014;125:193–202. doi: 10.1016/j.exer.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Kuwana T., Mackey M.R., Perkins G., Ellisman M.H., Latterich M., Schneiter R., Green D.R., Newmeyer D.D. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Li P., Nijhawan D., Budihardjo I., Srinivasula S.M., Ahmad M., Alnemri E.S., Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Li D., Yang F., Cheng H., Liu C., Sun M., Wu K., Ai M. Protective effects of total flavonoids from Flos Puerariae on retinal neuronal damage in diabetic mice. Mol. Vision. 2013;19:1999–2010. [PMC free article] [PubMed] [Google Scholar]
- Lopes C.R., Ferreira P.E., Zanoni J.N., Alves A.M., Alves E.P., Buttow N.C. Neuroprotective effect of quercetin on the duodenum enteric nervous system of streptozotocin-induced diabetic rats. Dig. Dis. Sci. 2012;57:3106–3115. doi: 10.1007/s10620-012-2300-7. [DOI] [PubMed] [Google Scholar]
- Mohr S., Xi X., Tang J., Kern T.S. Caspase activation in retinas of diabetic and galactosemic mice and diabetic patients. Diabetes. 2002;51:1172–1179. doi: 10.2337/diabetes.51.4.1172. [DOI] [PubMed] [Google Scholar]
- Muriach M., Bosch-Morell F., Alexander G., Blomhoff R., Barcia J., Arnal E., Almansa I., Romero F.J., Miranda M. Lutein effect on retina and hippocampus of diabetic mice. Free Radical Biol. Med. 2006;41:979–984. doi: 10.1016/j.freeradbiomed.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Nagahara A.H., Tuszynski M.H. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2011;10:209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- Navaratna D., Guo S.Z., Hayakawa K., Wang X., Gerhardinger C., Lo E.H. Decreased cerebrovascular brain derived neurotrophic factor-mediated neuroprotection in the diabetic brain. Diabetes. 2011;60:1789–1796. doi: 10.2337/db10-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez G., del Peso L. Linking extracellular survival signals and the apoptotic machinery. Curr. Opin. Neurobiol. 1998;8:613–618. doi: 10.1016/s0959-4388(98)80089-5. [DOI] [PubMed] [Google Scholar]
- Ola M.S., Alhomida A.S. Neurodegeneration in diabetic retina and its potential drug targets. Curr. Neuropharmacol. 2014;12:380–386. doi: 10.2174/1570159X12666140619205024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ola M.S., Nawaz M.I., Khan H.A., Alhomida A.S. Neurodegeneration and neuroprotection in diabetic retinopathy. Int. J. Mol. Sci. 2013;14:2559–2572. doi: 10.3390/ijms14022559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ola M.S., Nawaz M.I., El-Asrar A.A., Abouammoh M., Alhomida A.S. Reduced levels of brain derived neurotrophic factor (BDNF) in the serum of diabetic retinopathy patients and in the retina of diabetic rats. Cell. Mol. Neurobiol. 2013;33:359–367. doi: 10.1007/s10571-012-9901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ola M.S., Aleisa A.M., Al-Rejaie S.S., Abuohashish H.M., Parmar M.Y., Alhomida A.S., Ahmed M.M. Flavonoid, morin inhibits oxidative stress, inflammation and enhances neurotrophic support in the brain of streptozotocin-induced diabetic rats. Neurol. Sci. 2014;35:1003–1008. doi: 10.1007/s10072-014-1628-5. [DOI] [PubMed] [Google Scholar]
- Ola M.S., Ahmed M.M., Ahmad R., Abuohashish H.M., Al-Rejaie S.S., Alhomida A.S. Neuroprotective effects of rutin in streptozotocin-induced diabetic rat retina. J. Mol. Neurosci. 2015;56:440–448. doi: 10.1007/s12031-015-0561-2. [DOI] [PubMed] [Google Scholar]
- Park K.S., Kim S.S., Kim J.C., Kim H.C., Im Y.S., Ahn C.W., Lee H.K. Serum and tear levels of nerve growth factor in diabetic retinopathy patients. Am. J. Ophthalmol. 2008;145:432–437. doi: 10.1016/j.ajo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Patapoutian A., Reichardt L.F. Trk receptors: mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Podestà F., Romeo G., Liu W.H., Krajewski S., Reed J.C., Gerhardinger C., Lorenzi M. Bax is increased in the retina of diabetic subjects and is associated with pericyte apoptosis in vivo and in vitro. Am. J. Pathol. 2000;156:1025–1032. doi: 10.1016/S0002-9440(10)64970-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B., Korenbrot J.I., LaVail M.M., Reichardt L., Xu B. Role of neurotrophin receptor TrkB in the maturation of rod photoreceptors and establishment of synaptic transmission to the inner retina. J. Neurosci. 1999;19:8919–893002. doi: 10.1523/JNEUROSCI.19-20-08919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Ozawa Y., Kurihara T., Kubota S., Yuki K., Noda K., Kobayashi S., Ishida S., Tsubota K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia. 2010;53:971–979. doi: 10.1007/s00125-009-1655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M., Tanaka T., Sakai Y., Fukuchi T., Abe H., Nawa H., Takei N. Muller cells as a source of brain derived neurotrophic factor in the retina: noradrenaline upregulates brain derived neurotrophic factor levels in cultured rat Müller cells. Neurochem. Res. 2005;30:1163–1170. doi: 10.1007/s11064-005-7936-7. [DOI] [PubMed] [Google Scholar]
- Silva K.C., Rosales M.A., Hamassaki D.E., Saito K.C., Faria A.M., Ribeiro P.A., Faria J.B., Faria J.M. Green tea is neuroprotective in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2013;54:1325–1336. doi: 10.1167/iovs.12-10647. [DOI] [PubMed] [Google Scholar]
- Suchting S., Bicknell R., Eichmann A. Neuronal clues to vascular guidance. Exp. Cell Res. 2006;312:668–675. doi: 10.1016/j.yexcr.2005.11.009. [DOI] [PubMed] [Google Scholar]
- von Bartheld C.S. Neurotrophins in the developing and regenerating visual system. Histol. Histopathol. 1998;13:437–459. doi: 10.14670/HH-13.437. [DOI] [PubMed] [Google Scholar]
- Wang Q., Pfister F., Dorn-Beineke A., vom Hagen F., Lin J., Feng Y., Hammes H.P. Low-dose erythropoietin inhibits oxidative stress and early vascular changes in the experimental diabetic retina. Diabetologia. 2010;53:1227–1238. doi: 10.1007/s00125-010-1727-7. [DOI] [PubMed] [Google Scholar]
- Xu S.L., Bi C.W., Choi R.C. Flavonoids induce the synthesis and secretion of neurotrophic factors in cultured rat astrocytes: a signaling response mediated by estrogen receptor. Evid. Based Complement Alternat. Med. 2013;2013:127075. doi: 10.1155/2013/127075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka M., Itakura Y., Ono-Kishino M., Tsuchida A., Nakagawa T., Taiji M. Intermittent administration of brain derived neurotrophic factor (BDNF) ameliorates glucose metabolism and prevents pancreatic exhaustion in diabetic mice. J. Biosci. Bioeng. 2008;105:395–402. doi: 10.1263/jbb.105.395. [DOI] [PubMed] [Google Scholar]
- Yao R.Q., Qi D.S., Yu H.L., Liu J., Yang L.H., Wu X.X. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem. Res. 2012;37:2777–2786. doi: 10.1007/s11064-012-0871-5. [DOI] [PubMed] [Google Scholar]
- Yoshii A., Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev. Neurobiol. 2010;70:304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]