Abstract
Introduction
Deep dyspareunia negatively affects women’s sexual function. There is a known association between deep dyspareunia and endometriosis of the cul-de-sac or uterosacral ligaments in reproductive-age women; however, other factors are less clear in this population.
Aim
To identify anatomic sites and associated clinical factors for deep dyspareunia in reproductive-age women at a referral center.
Methods
This study involved the analysis of cross-sectional baseline data from a prospective database of 548 women (87% consent rate) recruited from December 2013 through April 2015 at a tertiary referral center for endometriosis and/or pelvic pain. Exclusion criteria included menopausal status, age at least 50 years, previous hysterectomy or oophorectomy, and not sexually active. We performed a standardized endovaginal ultrasound-assisted pelvic examination to palpate anatomic structures for tenderness and reproduce deep dyspareunia. Multivariable regression was used to determine which tender anatomic structures were independently associated with deep dyspareunia severity and to identify clinical factors independently associated with each tender anatomic site.
Main Outcome Measure
Severity of deep dyspareunia on a numeric pain rating scale of 0 to 10.
Results
Severity of deep dyspareunia (scale = 0–10) was independently associated with tenderness of the bladder (b = 0.88, P = .018), pelvic floor (levator ani) (b = 0.66, P = .038), cervix and uterus (b = 0.88, P = .008), and cul-de-sac or uterosacral ligaments (b = 1.39, P < .001), but not with the adnexa (b = −0.16, P = 0.87). The number of tender anatomic sites was significantly correlated with more severe deep dyspareunia (Spearman r = 0.34, P < .001). For associated clinical factors, greater depression symptom severity was specifically associated with tenderness of the bladder (b = 1.05, P = .008) and pelvic floor (b = 1.07, P < .001). A history of miscarriage was specifically associated with tenderness of the cervix and uterus (b = 2.24, P = .001). Endometriosis was specifically associated with tenderness of the cul-de-sac or uterosacral ligaments (b = 3.54, P < .001).
Conclusions
In reproductive-age women at a tertiary referral center, deep dyspareunia was independently associated not only with tenderness of the cul-de-sac and uterosacral ligaments but also with tenderness of the bladder, pelvic floor, and cervix and uterus.
Yong PJ, Williams C, Yosef A, et al. Anatomic Sites and Associated Clinical Factors for Deep Dyspareunia. Sex Med 2017;5:e184–e195.
Key Words: Bladder, Depression, Dyspareunia, Endometriosis, Miscarriage, Pelvic Floor
Introduction
Endometriosis affects 10% of reproductive-age women, and approximately half of women with endometriosis have deep dyspareunia (pelvic pain with intercourse).1, 2, 3 The consequences of deep dyspareunia have been demonstrated in multiple studies, including negative effects on sexual function, relationships, and quality of life.4, 5, 6, 7 Deep dyspareunia is differentiated from superficial dyspareunia (introital pain with intercourse),6 which is due primarily to vulvodynia.
There has been a call for more research into the pathophysiology of deep dyspareunia.2, 8 Endometriosis of the cul-de-sac or uterosacral ligaments, especially deep (infiltrating) endometriosis of this region, is a known risk factor for deep dyspareunia.9, 10, 11 Treatment trials also have shown that management of endometriosis can alleviate deep dyspareunia in some patients.6, 12, 13, 14 However, there is still phenotypic variability in deep dyspareunia that cannot be accounted for by the endometriosis alone. For example, clinicians will observe that one patient with cul-de-sac or uterosacral endometriosis might have severe deep dyspareunia, whereas another patient with the same cul-de-sac or uterosacral endometriosis might have minimal pain with intercourse. This was demonstrated in a study of women with cul-de-sac or uterosacral endometriosis: although cul-de-sac or uterosacral endometriosis increases the risk for deep dyspareunia, there was still wide variability in severity of deep dyspareunia reported by this population of women.15
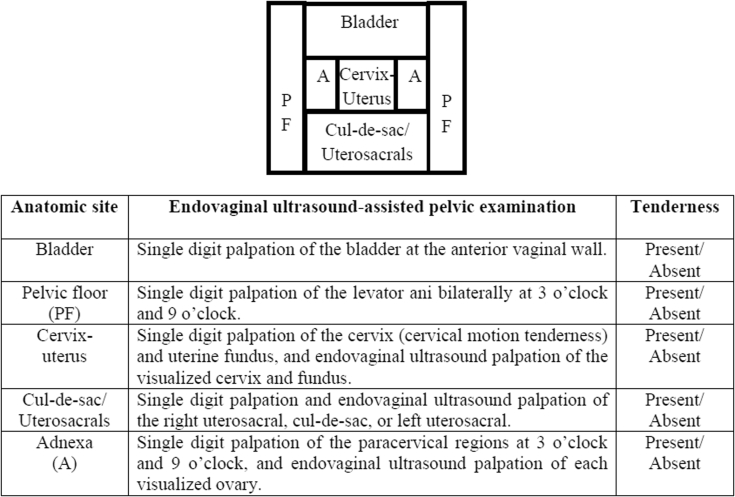
Thus, treatment of cul-de-sac or uterosacral endometriosis, although important, cannot be the sole management approach for deep dyspareunia. That is, there must be other causes of deep dyspareunia in this population of women. In addition to the cul-de-sac or uterosacral ligaments, deep dyspareunia could arise from contact with several other pelvic structures with proximity to the vagina (Figure 1): the bladder, the pelvic floor musculature, the cervix and uterus, and the adnexa. In some women, at least one of these anatomic structures can become tender and contact during deep penetration can lead to pain and deep dyspareunia. However, it is important to empirically validate whether tenderness of each anatomic site is associated with deep dyspareunia, similar to the cul-de-sac or uterosacral ligaments, so that clinicians know whether tenderness of each site can be a contributor to a patient’s deep dyspareunia and thus a potential treatment target. It also is important to provide some insight into the underlying etiologic factors for each tender anatomic site, which in turn would guide management. For example, it is clear that surgical excision of cul-de-sac or uterosacral endometriosis can be a treatment for deep dyspareunia.12 However, what is less clear are the clinical conditions or risk factors that could give rise to tender bladder, pelvic floor, cervix and uterus, or adnexa, which also could be potential therapeutic targets for deep dyspareunia.
Figure 1.
Putative anatomic sites in the vaginal canal that could be contacted during deep penetration and lead to deep dyspareunia. A standardized endovaginal ultrasound-assisted pelvic examination was carried out to palpate each anatomic site for tenderness (present vs absent) to objectively reproduce deep dyspareunia, as previously published.16, 17, 18
Therefore, our primary research question was whether tenderness in other pelvic structures (bladder, pelvic floor, cervix and uterus, and adnexa)—in addition to the known importance of tenderness of the cul-de-sac or uterosacral ligaments in endometriosis—is associated with severity of deep dyspareunia. Using multivariable regression, our hypothesis was that each of these pelvic structures would have an independent association with severity of deep dyspareunia. The clinical importance of this analysis is that it establishes whether tenderness of each anatomic site potentially has an independent contribution to deep dyspareunia. This guides the clinician in formulating a differential diagnosis for the deep dyspareunia. For example, in a given patient, deep dyspareunia could be due to independent contributions from the cul-de-sac or uterosacral ligaments, the bladder, the pelvic floor, and the uterus and cervix, each of which might require specific management, as described below.
Our secondary question was whether there are specific clinical factors associated with tenderness of each pelvic structure to provide insight into possible etiologic mechanisms for the development of tenderness. Endometriosis commonly grows in the cul-de-sac or uterosacral ligaments, and thus we hypothesized that endometriosis would be associated with tenderness of the cul-de-sac or uterosacral ligaments as described earlier. However, endometriosis rarely invades the bladder or reaches the pelvic floor or grows on the surface of the cervix and uterus. Therefore, we hypothesized that there would alternative mechanisms for tenderness of these structures. Alternative mechanisms could include painful bladder syndrome, psychological comorbidities such as depression, or dietary factors. In addition to endometriosis, these alternative mechanisms could be modifiable as part of a multidisciplinary treatment plan for deep dyspareunia.
Aims
To provide a systematic assessment of anatomic tenderness in deep dyspareunia and the associated clinical factors, a standardized endovaginal ultrasound-assisted pelvic examination was used to palpate each anatomic structure to detect tenderness and reproduce deep dyspareunia (Figure 1).
Methods
Study Setting
In this study, we analyzed cross-sectional baseline data from a prospective database at a tertiary referral center for endometriosis and/or pelvic pain. The details of this database have been previously described.16 Participants in the prospective database were recruited from new or re-referrals to the center who were seen from December 2013 through April 2015. All participants provided prospective informed consent, and the study received institutional ethics approval from the University of British Columbia (Vancouver, BC, Canada; H11-02882). All data were entered prospectively into an online Research Electronic Data Capture (REDCap) database. The prospective database was designed to study baseline features associated with severity of different types of pelvic pain (including deep dyspareunia) and predictors of change in pain severity over 5 years (currently in progress).
Study Population
Inclusion criteria were (i) patient completion of a standardized online questionnaire before assessment by a gynecologist at the center and (ii) full assessment by a gynecologist, including review of the patient questionnaire and patient medical records and performance of a standardized endovaginal ultrasound-assisted pelvic examination to palpate anatomic structures and reproduce deep dyspareunia (Figure 1).17, 18, 19 On average, the questionnaires were completed 2 weeks before the full assessment.
Exclusion criteria were (i) menopause; (ii) age at least 50 years; (iii) previous hysterectomy; (iv) previous oophorectomy; (v) incomplete examination; and/or (vi) patient stated that the primary outcome (severity of deep dyspareunia) was not applicable to her (ie, not sexually active). The reason for exclusion criteria i and ii was to exclude atrophy as a cause of dyspareunia. In addition, none of the participants had rare causes of deep dyspareunia such as anatomic abnormality (eg, vaginal septum) or active infection (eg, cervicitis or pelvic inflammatory disease).
Primary Outcome
Primary outcome from the patient online questionnaire was severity of deep dyspareunia rated on a numeric pain rating scale from 0 to 10 according to recommendations for endometriosis research.20 Deep dyspareunia was specifically differentiated from superficial dyspareunia and other pelvic pain symptoms (dysmenorrhea, chronic pelvic pain, dyschezia, and back pain) using a series of standardized questions.16
Anatomic Sites of Tenderness
Gynecologists at the center (n = 4) performed an endovaginal ultrasound-assisted pelvic examination for tenderness using a standardized approach described in previous studies17, 18, 19 (Figure 1). The reason for the use of endovaginal ultrasound palpation is to directly visualize palpated structures and to access difficult-to-reach structures at pelvic examination.16 Each anatomic site that putatively could be contacted at deep penetration and lead to deep dyspareunia was palpated for tenderness at examination to objectively reproduce the deep dyspareunia (Figure 1): bladder, pelvic floor (levator ani), cervix and uterus, adnexa, and cul-de-sac or uterosacral ligaments. Pressure applied at each site was approximately 0.5 to 1 kg force, measured with a thimble algometer system (outside of patients). However, the algometer could not be attached to the endovaginal ultrasound probe because it disrupts the ultrasound image; thus, pressure was not measured for the endovaginal ultrasound-assisted palpation in the patients in the study. In response to the palpation, tenderness at each anatomic site was rated by the patient as absent, mild, moderate, or severe. Because severity of deep dyspareunia was weakly correlated with the mild, moderate, and severe tenderness ratings (data not shown), we classified tenderness at each anatomic site as a binary variable: “tender” if any tenderness was present or “non-tender” if tenderness was absent.
Associated Clinical Factors
Potential clinical factors associated with each tender anatomic site were identified from the patient standardized online questionnaire and from review of medical charts.
Endometriosis was classified into three groups: (i) endometriosis present (previous surgical diagnosis); (ii) endometriosis excluded (negative surgical result or no clinical suspicion by the gynecologist); or (iii) endometriosis suspected (no previous surgery, but clinically suspected by the gynecologist based on current palpable cul-de-sac or uterosacral nodule, current ovarian endometrioma at endovaginal ultrasound, or other features at history and examination).21, 22 The reason for this classification is that Canadian guidelines recommend empiric medical therapy for suspected endometriosis, and therefore only a proportion of such patients undergo surgery.23 At the time of surgical diagnosis (group 1), surgical treatment (ablation or excision) also had been carried out.
Irritable bowel syndrome was diagnosed using the Rome III criteria,24 and painful bladder syndrome was based on criteria from the American Urological Association or the International Continence Society.25, 26 Depression, anxiety, and pain catastrophizing symptoms were assessed using the validated Patient Health Quality–9 questionnaire,27 the Generalized Anxiety Disorder–7 questionnaire,28 and the Pain Catastrophizing Scale,29 respectively.
Other clinical factors included elements from patient demographics, reproductive history, medical-surgical history, family history, and social-behavioral variables, as previously described.16
Statistical Analysis
Anatomic Sites of Tenderness
Tenderness at each anatomic site (bladder, pelvic floor, cervix and uterus, adnexa, and cul-de-sac or uterosacral ligaments) was coded as a binary variable (tender vs non-tender) and tested for associations with the primary outcome (severity of deep dyspareunia from 0 to 10; Mann-Whitney U-test). All anatomic sites with a significant association (P < .05, uncorrected) were entered into a multivariable linear regression model. Sequential backward elimination was carried out with a threshold of a P equal to .05 until the final regression model (all anatomic sites with P < .05). The goal of the multivariable linear regression was to identify anatomic sites with an independent association with severity of deep dyspareunia. In the multivariable linear regression modeling, we also considered possible confounding by referral status (re-referral or new referral), the attending gynecologist (n = 4), and a non-pelvic examination finding (abdominal wall pain assessed by the Carnett test,16 which is typically secondary to myofascial trigger points) to ensure the pelvic anatomic sites had a specific relation with deep dyspareunia.
Associated Clinical Factors
For each anatomic site with an independent association with severity of deep dyspareunia in the final multivariable linear regression model, we tested for associations with the clinical factors (Mann-Whitney, χ2, or Fisher exact test). All clinical factors with a significant association (P < .05, without adjustment for multiple testing) were entered into a multivariable logistic regression model for each anatomic site. Sequential backward elimination was performed using likelihood ratio modeling, until the final logistic regression model was reached (all clinical factors with P < .05). The goal was to identify clinical factors with an independent association with each tender anatomic site.
Sample Size
The analyses were performed on the first cohort of the ongoing prospective database at our center. The sample size of this cohort is 548. Based on data from a previous retrospective dataset,15 for the association between pelvic floor tenderness and severity of deep dyspareunia (with power = 0.99, α = 0.05, SD = 2.57, mean difference = 1.15, and incidence of pelvic floor tenderness = 33%), a sample size of 480 would be required.30
Other
The α value was a P value less than .05 (two-sided), with means described ± one SD and medians provided with the range. Missing data were excluded. Assumptions of multivariable regression were checked. We used SPSS 22.0 (IBM Corporation, Armonk, NY, USA).
Main outcome measure
Severity of deep dyspareunia was rated on a numeric pain rating scale from 0 to 10, in accord with recommendations for endometriosis research20 and as we previously described.16 In particular, pain with deep penetration was differentiated from superficial dyspareunia (introital or initial entry pain), and rated from 0 (no pain) to 10 (worst pain imaginable).16
Results
Study Sample
After an 87% consent rate, there were 548 participants who met the study criteria (see flowchart in the Appendix). Demographics of the sample are presented in Table 1.
Table 1.
Demographics
| Variable | Mean ± SD (range) or % |
|---|---|
| Age (y) | 34.5 ± 7.4 |
| BMI (kg/m2) | 25.3 ± 5.7 |
| Severity of deep dyspareunia (0–10) | 5.9 ± 3.2 (0–10) |
| New referral vs re-referral | 76 (416/548) vs 24 (132/548) |
| Endometriosis | |
| Prior surgical diagnosis | 54 (293/548) |
| Clinically suspected | 16 (88/548) |
| Excluded | 30 (167/548) |
| Stage (if prior surgical diagnosis) | |
| I–II | 44 (128/293) |
| III–IV | 45 (133/293) |
| Unknown∗ | 11 (32/293) |
| Cul-de-sac or uterosacral nodule at current examination | 11 (62/548) |
| Currently on hormonal suppression | 37 (204/548) |
| Previous laparoscopies, median (range) | 1 (0–14) |
| Currently taking neuromodulator (pain adjuvant) | 22 (123/548) |
| Painful bladder syndrome | 42 (230/548) |
| Currently on Elmiron† | 3 (6/230) |
| Irritable bowel syndrome | 53 (290/548) |
| Ethnicity (Caucasian) | 75 (403/536) |
| Sexual orientation (heterosexual) | 95 (498/535) |
| Marital status (married) | 46 (247/534) |
| Previous pregnancy | 49 (264/534) |
| Currently employed | 75 (399/534) |
| Education level, median | 2 y of college |
| Income, median (range) | $60,000–$79,000 |
| Current smoking | 13 (71/534) |
| Current drug use | 11 (59/530) |
BMI = body mass index.
Unable to determine from operative note.
Pentosan polysulfate sodium (Janssen Pharmaceuticals, Titusville, NJ, USA).
Anatomic Sites
Nineteen percent (102 of 548) of participants had a tender bladder, 28% (155 of 548) had a tender pelvic floor, 31% (168 of 548) had a tender cervix and uterus, 38% (209 of 548) had a tender adnexa, and 57% (313 of 548) had tender cul-de-sac or uterosacral ligaments. Seven percent (37 of 547) of participants had five tender anatomic sites, 9% (51 of 548) had four tender anatomic sites, 12% (64 of 548) had three tender anatomic sites, 19% (104 of 548) had two tender anatomic sites, 29% (158 of 548) had one tender anatomic site, and 24% (134 of 548) had no tenderness.
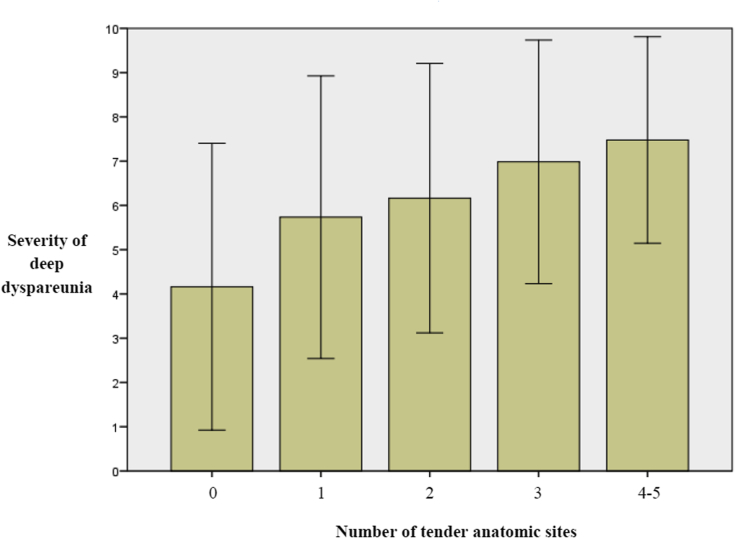
Severity of deep dyspareunia was significantly associated with tenderness of each anatomic site: bladder (P < .001), pelvic floor (P < .001), cervix and uterus (P < .001), adnexa (P < .001), and cul-de-sac or uterosacral ligaments (P < .001; Table 2). In addition, the number of tender anatomic sites in a patient was significantly correlated with a greater severity of deep dyspareunia (Spearman r = 0.34, n = 548, P < .001; Figure 2).
Table 2.
Anatomic site tenderness
| Anatomic site | Severity of deep dyspareunia (0–10) |
|||
|---|---|---|---|---|
| n | Mean ± SD | Mann-Whitney U | P value | |
| Bladder | ||||
| Tender | 102 | 7.4 ± 2.5 | 30,395.5 | <.001 |
| Non-tender | 446 | 5.5 ± 3.2 | ||
| Pelvic floor | ||||
| Tender | 155 | 7.0 ± 2.6 | 38,570.5 | <.001 |
| Non-tender | 393 | 5.4 ± 3.3 | ||
| Cervix-uterus | ||||
| Tender | 168 | 7.0 ± 2.8 | 41,181.5 | <.001 |
| Non-tender | 380 | 5.4 ± 3.3 | ||
| Cul-de-sac or uterosacral ligaments | ||||
| Tender | 313 | 6.6 ± 2.8 | 48,014.5 | <.001 |
| Non-tender | 235 | 4.8 ± 3.4 | ||
| Adnexa | ||||
| Tender | 209 | 6.4 ± 3.1 | 41,899.5 | <.001 |
| Non-tender | 339 | 5.5 ± 3.2 | ||
Figure 2.
Severity of deep dyspareunia (mean ± SD) with an increasing number of tender anatomic sites (bladder, pelvic floor, cervix and uterus, cul-de-sac and uterosacral, and adnexa). Samples were no tender anatomic sites (24%; 134 of 548), one tender anatomic site (29%; 158 of 548), two tender anatomic sites (19%; 104 of 548), three tender anatomic sites (12%; 64 of 548), four tender anatomic sites (9%; 51 of 548), and five tender anatomic sites (7%; 37 of 547) (cases with four to five tender anatomic sites were grouped together because of samples < 10%).
Multivariable linear regression showed that the severity of deep dyspareunia was independently associated with tenderness of the bladder (P = .018), pelvic floor (P = .038), cervix and uterus (P = .008), and cul-de-sac or uterosacral ligaments (P < .001), but not with tenderness of the adnexa (P = .92; Table 3). These independent associations were present even after controlling for possible confounding by referral status, the attending gynecologist, and a non-pelvic examination finding (abdominal wall pain by Carnett test; Table 3).
Table 3.
Anatomic site tenderness (multivariable regression)
| Severity of deep dyspareunia (0–10) |
||||
|---|---|---|---|---|
| n | Multivariable linear regression∗ |
|||
| b (95% CI)∗ | t | P value | ||
| Anatomic site | ||||
| Bladder | ||||
| Tender | 102 | 0.88 (0.15–1.60) | 2.38 | .018 |
| Non-tender | 446 | |||
| Pelvic floor | ||||
| Tender | 155 | 0.66 (0.04–1.28) | 2.08 | .038 |
| Non-tender | 393 | |||
| Cervix-uterus | ||||
| Tender | 168 | 0.84 (0.22–1.43) | 2.68 | .008 |
| Non-tender | 380 | |||
| Cul-de-sac or uterosacral ligaments | ||||
| Tender | 313 | 1.39 (0.85–1.93) | 5.05 | < .001 |
| Non-tender | 235 | |||
| Adnexa | ||||
| Tender | 209 | −0.05† (−0.64 to 0.54) | −0.10 | .92 |
| Non-tender | 339 | |||
| Referral | ||||
| Re-referral | 132 | 0.20† (−0.41 to 0.82) | 0.87 | .39 |
| New referral | 416 | |||
| Attending gynecologist | ||||
| 1 | 185 | 0.86† (−0.03 to 1.75) | 1.44 | .15 |
| 2 | 203 | 0.61 (0.08–1.14) | 2.26 | .024 |
| 3 | 100 | 0.62† (−0.35 to 1.59) | −0.01 | 1.00 |
| 4 | 60 | reference | — | — |
| Extrapelvic examination (abdominal wall pain by Carnett test) | ||||
| Yes | 153 | 0.14† (−0.50 to 0.77) | 0.20 | .84 |
| No | 395 | |||
CI = confidence interval.
Analysis of variance (F5,542 = 17.0, P < .001) for the final model after backward elimination (significant variables in final model: bladder, pelvic floor, cervix and uterus, cul-de-sac or uterosacral ligaments, and gynecologist 2).
These b coefficients were from the initial model before backward elimination.
Associated Clinical Factors
For the four anatomic sites with an independent association with severity of deep dyspareunia (bladder, pelvic floor, cervix and uterus, and cul-de-sac or uterosacral ligaments), associations between tenderness at each anatomic site and clinical factors are presented in Table 4. Notably, tenderness of each anatomic site was not associated with the timing of the examination in the menstrual cycle or whether the patient was amenorrheic (Table 4). In addition, tenderness of the bladder, pelvic floor, and cervix and uterus was similarly present in women with and without endometriosis, whereas tenderness of the cul-de-sac or uterosacral ligaments was much more frequent in women with endometriosis (Table 4).
Table 4.
Associations with clinical factors∗
| Clinical factor (binary and categorical variables)† | Bladder (% tender) | Pelvic floor (% tender) | Cervix and uterus (% tender) | Cul-de-sac or uterosacral ligaments (% tender) |
|---|---|---|---|---|
| Endometriosis‡ | NS | NS | NS | P < .001 |
| Painful bladder syndrome | P < .001§ | P = .001 | P < .001 | P = .004 |
| Irritable bowel syndrome | P = .004 | NS | NS | P = .015 |
| Sexual orientation | P = .048 | NS | NS | NS |
| Miscarriage | NS | NS | P < .001 | NS |
| Antidepressant use (current)‖ | P = .002 | P = .008 | P = .033 | NS |
| Family history of chronic pain¶ | P = .001 | P = .013 | NS | P = .012 |
| Marital status | P = .005 | NS | NS | NS |
| Smoking | P = .022 | NS | P = .008 | NS |
| Drug use | NS | NS | NS | P = .011 |
| Adult physical assault# | P = .020 | NS | NS | NS |
| Adult sexual assault# | P = .005 | NS | NS | NS |
| Clinical factor (continuous and ordinal variables)∗∗ | Bladder |
Pelvic floor |
Cervix and uterus |
Cul-de-sac or uterosacral ligaments |
||||
|---|---|---|---|---|---|---|---|---|
| Tender (n = 102) | Non-tender (n = 446) | Tender (n = 155) | Non-tender (n = 393) | Tender (n = 168) | Non-tender (n = 380) | Tender (n = 313) | Non-tender (n = 235) | |
| Age (y) | P < .001†† | P < .001 | NS | NS | ||||
| BMI (kg/m2) | NS | P = .027 | NS | NS | ||||
| Depression (PHQ-9) | P < .001 | P < .001 | P = .001 | P = .009 | ||||
| Anxiety (GAD-7) | P = .003 | P < .001 | NS | NS | ||||
| Pain Catastrophizing Scale | P < .001 | P < .001 | P < .001 | P = .002 | ||||
| Age pelvic pain started (y) | NS | P = .001 | NS | P = .036 | ||||
| Laparoscopies (n) | NS | P = .048 | NS | NS | ||||
| Age at menarche (y) | NS | P = .027 | NS | NS | ||||
| Severity of dysmenorrhea (0–10) | NS | P = .006 | NS | NS | ||||
| Education‡‡ | P = .004 | P = .001 | P = .004 | P < .001 | ||||
| Income§§ | P = .001 | P < .001 | NS | NS | ||||
| Red meat consumption‖‖ | NS | NS | P = .006 | NS | ||||
| Alcohol (drinks/wk) | NS | P = .009 | P = .006 | NS | ||||
BMI = body mass index; GAD-7 = Generalized Anxiety Disorder–7; NS = not significant; PHQ-9 = Patient Health Quality–9.
See eTable 1 for descriptive statistics (eg, proportions and means).
Binary and categorical variables without any significant associations: ethnicity (Caucasian vs other), current hormonal suppression, previous intrauterine device, tubal ligation, previous chlamydia, previous gonorrhea, previous pelvic inflammatory disease, retroverted uterus, adenomyosis, fibroid larger than 4 cm, vaginal birth, cesarean birth, termination, fibromyalgia, ruptured appendicitis, hernia surgery, memorable fall on the pelvis, currently working, child physical abuse, child sexual abuse, examination performed no more than or more than 14 days after last menstrual period, or patient is amenorrheic.
See Methods for description.
Fisher exact test.
Selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors, but excluding bupropion because of its known protective effects on sexual function.
Categorical variable: yes, no, don’t know.
Categorical variable: yes, no, no answer.
Continuous and ordinal variables without any significant associations: number of bleeding days in past 3 months, number of laparotomies, dairy product consumption, vegetable and fruit consumption, fish consumption, fiber consumption, fatty food consumption, sugary food consumption, exercise, and caffeine intake.
Mann-Whitney U test.
Education: some high school = 1, graduate high school = 2, some college = 3, graduate 2-year college = 4, graduate 4-year college = 5, postgraduate = 6.
Income: less than $20,000 = 1, $20,000 to $40,000 = 2, $40,000 to $60,000 = 3, $60,000 to $80,000 = 4, $80,000 to $100,000 = 5, more than $100,000 = 6.
Red meat: consumed never = 1, rarely = 2, few times per week = 3, or daily = 4.
Multivariable logistic regression was used to identify the clinical factors with an independent association with a tender bladder, pelvic floor, cervix and uterus, or cul-de-sac or uterosacral ligaments (Table 5).
Table 5.
Associated clinical factors (multivariable regression)
| Risk factor | Anatomic site |
|||||||
|---|---|---|---|---|---|---|---|---|
| Bladder (tender vs non-tender) |
Pelvic floor (tender vs non-tender) |
Cervix and uterus (tender vs non-tender) |
Cul-de-sac or uterosacral ligaments (tender vs non-tender) |
|||||
| Exp[b] (95% CI) | P value | Exp[b] (95% CI) | P value | Exp[b] (95% CI) | P value | Exp[b] (95% CI) | P value | |
| Endometriosis∗ | <.001 | |||||||
| Present | 3.54 (2.00–6.25) | <.001 | ||||||
| Suspected | 6.87 (3.69–12.8) | <.001 | ||||||
| Excluded | reference | — | ||||||
| Painful bladder syndrome† | 2.90 (1.77–4.76) | <.001 | 1.68 (1.12–2.50) | .011 | 1.62 (1.11–2.38) | .013 | ||
| Age‡ | 0.94 (0.91–0.97) | .001 | 0.96 (0.94–0.99) | .012 | ||||
| Depression‡ (PHQ-9) | 1.05 (1.01–1.09) | .008 | 1.07 (1.03–1.10) | <.001 | ||||
| Pain Catastrophizing Scale‡ | 1.03 (1.01–1.04) | <.001 | ||||||
| Miscarriage† | 2.24 (1.42–3.53) | .001 | ||||||
| Family history of chronic pain∗ | .009 | .016 | ||||||
| Yes | 1.81 (1.01–3.24) | .045 | 1.96 (1.23–3.11) | .005 | ||||
| Don’t know | 2.45 (1.37–4.39) | .003 | 1.40 (0.87–2.24) | .16 | ||||
| No | reference | — | reference | — | ||||
| Education§ | 0.84 (0.73–0.97) | .015 | 0.80 (0.70–0.91) | .001 | ||||
| Income§ | 0.87 (0.76–0.98) | .025 | ||||||
| Red meat consumption§ | 1.41 (1.06–1.89) | .019 | ||||||
CI = confidence interval; PHQ-9 = Patient Health Quality–9.
Categorical variable.
Binary variable.
Continuous variable.
Ordinal variable (see Table 4).
For the bladder, the following clinical factors had an independent association with a tender bladder: more severe depression symptoms (P = .008), painful bladder syndrome (P < .001), family history of chronic pain (P = .009), and younger age (P < .001; Table 5). Antidepressant usage (selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors, but excluding bupropion; Table 4) was not in the final regression model.
For the pelvic floor, the following clinical factors had an independent association with a tender pelvic floor: more severe depression symptoms (P = .001), younger age (P = .012), lower education level (P = .015), and lower income (P = .025; Table 5). Similarly, antidepressant usage was not in the final regression model.
For the cervix and uterus, the following clinical factors had an independent association with a tender cervix and uterus: history of at least one miscarriage (P = .001), higher red meat consumption (P = .019), more pain catastrophizing (P < .001), and painful bladder syndrome (P = .011; Table 5). Furthermore, the number of miscarriages was higher in women with a tender cervix and uterus compared with women with a non-tender cervix and uterus in the entire sample (Mann-Whitney U = 34273, n = 532, P < .001) and in the subset who had been pregnant at least once (Mann-Whitney U = 9223, n = 262, P = .010).
For the cul-de-sac or uterosacral ligaments, the following clinical factors had an independent association with tenderness of the cul-de-sac or uterosacral ligaments: endometriosis (P < .001), painful bladder syndrome (P = .013), family history of chronic pain (P = .016), and lower level of education (P = .001; Table 5). A cul-de-sac or uterosacral nodule at examination (ie, evidence of deep endometriosis) was further associated with tenderness: 69% (43 of 62) of those with a nodule had tenderness of the cul-de-sac or uterosacral ligaments, whereas 55% (142 of 257) of those without a nodule had tenderness of the cul-de-sac or uterosacral ligaments (Fisher exact test, P = .046).
Discussion
In this study of baseline features of a prospective cohort of women at a tertiary referral center for endometriosis and pelvic pain, we found that severity of deep dyspareunia was independently associated with tenderness of four anatomic sites: the bladder, pelvic floor (levator ani), cervix and uterus, and cul-de-sac or uterosacral ligaments. Thus, we propose that each of these four anatomic sites could be contacted during deep penetration and lead to deep dyspareunia (Figure 1). In addition, the number of tender anatomic sites in a patient was associated with more severe deep dyspareunia (Figure 2), suggesting that the association between the four anatomic sites and deep dyspareunia is cumulative. In contrast, there was no independent association with the adnexa, which makes anatomic sense because the ovaries are usually situated farther superiorly from the vaginal apex and are the least likely structure to be contacted during intercourse.
Strengths of the study include its sample size and consent rate (≥500 subjects and 87%), controlling for confounding, validated questionnaires, and comprehensive approach that assessed factors ranging from anatomic to dietary. The study also should be generalizable to other referral centers for endometriosis or pelvic pain, although not to patients who were excluded (eg, postmenopausal or after hysterectomy). A limitation is that we assessed for tenderness of only one pelvic floor component (levator ani); we did not record muscle tone or palpable trigger points and we did not use algometer testing to assess multiple pelvic floor muscles, which has been reported for chronic pelvic pain in general.31 Another limitation is that not all subjects had previous surgery to assess for endometriosis.
Each of the four anatomic sites had specific associated clinical factors at multivariable logistic regression (Table 5). As expected, we found that tenderness of the cul-de-sac or uterosacral ligaments was more frequent in women with endometriosis, in particular those with a cul-de-sac or uterosacral nodule (evidence of deep endometriosis).10, 11, 32 Tenderness of the cul-de-sac or uterosacral ligaments in endometriosis can arise from local neuroinflammation,33 and in previous work we found that local nerve density correlates to deep dyspareunia severity in cul-de-sac or uterosacral endometriosis.15 However, we also found that tenderness of the bladder, pelvic floor, and cervix and uterus was similarly present in women with and without endometriosis. This could account for why women with the same type and location of endometriosis can have wide variability in deep dyspareunia symptoms. For example, the patient with endometriosis who has tenderness of the cul-de-sac or uterosacral ligaments and the bladder, pelvic floor, and cervix and uterus is likely to have more severe deep dyspareunia compared with the patients with endometriosis who has tenderness of the cul-de-sac or uterosacral ligaments alone. This was demonstrated by the cumulative association of an increased number of tender sites with severity of deep dyspareunia (Figure 2).
For associated clinical factors, we also found that more severe depression symptoms were specifically associated with tenderness of the bladder and pelvic floor. This association was independent of antidepressant usage, indicating that the effect is not mediated by treatment side effects. Depression is associated with changes in genital physiology that predispose to tenderness (eg, less lubrication and genital blood flow).34, 35 Depression also can exhibit its effects centrally, because it might be a marker for central nervous system changes that amplify peripheral nociception36 and can worsen the emotional aspects of pain, thereby increasing distress that can produce higher pain ratings. It was interesting that among psychological factors, only depression (and not anxiety or abuse) had an independent association with bladder and pelvic floor tenderness, suggesting a depression-specific effect on sexual pain in these patients. In a study of women with vulvodynia, we also found that depression was the specific psychological factor associated with concurrent deep dyspareunia.37 Similarly, a study on fibroids found a specific relation between deep dyspareunia and depression.38 Given this evidence, clinical trials targeting depression (cognitively or pharmacologically) to treat deep dyspareunia might be of interest. However, it is important to emphasize that depression also can be a consequence of deep dyspareunia (in addition to being a cause). Prospective cohort studies, including interventional studies to treat deep dyspareunia, will help to clarify to what degree depression is a cause of deep dyspareunia (rather than vice versa).
In addition, a history of miscarriage and red meat consumption were specifically associated with a tender cervix and uterus. The process of miscarriage might sensitize the uterus through persistent uterine inflammation or neurogenesis. Uterine inflammation and neurogenesis have been described in conditions such as endometriosis and adenomyosis.39, 40 Miscarriage could be another trigger for these processes. Alternatively, clinical features of the miscarriage might be important, such as mode of pregnancy loss (spontaneous, medical, or surgical), gestational age, infection, or retained products, which should be a focus of future research. For instance, surgical evacuation of a miscarriage requires instrumentation of the uterus, which could increase the risk of subsequent inflammation or neurogenesis. An older gestational age (and thus larger conceptus) could involve a more physically and emotionally traumatic miscarriage process, which also could increase risk. History of infection after miscarriage and/or retained products of conception also could be expected to potentially trigger ongoing inflammation or neurogenesis.
Interestingly, increased red meat consumption also was independently associated with a tender cervix and uterus. In previous studies, meat intake has been associated with increased estrogen levels,41 fibroids,42, 43 and endometriosis,44 and increased estrogen levels could provoke local inflammation in the uterus.39 However, dietary information in our study came from a single questionnaire; thus, this finding requires confirmation with prospective daily dietary diaries. Pain catastrophizing also was increased in women a tender cervix and uterus, indicating that this subpopulation has greater magnification and rumination on pain symptoms. In contrast to a previous study, we did not find a relation between fibroids and deep dyspareunia.38 We also did not observe an association with adenomyosis at ultrasound, although the published literature on adenomyosis and deep dyspareunia is contradictory.45, 46, 47, 48 In our study, venography for pelvic congestion syndrome was not performed.
Several other observations were made. Painful bladder syndrome was most strongly associated with tenderness of the bladder, as expected,49 but also was associated with tenderness of the cervix and uterus and the cul-de-sac or uterosacral ligaments, likely because this syndrome might be a marker for central sensitization and organ cross-sensitization50 (or perhaps because of a shared nervous supply from close anatomic-embryologic relations). Central sensitization involves an increase in nociceptive signaling in the central nervous system (thereby amplifying pain signals from peripheral sources), whereas cross-sensitization involves organ cross-talk at the level of the spinal cord, which can link pain symptoms in adjacent organs.50 We hypothesize that these two mechanisms could explain some of the tender sites in a given patient; however, this study was not designed to objectively test for evidence of sensitization and thus these mechanisms should be a topic of future investigation. Family history of chronic pain was associated with tenderness of the bladder and cul-de-sac or uterosacral ligaments, but this finding requires confirmation because it is subject to recall bias.
This study could provide a standardized framework for evaluation and pathophysiology-guided management of deep dyspareunia. Patients could be evaluated for sites of tenderness (cul-de-sac or uterosacral ligaments, uterus and cervix, bladder, and pelvic floor), which would guide management options. For example, the patient with cul-de-sac or uterosacral tenderness only might respond best to laparoscopy and excision of endometriosis lesions. In contrast, the patient with bladder or pelvic floor tenderness might benefit from treatment of depression or painful bladder syndrome, if present. Sexual therapy also should be considered in patients with decreases in desire, arousal, and/or orgasm associated with the deep dyspareunia. Patients with multiple sites of tenderness, likely from nervous system sensitization, would be best managed in a multidisciplinary setting including physiotherapy and cognitive therapies. An ideal center would be one where the full range of options could be offered to patients with deep dyspareunia in an integrated fashion, from full surgical excision of endometriosis to multidisciplinary approaches.
Conclusions
In summary, we found that there were multiple anatomic sites (bladder, pelvic floor, and uterus and cervix) related to severity of deep dyspareunia in reproductive-age women at a referral center for endometriosis and pelvic pain, in addition to the known role of the cul-de-sac or uterosacral ligaments in those women with endometriosis. Thus, clinicians should be aware that there could be multiple anatomic factors for deep dyspareunia in this population (women with and without endometriosis). For example, if a patient has persistent deep dyspareunia even after surgical treatment of endometriosis of the cul-de-sac or uterosacral ligaments, the other anatomic sites should be assessed as potential contributors to the persistent deep dyspareunia. Currently, we are evaluating whether anatomic site of tenderness can predict response to surgery (or other treatments) in this ongoing prospective cohort.
Statement of authorship
Category 1
-
(a)Conception and Design
- Paul J. Yong; Christina Williams; Fontayne Wong; Mohamed A. Bedaiwy; Sarka Lisonkova; Catherine Allaire
-
(b)Acquisition of Data
- Paul J. Yong; Christina Williams; Ali Yosef; Fontayne Wong; Mohamed A. Bedaiwy; Sarka Lisonkova; Catherine Allaire
-
(c)Analysis and Interpretation of Data
- Paul J. Yong; Christina Williams; Ali Yosef; Fontayne Wong; Catherine Allaire
Category 2
-
(a)Drafting the Article
- Paul J. Yong
-
(b)Revising It for Intellectual Content
- Paul J. Yong; Christina Williams; Ali Yosef; Fontayne Wong; Mohamed A. Bedaiwy; Sarka Lisonkova; Catherine Allaire
Category 3
-
(a)Final Approval of the Completed Article
- Paul J. Yong; Christina Williams; Ali Yosef; Fontayne Wong; Mohamed A. Bedaiwy; Sarka Lisonkova; Catherine Allaire
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Funding: This work was supported by a Canadian Institutes of Health Research (CIHR) Operating Grant Priority Announcement (Reproductive & Child Health Start-up Grant) from the Institute of Human Development, Child and Youth Health [IHD-137431] and a CIHR Transitional Open Operating Grant [MOP-142273] to Paul J. Yong. This project was also supported by a VCHRI Investigator Award from the VGH and UBC Hospital Foundation (Mentored Clinician-Scientist Award) to Paul J. Yong.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.esxm.2017.07.001.
Supplementary data
References
- 1.Heim L.J. Evaluation and differential diagnosis of dyspareunia. Am Fam Physician. 2001;63:1535–1544. [PubMed] [Google Scholar]
- 2.Hummelshoj L., De Graaff A., Dunselman G. Let’s talk about sex and endometriosis. J Fam Plann Reprod Health Care. 2014;40:8–10. doi: 10.1136/jfprhc-2012-100530. [DOI] [PubMed] [Google Scholar]
- 3.Fauconnier A., Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11:595–606. doi: 10.1093/humupd/dmi029. [DOI] [PubMed] [Google Scholar]
- 4.Denny E., Mann C.H. Endometriosis-associated dyspareunia: the impact on women’s lives. J Fam Plann Reprod Health Care. 2007;33:189–193. doi: 10.1783/147118907781004831. [DOI] [PubMed] [Google Scholar]
- 5.Tripoli T.M., Sato H., Sartori M.G. Evaluation of quality of life and sexual satisfaction in women suffering from chronic pelvic pain with or without endometriosis. J Sex Med. 2011;8:497–503. doi: 10.1111/j.1743-6109.2010.01976.x. [DOI] [PubMed] [Google Scholar]
- 6.Ferrero S., Ragni N., Remorgida V. Deep dyspareunia: causes, treatments, and results. Curr Opin Obstet Gynecol. 2008;20:394–399. doi: 10.1097/GCO.0b013e328305b9ca. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez I., Reid C., Dziurawiec S. Living with endometriosis: the perspective of male partners. J Psychosom Res. 2006;61:433–438. doi: 10.1016/j.jpsychores.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Vercellini P., Meana M., Hummelshoj L. Priorities for endometriosis research: a proposed focus on deep dyspareunia. Reprod Sci. 2011;18:114–118. doi: 10.1177/1933719110382921. [DOI] [PubMed] [Google Scholar]
- 9.Vercellini P., Somigliana E., Buggio L. “I can’t get no satisfaction”: deep dyspareunia and sexual functioning in women with rectovaginal endometriosis. Fertil Steril. 2012;98:1503–1511 e1501. doi: 10.1016/j.fertnstert.2012.07.1129. [DOI] [PubMed] [Google Scholar]
- 10.Chapron C., Barakat H., Fritel X. Presurgical diagnosis of posterior deep infiltrating endometriosis based on a standardized questionnaire. Hum Reprod. 2005;20:507–513. doi: 10.1093/humrep/deh627. [DOI] [PubMed] [Google Scholar]
- 11.Fauconnier A., Chapron C., Dubuisson J.B. Relation between pain symptoms and the anatomic location of deep infiltrating endometriosis. Fertil Steril. 2002;78:719–726. doi: 10.1016/s0015-0282(02)03331-9. [DOI] [PubMed] [Google Scholar]
- 12.Ferrero S., Abbamonte L.H., Giordano M. Deep dyspareunia and sex life after laparoscopic excision of endometriosis. Hum Reprod. 2007;22:1142–1148. doi: 10.1093/humrep/del465. [DOI] [PubMed] [Google Scholar]
- 13.Vercellini P., Somigliana E., Consonni D. Surgical versus medical treatment for endometriosis-associated severe deep dyspareunia: I. Effect on pain during intercourse and patient satisfaction. Hum Reprod. 2012;27:3450–3459. doi: 10.1093/humrep/des313. [DOI] [PubMed] [Google Scholar]
- 14.Fritzer N., Tammaa A., Salzer H., Hudelist G. Dyspareunia and quality of sex life after surgical excision of endometriosis: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2014;173:1–6. doi: 10.1016/j.ejogrb.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 15.Williams C., Hoang L., Yosef A. Nerve bundles and deep dyspareunia in endometriosis. Reprod Sci. 2016;23:892–901. doi: 10.1177/1933719115623644. [DOI] [PubMed] [Google Scholar]
- 16.Yosef A., Allaire A., Williams C. Multifactorial contributors to the severity of chronic pelvic pain in women. Am J Obstet Gynecol. 2016;215:760.e1–760.e14. doi: 10.1016/j.ajog.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Nourmoussavi M., Bodmer-Roy S., Mui J. Bladder base tenderness in the etiology of deep dyspareunia. J Sex Med. 2014;11:3078–3084. doi: 10.1111/jsm.12708. [DOI] [PubMed] [Google Scholar]
- 18.Yong P.J., Sutton C., Suen M. Endovaginal ultrasound-assisted pain mapping in endometriosis and chronic pelvic pain. J Obstet Gynaecol. 2013;33:715–719. doi: 10.3109/01443615.2013.821971. [DOI] [PubMed] [Google Scholar]
- 19.Yong P.J., Mui J., Allaire C. Pelvic floor tenderness in the etiology of superficial dyspareunia. J Obstet Gynaecol Can. 2014;36:1002–1009. doi: 10.1016/S1701-2163(15)30414-X. [DOI] [PubMed] [Google Scholar]
- 20.Vincent K., Kennedy S., Stratton P. Pain scoring in endometriosis: entry criteria and outcome measures for clinical trials. Report from the Art and Science of Endometriosis meeting. Fertil Steril. 2010;93:62–67. doi: 10.1016/j.fertnstert.2008.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Holsbeke C., Van Calster B., Guerriero S. Imaging in gynaecology: how good are we in identifying endometriomas? Facts Views Vis Obgyn. 2009;1:7–17. [PMC free article] [PubMed] [Google Scholar]
- 22.Hudelist G., Ballard K., English J. Transvaginal sonography vs clinical examination in the preoperative diagnosis of deep infiltrating endometriosis. Ultrasound Obstet Gynecol. 2011;37:480–487. doi: 10.1002/uog.8935. [DOI] [PubMed] [Google Scholar]
- 23.Jarrell J.F., Vilos G.A., Allaire C. Consensus guidelines for the management of chronic pelvic pain. J Obstet Gynaecol Can. 2005;27:781–826. doi: 10.1016/s1701-2163(16)30732-0. [DOI] [PubMed] [Google Scholar]
- 24.Longstreth G.F., Thompson W.G., Chey W.D. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 25.Drossman D.A. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Meijlink J.M. Interstitial cystitis and the painful bladder: a brief history of nomenclature, definitions and criteria. Int J Urol. 2014;21(Suppl 1):4–12. doi: 10.1111/iju.12307. [DOI] [PubMed] [Google Scholar]
- 27.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spitzer R.L., Kroenke K., Williams J.B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 29.Osman A., Barrios F.X., Kopper B.A. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20:589–605. doi: 10.1023/a:1025570508954. [DOI] [PubMed] [Google Scholar]
- 30.Dupont W.D. Power and sample size calculations: a review and computer program. Control Clin Trials. 1990;11:116–128. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 31.As-Sanie S., Harris R.E., Harte S.E. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet Gynecol. 2013;122:1047–1055. doi: 10.1097/AOG.0b013e3182a7e1f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vercellini P., Fedele L., Aimi G. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22:266–271. doi: 10.1093/humrep/del339. [DOI] [PubMed] [Google Scholar]
- 33.McKinnon B.D., Bertschi D., Bersinger N.A., Mueller M.D. Inflammation and nerve fiber interaction in endometriotic pain. Trends Endocrinol Metab. 2015;26:1–10. doi: 10.1016/j.tem.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Basson R. Sexual function of women with chronic illness and cancer. Womens Health (Lond Engl) 2010;6:407–429. doi: 10.2217/whe.10.23. [DOI] [PubMed] [Google Scholar]
- 35.Basson R., Rees P., Wang R. Sexual function in chronic illness. J Sex Med. 2010;7:374–388. doi: 10.1111/j.1743-6109.2009.01621.x. [DOI] [PubMed] [Google Scholar]
- 36.Kaya S., Hermans L., Willems T. Central sensitization in urogynecological chronic pelvic pain: a systematic literature review. Pain Physician. 2013;16:291–308. [PubMed] [Google Scholar]
- 37.Yong P.J., Sadownik L., Brotto L.A. Concurrent deep-superficial dyspareunia: prevalence, associations, and outcomes in a multidisciplinary vulvodynia program. J Sex Med. 2015;12:219–227. doi: 10.1111/jsm.12729. [DOI] [PubMed] [Google Scholar]
- 38.Moshesh M., Olshan A.F., Saldana T. Examining the relationship between uterine fibroids and dyspareunia among premenopausal women in the United States. J Sex Med. 2014;11:800–808. doi: 10.1111/jsm.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bulun S.E. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X., Lu B., Huang X. Endometrial nerve fibers in women with endometriosis, adenomyosis, and uterine fibroids. Fertil Steril. 2009;92:1799–1801. doi: 10.1016/j.fertnstert.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Harmon B.E., Morimoto Y., Beckford F. Oestrogen levels in serum and urine of premenopausal women eating low and high amounts of meat. Public Health Nutr. 2014;17:2087–2093. doi: 10.1017/S1368980013002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trivedi P., Abreo M. Predisposing factors for fibroids and outcome of laparoscopic myomectomy in infertility. J Gynecol Endosc Surg. 2009;1:47–56. doi: 10.4103/0974-1216.51910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiaffarino F., Parazzini F., La Vecchia C. Diet and uterine myomas. Obstet Gynecol. 1999;94:395–398. doi: 10.1016/s0029-7844(99)00305-1. [DOI] [PubMed] [Google Scholar]
- 44.Parazzini F., Vigano P., Candiani M. Diet and endometriosis risk: a literature review. Reprod Biomed Online. 2013;26:323–336. doi: 10.1016/j.rbmo.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Parazzini F., Mais V., Cipriani S. Determinants of adenomyosis in women who underwent hysterectomy for benign gynecological conditions: results from a prospective multicentric study in Italy. Eur J Obstet Gynecol Reprod Biol. 2009;143:103–106. doi: 10.1016/j.ejogrb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Ekin M., Cengiz H., Ozturk E. Genitourinary symptoms in patients with adenomyosis. Int Urogynecol J. 2013;24:509–512. doi: 10.1007/s00192-012-1903-z. [DOI] [PubMed] [Google Scholar]
- 47.Yeniel O., Cirpan T., Ulukus M. Adenomyosis: prevalence, risk factors, symptoms and clinical findings. Clin Exp Obstet Gynecol. 2007;34:163–167. [PubMed] [Google Scholar]
- 48.Sammour A., Pirwany I., Usubutun A. Correlations between extent and spread of adenomyosis and clinical symptoms. Gynecol Obstet Invest. 2002;54:213–216. doi: 10.1159/000068385. [DOI] [PubMed] [Google Scholar]
- 49.Teichman J.M., Parsons C.L. Contemporary clinical presentation of interstitial cystitis. Urology. 2007;69(Suppl):41–47. doi: 10.1016/j.urology.2006.08.1111. [DOI] [PubMed] [Google Scholar]
- 50.Hoffman D. Central and peripheral pain generators in women with chronic pelvic pain: patient centered assessment and treatment. Curr Rheumatol Rev. 2015;11:146–166. doi: 10.2174/1573397111666150619094524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.