Abstract
Sulfur and ammonia nitrogen are rich nutrient pollutants, after entering water can cause algal blooms, cause eutrophication of water body, the spread of them will not only pollute the environment, destroy the ecological balance, but also harm human health through food chain channels, especially drinking-water toxicosis. Acticarbon can adsorb harmful substances, it was beneficial for people’s health. In order to figure out the optimal adsorption condition and the intrinsic change of acticarbon, five chemicals were adsorbed by acticarbon and analyzed by FT-IR. The optimal adsorption condition of Fe2(SO4)3, Na2SO4, Na2S2O8, S and Na2SO3 was 9 g/1000 g at 80 min, 21 g/1000 g at 20 min, 15g/1000 g at 20 min, 21 g/1000 g at 60 min and 21 g/1000 g at 100 min, respectively. FT-IR spectra showed that acticarbon had eight characteristic peaks, such as S-S stretch, H2O stretch, O—H stretch, —C—H stretch, C O or C C stretch, CH2 bend, C—H were at 3850 cm−1, 3740 cm−1, 3435 cm−1, 2925 cm−1, 1630 cm−1, 1390 cm−1, 1115 cm−1, 600 cm−1, respectively. For FT-IR spectra of Fe2(SO4)3, the peaks at 3850 cm−1, 3740 cm−1, 2925 cm−1 achieved the maximum with 9 g/1000 g at 20 min. For Na2SO4, the peaks at 2925 cm−1, 1630 cm−1, 1390 cm−1, 1115 cm−1, 600 cm−1 achieved the maximum with 21 g/1000 g at 120 min. For ones of Na2S2O8, the peaks at 3850 cm−1, 3740 cm−1, 1390 cm−1, 1115 cm−1, 600 cm−1, achieved the maximum with 2 g/1000 g at 80 min. For ones of S, the peaks at 3850 cm−1, 3740 cm−1, 2925 cm−1 achieved the maximum with 19 g/1000 g at 100 min, the peaks at 1390 cm−1, 1115 cm−1, 600 cm−1 achieved the maximum with 19 g/1000 g at 20 min. For FT-IR spectra of Na2SO3, the peaks at 1630 cm−1, 1390 cm−1, 1115 cm−1, 600 cm−1 achieved the maximum with 2 g/1000 g at 100 min. It provided that acticarbon could adsorb and desulphurize from sulfur solution against drinking-water toxicosis.
Keywords: Acticarbon, Drinking-water toxicosis, Fe2(SO4)3, Na2S2O8, Na2SO3
1. Introduction
Sulfur and ammonia nitrogen mainly come from chemical fertilizers, processed meat, leather and other industry emissions of industrial waste water, and city life sewage and farmland irrigation and drainage (He et al., 2008, Mao et al., 2000, Chai et al., 2010). Sulfur and ammonia nitrogen are rich nutrient pollutants, after entering water can cause algal blooms, cause eutrophication of water body, the spread of them will not only pollute the environment, destroy the ecological balance, but also harm human health through food chain channels, such as drinking-water toxicosis. Ecological effects of acid precipitation can be determined from the timing of changes in lake chemistry or acid-sensitive micro fossils and metallic pollutants in sediments. This is thought to be a result of a few factors: increased construction of large power plants and smelters with tall smokestacks coupled with a decrease in use of coal for home heating, converting the local air pollution problem into along-range, transboundary one; emissions of NOx and other pollutants that aid in the oxidation of sulfur and nitrogen oxide have increased; and it took years for lakes, streams and their catchments to lose their buffering capabilities, so that lower pH levels were not recognized until sometime after the precipitation became acidic. Anthropogenic emissions are comparable to natural emissions on the global level, but regionally over 90% of sulfur deposited from the atmosphere is anthropogenic (Schindler, 1988).
Acticarbon can use almost any type of carbon materials, such as wood (Wang et al., 2009a), sawdust (Zhang et al., 2010), coal (Ahmadpour and Do, 1996), shells (Chandra et al., 2009), the stone of the fruit (Jumasiah et al., 2005), bagasse, oil waste, waste plastics (Zhou et al., 2007), paper and leather scrap (Yuan et al., 2004), waste tires and urban waste (Wang, 2004). Acticarbon with highly developed porous structure and huge specific surface area (Ding et al., 2002), good chemical stability and thermal stability, high mechanical strength and surface contains a variety of oxygen containing functional groups (Yu et al., 2005 and Pu and Jiang, 2005). What’s more, acticarbon, which contained potassium, calcium and other minerals, could have adsorption and filtration of extractives, oil, other matters (Peng et al., 2013a, Peng et al., 2013b, Peng et al., 2013c, Peng et al., 2014a, Peng et al., 2012, Peng et al., 2011, Xiao et al., 2013, Wang et al., 2013, Peng and Le, 2012, Peng et al., 2011, Liu et al., 2008, Zhang et al., 2008, Qi et al., 2012). The fabric inhibited bacterial metabolism causing fewer allergic skin reactions than other fibers sterilized with antimicrobial agents. Because the trait was due to the highly porous structure of the bamboo fabric, it could absorb sulfur-based compounds, nitrogen-based compounds and so on (Milena et al., 2003, Ikuo et al., 2001, Masakazu et al., 2003, Kei et al., 1994, Wang et al., 2006, Xue et al., 2014, Cui et al., 2014). In order to figure out the optimal adsorption condition and the intrinsic change of the acticarbon, five chemicals were adsorbed by acticarbon and were analyzed by FT-IR.
2. Materials and methods
2.1. Materials
Acticarbon, Fe2(SO4)3, Na2SO4, Na2S2O8, S, and Na2SO3 were purchased from the market.
2.2. Methods
The Fe2(SO4)3 powder was weighed in quantities of 5 g, 9 g, and 21 g . These powder and 4 g over dry acticarbon were put into beaker which equipped with 1000 ml water, respectively.It was stirred in a beaker for 20 min, 80 min and 120 min. The Na2SO4 powder was weighed in quantities of 11 g, 19 g, and 21 g. These powder and 4 g over dry acticarbon were put into beaker which equipped with 1000 ml water, respectively. It was stirred in a beaker for 20 min, 60 min and 120 min, respectively. The Na2S2O8 powder was weighed in quantities of 2 g, 11 g, and 15 g. These powder and 4 g over dry acticarbon were put into beaker which equipped with 1000 ml water, respectively. It was stirred in a beaker for 20 min, 80 min and 100 min. The S powder was weighed in quantities of 2 g, 19 g, 21 g. These powder and 4 g over dry acticarbon were put into beaker which equipped with 1000 ml water, respectively. It was stirred in a beaker for 20 min, 60 min and 100 min. The Na2SO3 powder was weighed in quantities of 2 g, 11 g, 21 g. These powders and 4 g over dry acticarbon were put into a beaker which contained 1000 ml water. It was stirred in a beaker for 20 min, 40 min and 100 min. Each acticarbon was removed, dried, and weighed.
FT-IR spectra. FT-IR spectra of the above samples were obtained using a Thermo Scientific Nicolet iN10 FT-IR microscope as previously (Lin et al., 2015, Peng et al., 2014b, Peng et al., 2015, Sun et al., 2014, Wang et al., 2009, Parag and Bhanu, 2013.
3. Results and analysis
Based on the above test, the results of adsorption were obtained and listed in Table 1.
Table 1.
Adsorption results.
| SC (%) | Fe2(SO4)3 | SC (%) | Na2SO4 | SC (%) | Na2S2O8 | SC (%) | S | SC (%) | Na2SO3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stir time (min) |
Stir time (min) |
Stir time (min) |
Stir time (min) |
Stir time (min) |
|||||||||||||||
| 20 | 80 | 120 | 20 | 60 | 120 | 20 | 80 | 100 | 20 | 60 | 100 | 20 | 40 | 100 | |||||
| 0.5 | 3.76 | 2.49 | 1 | 1.1 | 1.25 | 0.75 | 2.51 | 0.2 | 0.5 | 1.79 | 1.03 | 0.2 | 7.75 | 4.52 | 4.73 | 0.2 | 0 | 2.74 | 1.75 |
| 0.9 | 1.75 | 23.7 | 0.5 | 1.9 | 3.52 | 1.99 | 2.26 | 1.1 | 8.25 | 3.23 | 2.23 | 1.9 | 37.3 | 58.6 | 11.5 | 1.1 | 2.74 | 2.74 | 2.26 |
| 2.1 | 17.5 | 18.5 | 2.75 | 2.1 | 8.21 | 1.75 | 2.99 | 1.5 | 20.1 | 12.7 | 2.19 | 2.1 | 37.7 | 80.3 | 32.8 | 2.1 | 1.25 | 2.26 | 4.26 |
Note: SC – Concentration of sulfur solution.
3.1. SC Effect
Based on Table 1, when the concentrations of Fe2(SO4)3 were 5 g/1000 g, 9 g/1000 g, 21 g/1000 g, Fe2(SO4)3’s adsorption capacities were 3.76 g/100 g, 1.75 g/100 g, 1.75 g/100 g, 2.49 g/100 g, 23.7 g/100 g, 18.5 g/100 g, 1 g/100 g, 0.5 g/100 g, 2.75 g/100 g for stir times of 20 min, 80 min, 120 min, respectively. When the concentrations of Na2SO4 were 11 g/1000 g, 19 g/1000 g, 21 g/1000 g, Na2SO4’s adsorption capacities were 1.25 g/100 g, 3.52 g/100 g, 8.21 g/100 g, 0.75 g/100 g, 1.99 g/100 g, 1.75 g/100 g, 2.51 g/100 g, 2.26 g/100 g, 2.99 g/100 g for the stir time of 20 min, 60 min, 120 min, respectively. When the concentrations of Na2S2O8 were 2 g/1000 g, 11 g/1000 g, 15 g/1000 g, Na2S2O8’s adsorption capacities were 0.5 g/100 g, 8.25 g/100 g, 20.1 g/100 g, 1.79 g/100 g, 3.23 g/100 g, 12.7 g/100 g, 1.03 g/100 g, 2.23 g/100 g, 2.19 g/100 g for the stir time of 20 min, 80 min, 100 min, respectively. When the concentrations of S were 2 g/1000 g, 19 g/1000 g, 21 g/1000 g, S’s adsorption capacities were 7.75 g/100 g, 37.3 g/100 g, 37.7 g/100 g, 4.52 g/100 g, 58.6 g/100 g, 80.3 g/100 g, 4.73 g/100 g, 11.5 g/100 g, 32.8 g/100 g for the stir time of 20 min, 60 min, 100 min, respectively. When the concentrations of Na2SO3 were 2 g/1000 g, 11 g/1000 g, 21 g/1000 g, Na2SO3’s adsorption capacities were 0 g/100 g, 2.74 g/100 g, 1.25 g/100 g, 2.74 g/100 g, 2.74 g/100 g, 2.26 g/100 g, 1.75 g/100 g, 2.26 g/100 g, 4.26 g/100 g for the stir time of 20 min, 40 min, 100 min, respectively. It showed that adsorption capacity changed at regularity difference. It might be because rapid stirring lead to a small amount of chemical medicine that was adsorbed by the acticarbon. The Fe2(SO4)3’s optimal adsorption condition were the concentration was 9 g/1000 g and stir 80 min, the Na2SO4’s optimal adsorption condition was the concentration of 21 g/1000 g and stir time of 20 min, the Na2S2O8’s optimal adsorption condition was the concentration of 15 g/1000 g and stir time of 20 min, the S’s optimal adsorption condition was the concentration of 21 g/1000 g and stir time of 60 min and the Na2SO3’s optimal adsorption condition was the concentration of 21 g/1000 g and stir time of 100 min.
3.2. FT-IR analysis
FT-IR spectra were recorded to investigate the functional groups of acticarbon during adsorption of Fe2(SO4)3, Na2SO4, Na2S2O8, S, and Na2SO3.
Spectra of the samples were shown in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5. In the spectrum of adsorption, the S-S stretch, H2O stretch, O—H stretch, —C—H stretch, C O or C C stretch, CH2 bend, C—H, were observed at 3850 cm−1, 3740 cm−1, 3435 cm−1, 2925 cm−1, 1630 cm−1, 1390 cm−1, 1115 cm−1, 600 cm−1, respectively (listed in Table 2). For FT-IR spectra of Fe2(SO4)3, the transmissivity of the peaks at 3850 cm−1, 3740 cm−1, 2925 cm−1 achieved the maximum for 20 min and the concentration was 9 g/1000 g, the transmissivity of the peaks at 3435 cm−1, 600 cm−1 achieved the maximum for 120 min and the concentration was 5 g/1000 g, the transmissivity of the peaks at 1630 cm−1 achieved the maximum for 120 min and the concentration was 21 g/1000 g, the transmissivity of the peaks at 1390 cm−1, 1115 cm−1 achieved the maximum for 120 min and the concentration was 9 g/1000 g.
Figure 1.
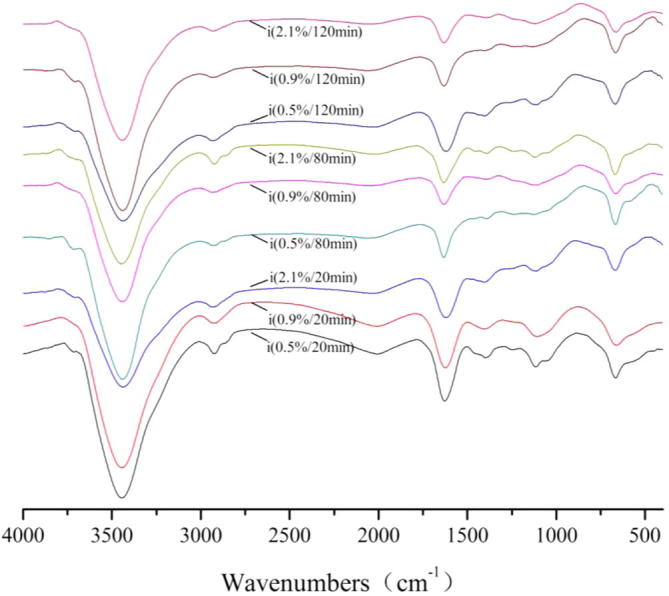
FT-IR spectra of acticarbon during adsorption of Fe2(SO4)3 solution.
Figure 2.
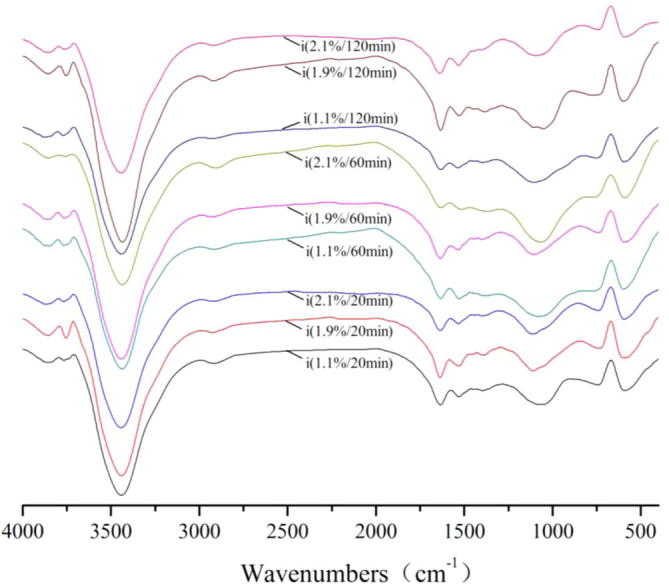
FT-IR spectra of acticarbon during adsorption of Na2SO4 solution.
Figure 3.
FT-IR spectra of acticarbon during adsorption of Na2S2O8 solution.
Figure 4.
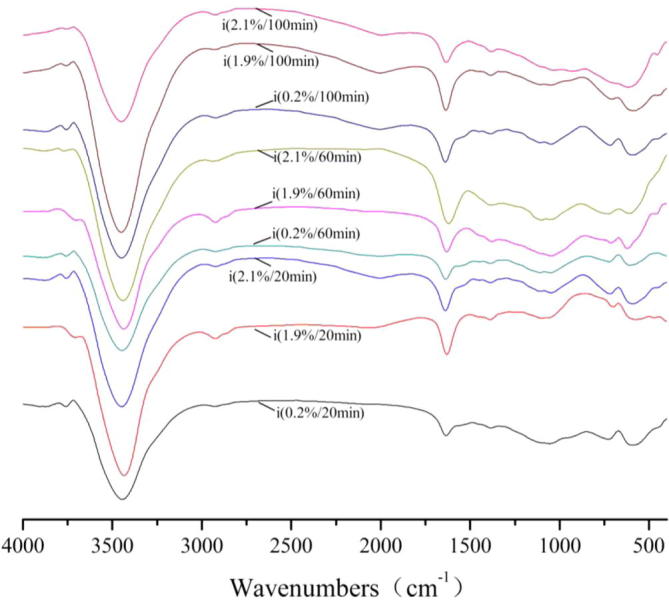
FT-IR spectra of acticarbon during adsorption of S solution.
Figure 5.
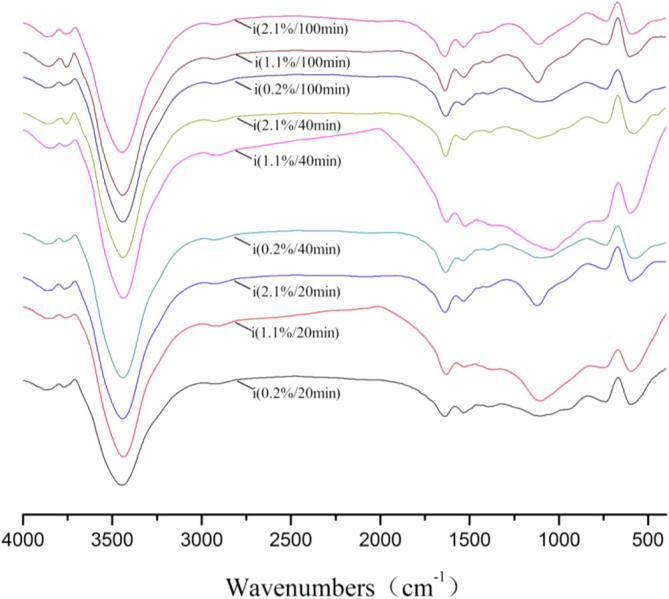
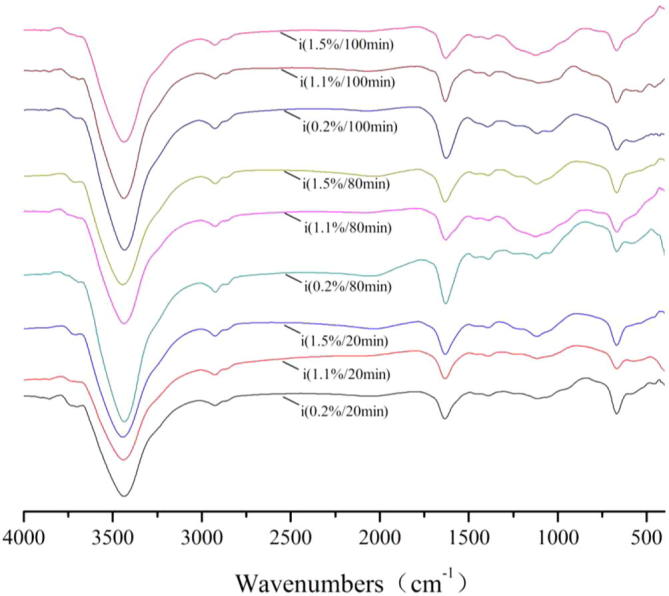
FT-IR spectra of acticarbon during adsorption of Na2SO3 solution.
Table 2.
Groups of acticarbon during adsorption of Fe2(SO4)3, Na2SO4, Na2S2O8, S and Na2SO3 (%).
| Kind | Peak (cm−1) | Adsorption time (min)/concentration (%) |
Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20/0.5 | 20/0.9 | 20/2.1 | 80/0.5 | 80/0.9 | 80/2.1 | 120/0.5 | 120/0.9 | 120/2.1 | |||
| Fe2(SO4)3 | 600 | 88.2 | 89.1 | 83.5 | 83.4 | 90.9 | 90.0 | 95.3 | 94.2 | 90.2 | C—H |
| 1115 | 86.8 | 88.2 | 82.5 | 80.1 | 88.8 | 89.5 | 92.2 | 92.6 | 89.9 | C—O stretch | |
| 1390 | 87.1 | 89.5 | 84.2 | 83.4 | 88.8 | 90.0 | 91.2 | 91.9 | 90.5 | CH2 bend | |
| 1630 | 84.7 | 84.2 | 82.0 | 82.6 | 86.1 | 86.6 | 86.8 | 87.2 | 87.3 | C O or C C | |
| 2925 | 88.8 | 89.5 | 88.6 | 88.2 | 88.8 | 88.6 | 87.9 | 88.8 | 89.0 | —C—H stretch | |
| 3435 | 76.1 | 73.4 | 74.6 | 72.6 | 76.4 | 77.4 | 79.1 | 73.5 | 76.4 | O—H stretch | |
| 3740 | 88.1 | 90.6 | 89.8 | 88.3 | 88.2 | 89.6 | 89.9 | 88.9 | 89.7 | H2O | |
| 3850 | 88.1 | 90.6 | 89.7 | 88.2 | 88.3 | 90.2 | 90.0 | 89.5 | 90.1 | S-S stretch | |
| Na2SO4 | Peak (cm−1) |
20/1.1 |
20/1.9 |
20/2.1 |
60/1.1 |
60/1.9 |
60/2.1 |
120/1.1 |
120/1.9 |
120/2.1 |
Group |
| 600 | 85.8 | 86.1 | 87.5 | 83.9 | 83.2 | 84.2 | 86.5 | 85.5 | 90.1 | C—H | |
| 1115 | 84.5 | 84.7 | 85.6 | 81.3 | 83.6 | 80.2 | 84.4 | 82.8 | 88.1 | C—O stretch | |
| 1390 | 85.7 | 86.2 | 87.5 | 83.2 | 86.0 | 83.0 | 86.2 | 84.4 | 88.7 | CH2 bend | |
| 1630 | 83.9 | 84.1 | 85.8 | 82.6 | 84.0 | 82.7 | 85.4 | 82.4 | 86.1 | C O or C C | |
| 2925 | 88.3 | 88.5 | 89.1 | 87.8 | 86.9 | 87.2 | 88.7 | 87.4 | 89.2 | —C—H stretch | |
| 3435 | 75.5 | 74.6 | 76.8 | 76.1 | 75.2 | 75.6 | 77.4 | 71.8 | 76.8 | O—H stretch | |
| 3740 | 88.5 | 88.1 | 89.0 | 87.9 | 89.5 | 87.9 | 89.2 | 87.9 | 88.5 | H2O | |
| 3850 | 88.4 | 88.1 | 88.7 | 87.9 | 89.8 | 88.0 | 88.8 | 88.0 | 88.4 | S-S stretch | |
| Na2S2O8 | Peak (cm−1) |
20/0.2 |
20/1.1 |
20/1.5 |
80/0.2 |
80/1.1 |
80/1.5 |
100/0.2 |
100/1.1 |
100/1.5 |
Group |
| 600 | 90.3 | 73.4 | 90.4 | 94.0 | 84.1 | 85.1 | 86.3 | 87.6 | 89.2 | C—H | |
| 1115 | 89.6 | 73.6 | 89.0 | 92.0 | 79.7 | 84.2 | 86.9 | 88.4 | 86.8 | C—O stretch | |
| 1390 | 89.9 | 73.8 | 90.0 | 91.9 | 83.2 | 85.9 | 87.8 | 89.3 | 88.7 | CH2 bend | |
| 1630 | 86.9 | 70.9 | 86.5 | 86.1 | 82.7 | 85.3 | 83.4 | 85.9 | 86.2 | C O or C C | |
| 2925 | 88.6 | 71.4 | 88.9 | 87.7 | 87.8 | 88.8 | 87.3 | 88.8 | 87.9 | —C—H stretch | |
| 3435 | 77.3 | 60.9 | 76.4 | 71.6 | 74.7 | 79.2 | 72.4 | 73.9 | 75.9 | O—H stretch | |
| 3740 | 88.6 | 70.7 | 89.5 | 89.8 | 87.7 | 88.8 | 89.5 | 89.2 | 89.4 | H2O | |
| 3850 | 89.5 | 71.0 | 90.1 | 90.2 | 87.7 | 88.7 | 89.7 | 89.9 | 89.9 | S-S stretch | |
| S | Peak (cm−1) |
20/0.2 |
20/1.9 |
20/2.1 |
60/0.2 |
60/1.9 |
60/2.1 |
100/0.2 |
100/1.9 |
100/2.1 |
Group |
| 600 | 85.9 | 90.8 | 87.5 | 89.2 | 86.8 | 83.5 | 89.3 | 86.3 | 84.9 | C—H | |
| 1115 | 86.1 | 90.8 | 88.6 | 88.3 | 85.7 | 82.5 | 89.0 | 88.8 | 86.9 | C—O stretch | |
| 1390 | 87.6 | 90.6 | 89.5 | 89.2 | 86.9 | 84.2 | 89.8 | 89.7 | 88.2 | CH2 bend | |
| 1630 | 86.7 | 86.8 | 86.7 | 87.5 | 85.6 | 82.0 | 86.6 | 85.9 | 87.1 | C O or C C | |
| 2925 | 89.7 | 88.6 | 91.1 | 90.4 | 88.7 | 88.6 | 88.8 | 92.3 | 92.0 | —C—H stretch | |
| 3435 | 80.5 | 75.0 | 77.2 | 80.6 | 78.1 | 74.6 | 74.8 | 74.2 | 81.4 | O—H stretch | |
| 3740 | 90.1 | 89.2 | 90.1 | 90.1 | 89.4 | 89.8 | 89.4 | 90.8 | 90.6 | H2O | |
| 3850 | 89.8 | 89.9 | 89.8 | 89.9 | 90.0 | 89.7 | 90.0 | 90.5 | 90.4 | S-S stretch | |
| Na2SO3 | Peak (cm−1) |
20/0.2 |
20/1.1 |
20/2.1 |
40/0.2 |
40/1.1 |
40/2.1 |
100/0.2 |
100/1.1 |
100/2.1 |
Group |
| 600 | 87.6 | 83.8 | 89.5 | 87.6 | 82.0 | 88.1 | 89.6 | 88.9 | 88.9 | C—H | |
| 1115 | 86.4 | 80.8 | 86.7 | 87.4 | 78.7 | 87.5 | 89.7 | 86.1 | 87.4 | C—O stretch | |
| 1390 | 87.2 | 83.9 | 88.7 | 88.1 | 80.7 | 88.0 | 90.0 | 88.4 | 88.8 | CH2 bend | |
| 1630 | 86.2 | 83.0 | 86.1 | 85.6 | 80.5 | 85.6 | 86.6 | 86.8 | 86.4 | C O or C C | |
| 2925 | 89.5 | 88.0 | 89.2 | 89.2 | 87.3 | 89.1 | 88.8 | 87.9 | 89.6 | —C—H stretch | |
| 3435 | 80.0 | 75.6 | 76.4 | 76.1 | 73.7 | 76.3 | 75.4 | 76.8 | 77.6 | O—H stretch | |
| 3740 | 89.2 | 88.5 | 88.4 | 88.9 | 87.7 | 89.0 | 89.1 | 89.4 | 88.3 | H2O | |
| 3850 | 88.9 | 88.6 | 88.5 | 88.7 | 87.8 | 88.8 | 89.9 | 90.1 | 88.4 | S-S stretch | |
For FT-IR spectra of Na2SO4, the transmissivity of the peaks at 3850 cm−1, 3740 cm−1, achieved the maximum for 60 min and the concentration was 11 g/1000 g, the transmissivity of the peaks at 3435 cm−1 achieved the maximum for 120 min and the concentration was 11 g/1000 g, the transmissivity of the peaks at 2925 cm−1, 1630 cm−1, 1390 cm−1, 1115 cm−1, 600 cm−1 achieved the maximum for 120 min and the concentration was 21 g/1000 g.
For FT-IR spectra of Na2S2O8, the transmissivity of the peaks at 3850 cm−1, 3740 cm−1, 1390 cm−1, 1115 cm−1, 600 cm−1, achieved the maximum for 80 min and the concentration was 2 g/1000 g, the transmissivity of the peaks at 3435 cm−1 achieved the maximum for 80 min and the concentration was 15 g/1000 g, the transmissivity of the peaks at 2925 cm−1 achieved the maximum for 20 min and the concentration was 15 g/1000 g, the transmissivity of the peaks at 1630 cm−1 achieved the maximum for 20 min and the concentration was 2 g/1000 g.
For FT-IR spectra of S, the transmissivity of the peaks at 3850 cm−1, 3740 cm−1, 2925 cm−1 achieved the maximum for 100 min and the concentration was 19 g/1000 g, the transmissivity of the peaks at 3435 cm−1 achieved the maximum for 100 min and the concentration was 21 g/1000 g, the transmissivity of the peaks at 1630 cm−1 achieved the maximum for 60 min and the concentration was 2 g/1000 g, the transmissivity of the peaks at 1390 cm−1, 1115 cm−1, 600 cm−1 achieved the maximum for 20 min and the concentration was 19 g/1000 g.
For FT-IR spectra of Na2SO3, the transmissivity of the peaks at 3850 cm−1, 3740 cm−1 achieved the maximum for 100 min and the concentration was 11 g/1000 g, the transmissivity of the peaks at 3435 cm−1 achieved the maximum for 20 min and the concentration was 2 g/1000 g, the transmissivity of the peaks at 2925 cm−1 achieved the maximum for 100 min and the concentration was 21 g/1000 g, the transmissivity of the peaks at 1630 cm−11390 cm−1, 1115 cm−1, 600 cm−1 achieved the maximum for 100 min and the concentration was 2 g/1000 g.
4. Conclusion
As we can see from the above methods, Fe2(SO4)3’s, Na2SO4’s, Na2S2O8’s, S’s, and Na2SO3’s adsorption capacity were different for several stir times and several concentrations, respectively. The Fe2(SO4)3’s optimal adsorption condition was the concentration was 9 g/1000 g and stir time of 80 min, the Na2SO4’s optimal adsorption condition was the concentration was 21 g/1000 g and stir 20 min, the Na2S2O8’s optimal adsorption condition was the concentration of 15 g/1000 g and stir time of 20 min, the S’s optimal adsorption condition was the concentration was 21 g/1000 g and stir time of 60 min and the Na2SO3’s optimal adsorption condition were the concentration of 21 g/1000 g and stir time of 100 min.
FT-IR spectra showed that acticarbon had the eight characteristic absorption band. And the S-S stretch, H2O stretch, O—H stretch, —C—H stretch, C O or C C stretch, CH2 bend, C—H, were observed at 3850 cm−1, 3740 cm−1, 3435 cm−1, 2925 cm−1, 1630 cm−1, 1390 cm−1, 1115 cm−1, 600 cm−1, respectively. For FT-IR spectra of Fe2(SO4)3, the transmissivity of the peaks at 3850 cm−1, 3740 cm−1, 2925 cm−1 achieved the maximum for 20 min and the concentration was 9 g/1000 g. For FT-IR spectra of Na2SO4, the transmissivity of the peaks at 2925 cm−1, 1630 cm−1, 1390 cm−1, 1115 cm−1, 600 cm−1 achieved the maximum for 120 min and the concentration was 21 g/1000 g. For FT-IR spectra of Na2S2O8, the transmissivity of the peaks at 3850 cm−1, 3740 cm−1, 1390 cm−1, 1115 cm−1, 600 cm−1, achieved the maximum for 80 min and the concentration was 2 g/1000 g. For FT-IR spectra of S, the transmissivity of the peaks at 3850 cm−1, 3740 cm−1, 2925 cm−1 achieved the maximum for 100 min and the concentration was 19 g/1000 g, the transmissivity of the peaks at 1390 cm−1, 1115 cm−1, 600 cm−1 achieved the maximum for 20 min and the concentration was 19 g/1000 g. For FT-IR spectra of Na2SO3, the transmissivity of the peaks at 1630 cm−1, 1390 cm−1, 1115 cm−1, 600 cm−1 achieved the maximum for 100 min and the concentration was 2 g/1000 g. In these states, the number of the transmissivity of the maximum peaks is the largest.
Acknowledgment
This work was financially supported by the National 948 Plan (2014-4-38).
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmadpour A., Do D.D. The preparation of activated carbon from coal by chemical and physical activation. Carbon. 1996;34(4):471–479. [Google Scholar]
- Chai X.W., Wang Y.N., Liao X.P., et al. Leather on the determination of ammonia nitrogen in wastewater and source analysis. China Leather. 2010;17(3):332–335. [Google Scholar]
- Chandra T.C., Mirna M.M., Sunarso J., et al. Activated carbon from durian shell: Preparation and characterization. J. Taiwan Inst. Chem. Eng. 2009;40(4):457–462. [Google Scholar]
- Cui L., Peng W.X., Sun Z.J. Weibull statistical analysis of tensile strength of vascular bundle in inner layer of moso bambooculm in molecular parasitology and vector biology. Pak. J. Pharm. Sci. 2014;27(4):1083–1087. [PubMed] [Google Scholar]
- Ding L.P., Bhatia, Liu, K. S.K., Liu K. Kinetics of adsorption on activated carbon: Application of heterogeneous vacancy solution theory. Chem. Eng. Sci. 2002;57(18):3909–3928. [Google Scholar]
- He Y., Zhao Y.C., Zhou G.M. Journal of the high concentration of ammonia nitrogen wastewater denitrification technology. Ind. Water Treat. 2008;28(1):1–4. [Google Scholar]
- Ikuo A., Tomoko F., Jun M., et al. Preparation of carbonaceous adsorbents for removal of chloroform from drinking water. Carbon. 2001;39:1069–1073. [Google Scholar]
- Jumasiah A., Chuah T.G., Gimbon J., et al. Adsorption of basic dye onto palm kernel shell activated carbon: Sorption equilibrium and kinetics studies. Desalination. 2005;186(1/3):57–64. [Google Scholar]
- Kei M., Toshitatsu M., Yasuo H. Removal of nitrate-nitrogen from drinking water using bamboo powder charcoal. Bioresour. Technol. 1994;95:255. doi: 10.1016/j.biortech.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Lin Z., Ge S.B., Li D.L., Peng W.X. Structure Characteristics of Acidic Pretreated Fiber and Self-bind Bio-boards for Public Health. J. Pure Appl. Microbiol. 2015;9:221–226. [Google Scholar]
- Liu Q.M., Luo Y.S., Yin S.P., et al. Liquid rheology study on refined rapeseed oil. J. Cent. South Univ. Technol. 2008;15:525–528. [Google Scholar]
- Mao Y.T., Liu Z.Q., Qiao X.Y., et al. The analysis and research of the aquatic products processing wastewater ammonia nitrogen removing abnormal. The Prevent. Control Environ. Pollut. 2000;31(8):101–106. [Google Scholar]
- Masakazu K., Shogo T., Hyun-Min K., et al. Preparation of antibacterial silver-doped silica glass microspheres N. J. Biomed. Mater. Res. 2003;66:266. doi: 10.1002/jbm.a.10547. [DOI] [PubMed] [Google Scholar]
- Milena I., David L., Sandrine L., Christine J. Immobilization of silver in polypyrrole/polyanion composite coatings: preparation, characterization, and antibacterial activity. Langmuir. 2003;19:8971–8979. [Google Scholar]
- Parag A.P., Bhanu R. The Ft-Ir spectrometric studies of vibrational bands of semecarpus anacardium Linn.F. leaf, stem powder and extracts. Asian J. Pharm. Clin. Res. 2013;6:159–198. [Google Scholar]
- Peng W.X., Le C. Crystal structure of 3-(3-bromophenyl)-4-(3,5-dichloro-phenylamino)furan-2(5H)-one C16H10BrCl2NO2. Z. Kristallogr. – New Cryst. Struct. 2012;227(2):267–268. [Google Scholar]
- Peng W.X., Wang L.S., Wu F.J., Xu Q. 3-(4-Bromophenyl)-4-(4-hydroxyanilino)furan-2(5H)-one. Acta Crystallogr. Sect. E – Struct. Rep. Online. 2011;67 doi: 10.1107/S1600536811031849. O2329-U206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W.X., Wu F.J., Wang L.S., Xu Q. Crystal structure of 3-(4-bromophenyl)-4-(4-chlorophenylamino)furan-2(5H)-one C16H11BrClNO2. Z. Kristallogr. – New Cryst. Struct. 2012;227(1):61–62. [Google Scholar]
- Peng W.X., Lin Z., Chang J.B., Gu F.L., Zhu X.W. Biomedical Molecular Characteristics of Ybsj Extractives from Illicium Verumfruit. Biotechnol. Biotechnol. Equip. 2013;27(6):4311–4316. [Google Scholar]
- Peng W.X., Wang L.S., Lin Z., et al. Identification and Chemical Bond Characterization of Wood Extractives in Three Species of Eucalyptus Biomass. J. Pure Appl. Microbiol. 2013;7:67–73. [Google Scholar]
- Peng W.X., Wang L.S., Zhang M.L., Lin Z. Molecule characteristics of eucalyptus hemicelluloses for medical microbiology. J. Pure Appl. Microbiol. 2013;7(2):1345–1349. [Google Scholar]
- Peng W.X., Ge S.B., Li D.L., Mo B., Daochun Q., Ohkoshi M. Molecular basis of antibacterial activities in extracts of Eucommia ulmoides wood. Pak. J. Pharm. Sci. 2014;27(6):2133–2138. [PubMed] [Google Scholar]
- Peng W.X., Wang L.S., Zhang M.L., Lin Z. Separation characteristics of lignin from Eucalyptus camaldulensis lignincelluloses for biomedical cellulose. Pak. J. Pharm. Sci. 2014;27:723–728. [PubMed] [Google Scholar]
- Peng W.X., Lin Z., Chen H., Wu J.G. Biochemical Group Characteristics of Self-Bonded Boards During Acidic Oxidation for Public Health. J. Pure Appl. Microbiol. 2015;9:307–311. [Google Scholar]
- Pu H.Z., Jiang J.C. Modified activated carbon materials used for flue gas desulfurization. Forest Chem. Commun. 2005;39(5):29–33. [Google Scholar]
- Qi H.C., Peng W.X., Wu Y.Q., Wu S.B., Xu G.J. Effects of alkaline extraction on micro/nano particles of eucalyptus camaldulensis biology. J. Comput. Theor. Nanosci. 2012;9(9):1525–1528. [Google Scholar]
- Schindler D.W. Effects of acid rain on freshwater ecosystems. Science. 1988;239(4836):149–157. doi: 10.1126/science.239.4836.149. [DOI] [PubMed] [Google Scholar]
- Sun Y.C., Lin Z., Peng W.X., et al. Chemical changes of raw materials and manufactured binderless boards during hot pressing: lignin isolation and characterization. Bioresources. 2014;9(1):1055–1071. [Google Scholar]
- Wang Y.X. Journal of the activated carbon desulfurization denitration technology. Power Syst. Eng. 2004;20(6):41–42. [Google Scholar]
- Wang J.X., Wen L.X., Wang Z.H., Chen J.F. Immobilization of silver on hollow silica nanospheres and nanotubes and their antibacterial effects. Mater. Chem. Phys. 2006;96(1):90–97. [Google Scholar]
- Wang T.H., Tan S.X., Liang C.H. Preparation and characterization of activated carbon from wood via microwave-induced ZnCl2 activation. Carbon. 2009;47(7):1880–1883. [Google Scholar]
- Wang Z.Z., Lv P., Hu Y., Hu K.L. Thermal degradation study of intumescent flame retardants by TG and FTIR: melamine phosphate and its mixture with pentaerythritol. J. Anal. Appl. Pyrolysis. 2009;86:207–214. [Google Scholar]
- Wang L.S., Peng W.X., Zhang M.L., Lin Z. Separation characteristics of lignin from eucalyptus lignincellulose for medicinal biocellulose preparation. J. Pure Appl. Microbiol. 2013;7:59–66. [Google Scholar]
- Xiao Z.P., Peng Z.Y., Dong J.J., et al. Synthesis molecular docking and kinetic properties of beta-hydroxy-beta-phenylpropionyl- hydroxamic acids as Helicobacter pylori urease inhibitors. Eur. J. Med. Chem. 2013;68:212–221. doi: 10.1016/j.ejmech.2013.07.047. [DOI] [PubMed] [Google Scholar]
- Xue Q., Peng W.X., Ohkoshi M. Molecular bonding characteristics of Self-plasticized bamboo composites. Pak. J. Pharm. Sci. 2014;27:975–982. [PubMed] [Google Scholar]
- Yu G.X., Lu S.X., Chen H., et al. Diesel fuel desulfurization with hydrogen peroxide promoted by formic acid and catalyzed by activated carbon. Carbon. 2005;43(11):2285–2294. [Google Scholar]
- Yuan C.S., Lin H.Y., Wu C.H., et al. Preparation of sulfurized powdered activated carbon from waste tires using an innovative compositive impregnation process. J. Air Waste Manag. Assoc. 2004;54(7):862–870. doi: 10.1080/10473289.2004.10470954. [DOI] [PubMed] [Google Scholar]
- Zhang D.Q., Chen S.M., Peng W.X., et al. Rheology study of supercritically extracted tea-oil. J. Cent. South Univ. Technol. 2008;15:506–508. [Google Scholar]
- Zhang H.O., Yan Y., Yang L.C. Preparation of activated carbon from sawdust by zinc chloride activation. Adsorption. 2010;16(3):161–166. [Google Scholar]
- Zhou P.Y., Qian Q.R., Xiao L.R., et al. Preparation of polyvinyl chloride (PVC) plastic waste activated carbon fiber. Plast. Ind. 2007;10(1):34–37. [Google Scholar]


