Abstract
The middle temporal area (MT) is a visual area in primates with direct and indirect inputs from the primary visual cortex (V1), a role in visual motion perception, and a suggested role in “blindsight.” When V1 is deactivated, some studies report continued activation of MT neurons, which has been attributed to an indirect pathway to MT from the superior colliculus. Here we used muscimol to deactivate V1 while optically imaging visually evoked activity in MT in two primates, owl monkeys and galagos, where MT is exposed on the brain surface. The partial loss of V1 inputs abolished all or nearly all evoked activity in the retinotopically matched part of MT. Low levels of activation that persisted in portions of MT that were unstimulated or retinotopically congruent with the blocked portion of V1 appeared to reflect the spread of activity from stimulated to unstimulated parts of MT. Thus, a significant pathway based on the superior colliculus was not demonstrated.
Keywords: V1 lesion, blindsight, muscimol, intrinsic connections
The middle temporal area (MT, also known as V5) is a visual area in the upper temporal lobe of primates that contains a very high proportion of neurons selective for the direction of motion (1, 2) and the orientation of moving gratings (3, 4) and clearly influences motion perception (5). Although major inputs to MT are from the primary visual cortex (V1) and areas dependent on V1 for visual activation, such as V2 (6, 7), the effects of lesions or cooling of V1 on the responsiveness of neurons in MT have been inconsistent and difficult to interpret. In early experiments in macaque monkeys, lesions of V1 appeared to only partially inactivate MT (8); an additional lesion of the superior colliculus rendered MT neurons unresponsive to visual stimulation (9). Similarly, a cooling block of part of V1 failed to eliminate all visually evoked activity in the retinotopically matched portion of MT (10). In addition, functional MRI of the visual cortex in a well studied patient with V1 damage that is known for blindsight suggested that visually evoked activity in MT can be independent of V1 (11, 12). Thus, a possible source of blindsight (13), the ability to respond to visual information without awareness of the stimuli, is a relay of visual information from the superior colliculus to the pulvinar and then to extrastriate cortex (8–10, 14).
In other experiments on New World owl monkeys, MT was found to be completely dependent on V1 for visual activation, whether the lesion was recent (15) or long standing (16). In contrast, recordings from MT of the marmoset, another New World monkey, suggested that some neurons do not depend on V1 (14; for contrast, see ref. 16). These varying results suggest that species differ in dependencies of MT on V1 or that visually evoked responses in deprived parts of MT have been incorrectly attributed to an extrastriate (non-V1) source of activation.
The present experiments allowed us to visualize global patterns of visual activation in MT after a block of relayed information from the part of V1 representing central vision. In both owl monkeys and prosimian galagos, nearly all of MT is exposed on the dorsolateral surface of the cerebral hemisphere, and the functional organizations of MT have been explored with optical imaging techniques in both of these primates (3, 4). MT contains a systematic representation of the contralateral visual hemifield in these (17, 18) and other primates (19) that is a mirror reversal of the representation in V1, so that central vision is caudal in MT and on the exposed dorsolateral surface in V1. We sought to block the output of dorsolateral V1 representing the central 10° of vision in these primates while observing the evoked activity in MT to moving, high-contrast gratings, which are highly effective stimuli for MT neurons. By varying the grating orientation, it was possible to reveal the domains of orientation-selective neurons, and by restricting gratings to parts of the visual field, it was possible to selectively activate parts of V1 and MT. The main goal was to see whether a loss of input from dorsolateral V1 would reduce or eliminate visually evoked activity in a retinotopically matched portion of MT. In addition, we wanted to see whether visually evoked activity would spread to parts of MT that were not retinotopically matched with the excited part of V1. Such activity could be based on divergent connections from V1 that go beyond retinotopically matched locations, either directly (20) or indirectly (21), or on the dense network of intrinsic connections within MT that could spread visually evoked activity from stimulated to unstimulated portions of MT (22). Optical imaging allowed the activities of groups of interlinked neurons to be visualized, in contrast to the limited sampling of activity provided by microelectrode mapping techniques.
Materials and Methods
The five animals used in these experiments were handled according to an approved protocol from the Vanderbilt University Animal Care and Use Committee. In these animals, we imaged both MT and V1 from two hemispheres in an owl monkey (Aotus trivirgatus) and five hemispheres in four galagos (Otolemur garnetti). Normal features of MT response patterns and retinotopy were explored with optical imaging in additional galagos (4) and owl monkeys.∥ The animals were prepared for surgery, paralyzed, and anesthetized as described in refs. 4 and 23. In these experiments, a larger craniotomy was made to expose V1, MT, and surrounding visual areas. The opening was sealed with 1% agarose under a cover glass.
After initial images were collected of MT and of the exposed portion of V1 in each cortical hemisphere by using full-screen and topographically limited stimuli to determine retinotopic layout, the camera was refocused over MT, and preblocking images were collected by using the same stimuli. When sufficient baseline images were obtained, the agarose overlying V1 was carefully removed, and a piece of gelfoam cut to fit the exposed portion of V1 was soaked in a 50 mM muscimol solution and applied to the surface of V1 (24, 25). Fast green dye was added to the muscimol solution to mark the exact location of the gelfoam on the cortex and to reveal any muscimol leakage to other parts of cortex. No spreading of muscimol solution was observed in any of the imaged hemispheres. While in place, the gelfoam pad was frequently remoistened with muscimol solution to maintain cortical hydration. The gelfoam pad was removed after 20–40 min of muscimol blocking, the cortex was rinsed with saline, and a new saline-soaked gelfoam pad was applied to maintain hydration. After ≈3–5 h of data collection in MT after blockade of V1 activity, V1 was re-covered with 1% agarose and reimaged to assess the level of remaining activity, if any. Optical imaging and analysis, visual stimuli, and histological reconstruction procedures were performed as described in ref. 23.
Results
The results indicate that MT is highly dependent on inputs from V1 for activation and suggest that such activation is not limited to retinotopically matched locations. Results are presented first for the owl monkey and then for galagos.
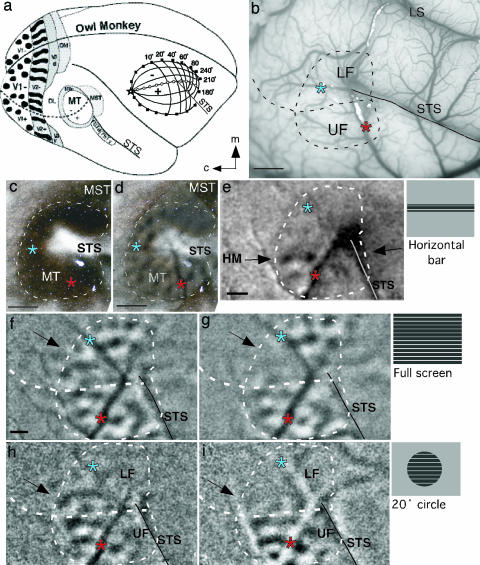
The V1 dependence of visually evoked activity in MT of an adult owl monkey was revealed by stimulating most or part of the contralateral visual hemifield with drifting square wave gratings before and after deactivation of central vision with muscimol (Fig. 1). In owl monkeys, MT is located in the middle of the upper temporal lobe near the tip of the superior temporal sulcus (STS) (Fig. 1a). Central vision is represented caudally in MT, with the upper quadrant located ventrally and the lower quadrant located dorsally (Fig. 1 a Inset and b). V1 and V2 are caudal to MT (Fig. 1a). During stimulation, MT stands out as an area that is highly responsive to moving stimuli (Fig. 1 d–i); surrounding visual areas are much less responsive. MT can be easily identified by its characteristic dark stain in brain sections processed for myelinated fibers (17) (Fig. 1 c and d) or for cytochrome oxidase (26).
Fig. 1.
Loss of MT responses in the visuotopically matched location of deactivated V1 in the owl monkey. Asterisks indicate blood vessel bifurcations used to align brain sections to imaging data. (a) Drawing of owl monkey brain with positions of some visual areas indicated. (Inset) Visuotopic map of MT. (b) Photograph of the surface blood vessel pattern from owl monkey 04-25. Dotted line through MT indicates the position of the horizontal meridian (HM). (Scale bar, 2 mm.) (c) Flattened section of cortex from owl monkey 04-25 stained for myelinated fibers to distinguish MT boundaries. (Scale bar, 2 mm.) (d) The same section in c from owl monkey 04-25 stained for myelinated fibers aligned with a 50% transparent image of the MT activation pattern. (e) The region of the representation of the HM in MT was revealed by the pattern of activation resulting from a 3°-high, 74°-wide horizontal bar grating centered at the HM (stimulus schematic adjacent to e). (Scale bar, 1 mm.) (f) OD image (0°/90°) produced with binocular stimulation using wide-field square wave gratings. Black arrow marks location of paracentral lower visual field (LF) representation. (Scale bar, 1 mm, applies to f–i.) (g) OD image (0°/90°) 3 h after muscimol deactivation of the central LF representation of V1. The retinotopically matched portion of MT shows dramatically reduced activation. (h) OD image (0°/90°) resulting from monocular stimulation using topographically restricted gratings within a 20° circle centered at the area centralis (AC). The representation of the central 10° of paracentral LF demonstrates modular activation. Unstimulated, rostral parts of MT representing peripheral vision show little activation. (i) OD image (0°/90°) (stimulus as in h) centered at AC 1 h after muscimol deactivation of paracentral V1. Modular pattern of activation in the LF representation is dramatically reduced, and activation in the paracentral upper visual field (UF) is weakened.
Before the deactivation of V1, moving gratings were highly effective in activating MT. With a wide-field drifting grating of 74° × 62° centered on the area centralis, most of MT was clearly activated (Fig. 1f). The orientation difference (OD) image (0°/90°) revealed an array of dot-like modules that were either highly activated by vertical gratings (white dots) or by horizontal gratings (black dots). Only the portion of MT representing peripheral vision (≈40–90°) lying just rostral to the superior temporal sulcus was poorly activated. Although a total lack of activation might be expected in this part of MT given that only 37° of the contralateral hemifield was stimulated, a weak pattern of activation was apparent. Thus, activation can spread beyond visually stimulated parts of MT to visually unstimulated parts of MT. However, the activation level in the unstimulated sector of MT is relatively weak. The activation pattern in parts of MT representing more central vision was consistent with previous optical imaging observations in MT of owl monkeys (3) and galagos (4).
Blocking the output from V1 with muscimol greatly altered the activity pattern in MT. After dorsolateral V1 (the portion representing the central 10° of vision largely in the lower quadrant) was blocked, most of the activity in the retinotopically matched part of MT disappeared (arrow in Fig. 1g), whereas activity in other parts of MT remained. Yet the border between the active and inactive zones in MT was not completely sharp, and several foci of low levels of activation persisted. This observation suggests that some activation based on the horizontal spread of excitation remains in the deprived portion of MT, especially near the edges of the deprived zone, as it does in the unstimulated portion of MT devoted to peripheral vision. A more substantial deactivation of MT occurred when visual stimuli were restricted to the central 10° (Fig. 1 h and i) of the visual hemifield. In the dorsal representation of the lower visual quadrant in MT, the stimulus was largely or completely within the deactivated part of V1. As a result, both deprived and unstimulated parts of dorsal MT were unresponsive (compare Fig. 1 f and g with h and i). However, as ventral V1, representing central vision of the upper visual quadrant (27, 28), was unblocked and stimulated, caudoventral MT remained responsive. These results are consistent with the interpretation that visually evoked activations of MT neurons are highly dependent on retinotopically matched inputs from V1. Weak activation in retinotopically incongruent locations occurs, but it is also dependent on V1 inputs.
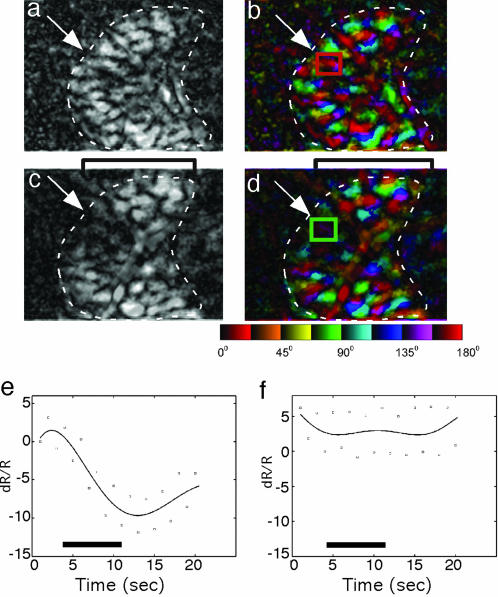
The dependence of MT on V1 for visual activation is demonstrated further in orientation polar maps and magnitude maps before (Fig. 2 a and b) and after (Fig. 2 c and d) partial V1 deactivation in an owl monkey. After deactivation of the representation of the central 10° in V1, activity was greatly reduced or absent in the retinotopically matched portion of MT (Fig. 2d), but some selective activation remained (Fig. 2c). Measurement of the intrinsic responses in the boxed-in portion of V1-deprived MT (Fig. 2 b and d) revealed no modulation of activity after the muscimol block (Fig. 2f), whereas normal levels were observed before V1 deactivation (Fig. 2e). The ΔR/R (intensity of optical reflectance R during the stimulus condition relative to the intensity during the blank control) across four orientations inside the deactivation zone was –.137 ± 0.23% (mean ± SD) and –0.049 ± 0.026% before and after V1 block, respectively, which differed significantly (P < 0.0001, Student's t test). However, when a larger portion of depressed MT was included in this sample, weak modulation was evident. The modular distribution of the remaining activation is consistent with the anatomical evidence that an intrinsic network of horizontal connections in MT binds together foci of similar orientation selectivities. Thus, the orientation domains may be weakly activated by retinotopically incongruent stimuli of appropriate orientations. In addition, in Fig. 2 c and d, the brackets highlight an area of increased activation outside the predeactivation boundaries of MT, perhaps resulting from a decrease in lateral inhibition in the area adjacent to that affected by the deactivation.
Fig. 2.
Response magnitude changes in a visuotopically correspondent portion of MT after V1 deactivation in the owl monkey. (a and b) Magnitude (a) and orientation polar (b) maps produced by using full-field gratings at four orientations. The magnitude map shows the overall strength of orientation response (light = stronger; dark = less strong). The polar map contains information about both orientation preference (color) and magnitude of orientation selectivity (brightness) (see the color key below d). (c and d) Magnitude (c) and orientation polar (d) maps after V1 deactivation with muscimol. Reduced activation is apparent after V1 deactivation. Brackets highlight an area of increased activation outside the predeactivation boundaries of MT, perhaps resulting from a decrease in lateral inhibition in the area adjacent to that affected by deactivation. (e and f) Time courses of intrinsic signal strength to 8 s of stimulation (indicated by the solid black line above the x axis) using a 0° full-field drifting grating for the small square regions [red square in b and green square in d, which are the regions of interest (ROIs)] on the maps before and after V1 deactivation, respectively. The plots are polynomial fits of the average data points (squares) from 10 trials. The control ROI plot (e) shows a typical intrinsic response curve; the optical reflectance decreased with stimulus onset, dipped to its lowest point ≈7 s after onset, and then rebounded. On the graphs, the x axis shows time in s, and the y axis shows the change (in 10–4) in the ROI relative to the blank (ΔR/R). The control ROI peak magnitude ΔR/R (dR/R) = –0.119 ± 0.017% (mean ± SD). The post-blocking ROI plot (f) does not show any stimulation-related response, and the optical reflectance appears to remain constant during stimulation, as indicated by the ROI peak magnitude ΔR/R = –0.008 ± 0.027%.
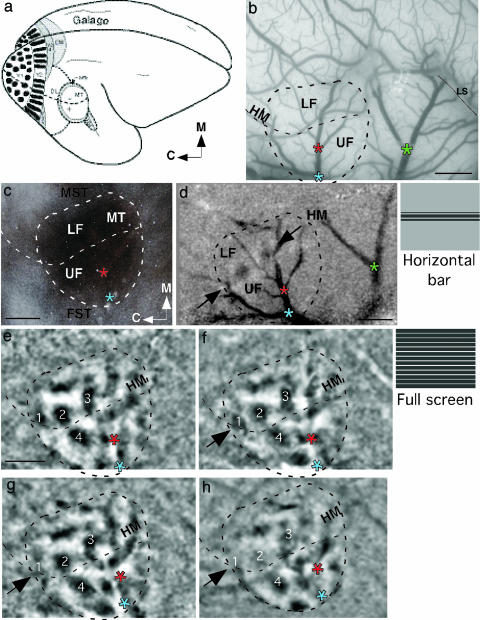
Similar but more extensive results were obtained from five hemispheres from four galagos. As in the owl monkey, the dorsolateral portion of area 17 was exposed for the application of muscimol. Previous microelectrode mapping of V1 in galagos indicated that most of dorsolateral V1 is devoted to the central 10° of vision, primarily of the lower visual quadrant (28). As in owl monkeys, central vision is represented caudally in MT of galagos, with the lower visual quadrant dorsal to the upper quadrant (18). Our previous optical imaging study of MT in galagos (4) revealed a functional organization much like that observed in owl monkeys (3). Some of these observations were repeated and confirmed here. Thus, wide-field (≈0–40°) stimulation of the contralateral visual hemifield with a drifting, high-contrast grating evoked high levels of activity over most of MT but little activity in the immediately surrounding cortex (Fig. 3). As a result, MT was easily identified and distinguished from the surrounding cortex. The physiologically identified MT was later found to be congruent with the histologically identified MT, as distinguished in sections cut parallel to the brain surface and processed for myelin (Fig. 3c) or cytochrome oxidase.
Fig. 3.
Loss of MT responses at the visuotopically matched location of deactivated V1 in a prosimian galago. (a) Drawing of a galago brain with positions of some visual areas indicated. MT is entirely exposed on the brain surface. (b) Photograph of the surface blood vessel pattern from galago 04-10. The boundaries of MT are indicated relative to the lateral sulcus (LS) and blood vessel landmarks. Black dotted line through MT indicates the position of the HM, separating the representation of the upper visual field (UF) from the lower visual field (LF). Asterisks mark blood vessel bifurcations used to align brain sections to imaging data. (Scale bar, 2 mm.) (c) A section of flattened cortex from galago 04-10 stained for myelinated fibers allows anatomical confirmation of MT boundaries based on imaging. (Scale bar, 2 mm.) (d) The location of the HM is revealed by the pattern of activation resulting from a 3°-high, 74°-wide screen horizontal bar stimulus centered along the HM in the left visual hemifield. Stimulus schematic adjacent to d. Three asterisks provide alignment points for the brain surface photograph in b. (Scale bar, 2 mm.) (e) OD image (0°/90°) produced with monocular stimulation using wide-field square wave gratings 3 h before blocking V1 with muscimol. Four clearly visible modules are numbered 1–4 for comparison with subsequent images. Modules 1–3 are in the paracentral lower visual field (LF) representation, and module 4 is in the central UF representation. (Scale bar, 2 mm, applies to e–h.) (f) OD image (0°/90°) produced with monocular stimulation minutes before blocking V1 with muscimol. The four modules marked in e remain clearly visible, and the overall pattern of activity is extremely stable. (g) OD image (0°/90°) produced with monocular stimulation using wide-field square wave gratings 40 min after blocking V1 with muscimol. Module 1 is mostly lost, but 2, 3, and 4 remain normal. (h) OD image (0°/90°) 2 h after blocking V1. Modules 1 and 2 are no longer discernible, and 3 and 4 are reduced in size. Parts of MT with intact V1 inputs remain normally responsive, with a pattern of activation similar to that in e.
The dorsolateral portion of V1 representing the central 10–15° of vision, mainly in the lower visual quadrant, was deactivated in one or both hemispheres of all four galagos. Wide-field stimulation before and after the application of muscimol to V1 indicated that MT organization was stable over several hours. An OD image (horizontal vs. vertical) obtained 3 h before muscimol application was very similar to the image obtained immediately before application (Fig. 3 e and f). With no manipulations to the cortex, the pattern of activation revealed by optical imaging is extremely consistent over several hours. The contrasting modules denoting the two orthogonal grating orientations covered most of MT, and they changed very little over time. In the rostral portion of MT representing the unstimulated portion of the contralateral visual hemifield beyond 40°, the activation at both times was weak but apparent. As in the owl monkey, the low level of activity in this portion of MT likely depends on a network of intrinsic connections between stimulated and unstimulated sectors of MT. After application of muscimol on the surface of V1, there was no immediate depression of activity in MT. Even after 40 min, activity levels remained high, although some modules began to disappear (Fig. 3g). However, after 2 h, little or no activity was apparent in the caudal sector of MT (arrow in Fig. 3h), corresponding to the portion of V1 deactivated by muscimol. The time course of deactivation occurred faster in some cases because of variation in the time of penetration of muscimol from the cortical surface to the middle and deep layers of the cortex, where neurons projecting to MT are located (29).
The gradually increasing effectiveness of the muscimol block of V1 on MT is apparent in a sequence of OD images (0°/90°) obtained before deactivation (Fig. 4a, which is published as supporting information on the PNAS web site), 45 min after application (Fig. 4b), and 80 min after application (Fig. 4c). Gratings were restricted to the central 20° of vision, activating the representation of the central 10° of vision in MT. Three of the activated modules were numbered for reference. Forty-five minutes after the application of muscimol to V1, the modules in the representation of the lower visual quadrant were no longer activated, and module 3 in the upper quadrant representation was only weakly activated. After 80 min, only module 3 remained weakly active. Thus, the muscimol block was completely effective for the part of MT representing the lower quadrant, but it was only partially effective for the part of MT devoted to central vision of the upper quadrant. The totally deactivated part of MT corresponds retinotopically to the dorsolateral part of V1 where the muscimol was applied.
The modular activation of MT and the dependence on V1 for that activation was further shown when the stimulating grating was restricted to a 10° circle centered at the area centralis (Fig. 5, which is published as supporting information on the PNAS web site). This stimulus would activate the representation of the central 5° of MT. Before the muscimol block of central V1, this stimulus revealed three clear modules in caudal MT in the OD image (0°/90°). Two of the modules were in the representation of central vision of the lower visual quadrant in MT (black arrows in Fig. 5a); one was in the representation of the upper visual quadrant (white arrow in Fig. 5a). After the deactivation of central V1 with muscimol, the two orientation modules in the representation of the lower visual quadrant in MT were no longer activated (black arrows in Fig. 5b), and the visible module in the representation of the upper visual quadrant remained responsive (white arrow in Fig. 5b). This finding suggests that the muscimol effectively blocked dorsomedial V1 representing the lower quadrant, where the muscimol was applied, but very little of lateroventral V1, representing the upper quadrant. Again, MT appears to depend completely on V1 for activation.
In some of these experiments, the effectiveness of the muscimol block of V1 was assessed with optical imaging. In one example, dorsolateral V1 was imaged in galago 04-08 before and after deactivation by muscimol. Before deactivation, an OD image (0°/90°) demonstrated a normal pattern of activation of orientation modules in V1 (Fig. 6a, which is published as supporting information on the PNAS web site). More than 5 h after the muscimol was applied to V1, the activity pattern in V1 was reimaged, and no evoked activity was apparent (Fig. 6b). Thus, the deactivation in MT could be attributed to a thorough and persisting deactivation of V1. Similar procedures were followed to determine the rostral border of V1 in the owl monkey. Resulting OD images were used to limit muscimol application to V1 and were consistent with previously published optical images from V1 of owl monkeys (23).
Discussion
The present results support two main conclusions. First, in both adult owl monkeys and galagos, evoked visual activity in MT is highly dependent on V1. When the central 10–15° of V1 was blocked with muscimol and visual stimuli were restricted to central vision, no detectable visual stimulation of MT was observed. Thus, the results provide no evidence for significant activation of MT by means of a superior colliculus-to-pulvinar-to-cortex pathway or significant activation by means of the sparse direct input pathway from the lateral geniculate nucleus (30–32). Second, activity based on V1 spreads from retinotopically matched parts of MT to other parts of MT, although the level of this activation is greatly reduced. This spread of activation is specific to orientation domains in MT that extend the pattern of the highly active orientation domains. Visual stimuli restricted to the central 20° of the visual hemifield produced high levels of activation in the matched caudal three-fourths of MT, and low levels of activation in orientation domain-sized patches in the unmatched rostral one-fourth of MT. In addition, while V1 was partially blocked, wide-field stimulation produced high levels of activation in rostral MT, corresponding to unblocked V1, and low levels of patchy activation in caudal MT, corresponding to blocked V1. Thus, low levels of visually evoked activity may be based on the lateral spread of excitation from other parts of MT in the absence of retinotopically appropriate visual stimuli. The implications of these conclusions and their relationship to previous findings and theories of blindsight are discussed below.
Visually Evoked Activity in MT Depends on V1. The present results support the conclusion that visually evoked activity in MT depends on a relay from V1, at least in some primates. This conclusion is consistent with previous evidence that V1 lesions abolish visually evoked activity in MT of owl monkeys and marmosets (15, 16). Although V1 lesions appeared to incompletely deactivate the retinotopically matched portion of MT in marmosets in one recent study (14), many of the responsive neurons had receptive fields that were displaced to parts of the visual hemifield corresponding to intact parts of V1. Thus, at least part of the preserved activity depended on V1. The displaced receptive fields provide further evidence for a system of intrinsic horizontal connections that spread activity from highly active portions of MT to deprived portions of MT. A similar horizontal spread of activation in MT may have been responsible for some of the neuronal responses in deprived portions of MT in other studies with V1 lesions or cooling in monkeys (8, 10). In a patient with blindsight, inadvertent stimulation of preserved parts of V1 or callosally connected parts of V1 of the intact hemisphere possibly accounted for or contributed to the activation of the MT region in functional MRI studies (11, 12, 33, 34).
Although some caution is needed in making strong conclusions, it seems justified to conclude that MT in some primates is highly dependent on V1 and that other sources of visual activation are not effective. The phenomenon of blindsight and visual capacities that are independent of V1 seem well established (13), but they may not involve MT.
The Domain-Specific Spread of Activation in MT. One of the more interesting findings was of low levels of activation of unstimulated portions of MT when other portions of MT were highly stimulated by drifting gratings. Such gratings activated different sets of orientation (or axis of motion) domains, depending on the orientation of the grating. The domain pattern of activation extended from the regions of high activation into regions of low activation, suggesting high and low levels of activation in the domain-specific network. The regions of high levels of activation corresponded retinotopically to visually stimulated parts of V1; the regions of low activation included the representation of peripheral vision in MT that was beyond the 40° drifting grating or the central 10–15° of vision that was blocked in V1. Such a spread of activation could be attributed to either a divergence of projections from V1 to MT, so that low levels of activation resulted from projections that were domain-specific but heterotopic rather than retinotopic, or the spread of activation in MT by means of a domain-specific network of intrinsic connections. What is known about connectional anatomy favors the second interpretation. Although projections of V1 and V2 to MT are somewhat divergent and patchy (20, 21), they appear to be too topographically precise (35–37) to account for all of the spread of activation. In contrast, small injections in MT produce a widespread pattern of patches of intrinsic horizontal connections that tend to favor orientation domains that match the domain of the injection site and avoid orthogonal domains (22). As Malach et al. (22) point out, the tendency for orientation domains of similar orientation preference to be connected “appears to be a common feature across species and cortical areas” (38–40). Thus, highly activated parts of MT in specific orientation domains would activate neurons at low levels in matching orientation domains in parts of MT not directly activated by V1.
Supplementary Material
Acknowledgments
We thank Dr. Anna Roe for helpful comments on the manuscript and Laura Trice, Mary Varghese, and Julia Mavity-Hudson for assistance with histology and image processing. This work was supported by National Eye Institute Grants EY002686 (to J.H.K.) and EY01778 (to V.A.C.) and National Center for Research Resources Grant IS10RR13947 (to V.A.C.).
Author contributions: C.E.C., X.X., P.M.K., V.A.C., and J.H.K. designed research; C.E.C., X.X., I.K., P.M.K., and V.A.C. performed research; C.E.C., X.X., and I.K. analyzed data; and C.E.C., X.X., V.A.C., and J.H.K. wrote the paper.
Abbreviations: MT, middle temporal area; V1, primary visual cortex; OD, orientation difference; HM, horizontal meridian; ROI, region of interest.
Footnotes
Khaytin, I., Xu, X., Collins, C. E., Kaskan, P. M., Shima, D. W., Kaas, J. H. & Casagrande, V. A. (2004) J. Vision 4, 279a (abstr.).
References
- 1.Zeki, S. (1980) Proc. R. Soc. London Ser. B 207, 239–248. [DOI] [PubMed] [Google Scholar]
- 2.Felleman, D. J. & Kaas, J. H. (1984) J. Neurophysiol. 52, 488–513. [DOI] [PubMed] [Google Scholar]
- 3.Malonek, D., Tootell, R. B. & Grinvald, A. (1994) Proc. R. Soc. London Ser. B 258, 109–119. [DOI] [PubMed] [Google Scholar]
- 4.Xu, X., Collins, C. E., Kaskan, P. M., Khaytin, I., Kaas, J. H. & Casagrande, V. A. (2004) Proc. Natl. Acad. Sci. USA 101, 2566–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salzman, C. D., Murasugi, C. M., Britten, K. H. & Newsome, W. T. (1992) J. Neurosci. 12, 2331–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiller, P. H. & Malpeli, J. G. (1977) Brain Res. 126, 366–369. [DOI] [PubMed] [Google Scholar]
- 7.Girard, P. & Bullier, J. (1989) J. Neurophysiol. 62, 1287–1302. [DOI] [PubMed] [Google Scholar]
- 8.Rodman, H. R., Gross, C. G. & Albright, T. D. (1989) J. Neurosci. 9, 2033–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodman, H. R., Gross, C. G. & Albright, T. D. (1990) J. Neurosci. 10, 1154–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girard, P., Salin, P. A. & Bullier, J. (1992) J. Neurophysiol. 67, 1437–1446. [DOI] [PubMed] [Google Scholar]
- 11.Barbur, J. L., Watson, J. D., Frackowiak, R. S. & Zeki, S. (1993) Brain 116, Part 6, 1293–1302. [DOI] [PubMed] [Google Scholar]
- 12.Baseler, H. A., Morland, A. B. & Wandell, B. A. (1999) J. Neurosci. 19, 2619–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiskrantz, L. (2004) Prog. Brain. Res. 144, 229–241. [DOI] [PubMed] [Google Scholar]
- 14.Rosa, M. G., Tweedale, R. & Elston, G. N. (2000) J. Neurosci. 20, 5552–5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaas, J. H. & Krubitzer, L. A. (1992) Visual Neurosci. 9, 399–407. [DOI] [PubMed] [Google Scholar]
- 16.Collins, C. E., Lyon, D. C. & Kaas, J. H. (2003) J. Neurosci. 23, 2251–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allman, J. M. & Kaas, J. H. (1971a) Brain Res. 85, 85–105. [DOI] [PubMed] [Google Scholar]
- 18.Allman, J. M., Kaas, J. H. & Lane, R. H. (1973) Brain Res. 57, 197–202. [DOI] [PubMed] [Google Scholar]
- 19.Gattass, R. & Gross, C. G. (1981) J. Neurophysiol. 46, 621–638. [DOI] [PubMed] [Google Scholar]
- 20.Rockland, K. S. (1989) Visual Neurosci. 3, 155–170. [DOI] [PubMed] [Google Scholar]
- 21.Rockland, K. S. (1995) J. Comp. Neurol. 355, 15–26. [DOI] [PubMed] [Google Scholar]
- 22.Malach, R., Schirman, T. D., Harel, M., Tootell, R. B. & Malonek, D. (1997) Cereb. Cortex 7, 386–393. [DOI] [PubMed] [Google Scholar]
- 23.Xu, X., Bosking, W., Sary, G., Stefansic, J., Shima, D. & Casagrande, V. (2004) J. Neurosci. 24, 6237–6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chapman, B., Zahs, K. R. & Stryker, M. P. (1991) J. Neurosci. 11, 1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chatterjee, S. & Callaway, E. M. (2003) Nature 426, 668–671. [DOI] [PubMed] [Google Scholar]
- 26.Tootell, R. B., Hamilton, S. L. & Silverman, M. S. (1985) J. Neurosci. 5, 2786–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allman, J. M. & Kaas, J. H. (1971b) Brain Res. 35, 89–106. [DOI] [PubMed] [Google Scholar]
- 28.Rosa, M. G., Casagrande, V. A., Preuss, T. & Kaas, J. H. (1997) J. Neurophysiol. 77, 3193–3217. [DOI] [PubMed] [Google Scholar]
- 29.Casagrande, V. A. & Kaas, J. H. (1994) in Cerebral Cortex: Primary Visual Cortex in Primates, eds. Peters, A. & Rockland, K. S. (Plenum, New York), Vol. 10, pp. 201–259. [Google Scholar]
- 30.Fries, W. (1981) Proc. R. Soc. London Ser. B 213, 73–86. [DOI] [PubMed] [Google Scholar]
- 31.Stepniewska, I., Qi, H. X. & Kaas, J. H. (1999) Eur. J. Neurosci. 11, 469–480. [DOI] [PubMed] [Google Scholar]
- 32.Sincich, L. C., Park, K. F., Wohlgemuth, M. J. & Horton, J. C. (2004) Nat. Neurosci. 7, 1123–1128. [DOI] [PubMed] [Google Scholar]
- 33.Zeki, S. & Ffytche, D. H. (1998) Brain 121, Part 1, 25–45. [DOI] [PubMed] [Google Scholar]
- 34.Fendrich, R., Wessinger, C. M. & Gazzaniga, M. S. (2001) Prog. Brain Res. 134, 353–366. [DOI] [PubMed] [Google Scholar]
- 35.Lin, C. S., Weller, R. E. & Kaas, J. H. (1982) J. Comp. Neurol. 211, 165–176. [DOI] [PubMed] [Google Scholar]
- 36.Cusick, C. G. & Kaas, J. H. (1988a) Brain Res. 458, 383–388. [DOI] [PubMed] [Google Scholar]
- 37.Cusick, C. G. & Kaas, J. H. (1988b) Visual Neurosci. 1, 211–237. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert, C. D. & Wiesel, T. N. (1989) J. Neurosci. 9, 2432–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malach, R., Amir, Y., Harel, M. & Grinvald, A. (1993) Proc. Natl. Acad. Sci. USA 90, 10469–10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malach, R., Tootell, R. B. & Malonek, D. (1994) Cereb. Cortex 4, 151–165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.