Abstract
Schistosomiasis is still one of the main parasitic diseases that affect human health in tropical regions. Whilst praziquantel (PZQ) is the main classic antischistosomal drug, the need for new drugs is still a must due to the low effectiveness of the drug on the schistosome young worms, and the evolving of PZQ resistant strains. Nanotechnology is one of the most important recent and current methods used to treat human diseases including parasitic ones. Therefore, the present study aimed to examine the curative role of gold nanoparticles (GNPs) on splenic tissue of mice infected with Schistosoma mansoni Sambon, 1907. High-resolution transmission electron microscopy was used for characterization of nanoparticles (NP). GNPs of 1 mg/kg mice body weight were inoculated into mice infected with S. mansoni. The parasite caused deteriorations in histological architecture of the spleen tissue, and splenomegaly. Additionally, the parasite induced a significant reduction in splenic tissue glutathione levels; however, the concentrations of nitric oxide and malondialdehyde were significantly increased. Treatment of mice with GNPs reduced the extent of histological impairment and oxidative stress in spleen tissue. Therefore, our results demonstrate the protective role of GNPs against splenic damage in mice infected with S. mansoni.
Keywords: Gold nanoparticles, Schistosomiasis, Splenic damage, Histopathology, Oxidative stress, Mice
1. Introduction
Schistosomiasis, a parasitic disease caused by blood helminthes of genus Schistosoma, is still considered one of the major threatening health diseases in water based world's tropical regions (Steinmann et al., 2006), particularly the developing countries with significant poverty and public health problems (Barbosa et al., 2001), and in 2013, WHO estimated the need of treatment to at least 230 million people per year against schistosomiasis.
Adult flukes of the parasite are not the main cause of the injuries in the mammalian host body (Gryseels et al., 2006), but the massive egg production by the female worms, that reaches hundreds to thousands of eggs per day, is the major stimulant of chronic lesions, as it results in formation of granuloma in different body organs such as gut, intestine, liver, spleen, bladder and lungs (Araújo et al., 2010, Wang et al., 2015).
Enlargement of both the liver (Chen, 1993) and spleen (Bauomy et al., 2014, Chen, 1993) is called hepatosplenomegaly, a major pathological symptom of chronic schistosomiasis (Burke et al., 2009). Spleen, the biggest and the main peripheral immune system organ, is one of the important affected organs by schistosomiasis in variable degrees correlated with the hepatic portal circulation (Wang et al., 2015).
Treatment of schistosomiasis is still based on praziquantel (PZQ), a drug known by its effectiveness against different Schistosoma specie (Hotez et al., 2007), however, its low absorption through the intestine because it is less soluble in water (Lindenberg et al., 2004) and its metabolism results in production of a less effective compound and thus must be administrated in higher concentrations (Mourão et al., 2005), additionally, the influence of the drug is inadequate against schistosomules and immature worms, so it is unsuitable for mass treatment in high endemic regions (Doenhoff et al., 2008). These factors in addition to the parasite resistance develops against the drug (Botros and Bennett, 2007) were of the reasons that motivated scientists and physicians to search for alternative safe and more effective treatments to improve oral absorption of PZQ and/or replace it (Yang et al., 2009, Almeida et al., 2012).
Recently, nanotechnology is being used globally in different fields, nanoparticles (NPs) of some metals or metal oxides are now widely used as drugs to treat different diseases and improve human health due to their antimicrobial actions; these tiny molecules of specific chemical and physical features have shown antibacterial, antiviral and antiparasite activities (Elechiguerra et al., 2005, Jebali and Kazemi, 2013). Gold and silver NPs and oxide NPs of zinc, titanium and magnesium have presented antileishmanial activities (Jebali and Kazemi, 2013), in addition, Dkhil et al., 2015a, Dkhil et al., 2015b have demonstrated the antischistosomal activity of gold nanoparticles (GNPs) in liver and brain of mice respectively. Therefore, the present study is a continuation of serial studies of Dkhil et al., 2015a, Dkhil et al., 2015b to emphasize the antischistosomal action of GNPs on the splenic tissue of mice infected with the Schistosoma mansoni.
2. Materials and methods
2.1. Animals and infection
Male Swiss albino mice were bred in Schistosome Biological Supply Center at Theodor Bilharz Research Institute, Imbaba, Giza, Egypt. In our study, 24 mice were maintained under specified pathogen-free conditions and fed on standard diet. Diet and water were provided ad libitum.
By subcutaneous injection; mice (9–11) weeks of age, were infected with 100 ± 10 S. mansoni cercarie (Oliver and Stirewalt, 1952). Cercarie were obtained from Schistosome Theodor Bilharz Research Institute.
2.2. Gold Nano-Particles (GNPs)
According to Turkevich et al. (1951), GNPs have been prepared by chemical reduction method. “HAuCl4” solution has been used as Au3+ ions precursor; while sodium citrate has been used as both of mild reducing and stabilizing agent. The color of the solution slowly turned into faint pink color, indicating the reduction of the Au3+ ions to Au NPs.
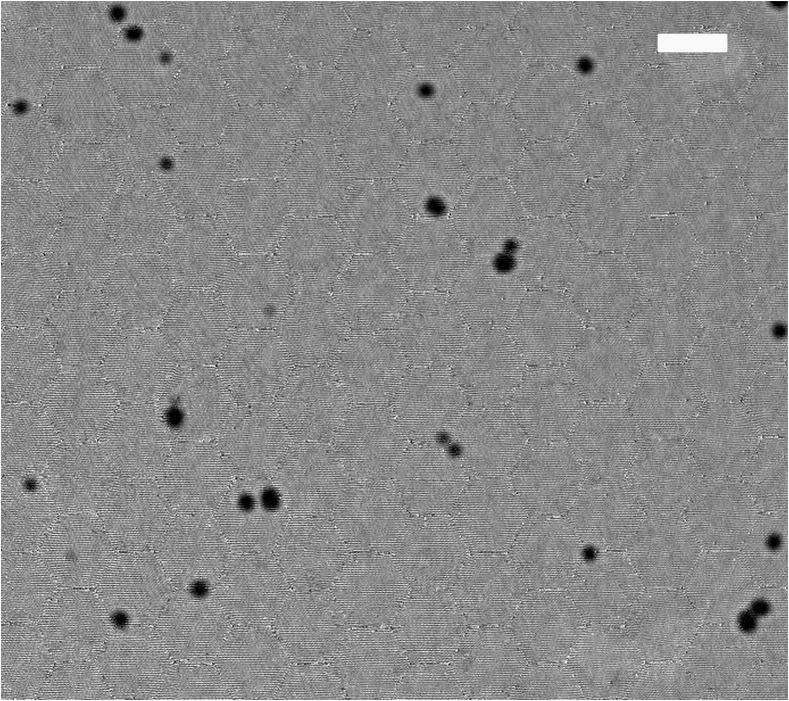
By transmission electron microscopy (TEM); size and morphology of GNPs were determined (Fig. 1). Samples for TEM were prepared using the clear solution of NPs. The sample solution was put on a formvar coated grid. On this grid, a drop of the sample solution (containing dispersed NPs) was placed and allowed to air-dry. A TEM picture was taken by a JOEL JEM 2000 EX200 microscope.
Figure 1.
Transmission electron microscopy image of gold nanoparticles illustrates their shape and size. Scale bar indicates 100 nm.
2.3. Animal groups
Experimental animals were divided into 4 groups; each of 6 mice. First group was considered as non-infected control group where the animals were orally received 100 μl water/mouse for 10 days. The remaining animals were infected with 100 ± 10 S. mansoni cercarie then divided into 3 groups, 46 days post-infection (p.i.).
One of these was infected (untreated). In addition, one of the remaining 2 infected groups received i.p. injection of 100 μl GNPs (1 mg/kg mice body weight), twice per week on day 46 and day 49 p.i.. On day 46 p.i. at an interval of 24 h for 2 days; the infected animals of last fourth group were orally administered 100 μl of PZQ (600 mg/kg body weight).
2.4. Histological investigations
On day 56 p.i. and GNPs injection, the animals were killed by fast decapitation. Fragments of spleen were immediately removed, fixed in 10% formalin, dehydrated, embedded in paraffin, sectioned and stained with haematoxylin and eosin for histopathological studies.
2.5. Biochemical parameters
Spleen was immediately excised and weighted then homogenized immediately to give 50% (w/v) homogenate in ice-cold Tis-HCl buffer (Tsakiris et al., 2004). The homogenate was centrifuged at 500 × g for 10 min. The supernatant (10%) was used for estimation of glutathione, nitrite/nitrate and malondialdehyde.
The glutathione (GSH) level in intestine and spleen was determined by the method of Ellman (1959), in addition, the nitrite/nitrate and malondialdehyde (MDA) levels were assayed according to the method of Green et al. (1982) and Ohkawa et al. (1979), respectively.
2.6. Spleen index
The spleen index was calculated by (ratio of spleen weight in mg/mouse to body weight in g/mouse), where; in all groups each mouse and its spleen were weighed.
2.7. Statistical analysis
The obtained data were presented as means ± standard error. One-way ANOVA was carried out, and the statistical comparisons among the groups were performed with Duncan’s test using a statistical package program (SPSS version 17.0). P ⩽ 0.05 was considered as low, moderate and highly significant for all statistical analysis in this study.
3. Results
S. mansoni induced a significant reduction in splenic GSH levels versus non-infected group as shown in Table 1. Moreover, GNPs treatment at a dose level of (1 mg/kg mice body weight), twice per week on day 46 and day 49 p.i.; elevated GSH level significantly (P ⩽ 0.05) in the investigated spleen tissue as compared to schistosome infected mice. Likewise, PZQ gavage on day 46 p.i.at an interval of 24 h for 2 days resulted in a significant increase in splenic GSH level versus infected group (see Table 1).
Table 1.
Effect of GNPs on glutathione (GSH), nitrite/nitrate, and malondialdehyde (MDA) levels in splenic homogenate of mice infected with S. mansoni.
| Group | Splenic GSH (mg/g) | Splenic nitrite/nitrate (μmol/g) | Splenic MDA (nmol/g) |
|---|---|---|---|
| Non-infected (-GNPs) | 52.32 ± 1.89 | 96.58 ± 2.33 | 28.31 ± 1.24 |
| Infected (-GNPs) | 16.26 ± 1.05a | 243.6 ± 3.24a | 79.68 ± 2.00a |
| Infected (+1 mg/kg GNPs) | 26.91 ± 1.95ab | 189.2 ± 2.59ab | 58.07 ± 2.23ab |
| Infected (+PZQ) | 22.69 ± 1.23ab | 200.4 ± 4.89ab | 53.72 ± 3.39ab |
Values are means ± SE. a: Significant against (non-infected) control group at P ⩽ 0.05, b: Significant against infected control group at P ⩽ 0.05, n = 6.
Data tabulated in Table 1 revealed that S. mansoni infection caused a significant elevation at P ⩽ 0.05 in levels of nitrite/nitrate and MDA in spleen as compared to non-infected group. On the other hand, the levels of nitrite/nitrate and MDA showed a significant reduction in splenic tissues as a result of GNPs and PZQ treatment versus infected group.
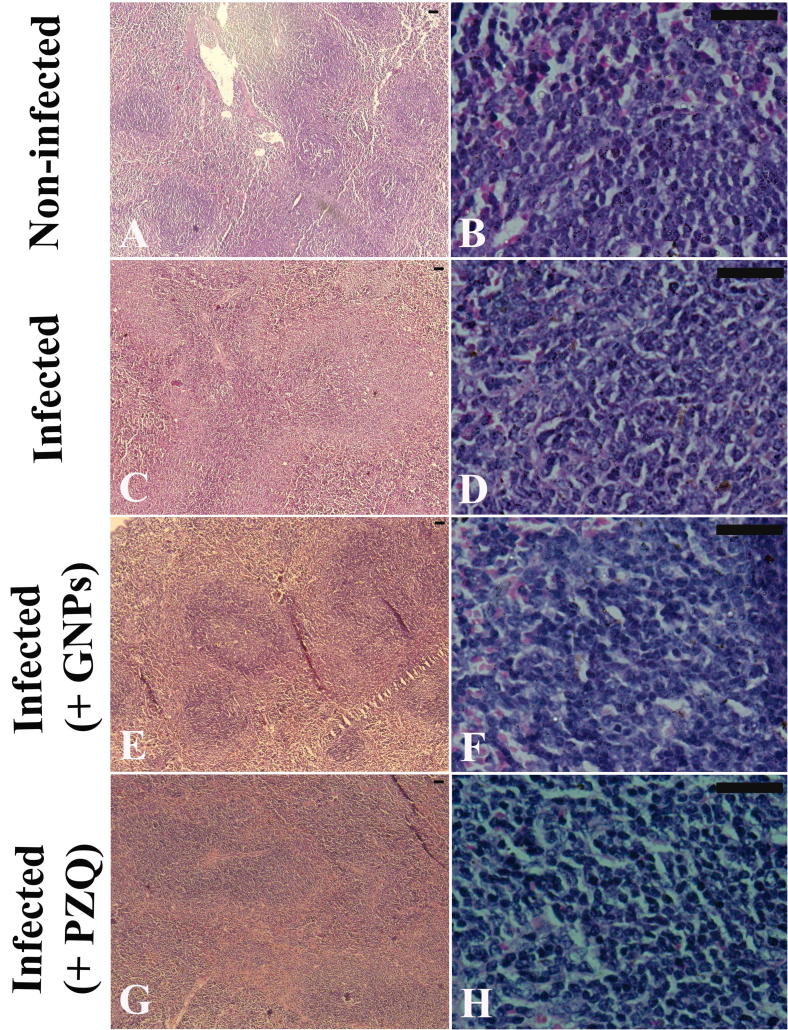
Fig. 2 showed the histological picture of spleen; Fig.2A & B revealed normal spleen architecture where white and red pulps surrounded by a capsule of dense connective tissue. Since, white pulp is composed of a central, T-cell rich zone, and a periarterial lymphoid sheath surrounded by B-cell-rich primary follicles; the white pulp enlarged due to cellular proliferation and the limit between white and red pulps started to disappear; moreover, splenomegaly was observed as a result of schistosome infection (Fig.2C & D). Gold nanoparticles (1 mg/kg b. wt.) injection to schistosome infected induced significant improvements in the histological picture of spleen; with some histological impairment was still observed (Fig.2E & F). In the same manner, PZQ treatment induced significant improvements in spleen architecture of schistosome infected mice (Fig.2G & H).
Figure 2.
Histological changes in splenic tissue of uninfected, and untreated and treated mice infected with Schistosoma mansoni on day 56 post-infection. (A & B) Uninfected spleen with normal architecture. (C & D) Spleen tissue of mice in S. mansoni infected group, showing splenomegaly, and enlargement of the white pulp indicated by cellular prolifertation, and gradual disappearance of the boundary between white and red pulps. (E&F) Spleen tissue of mice in S. mansoni infected groups treated with 1 mg GNPs/kg b. wt., exhibiting significant improvements in the histological architecture of the spleen, and less tissue damage. (G & H) Spleen of mice in infected group treated with PZQ, showing significant improvements in the spleen tissue with fewer lesions. Sections were stained with hematoxylin and eosin; scale bar = 50 μm.
Spleen index was calculated in Table 2, infection resulted in a significant increase in spleen index as compared to non-infected control group. On the other hand, a significant decrease was recorded in spleen index as a result of GNPs injection and PZQ treatment versus schistosome-infected group.
Table 2.
Effect of GNPs on the spleen index of mice infected with S. mansoni.
| Group | Spleen index (mg/g) |
|---|---|
| Non-infected (-GNPs) | 4.18 ± 0.34 |
| Infected (-GNPs) | 15.13 ± 1.18a |
| Infected (+1 mg/kg GNPs) | 9.80 ± 0.79ab |
| Infected (+PZQ) | 9.16 ± 0.77ab |
Values are means ± SE. a: Significant against (non-infected) control group at P ⩽ 0.05, b: Significant against infected control group at P ⩽ 0.05, n = 6.
4. Discussion
In the present study, GNPs treatment decreased splenic levels of nitrite/nitrate and MDA significantly; moreover, the nanoparticles increased GSH level significantly in intestine and spleen of the schistosome infected mice. Our results are in agreement on the protective role of GNPs on body tissues with (La Flamme et al., 2001, El-Sokkary et al., 2002, Facundo et al., 2004, de Oliveira et al., 2013, Bauomy et al., 2014, Dkhil et al., 2015a, Dkhil et al., 2015b).
The oxidative status in bilharziasis was previously studied in organs such as liver, kidneys, gut and, to a lesser extent, spleen (La Flamme et al., 2001, El-Sokkary et al., 2002, Facundo et al., 2004, Bauomy et al., 2014). Since; it was previously observed that S. mansoni infection may induce oxidative stress in spleen (El-Sokkary et al., 2002, Facundo et al., 2004, Bauomy et al., 2014).
El-Sokkary et al. (2002) and Bauomy et al. (2014) deduced that spleen of schistosome-infected mice showed a significant increment in lipid peroxidation and nitric oxide levels and a significant decrease in vitamin E, GSH levels, and superoxide dismutase activity, where the oxidative processes occur at the inflammation site and are involved in the damaging effects of schistosomiasis so free radicals may be a major component of the disease. In the same manner; de Oliveira et al. (2013) observed that decreased non-enzymatic antioxidant capacity in spleen.
Spleen is considered as one of the main affected organs during the schistosomiasis due to increased eosinophil peroxidase activity and imbalance in the antioxidant defense mechanisms (Gharib et al., 1999). In addition, some reports cleared that S. mansoni infection may imbalance oxidative parameters by different causes or mechanisms such as egg deposition, changes in vascular tone and soluble immune mediators (Wynn et al., 2004, Pearce, 2005, Wilson et al., 2011). Dkhil et al., 2015a, Dkhil et al., 2015b found that GNPs showed an anti-schistosomal effect in brain and liver of mice. GNPs decreased nitrite/nitrate and MDA levels while, it increased the level of GSH in brain and liver homogenates; where, attributed these results to GNPs ability to scavenge free radicals.
Our results revealed that GNPs improved significantly; the splenic impairments induced by schistosomiasis. Likewise, GNPs showed a significant decrease in spleen index as compared to infected group. El-Sokkary et al. (2002) reported that histopathological observations revealed a large number of megakaryocytes in the spleen, and Bauomy et al. (2014) deduced that the infection induced splenic enlargement. In addition, disorganized red and white pulps were found in splenic tissue of the schistosomal infected mice. Moreover, GNPs at different doses showed an anti-bilharziasis activity in our previous work, where, GNPs ameliorated effects on schistosomiasis and improved the histopathological changes in both investigated organs “brain and liver” (Dkhil et al., 2015a, Dkhil et al., 2015b).
5. Conclusion
This study provides information for raising the interesting possibility that gold nanoparticles eventually have therapeutic potential against splenic disorders induced by S. mansoni infection. Therefore, the loading of antischistosomal drugs on nanocarriers may be the aim for the future work in this field to evaluate the therapeutic possibility of such drug delivery system.
Acknowledgment
The authors extend their appreciations to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RGP-198.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohamed A. Dkhil, Email: mohameddkhil@yahoo.com.
Mona F. Khalil, Email: dr.monakhalil@gmail.com.
Marwa S.M. Diab, Email: marwa.db@gmail.com.
Saleh Al-Quraishy, Email: guraishi@yahoo.com.
References
- Almeida A.E., Souza A.L.R., Cassimiro D.L., Gremião M.P.D., Ribeiro C.A., Crespi M.S. Thermal characterization of solid lipid nanoparticles containing praziquantel. J. Therm. Anal. Calorim. 2012;108:333–339. [Google Scholar]
- Araújo A.P., Frezza T.F., Allegretti S.M., Giorgio S. Hypoxia, hypoxia-inducible factor-1α and vascular endothelial growth factor in a murine model of Schistosoma mansoni infection. Exp. Mol. Pathol. 2010;89:327–333. doi: 10.1016/j.yexmp.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Barbosa C.S., Montenegro S.M.L., Abath F.G.C., Domingues A.L.C. Specific situations related to acute schistosomiasis in Pernambuco, Brazil. Memóris do Instituto Oswalo Cruz. 2001;96:169–172. doi: 10.1590/s0074-02762001000900026. [DOI] [PubMed] [Google Scholar]
- Bauomy A.A., Dkhil M.A., Diab M.S.M., Amer O.S.O., Zrieq R.M., Al-Quraishy S. Response of spleen and jejunum of mice infected with Schistosoma mansoni to Mulberry treatment. Pak. J. Zool. 2014;46(3):753–761. [Google Scholar]
- Botros S.S., Bennett J.L. Praziquantel resistance. Expert Opin. Drug Discovery. 2007;2:S35–S40. doi: 10.1517/17460441.2.S1.S35. [DOI] [PubMed] [Google Scholar]
- Burke M.L., Jones M.K., Gobert G.N., Li Y.S., Ellis M.K., Mcmanus D.P. Immunopatho-genesis of human schistosomiasis. Parasite Immunol. 2009;3:163–176. doi: 10.1111/j.1365-3024.2009.01098.x. [DOI] [PubMed] [Google Scholar]
- Chen M.G. Schistosoma japonicum and S. japonicum-like infections: epidemiology, clinical and pathological aspects. In: Jordan P, Webbe, Webbe G., Sturrock F.S., editors. CAB International; Wallingford: 1993. pp. 237–270. (Human Schistosomiasis). [Google Scholar]
- de Oliveira R.B., Senger M.R., Vasques L.M., Gasparotto J., Dos Santos J.P.A., Depasquali M.A., Moreira J.C.F., Jr F.P.S., Gelain D.P. Schistosoma mansoni infection causes oxidative stress and alters receptor for advanced glycation end product (RAGE) and tau levels in multiple organs in mice. Int. J. Parasitol. 2013;43:371–379. doi: 10.1016/j.ijpara.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Dkhil M.A., Bauomy A.A., Diab M.S., Al-Quraishy S. Antioxidant and hepatoprotective role of gold nanoparticles against murine hepatic schistosomiasis. Int. J. Nanomed. 2015;10:7467–7475. doi: 10.2147/IJN.S97622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dkhil M.A., Bauomy A.A., Diab M.S., Wahab R., Delic D., Al-Quraishy S. Impact of gold nanoparticles on brain of mice infected with Schistosoma mansoni. Parasitol. Res. 2015;114:3711–3719. doi: 10.1007/s00436-015-4600-2. [DOI] [PubMed] [Google Scholar]
- Doenhoff M.J., Cioli D., Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 2008;21(6):659–667. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- Elechiguerra J.L., Burt J.L., Morones J.R. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005;3:6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- El-Sokkary G.H., Omar H.M., Hassanein A.F., Cuzzocrea S., Reiter R.J. Melatonin reduces oxidative damage and increases survival of mice infected with Schistosoma mansoni. Free Radical Biol. Med. 2002;32:319–332. doi: 10.1016/s0891-5849(01)00753-5. [DOI] [PubMed] [Google Scholar]
- Facundo H.T., Brandt C.T., Owen J.S., Lima V.L. Elevated levels of erythrocyte-conjugated dienes indicate increased lipid peroxidation in schistosomiasis mansoni patients. Braz. J. Med. Biol. Res. 2004;37:957–962. doi: 10.1590/s0100-879x2004000700003. [DOI] [PubMed] [Google Scholar]
- Gharib B., Abdallahi O.M., Dessein H., Dereggi M. Development of eosinophil peroxidase activity and concomitant alteration of the antioxidant defenses in the liver of mice infected with Schistosoma mansoni. J. Hepatol. 1999;30:594–602. doi: 10.1016/s0168-8278(99)80189-5. [DOI] [PubMed] [Google Scholar]
- Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Gryseels B., Polman K., Clerinx J., Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- Hotez P.J., Molyneux D.H., Fenwick A., Kumaresan J., Sachs S.E., Sachs J.D., Savioli L. Control of neglected tropical diseases. N. Engl. J. Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- Jebali A., Kazemi B. Nano-based antileishmanial agents: a toxicological study on nanoparticles for future treatment of cutaneous leishmaniasis. Toxicol. In Vitro. 2013;27(6):1896–1904. doi: 10.1016/j.tiv.2013.06.002. [DOI] [PubMed] [Google Scholar]
- La Flamme A.C., Patton E.A., Bauman B., Pearce E.J. IL-4 plays a crucial role in regulating oxidative damage in the liver during schistosomiasis. J. Immunol. 2001;166:1903–1911. doi: 10.4049/jimmunol.166.3.1903. [DOI] [PubMed] [Google Scholar]
- Lindenberg M., Kopp S., Dressman J.B. Classification of orally administered drugs on the World Health Organization model list of essential medicines according to the biopharmaceutics classification system. Eur. J. Pharm. Bio-pharm. 2004;58:265–278. doi: 10.1016/j.ejpb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Mourão S.C., Costa P.I., Salgado H.R.N., Gremião M.P.D. Improvement of antischistosomal activity of praziquantel by incorporation into phosphatidylcholine-containing liposomes. Int. J. Pharm. 2005;295:157–162. doi: 10.1016/j.ijpharm.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Oliver L., Stirewalt M.A. An efficient method for the exposure of mice to cercaria of Schistosoma mansoni. J. Parasitol. 1952;38:19–23. [PubMed] [Google Scholar]
- Pearce E.J. Priming of the immune response by schistosome eggs. Parasite Immunol. 2005;27(7–8):265–270. doi: 10.1111/j.1365-3024.2005.00765.x. [DOI] [PubMed] [Google Scholar]
- Steinmann P., Keiser J., Bos R., Tanner M., Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- Tsakiris S., Schulpis K.H., Marinou K., Behrakis P. Protective effect of l-cysteine and glutathione on the modulated suckling rat brain Na+, K+, -ATPase and Mg2+ -ATPase activities induced by the in vitro galactosaemia. Pharmacol. Res. 2004;49:475–479. doi: 10.1016/j.phrs.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Turkevich J., Stevenson P.C., Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Faraday Soc. 1951;11:55–75. [Google Scholar]
- Wang Y., Zhang J., Yin J., Shen Y., Wang Y., Xu Y., Cao J. The formation of egg granulomas in the spleens of mice with late Schistosoma japonicum infection alters splenic morphology. Parasites Vectors. 2015;8:375. doi: 10.1186/s13071-015-0988-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2013. Schistosomiasis.
- Wilson S., Vennervald B.J., Dunne D.W. Chronic hepatosplenomegaly in African school children: a common but neglected morbidity associated with schistosomiasis and malaria. PLOS Negle. Trop. Dis. 2011;5:e1149. doi: 10.1371/journal.pntd.0001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T.A., Thompson R.W., Cheever A.W., Mentink-Kane M.M. Immunopathogenesis of schistosomiasis. Immunol. Rev. 2004;201:156–167. doi: 10.1111/j.0105-2896.2004.00176.x. [DOI] [PubMed] [Google Scholar]
- Yang L., Geng Y., Li H., Zhang Y., You J., Chang Y. Enhancement the oral bioavailability of praziquantel by incorporation into solid lipid nanoparticles. Pharmazie. 2009;64:86–89. [PubMed] [Google Scholar]