Summary
Precise control of sister chromatid separation during mitosis is pivotal to maintaining genomic integrity. Yet, the regulatory mechanisms involved are not well-understood. Remarkably, we discovered that linker histone H1 phosphorylated at S/T18 decorated the inter-chromatid axial DNA on mitotic chromosomes. Sister chromatid resolution during mitosis required the eviction of such H1S/T18ph by the chaperone SET, with this process being independent of and most likely downstream of arm-cohesin dissociation. SET also directed the disassembly of Shugoshins in a polo-like kinase 1 augmented manner, aiding centromere resolution. SET ablation compromised mitotic fidelity as evidenced by unresolved sister chromatids with marked accumulation of H1S/T18ph and centromeric Shugoshin. Thus, chaperone-assisted eviction of linker histones and Shugoshins is a fundamental step in mammalian mitotic progression. Our findings also elucidate the functional implications of the decades-old observation of mitotic linker histone phosphorylation, serving as a paradigm to explore the role of linker histones in bio-signaling processes.
Keywords: Histone chaperone, linker histone, Shugoshin, mitosis, chromatid resolution, chromosome integrity, phosphorylation
Introduction
Linker histones are a fundamental unit of chromatin that bind to linker DNA adjoining mononucleosomes. Some of their most well-characterized functions include the formation and maintenance of higher-order chromatin structures and transcriptional repression (Hergeth and Schneider, 2015). Linker histones display the largest number of variants (over 11) among histones, some of which are expressed in a cell-type and development-stage specific manner, endowing them with a remarkable functional plasticity (Harshman et al., 2013; Hergeth and Schneider, 2015). Similar to core histones, linker histones are also extensively decorated with chemical modifications such as acetylation, phosphorylation and ubiquitination, which have been implicated in various cellular processes such as transcription, replication, DNA repair and mitosis (Harshman et al., 2013; Hergeth and Schneider, 2015). Phosphorylated H1s have been broadly implicated in dynamic binding to mitotic chromosomes, yet information about where on the chromosome they are enriched and the role of linker histone chaperones in modulating such dynamicity remains largely unknown.
Histone chaperones aid in the transport, assembly and disassembly of histones during various cellular processes, such as DNA replication, transcription and DNA repair (Burgess and Zhang, 2013; Das et al., 2010; Gurard-Levin et al., 2014). Chaperones like Chromatin assembly factor −1 (CAF-1) and Anti-silencing function protein 1 (ASF1) have been shown to transport and assemble core histones H3-H4 during DNA replication (Campos et al., 2010; Tagami et al., 2004), while chaperones like FACT (Facilitates Chromatin Transcription) play pivotal roles in the exchange of core histones H2A-H2B to allow RNA polymerase II to traverse nucleosomes (Belotserkovskaya et al., 2003). While core histone chaperones have been generally well characterized, there are fewer mechanistic studies on linker histone chaperones. Chaperones like Nucleophosmin 1 (NPM1), Nuclear autoantigenic sperm protein (NASP) and members of the Nucleosome Assembly Protein 1 (NAP1) family and SET nuclear proto-oncogene have been implicated in H1 chaperoning (Gadad et al., 2011; Kato et al., 2011; Kepert et al., 2005; Richardson et al., 2000; Shintomi et al., 2005), but whether these are bona fide H1 chaperones in cells remains vague. A related unresolved issue is whether these are general H1 chaperones or whether they function in specific contexts or conditions.
SET has structural homology to NAP1 and has been reported to possess histone chaperone activity for all core histones and linker histones in vitro (Kato et al., 2011; Muto et al., 2007). Cellular studies on SET have revealed a wide range of functions including transcriptional regulation, modulation of chromosome condensation and cohesion during meiosis, as well as in anti-apoptotic and DNA repair signaling pathways (Chambon et al., 2013; Fan et al., 2003; Kalousi et al., 2015; Matsumoto et al., 1999). Yet, the functional contribution of SET to these processes and whether/how its property as a chaperone integrates into these pathways is unclear. SET also has inhibitory activity towards protein phosphatase 2A (PP2A), however, the mechanistic basis for this activity remains unknown (Li and Damuni, 1998; Li et al., 1995; Li et al., 1996).
Here, we use an unbiased proteomic approach to identify SET-associated proteins in mammalian cells and characterized these interactions to better understand its cellular functions and define its role as a linker histone chaperone. We discovered that SET is a mitotic chaperone that associates with and disassembles a specific phospho-H1 species, H1S/T18ph, from prophase chromosomes. Additionally, SET also mediates the eviction of mitotic proteins called Shugoshins from centromeres and this step-wise, SET-assisted eviction of chromosomal proteins directly controls sister chromatid resolution during mammalian mitosis.
Results
Chaperone SET associates with linker histones and Shugoshins
We performed tandem affinity purification (TAP) of FLAG-HA tagged SET (NFH-SET) followed by mass spectrometry (MS) in asynchronously growing immortalized mouse embryonic fibroblasts (iMEFs) and human telomerase-immortalized retinal pigmented epithelial (hTERT-RPE1) cells (Figure 1A, 1B and Table S1). Linker histones were among the most abundantly associated histones, while core histones H2A and H2B were moderately enriched and histones H3 and H4 were barely detectable, consistent with published reports (Figure 1A, 1B, Table S1, Table S2) (Campos et al., 2010; Campos et al., 2015; Kato et al., 2011). SET association with histones was detected largely in the soluble nuclear extract suggesting that SET affects histone dynamics in the nucleus (Figure 1B, Table S1). Among non-histone proteins, we observed a marked enrichment, especially in the cytosolic extracts, of the mitotic proteins Shugoshin-like 1 (SGOL1), Shugoshin-like 2 (SGOL2), PP2A subunits (previously reported to associate with SGOL1 and SGOL2) (Kitajima et al., 2006) and replication initiator 1 (REPIN1), a protein reported to function at replication origins (Dailey et al., 1990) (Figure 1B, Table S1).
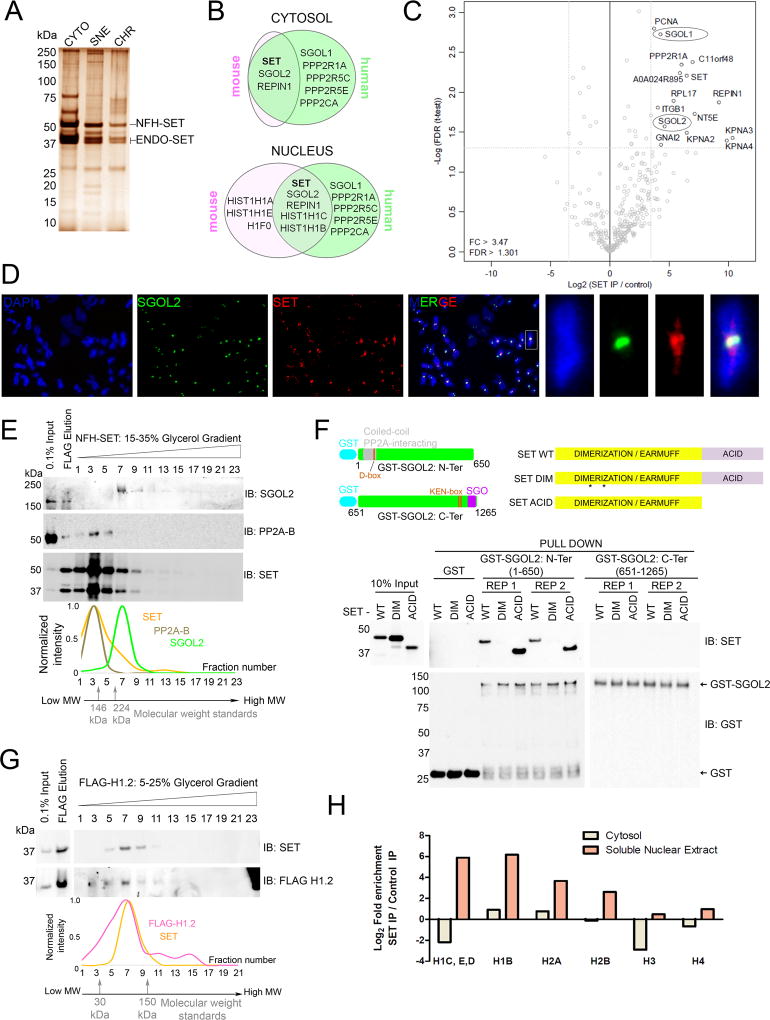
Figure 1. Chaperone SET associates with linker histones and Shugoshins.
(A) Silver stain of Tandem Affinity Purification (TAP) of SET-complexes from iMEFs. CYTO: cytosol, SNE: soluble nuclear extract, CHR: chromatin pellet, NFH-SET: N-terminal FLAG-HA-SET, ENDO-SET: endogenous SET. (B) Venn diagram depicting top proteins identified from TAP-MS analysis of cytosolic and nuclear extracts prepared from mouse (iMEFs) and human (hTERT-RPE1) cells. Raw peptide count numbers are presented in Table S1. Only proteins with a cumulative peptide count of 10 or higher were included in the Venn diagram. (C) Volcano plot of quantitative-MS analysis of SET-associated proteins from mitotic extracts. SGOL1 and SGOL2 are highlighted in ellipses. Note that the plot represents proteins enriched in cytosolic and nuclear fractions combined. Histones enriched in the nuclear extracts are depicted separately in Figure 1H. (D) IF analysis on human chromosomes from WT RPE1 cells depicting co-localization of SET and SGOL2 at centromeres. Chromosomal arm staining is also observed for SET. Also see Figure S1E for another zoomed in chromosome. Scale bar =5 µm. (E) Glycerol gradient analysis of SET-complexes indicating co-elution of SET and SGOL2 at fraction 7, independent of PP2A (fraction 3). Traces of peak normalized intensities for each fraction is shown below. Also see Figure S1E for the same analysis with an alternate PP2A-B subunit. (F) (Top) Domain organization and constructs of SGOL2 and SET used in the pull-down assay. D-box, KEN-box: putative APC/C recognition domains, SGO: SGO domain. The asterisks in SET DIM represent point mutations that impaired dimerization. (Bottom) In vitro pull-down assay between GST-tagged N-terminal (1–650 a.a.) or C-terminal (651–1265 a.a.) regions of SGOL2 with SET, either WT or mutant in its dimerization domain (DIM) or in its acidic tail (ACID), indicating direct association of SET with the N-terminal region of SGOL2. Dimerization, but not the acidic tail, is required for SET binding. REP1 and REP2: experimental replicates. (G) Glycerol gradient separation of H1.2 complexes indicating co-elution with SET at fractions 7–9. Traces of peak normalized intensities for each fraction is shown below. Fraction 23 was omitted for clarity. (See also Figure S1I). (H) Quantitative MS data for histones co-purifying with SET in mitotic extracts. Linker histones are the most abundant histones associated with SET in the nuclear extracts. (See also Figure S1).
SGOL1 and SGOL2 are mitotic proteins that aid in the protection of centromeric cohesin from the prophase pathway (Gutierrez-Caballero et al., 2012; Kitajima et al., 2006; Marston, 2015). This pathway elicits the phosphorylation of cohesin by polo-like kinase 1 (PLK1) on chromosomal arms, promoting arm resolution during prophase (Sumara et al., 2002). Centromeric cohesin is refractory to this pathway until all kinetochores are properly bi-oriented because Shugoshins counteract cohesin phosphorylation at the centromeres by recruiting PP2A. Interestingly, endogenous SGOL1 from HeLa cells and Ascidian oocytes has been previously shown to interact with SET (Chambon et al., 2013; Kitajima et al., 2006).
Since SGO proteins are expressed only during mitosis (Figure S1A), we performed TAP-quantitative MS of SET in mitotically synchronized cells and observed a significant enrichment of SGOL1, SGOL2, PP2A and REPIN1 in both cytosolic and nuclear extracts, indicating that these interactions occurred during mitosis (Figure 1C, Table S2). We observed a marked co-localization of SET and SGOL2 at human centromeres suggesting that the proteins interact on centromeric chromatin (Figure 1D, Figures S1B – S1D). In addition to centromeres, we also observed a significant chromosomal arm staining of SET (Figure 1D, Figure S6D). We verified that SET and SGOL2 existed in a stable complex by performing a glycerol gradient analysis of FLAG-SET complexes from mitotic cells, which demonstrated the co-elution of SET and SGOL2 (fraction 7, Figure 1E). Importantly, this complex lacked PP2A (both B55 and B56 subunits), suggesting that SET-PP2A and SET-SGOL2 are independent complexes and that the interaction of SET with PP2A does not mediate its interaction with SGOL2 (Figure 1E, Figure S1E). In accordance, SET purifications from iMEFs included SGOL2 as the most abundant SET interactor, even in the near complete absence of the majority of PP2A subunits (Figure 1B, Table S1).
To further scrutinize the direct interaction of SET and SGOL2, we performed pull-down assays in vitro, with GST-SGOL2 comprising either the N-terminal (1–650 a.a.) or C-terminal (651–1265 a.a.) regions of human SGOL2. Wild-type (WT) SET bound efficiently to the N-terminal, but not the C-terminal region of SGOL2 (Figure 1F). We also observed similar results when using full-length GST-SGOL2 (Figure S1F). The N-terminal region of SGOL2 contains its coiled-coil dimerization motif (Xu et al., 2009), and given the size of the SET-SGOL2 complex detected upon glycerol gradient analysis (>224kDa) (Figure 1E, Figure S1E), SET (a dimer itself) likely binds to a dimer of SGOL2. Importantly, the N-terminal region of SGOL2 was shown to directly interact with PP2A (Xu et al., 2009), in keeping with the likelihood that PP2A and SET bind in a mutually exclusive manner to SGOL2. The complex of SET-SGOL2 was resistant to salt washes up to 400 mM NaCl, highlighting that their interaction was strong and specific (Figure 1F). The related chaperone NAP1 did not associate with SGOL2 (Figure S1H), consistent with a previous study in Drosophila showing that NAP1 did not interact with the Shugoshin homolog, MEIS322 (Moshkin et al., 2013). Impairment of the SET dimerization domain (DIM mutant), but not its acidic C-terminal domain (ACID mutant, which can still form a dimer) completely abolished SGOL2 binding, showing that SET dimerization is necessary and sufficient for SGOL2 binding in vitro (Figure 1F, Figure S1F). However, in cells, both DIM and ACID mutants did not associate with SGOL2 (Figure S1G) likely because chaperone activity is compromised in both cases (see below).
The stable cellular association of SET with linker histone H1 was verified using glycerol gradient analysis, whereby FLAG-H1.2 and SET co-elute (fraction 7, Figure 1G) demonstrating that SET-H1 formed an intact complex. Interestingly, linker histones also co-purified with SET in the mitotic extracts, albeit only in the nucleus, showing that the SET-H1 interaction also occurs during mitosis (Figure 1H, Table S2). MS analysis of the peak fractions from the gradient analysis (Figure 1G) indicated that none of the other SET-associated proteins purified with the SET-H1 complex suggesting that SET-H1 exists independently (Figure S1I). Several chaperones such as NAP1 and nucleophosmin 1 (NPM1) also eluted in the same fraction as SET (owing to their similar molecular weights) suggesting that these proteins are also H1 chaperones in the cell (Figure S1I) (Kepert et al., 2005; Shintomi et al., 2005; Zhang et al., 2015). We also noticed the stable association of the PAF1 transcription complex with H1 in the high molecular weight fractions 11–13 (Figure S1I), consistent with previous studies (Kim et al., 2013). Taken together, these results indicated a direct association of SET-SGOL2 and SET-H1, in mutually exclusive complexes.
Localization of SET complexes is dynamic during the cell-cycle
Since SET-SGOL2 complexes were detected in both cytosolic and nuclear extracts, we next explored if localization depends on the mitotic stage. Endogenous SET was highly dynamic, displaying a centrosomal presence throughout the cell-cycle, as highlighted by its co-localization with the centrosomal marker γ-tubulin (Figure 2A). Nuclear SET was significantly enriched during G2 and prophase, also consistent with inception of nuclear SGOL2 expression during prophase (Figure 2A and Figure S1A). After nuclear-envelope breakdown and metaphase entry, the majority of SET became cytosolic (Figure 2A), indicating that SET undergoes a nuclear to cytosolic transition during progression from prophase to metaphase, which likely explains its significant association with importin proteins KPNA3 and KPNA4 (Figure 1C, Table S1).
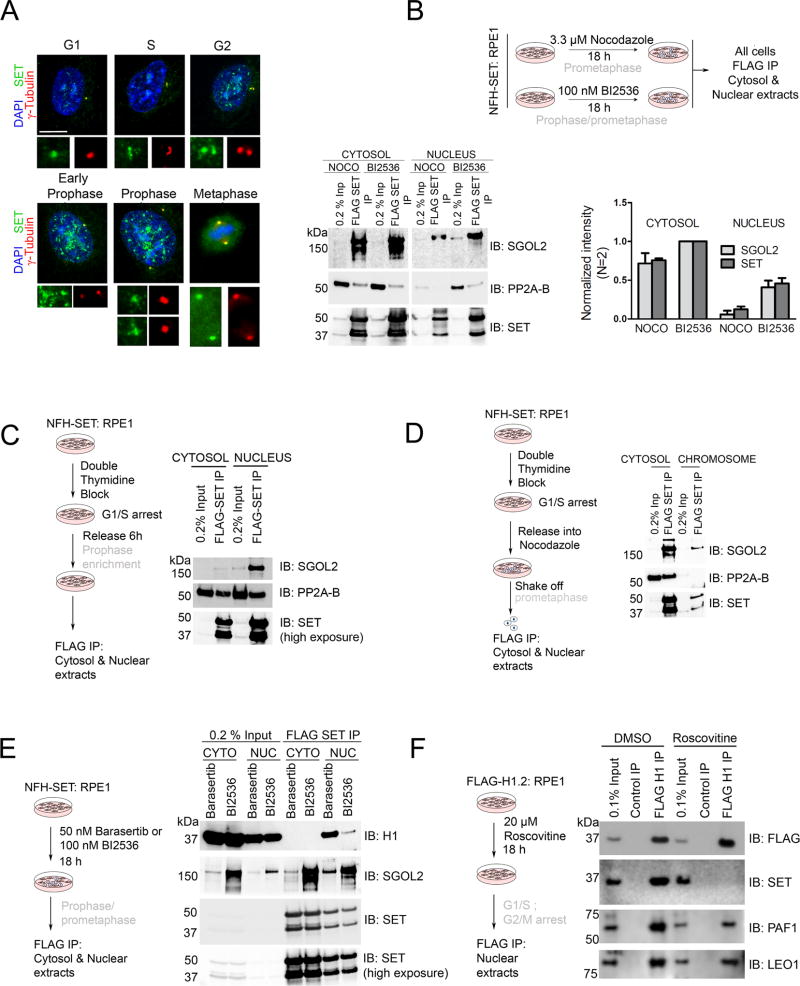
Figure 2. SET complexes display cell-cycle dependent dynamicity.
(A) IF analysis on WT RPE1 cells highlighting SET localization during the cell-cycle. Nuclear SET increases dramatically during G2-prophase, followed by its cytosolic presence at metaphase. Centrosomal/spindle pole localization is observed at all stages as depicted by co-staining with the centrosomal marker, γ-tubulin. Scale bar = 5 µm. (B) (Top) Schematic to study SET-SGOL2 complexes in FLAG-HA-SET expressing RPE1 cells, at different mitotic stages: Nocodazole (prometaphase arrest), BI2536 (PLK1 inhibitor; prophase and prometaphase arrest). (Bottom) FLAG-SET IP from cells treated with nocodazole (NOCO) and BI2536 indicating complex formation with SGOL2 in the cytosol (NOCO and BI2536) and nuclear fractions (BI2536-nucleus/chromosome). Quantification of the same is shown on the right. (C) (Left) Schematic to synchronize cells at prophase by double-thymidine block and release. (Right) FLAG-SET IP from prophase synchronized cells indicating SGOL2 complex formation predominantly in the nucleus (D) (Left) Schematic to synchronize cells at prometaphase by double-thymidine block and release into nocodazole. Cells were harvested by mitotic shake-off (Right) FLAG-SET IP showing that the SET-SGOL2 complex is largely cytosolic at this stage. (E) (Left) Schematic to study SET-H1 complexes using Barasertib and BI2536 (AURKB inhibitor; prophase and prometaphase arrest). (Right) FLAG-SET IP showing the enrichment of H1 complexes in the nucleus. (F) (Left) Schematic to study cell-cycle dependency of SET-H1 interaction using Roscovitine (CDK inhibitor; G1/S and G2/M arrest). (Right) FLAG H1.2 IP from RPE1 cells treated with Roscovitine show ablation of SET association with H1, but not of PAF1/LEO1, highlighting that progression through the cell-cycle is necessary for SET-H1 interaction. All complexes in (B), (C), (D), (E) and (F) were natively eluted using FLAG peptide. (See also Figure S2).
To assess the behavior of the SET-SGOL2 and SET-H1 complexes during this transition, we performed FLAG-SET IP and native peptide elution from cells treated with chemicals known to thwart mitotic progression at distinct phases: nocodazole (microtubule poison) that arrests cells at prometaphase (Tanno et al., 2010), and BI2536 (PLK1 inhibitor) and Barasertib (Aurora kinase B-AURKB inhibitor) each of which arrest cells at prophase and prometaphase (Lenart et al., 2007; Mortlock et al., 2007; Steegmaier et al., 2007) (Figure 2B, 2E (cartoons) and Figure S2A). In addition, we synchronized cells with a double thymidine block, with subsequent release either into thymidine-free media for 6 h (for prophase-enriched cells) (Figure 2C (cartoon) and Figure S2C) or media containing nocodazole and harvested cells by mitotic shake-off (for prometaphase-enriched cells) (Figure 2D (cartoon)).
Native FLAG peptide elution from cells expressing empty vector resulted in no enrichment of SET, SGOL2 or H1, as expected (Figure S2B). Upon prometaphase arrest, which occurs during treatment with each of the chemicals, we observed a cytosolic SET-SGOL2 complex (Figures 2B, 2D and 2E; cytosol panels) consistent with SET localization being cytosolic at this stage (Figure 2A). When cells were arrested in prophase (BI2536, Barasertib and synchronization), a marked nuclear presence of the complex was evident (Figures 2B, 2C and 2E; nucleus/chromosome panels). This finding is consistent with nuclear SET being enriched during prophase (Figure 2A) and with SGOL2 expression being observed first in the nucleus, during prophase (Figure S1A). We also monitored the SET-PP2A complexes under these conditions, which in some cases mirrored the SET-SGOL2 complex (Figure 2B), but not in others (Figure 2C and Figure 2D), again signifying that the SET-SGOL2 complex behaves independently from the SET-PP2A complex (also see Figure 3D).
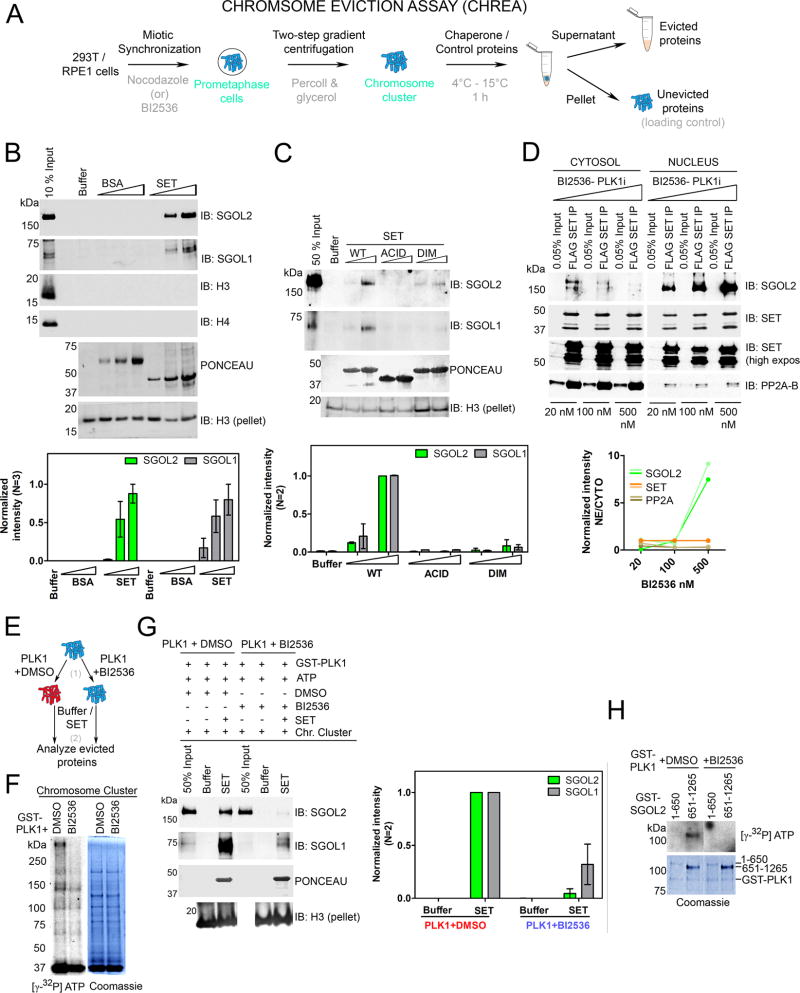
Figure 3. SET is a Shugoshin chaperone and PLK1 augments chaperone activity.
(A) Schematic of CHREA assay to study mitotic protein eviction from chromosomal clusters. (B) (Top) Western blot of evicted proteins reveals that addition of SET, but not BSA, or NAP1 (Figure S3), results in a dose-dependent eviction of SGOL1 and SGOL2 from chromosomes. Additional controls are shown in Figure S3A. Histones H3 and H4 are not evicted. Immunoblot for H3 from the ensuing pellets of the reaction serves as loading control to gauge chromosomal input across samples. (Bottom) H3-pellet and peak normalized intensities from left. (C) (Top) Chaperone assay with SET mutants indicating that both dimerization and acidic tail domains are required for chaperone activity. (Bottom) H3-pellet and peak normalized intensities from left. (D) (Top) NFH-SET RPE1 cells treated with increasing concentrations of BI2536 (20, 100 and 500 nM) display a dose-dependent retention of SET-SGOL2, but not SET-PP2A complexes in the nucleus. (Bottom) Quantification of SET-normalized intensities. Two lines reflect replicates of the experiment. See also Figure S3D for total levels of SGOL2 and SET under these conditions. (E) Schematic of the two-step CHREA in the presence of PLK1. (F) Results of a kinase assay [step 1 of (E)] visualized by autoradiography along with the corresponding Coomassie Blue staining. PLK1 phosphorylates chromosomal clusters used in the chaperone assay. (G) (Left) CHREA performed as in step 2 of (E) indicating that SET evicts SGOL1 and SGOL2 from PLK1 pre-phosphorylated chromosomal clusters, with higher efficiency than those phosphorylated with PLK1+BI2536. (Right) H3-pellet and peak normalized intensities from top. (H) PLK1 kinase assay with purified N-terminal and C-terminal domains of SGOL2. The C-terminal region of SGOL2 is preferentially phosphorylated by PLK1. (See also Figure S3).
Interestingly, we detected SET-H1 complexes in the nuclear but not cytosolic fractions of BI2536- and Barasertib-treated cells, consistent with our MS analyses (Figure 1B and 1H), suggesting that SET associates with H1 during prophase (Figure 2E). For these initial analyses of SET-H1 complexes, we utilized a pan-H1 antibody with broad specificity (both for H1 variants and modification-status). However, we describe below that SET immunoprecipitates from prophase-arrested cells were enriched for a specific-phosphorylated form of H1 (detected from MS analysis) and we performed similar analyses with an antibody specific to this mark (see Figure 5 and Figure S6). To test if the interaction of SET and H1 depends on active cell-cycle progression, we treated FLAG-H1.2 expressing cells with Roscovitine, a cyclin-dependent kinase inhibitor (Meijer et al., 1997), that impairs progression into S and M phases (Figure 2F and S2D). While this treatment did not impact H1 interaction with the PAF1 complex, the association of SET and H1 was completely abolished (Figure 2F), suggesting that active cell-cycle progression is required for the formation of SET-H1 complexes. These results indicate that SET complexes undergo dynamic changes in their localization whereby SET-SGOL2 transitions from the nucleus to the cytosol during prophase to prometaphase/metaphase, while SET-H1 is predominantly nuclear during prophase (Figure S2E). However, we cannot rule out the possibility that the inhibition of the kinases themselves (see below) and/or overexpression of SET (FLAG-HA-SET) might be responsible for some of the observed effects (see Discussion).
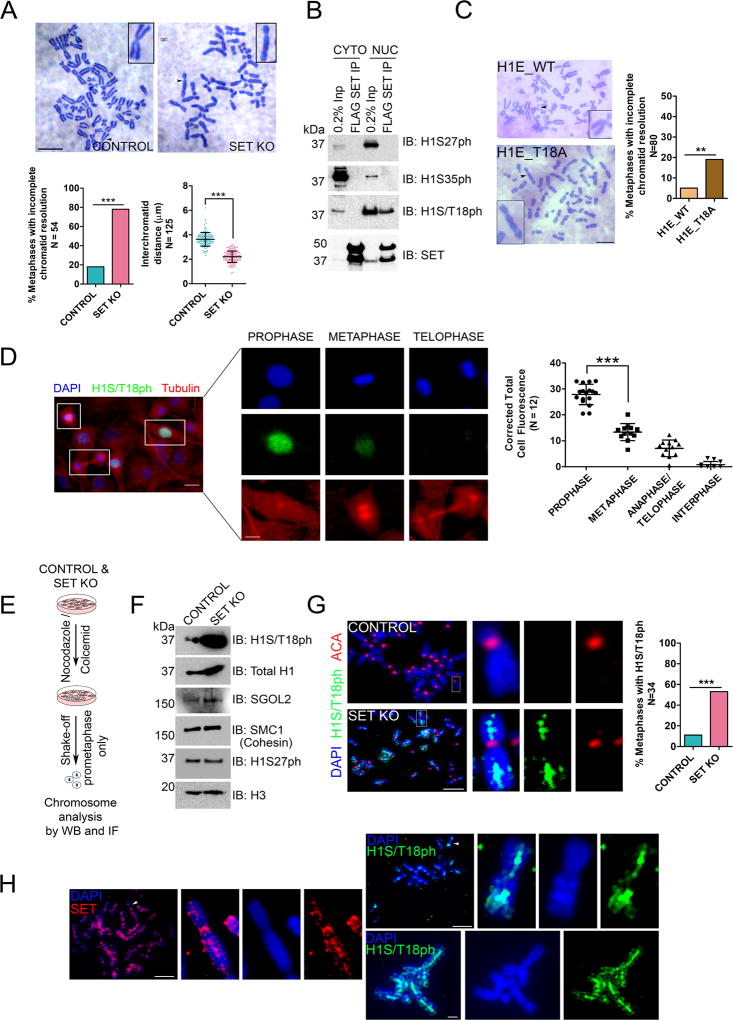
Figure 5. Phospho-H1 persists on inter-chromatid axis upon SET knockout.
(A) (Top) Giemsa staining of metaphase chromosomes showing unresolved sister-chromatids upon SET KO. Scale bar = 10 µm. (Bottom) Inter-chromatid distances are significantly reduced and percentage of metaphases with resolution defects are markedly increased. (B) FLAG-SET IP from BI2536-treated cells showing specific association with H1S/T18ph but not with other mitotic marks, H1S27ph or H1S25ph. Also see Figure S6C. CYTO: Cytosol; NUC: Nuclear Extract. (C) (Left) Giemsa staining of metaphase chromosomes isolated from cells overexpressing H1E_WT or H1E_T18A showing defective chromatid resolution in the latter case. Quantification is shown on right. (D) (Left) Dynamics of H1S/T18ph through mitosis was monitored by IF analysis. Images were captured on the same frame for comparing the different stages. Scale bar = 20 µm in the low magnification image and 10 µm on the zoomed panels. (Right) Quantification of H1S/T18ph expression by measurement of corrected total cell fluorescence. (E) Schematic to study H1 eviction from chromosomes upon SET KO. (F) Western blot analysis of chromosomes from control and SET KO cells depicting a dramatic increase in chromosomal H1S/T18ph upon SET KO. A modest increase in SGOL2 was also observed. Levels of other chromosomal proteins are largely unaltered. (G) IF analysis on metaphase chromosomes showing that H1S/T18ph is not evicted from the inter-chromatid axis upon SET KO. ACA: Anti centromere antigen. Scale bar = 10 µm. Quantification is shown on the right. The staining intensity with H1S/T18ph antibody was weak and the green channel was enhanced equally for all control and SET KO images. (H) Chromosome IF analysis of SET (Left) and H1S/T18ph (Right) in RPE1 WT cells depicting strong inter-chromatid axial staining. In addition, SET also localizes to centromeres. Scale bar = 10 µm. For quantification panels in 5A, 5C, 5D and 5G: N numbers are indicated on the y-axis. *** = p<0.0001, Fisher’s exact test. (See also Figures S5 and S6).
SET disassembles Shugoshins from mitotic chromosomes and PLK1 augments eviction
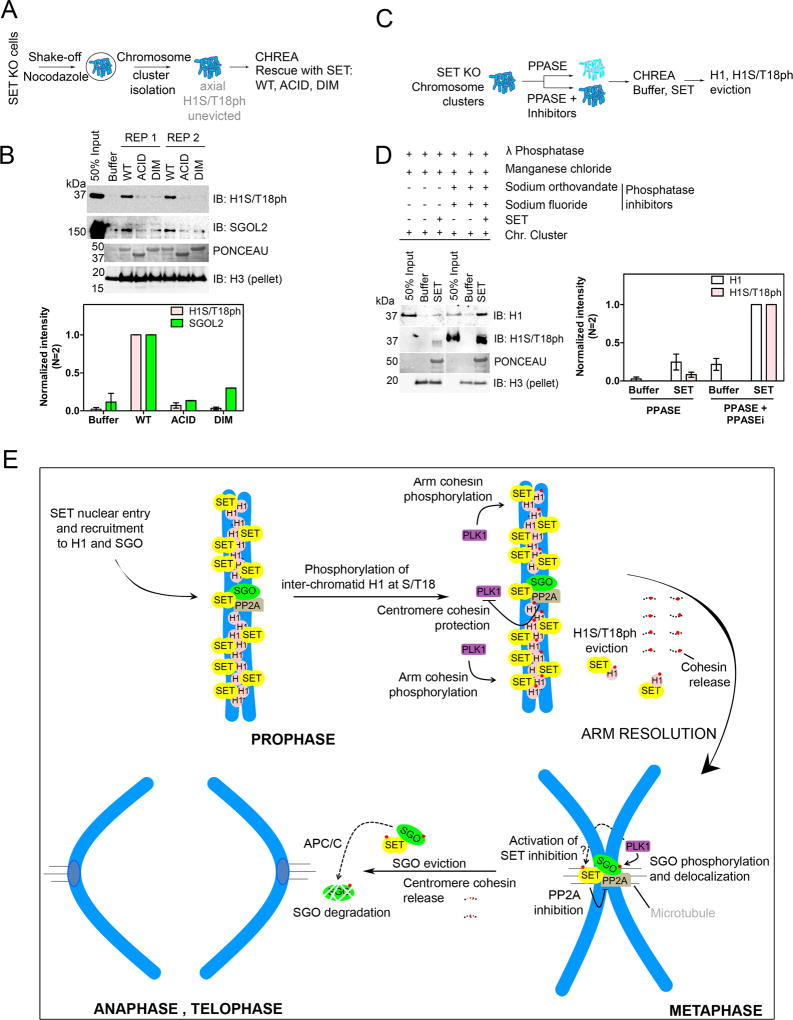
Given the transition of SET-SGOL2 from a nuclear to a cytosolic complex, we hypothesized that SET functions as a chaperone for SGOL2, facilitating its disassembly from chromosomes. To test this possibility, we developed an in vitro chaperone assay to monitor mitotic protein eviction, termed Chromosome Eviction Assay (CHREA) (Figure 3A). Chromosomal clusters were isolated from mitotic cells by a modified percoll-glycerol gradient centrifugation method (Gasser and Laemmli, 1987) and served as CHREA templates. These templates were incubated for one hour with buffer, chaperones or control proteins, after which the supernatant containing the evicted proteins was harvested from the pellet that retained the remainder of the chromosomal cluster (Figure 3A). Adding recombinant SET, but not BSA or NAP1, led to dissociation of SGOL1 and SGOL2 from the chromosomal clusters as evidenced by western blot analysis (Figure 3B and S3B). The specificity of eviction was validated by testing a number of other chromosomal proteins including SMC1 and SA1 (cohesin subunits), Topoisomerase II and Survivin, a subunit of the CPC, none of which were disassembled by SET, suggesting that SET-mediated eviction of Shugoshin was specific (Figure S3A). Moreover, histones H3 and H4 were not evicted, consistent with the MS data indicating a lack of association between SET and H3/H4. We also did not observe PP2A in the supernatant. A plausible explanation is that SET possesses binding and inhibitory activities towards PP2A (Li et al., 1995; Li et al., 1996), but not chaperone activity and efficient binding alone does not necessarily translate to chaperone activity (see below). The DIM mutant of SET did not evict SGOL1 and SGOL2 (Figure 3C), consistent with its lack of interaction with SGOL2 (Figure 1F). The ACID mutant was also defective in eviction (Figure 3C), despite being able to bind to recombinant SGOL2 (Figure 1F), underscoring that CHREA measures chaperone activity and not just binding activity. Results from cellular IP experiments confirmed that the lack of SET chaperone activity translated to an absence of SGOL2 (Figure S1G). Consistently, acidic domains of histone chaperones are critical for mediating histone dynamics on chromatin, highlighting a common eviction mechanism for non-histone proteins (Gurard-Levin et al., 2014; Kato et al., 2011). At this point, we did not detect eviction of H1 in the assay (data not shown), given that SET-H1 interaction occurs much earlier in mitosis, during prophase (Figure 2E and Figure 5), and that the chromosomal clusters used in the chaperone assay were prepared from cells arrested at prometaphase. However, we detected robust H1 eviction using chromosome templates from SET KO cells (see Figure 6).
Figure 6. SET directly evicts H1S/T18ph and regulates mitotic chromosome segregation.
(A) Schematic of CHREA to study H1S/T18ph eviction. (B) (Top) Western blot analysis of evicted proteins showing eviction of H1S/T18ph (as well as SGOL2) upon rescue with WT, but not ACID or DIM SET. REP1 and REP2 are two replicates of the experiment. (Bottom) Quantification of western blot on top. (C) Schematic of CHREA for analyzing effect of phosphorylation on H1 eviction by treatment with λ phosphatase (PPASE) or λ phosphatase with phosphatase inhibitors (PPASE+ Inhibitors). (D) (Right) Western blot analysis of evicted proteins showing a significant reduction in H1 eviction upon phosphatase treatment. Note that recognition by the H1S/T18ph mark is lost upon phosphatase treatment. (Left) Quantification of evicted proteins. (E) Model depicting the function of SET as a mitotic chaperone. See discussion for description.
Interestingly, our quantitative-MS analysis of SET-associated proteins revealed the presence of the activated form of PLK1 that is phosphorylated at T210 (Figure S3C) (Macurek et al., 2008). In Drosophila, PLK1 phosphorylates Shugoshin and promotes its dissociation from centromeres during the metaphase-anaphase transition (Clarke et al., 2005). We were intrigued by the possibility that PLK1-mediated phosphorylation on Shugoshin might partially destabilize its chromatin contacts, which could optimize SET-mediated disassembly of Shugoshin. Indeed, upon treatment with increasing BI2536 concentrations, we observed that SET-SGOL2 complexes displayed a dose-dependent retention in the nuclear fractions and a converse lack of eviction in the cytosolic fractions (Figure 3D), while total amounts of SGOL2 and SET were largely unchanged (Figure S3D). This effect was not observed with SET-PP2A complexes, again highlighting that the SET-SGOL2 complex behaves independently of SET-PP2A (Figure 3D). To test if phosphorylation by PLK1 might serve to partially dissociate Shugoshin from chromosomes to augment its eviction by SET, we treated chromosomal clusters with GST-PLK1 pre-incubated with either DMSO or BI2536 (Figure 3E and 3F). We readily observed phosphorylation of several chromosomal proteins in the presence of active PLK1, while inactivated PLK1 also resulted in phosphorylation of some of these proteins but at considerably lower levels (Figure 3F). Some proteins were equally phosphorylated in the two samples, most likely due to kinases pre-existing on the chromosomal clusters themselves (Figure 3F). When we subjected these chromosomes to CHREA, we detected a significant increase in SET-mediated eviction of SGOL1 and SGOL2 in the case of chromosomes treated with active as opposed to inactive PLK1, indicating that PLK1-mediated phosphorylation enhanced SET chaperone activity, rather than pre-existing kinases (Figure 3G). Moreover, PLK1 directly phosphorylated the C-terminal half of SGOL2 (Figure 3H), consistent with results from PLK1-phosphoproteomic studies wherein more than 90% of SGOL2 phospho-sites were present in its C-terminal domain (Santamaria et al., 2011). The C-terminal region of SGOL2 (and SGOL1), encompasses the conserved SGO domain, which is critical to Shugoshin recruitment and binding to chromosomes via its interaction with H2A-S121ph (Kawashima et al., 2010). Therefore, it is highly conceivable that phosphorylation in the vicinity of the SGO motif would weaken chromosomal interactions, rendering Shugoshins more susceptible to SET-mediated eviction.
Given that detection of labile modifications like phosphorylation requires significant amounts of protein and that Shugoshins are low in abundance, we were not able to observe Shugoshin phosphorylation in SET-IPs (Figure S3E). However, in proteins that were more abundant, such as REPIN1 and histone H1, we readily observed phosphorylation in mouse and human SET purifications, suggesting that SET-associated proteins were phosphorylated (Table S3, S4 and see below for H1). Furthermore, the proteins obtained in SET-IPs were highly reactive with the Mitotic Phospho-protein Monoclonal 2 (MPM2) antibody, which detects phospho-epitopes enriched during mitosis (Figure S3F). These findings lead to a model whereby SET inhibition of PP2A fosters a prime environment in which kinases like PLK1 can phosphorylate client proteins such as SGOL2, which in turn enhances SET-mediated eviction of these proteins (Figure S3G). This arrangement is consistent with existing models wherein SET co-localizes with PP2A at centromeres during meiosis, and its ability to inhibit PP2A was hypothesized to create an environment that indirectly allows for effective Rec8 (cohesin equivalent in meiosis) phosphorylation and its subsequent removal (Chambon et al., 2013).
SET is required for mitotic-fidelity
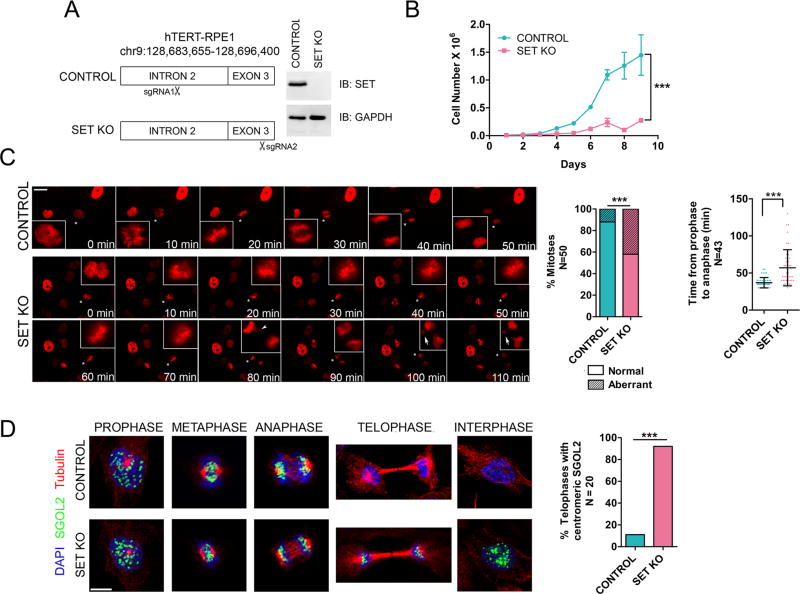
To delineate the role of SET as a mitotic chaperone in a cellular context, we deleted SET from RPE1 cells using CRISPR-Cas9 (Doudna and Charpentier, 2014). A guide RNA targeting exon 3 of SET was designed to abrogate the expression of all SET isoforms, while a guide RNA targeting an upstream intron served as control (Figure 4A). A clone bearing a single base deletion in exon 3 resulted in the complete knockout of SET (SET KO) and a control clone bearing a single base insertion in intron 2 was selected (Figure 4A and Figure S4A). The growth rate of SET KO cells was dramatically slower than that of the control cells (Figure 4B). Live-cell imaging on control and SET-KO cells stably expressing H2B-RFP revealed a significant delay in the SET KO prophase to anaphase transition (mean = 57 minutes) compared to control cells (mean = 37 minutes), with 25% of these mitoses averaging >90 minutes (Figure 4C). Moreover, we observed a significant increase in aberrant mitoses in SET KO cells, including the presence of lagging chromosomes, chromosomes trapped in cleavage furrows and the formation of micronuclei [Figure 4C, arrowheads and arrows, Figure S4B and movies S1 (control) and S2 (SET KO)]. To understand the effect of SET KO on Shugoshin, we monitored the localization of SGOL1 and SGOL2, which are expressed in WT cells during prophase, metaphase and anaphase and degraded by telophase, potentially due to the activity of the anaphase promoting complex/cyclosome (APC/C) (Fu et al., 2007; Karamysheva et al., 2009; Salic et al., 2004; Zachariae and Nasmyth, 1999) (Figure S1A). The expression and localization of SGOL1 and SGOL2 during prophase, metaphase and early anaphase were largely similar between control and SET KO cells (Figure 4D, Figures S4C and S4D). However, a striking deviation was evident during telophase, wherein a significant number of SET KO cells retained centromeric SGOL1 and SGOL2 during telophase and into ensuing interphases, in contrast to the control cells (Figure 4D and S4C). This finding indicates that SET is indeed a Shugoshin chaperone in cells and suggests a mechanism whereby SET-mediated Shugoshin eviction precedes degradation by the APC/C. Moreover, defects in Shugoshin degradation have been reported to result in mitotic delays and in the occurrence of lagging chromosomes (Fu et al., 2007), suggesting that some of the mitotic defects in SET KO cells are due to defects in Shugoshin eviction, although it is unlikely to be the only cause (see below). Of note, SET KO cells phenocopy Drosophila PLK1 mutants, displaying Shugoshin-retention past anaphase into interphase and exhibiting lagging chromosomes and chromosome segregation defects (Clarke et al., 2005), corroborating that SET and PLK1 indeed function in the same pathway (also see Figure 5A and S5D), such that both activities are likely required to enable Shugoshin dissociation from centromeres.
Figure 4. SET knockout results in aberrant and delayed mitotic progression with retention of centromeric Shugoshin.
(A) (Left) Schematic of CRISPR-Cas9 mediated SET knockout (SET KO) in RPE1 cells. (Right) Western blot of whole-cell lysate confirming the knockout. (B) Growth curve of control and SET KO cells indicating severe growth-impairment upon SET KO. Number of replicates = 3 for each time point. *** = p <0.0001, two-way ANOVA test. (C) (Left) Panels from live-cell imaging of control and SET KO cells expressing H2B-RFP. Mitoses marked with asterisks are zoomed on the insets. Scale bar = 10 µm. SET KO cells display a significant delay in prophase-metaphase transition and increased frequencies of abnormal mitoses (quantification shown on right), including the presence of lagging chromosomes (arrowheads on SET KO panel- 80 min) and occurrence of micronuclei (arrows on SET KO panels −100 min and 110 min). (D) IF analysis at various mitotic stages showing that SET KO results in SGOL2 and SGOL1 (Figure S4C), persistence beyond anaphase into telophase and interphase. Scale bar = 5 µm. Quantification is shown on the right. For all quantification panels in (C) and (D): ***=p<0.0001, Fisher’s exact test, N numbers are indicated on the y-axis. (See also Figure S4, movies S1 and S2).
Phospho-H1 eviction is requisite for chromatid resolution
In a striking number of SET KO cells, sister chromatids failed to resolve along the entire length of the chromosomes and inter-chromatid distances were significantly reduced from an average of 3.6 µm in control cells to 2.2 µm (Figure 5A). Consistent with published reports, we observed a similar failure to resolve arms when PLK1 was inhibited by BI2536 (Figure S5D). Moreover, defects/delay in sister chromatid resolution have also been shown to result in lagging chromosomes during anaphase (Coelho et al., 2003; Strunnikov, 2010), suggesting that at least part of the mitotic anomalies observed upon SET KO could be mediated by untimely arm-resolution. Since SET-H1 interactions occur during prophase (Figure 2E), during which chromatid arms are resolved via the prophase-pathway, we hypothesized that defects in arm resolution were due to H1 retention on chromosomes. Indeed, in vitro biochemical assays demonstrated that SET, as well as NAP1, can robustly disassemble H1 (Figure S5A–C). However, similar to core histones, H1 is ubiquitous on chromosomes and thus, we searched for H1 modifications that might be linked specifically to SET-activity. Our quantitative MS data from SET IP gave evidence of a single phosphorylation mark, H1S/T18ph (referred to as S/T17 in some cases) on two H1 isoforms (Figure S5E, Table S4). We also detected the same phospho-site on H1 in SET purifications from mouse cells (data not shown). Indeed, H1S/T18ph was shown to be enriched in mitotic phospho-proteomes (Olsen et al., 2010; Santamaria et al., 2011), but its functional relevance is unknown. We validated the interaction between H1S/T18ph and SET by a FLAG-SET IP from BI2536-arrested cells and prophase-synchronized cells (Figure 5B and Figure S6C), using an antibody we thoroughly validated (Figure S6A and S6B). Importantly, SET did not co-IP with other mitotic phospho-H1 marks, namely H1S35ph and H1S27ph (Chu et al., 2011; Hergeth et al., 2011), signifying that SET specifically associates with H1S/T18ph during mitosis (Figure 5B). In accordance, the behavior of H1S/T18ph largely mimicked that of SET during prophase wherein it was highly enriched in the nucleus, but exhibited a significant decrease in metaphase and thereon into telophase (Figure 5D). A large amount of cytosolic H1S/T18ph was observed at metaphase suggesting that this histone species is evicted off chromosomes during the prophase-metaphase transition. This behavior is also consistent with that of another mitotic phospho-H1, H1S35ph (Chu et al., 2011). These results indicate the possibility that SET mediates the eviction of H1S/T18ph during the prophase-metaphase transition, during which chromatid arm resolution occurs. That H1S/T18ph could play a direct role in arm resolution is also supported by experiments in which we overexpressed an H1E bearing a T18A mutation (phospho-null) and observed a significant number of cells with defective arm resolution (Figure 5C); a phenocopy of SET KO (Figure 5A).
To test if H1S/T18ph was the species targeted by SET to promote arm resolution, we harvested prometaphase cells from control and SET KO cells, and isolated the chromosomes for western blot analyses (Figure 5E). Remarkably, H1S/T18ph was 20- to 30-fold enriched on metaphase chromosomes in SET KO cells compared to the control cells, while total H1 (as detected using a pan-H1 antibody) was enriched by less than 3-fold, highlighting that the H1S/T18ph was specifically retained on SET KO chromosomes (Figure 5F and Figure S6E). We detected only a modest two-fold increase in SGOL2, suggesting that a defective eviction of H1 as opposed to SGOL2 may have resulted in chromosome arms being unresolved (Figure 5F). We also did not detect changes in other mitotic phospho-H1 marks like H1S27ph (Figure 5F), pointing to H1S/T18ph being specifically retained on SET-KO chromosomes. The retention of H1S/T18ph on unresolved chromosomes is analogous to effects observed with cohesin, whereby perturbation of proteins like PLK1 or Wap1 resulted in cohesin accumulation on the arms of prophase chromosomes (Kueng et al., 2006; Sumara et al., 2002). However, cohesin levels (SMC1) from SET KO and control chromosomes were largely similar despite that SET KO chromosomes were unresolved while the controls were largely resolved (Figure 5F). Arm-resolved chromosomes retain cohesin in the centromeres until anaphase commitment and therefore, the remnant cohesin observed in control and SET KO chromosomes is most likely centromeric. Thus, the pathways controlling cohesin inactivation/removal along the arms are largely independent of H1S/T18ph eviction by SET pointing to an interesting possibility that cohesin release from the arms could occur upstream of H1S/T18ph eviction.
To determine the precise location of H1S/T18ph retention on mitotic chromosomes, we performed immunofluorescence (IF) analysis on metaphase chromosomes of control and SET KO cells. Strikingly, H1S/T18ph was present along the entire length of the inter-chromatid DNA in SET KO cells in a significant number of metaphases, while this percentage was much lower in the control cells (Figure 5G), confirming our observations from western blot analysis (Figure 5F). Indeed, phosphorylated histones such as histone H3 phosphorylated at threonine 3 localize to the inter-chromatid axis during prophase and recruit the CPC proteins to chromosomes (Yamagishi et al., 2010), highlighting that phosphorylation of histones in the inter-chromatid region could specify defined processes during mitotic progression. Importantly, in WT cells, SET and H1S/T18ph localized to the inter-chromatid axis, strongly suggesting that SET indeed exerts its function at the inter-chromatid region via H1S/T18ph eviction (Figures 1D and 5H). Our attempts to perform co-localization analysis of SET and H1S/T18ph on chromosomes were only moderately successful because of technical difficulties with the antibodies. Nonetheless, pixel intensity correlation analysis allowed us to observe on a few chromosomes, co-localization of SET and H1S/T18ph at the inter-chromatid axis (Figure S6D). Taken together, these data indicate that both SET and H1S/T18ph localize to the inter-chromatid axis and that ablation of SET results in the marked accumulation of this mark in the axial region.
To test if SET directly evicts H1S/T18ph, we isolated prometaphase chromosomes from SET KO cells and performed CHREA in the presence of WT SET, ACID or DIM mutants (Figure 6A). Strikingly, WT SET, but not the ACID and DIM mutants evicted H1S/T18ph from SET KO chromosomes (Figure 6B). We also observed efficient SGOL2 eviction under these conditions (Figure 6B). Importantly, we did not detect such eviction activity when testing for another mitotic phospho-mark H1S27ph (Figure S3A), suggesting that the eviction activity was specific to H1S/T18ph. To test if eviction depended on phosphorylation, we dephosphorylated the assay templates with λ-phosphatase (a control template was treated with both the phosphatase and phosphatase inhibitors) and subjected these differentially phosphorylated substrates to CHREA (Figure 6C). We used a pan-H1 antibody to gauge H1 eviction because treatment with the phosphatase precluded detection by the H1S/T18ph antibody. Upon SET addition, we observed a striking 4- to 6-fold reduction in H1 eviction (Figure 6D) indicating that phosphorylation plays a vital role in enhancing eviction efficiency, similar to our observations with Shugoshin (Figure 3G). In summary, these results indicate that SET is necessary and sufficient for the eviction of H1S/T18ph from chromosomes in vivo and in vitro respectively, and elucidate a direct functional outcome of H1 site-specific phosphorylation.
Discussion
Taken together, our studies demonstrated that SET mediates the timely resolution of sister chromatids during mitosis. We propose that this happens in two stages (Figure 6E). In the first stage, SET-H1 complexes are formed on chromosomes but are not evicted, prior to the phosphorylation of axial H1 at S/T18ph. This phosphorylation aids in the site-specific eviction of H1 and we believe this to be a critical step in the prophase signaling cascade that includes cohesin phosphorylation by PLK1, release and arm resolution. SET also localizes to centromeres and associates with Shugoshins as early as prophase. In the second stage, after chromosome bi-orientation and as cells proceed to enter anaphase and telophase, SET inhibitory activity towards PP2A creates an optimal environment for phosphorylation by PLK1. An additional step whereby SET is itself phosphorylated might be required for the activation of SET inhibitory activity towards PP2A (Vasudevan et al., 2011) and indeed, our quantitative-MS data revealed the presence of a number of phosphorylation sites on SET (Table S3 and S4). This process likely creates a positive-feed forward loop that, in addition to tension-mediated pathways, leads to the partial delocalization and subsequent SET-mediated eviction of Shugoshin. The evicted Shugoshin is most likely targeted for degradation by APC/C.
While it is generally acknowledged that phosphorylation of H1 at specific sites progressively increases during S and M phase transition with resultant H1 dynamicity, the functional implications of site-specific H1 phosphorylation were largely unknown for decades (Hohmann, 1983). Here, we showed that H1S/T18ph localizes at the inter-chromatid axis, and that S/T18ph serves as the target for appropriate H1 removal by SET. Our analysis of SET KO chromosomes is strongly suggestive of a mechanism whereby arm-cohesin release is independent of and precedes H1S/T18ph eviction. Therefore, the resolution of sister chromatids during mammalian mitosis likely involves the successive disassembly of various proteins like cohesin, H1 (and maybe others) in a manner that impinges on the local-chromatin structure, kinase and chaperone activity. The emerging theme is that H1 modifications can broadly target cellular factors and their activities to specific regions on chromatin and likely represent an early event in the chromatin signaling cascade. Indeed, it was shown recently that H1 ubiquitination serves as a key signaling intermediate to recruit factors to sites of double-strand DNA breaks (Thorslund et al., 2015). Future experiments should aim to address the roles of these abundant, yet poorly understood class of histones as pivotal modulators of bio-signaling processes.
SET was detected as one of the interactors in Shugoshin immunoprecipitation experiments in meiotic and mitotic cells (Chambon et al., 2013; Kitajima et al., 2006). But, the role of SET as a chaperone in this context was not known. Here we showed that SET is a Shugoshin chaperone, required for its the timely eviction from mitotic chromosomes. It will be interesting to uncover in the future if other histone chaperones like NAP1 can evict non-histone proteins like cohesin. To address this and other pertinent questions, the viable experimental tool we developed and designated CHREA in this report, may prove valuable as we have tested its robustness on chromosomes isolated from different cell lines and contexts.
While in normal cells, Shugoshin removal likely occurs during the metaphase to telophase transition, the presence of SET-SGOL2 in the cytosolic fractions from metaphase-arrested FLAG-HA-SET cells (Figure 2), is most likely a result of SET overexpression leading to a premature eviction of SGOL2. Indeed, SET overexpression can cause a premature loss of sister chromatid cohesion in mouse oocytes (Qi et al., 2013) and knockdown of Shugoshin in mammalian cells results in untimely separation of sister chromatids (Tanno et al., 2010). Extending these findings further, our studies provide a tenable mechanistic perspective regarding the oncogenic SET-NUP214 fusion in Acute Lymphoblastic Leukemia (ALL) and Acute Myeloid Leukemia (AML) (Adachi et al., 1994; Ozbek et al., 2007; Quentmeier et al., 2009; Rosati et al., 2007). This fusion results in the constitutive nuclear localization of SET (Saito et al., 2004) and based on our studies, the sub-cellular localization of SET is tightly regulated during the cell cycle (Figure 2A). Therefore, the precocious nuclear presence of SET could lead to the untimely eviction of Shugoshin and phospho-H1 from chromosomes, thereby compromising sister chromatid cohesion with resultant aneuploidy, a potent driver of tumorigenesis.
In summary, our study revealed that SET is a mitotic chaperone that promotes chromatid resolution, providing a new dimension to the cellular properties of histone chaperones. Our findings also demonstrated that site specific phosphorylation of linker histones constitutes a fundamental step in mediating chromatid resolution during mitosis, opening avenues to explore the roles of linker histones and their modifications in cellular biology.
STAR methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Danny Reinberg (Danny.Reinberg@nyumc.org).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
The following cells were used in this study: Human telomerase immortalized retinal pigmented epithelial cells (RPE1), Human embryonic kidney 293T (293T), Human embryonic kidney 293GP2 (293 GP2), immortalized mouse embryonic fibroblasts (iMEFs). HEK293T, HEK293 GP2 and iMEFs were grown with DMEM supplemented with 10% FBS, 1% FBS and 2 mM glutamine. For RPE1 cells, the same media was additionally supplemented with 15 mM HEPES and sterilized through a 0.22 µM vacuum filter. All cells were cultured in standard cell culture incubators (37°C, 5% CO2).
METHOD DETAILS
Antibodies
Antibodies for western blots were used at the following dilutions: rabbit anti-SET and goat anti-SET (Santa Cruz, 1:1000), rabbit anti-SGOL2 (Bethyl, 1:1000), mouse anti-SGOL1 (Abcam, 1:1000), rabbit anti-PP2A-B (Cell Signaling, 1:1000), rabbit anti-PP2A-B’ (Bethyl, 1:1000), mouse anti-H1 (Santa Cruz, 1:2500), rabbit anti-H3 and anti-H4 (Abcam, 1:5000), mouse anti-H3S10ph (Millipore, 1:1000), mouse anti-GAPDH (Genetex, 1:5000), rabbit anti-REPIN1 (Sigma, 1:1000), mouse anti-MPM2 (Millipore, 1:1000), rabbit anti-H1S/T18ph (Abcam, 1: 1000), rabbit anti-PAF1 and anti-LEO1 (Reinberg lab, 1:1000), Rabbit anti-H1S27ph (Sigma 1:1000), anti-H1S35ph (genetex, 1:1000). For IF studies, the following dilutions were used: rabbit anti-SET and goat anti-SET (Santa Cruz, 1:100 and 1:50, respectively), rabbit anti-SGOL2 (Bethyl, 1:100), mouse anti-SGOL1 (Abcam, 1:100), rabbit anti-H1S/T18ph (Abcam ab188294, 1:50 for chromosome spreads, 1:20,000 for whole cell IF), human ACA (Immunovision, 1:50), rat tubulin (Abcam, 1:500), mouse γ-tubulin (Sigma, 1:500). We also tested another rabbit anti-H1T18ph which worked similar to the abcam antibody for western blots and moderately better with less background staining (compared to the abcam antibody) for whole cell IF (Aviva biosystems, 1:1000 western blots, 1:50 for chromosome spreads, 1:10,000 for whole cell IF, data not shown) All fluorophore conjugated secondary antibodies were used at a dilution of 1:500 (Life Technologies).
Cell synchronization
For synchronization of cells in mitosis, cells were treated with inhibitors as mentioned in Figure 2B. FACS analysis was performed by harvesting the cells, fixing with ethanol and staining with propidium iodide. For double thymidine block (Figure 2A), RPE1 cells were treated with 5 mM thymidine for 19 h, washed and released into thymidine-free media for 9 h and blocked for a second time with 5 mM thymidine for 9 h and released into thymidine-free media.
Cell line generation
The cDNA for human SET was cloned into the pINTO-NFH (for expression in iMEFs) and the cDNA for human SET, H1.2 and H1.4 were cloned into pBABE-puro (for expression in RPE1 cells). For iMEFs, expression vectors were transfected using Lipofectamine 2000 (Life technologies) and stable clones were selected with 400 µg/ml Zeocin for three weeks. For RPE1, retroviral particles (generated in the GP2-293, Clonetech) were transduced and stable clones were selected using 2.5 µg/ml Puromycin for one week. Clones were tested for expression of the tagged protein using mouse anti-HA antibody (Covance, 1:1000) or mouse anti-FLAG antibody (Sigma, 1:1000).
Subcellular fractionation
All fractionation and purification experiments were performed on ice or 4 °C, with all the extraction and wash buffers containing 5 µg/ml each of Pepstatin, Aprotonin and Leupeptin, 1 mM PMSF and 20 mM sodium fluoride. Cytosolic extracts were prepared by incubating pelleted and washed cells with PBS+0.05% IGEPAL-CA630 for 5 min and spun at 5000g for 5 min. The supernatant was carefully harvested as the cytosolic extract. Pellets were treated with Dignam buffer (20 mM Tris, pH 7.9, 25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.05% IGEPAL-CA630), incubated with rotation at 4 °C for 30 min followed by centrifugation at 30,000g for 30 min. Supernatant was isolated carefully as the soluble nuclear extract. For immunoprecipitation experiments, the salt concentration of this extract was diluted to 0.3 M NaCl by adding appropriate amounts of a no-salt buffer.
Immunoprecipitation, MS and glycerol gradient
Cytosolic and soluble nuclear extracts were incubated with FLAG-M2 agarose beads (Sigma) overnight, with rotation at 4 °C. Beads were washed five times with FLAG wash buffer containing 20 mM Tris, pH 7.9, 0.3 M NaCl, 1 mM MgCl2, 0.2 mM EDTA, 0.05% IGEPAL-CA630 and inhibitors mentioned in the previous section. A final wash with the above buffer containing 0.1 M NaCl was performed and bound complexes were eluted with 700–800 µl of 500 µg/ml FLAG peptide (Sigma) in the same buffer. For TAP, two elutions were performed with rotation at 4 °C for 2 h for a total of 4 h of elution. For FLAG immunoprecipitation experiments, a single elution for 3 h was performed. The eluates were gently harvested to avoid FLAG beads and transferred to HA beads (Sigma) (for TAP) or concentrated in Amicon ultra 0.5 ml centrifugal units and mixed with SDS loading buffer (for IP). Glycerol gradient with FLAG eluates were carried out as previously described (Gao et al., 2012). For TAP, incubation in HA beads was carried out for 4 h, and the beads were washed three times with the FLAG wash buffer. Subsequently, three washes were carried out with a modified FLAG wash buffer containing 0.1 M NaCl without IGEPAL-CA630. Bound complexes were eluted with 0.1 M glycine on ice for 10 min, neutralized with 1 M Tris, pH 7.9 and subjected to mass spectrometric analysis. Mass spectrometric analysis for initial studies of SET-associated proteins in iMEFs and RPE1 cells were carried out as previously described (Gao et al., 2012). Quantitative mass spectrometry experiments in mitotic cells were performed and analyzed as previously described (Campos et al., 2015), with the exception that mass spectra were recorded on a Q Exactive mass spectrometer (Thermo Scientific) using higher-energy collisional dissociation (HCD) fragmentation on the top 10 most intense precursor ions and that phosphorylation was included as a variable modification in data analysis.
Immunofluorescence and chromosome spreads
For immunofluorescence on whole cells, RPE1 cells were grown on sterile coverslips. Cells were washed with PBS for 10 min (two washes of 5 min each) and fixed with 4% paraformaldehyde in PBS for 10 min at room temperature. Cells were washed again with PBS for 15 min (three washes of 5 min each) and permeabilized with PBS containing 0.3% Triton X100 for 5 min. Following extensive washes with PBS containing 0.05% Tween 20 (PBST), cells were blocked with PBST+5% BSA or PBST+donkey serum, for 1 h and primary antibody was applied at an appropriate dilution and incubated overnight at 4 °C. Cells were washed with PBST and incubated with fluorophore-conjugated secondary antibodies in PBST in dark for 1 h. After washing with PBST, cells were counter-stained with DAPI and mounted with aquamount and dried overnight. Imaging was performed on a Zeiss LSM710 Confocal microscope using 63X objective. For immunofluorescence on chromosomes (SET and SGOL2), cells were cytospun on glass slides and processed as described previously (Jeppesen, 2000). For chromosomal staining for H1S/T18ph and ACA, cells were treated with 0.2 M Karyomax (Gibco) for 1 h and chromosomes were fixed and dropped as per (Theunissen and Petrini, 2006). After drying for 5 min, slides were rehydrated in water for 10 min, and transferred to PBST for 30 min, after which they were blocked in PBST+5%BSA for 1 h. Subsequent steps were performed as per the protocol used for IF on whole cells. Using this protocol, the intensity of staining with the H1S/T18ph antibody was sometimes weak, and the green channel was enhanced equally, in all control and SET KO images shown and quantified in Figure 5G. Using other protocols (like cytospin) for staining of H1S/T18ph usually resulted in some amount of background staining, in addition to the inter-chromatid axis. Co-localization analysis for SET and H1S/T18ph was performed using the two independent co-localization plugins available for ImageJ with default settings (https://imagej.nih.gov/ij/plugins/colocalization.html and https://imagej.nih.gov/ij/plugins/colocalization-finder.html).
For giemsa staining of chromosomes, cells were treated with 0.2 M Karyomax (Gibco) for 1 h and fixed and stained as previously described (Theunissen and Petrini, 2006). Briefly, cells were treated with 0.2 µM colcemid for 1 h, harvested and washed with PBS. Cells were swollen in a hypotonic 75 mM KCl solution and incubated at 37°C for 15 min. Without mixing, 5 ml of ice-cold fixative prepared fresh (3:1- Methanol: Acetic Acid) was added. Cells were spun at 1000 rpm for 5 min, and supernatant was carefully removed. 10 ml of fixative was added to the cells with the first 2 ml added dropwise with intermittent mixing. This step was repeated twice and cells were resuspended in a final volume of 50–100 µl. Cold slides (previously incubated at −20 °C) were rested inside a 37 °C water bath and allowed to moisten for 2–3 min before the cell suspension was dropped on the slide. Slides were dried overnight, stained with giemsa stain and destained with water. Interchromatid distances were calculated as previously described (Kueng et al., 2006) with the exception that ImageJ software was used.
Live-cell imaging and growth analysis
For live-cell imaging, cells were transduced with H2B-RFP lentiviral particles and plated on Nunc 8-well chamber slides. Imaging was performed on a Zeiss Axio Observer microscope (20X objective), in a temperature (37 °C) and CO2 controlled chamber and DIC and RFP channel images were captured every 5 min for a period of 30 h. All resultant images were processed using ImageJ. For growth analysis, 1 × 104 cells/ well were plated on 9 × 6 well plates (one plate to be counted per time point). Three wells per cell line were counted every day for a period of 9 days. Media was changed every 3rd day.
Protein purification and pull-down assays
cDNA for human SET (WT and mutants) was cloned into a pST4 Strep(II) tag vector and protein expression was performed as described previously (Krishnan et al., 2012). Briefly, Rosetta 2 competent cells (Novagen) were transformed with expression vector. Colonies were inoculated in 50 ml 2XYT media and grown at 37 °C, until saturation (OD=1). From this starter culture, 10 ml was added to 1.5 liters of 2XYT media and grown at 37 °C until OD=0.4. Temperature of the incubator was reduced to 16 °C and expression was induced by adding 1mM IPTG. Cells were lysed by sonication, clarified by centrifugation at 50,000g for 20 min. The supernatant supplemented with protease inhibitors was incubated overnight with Streptactin macroprep resin (IBA life sciences). Resin was washed in at least 20 column volumes of 50 mM Tris pH, 7.9, 0.5 M NaCl and eluted with 2.5 mM biotin in the same buffer. Peak fractions (as analyzed by SDS PAGE) were pooled and dialyzed against a buffer of 50 mM Tris, pH 7.9, 100 mM NaCl and 5% glycerol. The recombinant protein at this step is ~90% pure as judged by SDS-PAGE. An additional chromatography step offered a modest increase in purity and can be deemed optional. After dialysis, ion exchange chromatography (Mono Q) step was performed with a linear gradient of the 50 mM Tris, pH 7.9, 5% glycerol buffer prepared with 0.1 M NaCl and 1 M NaCl. (As an alternative method, we also performed gel filtration after the Streptactin elution but this did not produce the same purity as a MonoQ column). Peak fractions from the MonoQ column were dialyzed in 20 mM Tris, pH 7.9, 50 mM NaCl and 5% glycerol. For use in chromosome eviction assays, the pooled fractions were supplemented with 0.05% IGEPAL-CA630 and dialyzed in 1X buffer D (see below, 15 mM Tris, pH 7.5, 80 mM KCl, 2 mM EDTA, 0.05% IGEPAL-CA630).
cDNA for human SGOL2 and human PLK1 was cloned into a pGEX6P2 expression vectors and protein expression was carried out as for SET. For purification of GST-SGOL2 and GST-PLK1, cell lysates were supplemented with 5% glycerol and 0.1% Triton X-100 and these conditions were maintained throughout. Binding to Glutathione-Sepharose beads was carried out for 2 h, followed by washing with buffer containing 50 mM Tris, 0.3 M NaCl, 5% glycerol and 0.1% Triton X100. Elution was carried out with 20 mM glutathione in 100 mM Tris, pH 8.8 containing 5% glycerol and 0.1% Triton X-100. Peak fractions were dialyzed in 20 mM Tris, pH 7.9, 50 mM NaCl, 5% glycerol and 0.1% Triton X-100.
For GST pull-down assays, 300 pmoles of GST or GST-SGOL2 was immobilized on glutathione beads for 15 min at 4 °C following which, 30 pmoles of SET /NAP1 was added to the reaction and incubated for an additional 30 min. Beads were washed 5 times with 20 mM Tris, pH 7.9, 0.4 M NaCl and 0.1% Triton X-100. Bound proteins were released by boiling the beads with SDS-PAGE loading buffer.
Preparation of chromosome clusters
For the isolation of chromosomal clusters, 25 × 150mm plates of 293T cells were synchronized in mitosis by the addition of 3.3 µM Nocodazole for 16 h. Synchronization with 10 nMBI2536 was also tested and could be utilized. For RPE1 cells, 50 × 150mm plates of cells were used and mitotic cells were harvested by mitotic shake-off. Shake-off was not necessary when using 293T cells. Chromosome cluster isolation was modified from a previous protocol (Gasser and Laemmli, 1987). All steps were performed on ice and buffers were supplemented with protease and phosphatase inhibitors. Cells were harvested and washed twice with PBS. Cell pellets were resuspended in 25ml of 1X buffer D (15 mM Tris, pH 7.5, 80 mM KCl, 2 mM EDTA, 0.05% IGEPAL-CA630), incubated on ice for 5 min and homogenized 15X with a dounce homogenizer with a tight pestle. Six percoll-glycerol gradients were prepared using two solutions: (1) 25% v/v glycerol solution was prepared using 1X buffer D (2) Percoll solution was prepared with 60% v/v Percoll, 15% v/v glycerol and 0.5X buffer D. Six gradients were prepared in 50 ml tubes by adding 20 ml of solution (1) and layering 5 ml of solution (2) underneath. Sharp gradient separation may not be visible at this point between (1) and (2). 4 ml of homogenized cells were gently layered on top of each of the six prepared gradients and spun at 1000g for 15 min in a swinging bucket centrifuge. The top layer of the gradient was gently removed and chromosome clusters located at the interphase along with the solution underneath were harvested into a dounce homogenizer. After douncing 15X, 100 ml of percoll solution (2) was added and mixed with the chromosomes, to reach a total volume of approximately 130–150 ml. This was split into 8 centrifuge tubes with 15–20 ml in each, spun first at 3000g for 5 min and increasing the speed in the same centrifuge to 35,000g for 30 more min. The chromosome clusters are observed as a mesh, floating on or close to the top of the solution, and are carefully harvested with large orifice tips (or cut tips) into a 15ml tube. The chromosomes were washed 3X with 10 ml of 1X Buffer D to remove residual percoll, by spinning at 5000g for 5 min. After the last wash, the chromosomes were resuspended in 3–5 ml of 1X buffer D, homogenized sequentially with 18G and 22G needles, aliquoted (200 – 300 µl) and stored at −80°C.
CHREA and kinase assay
Chromosome clusters were thawed gently on ice and homogenized briefly but thoroughly with an 18G needle followed by a 22G needle. This was the most crucial step in the assay for two reasons: (1) proper homogenization ensures ease of distribution of similar amounts of clusters between different reactions and (2) harsh homogenization and/or failure to keep the clusters on ice during the process resulted in spontaneous release of chromosomal proteins rendering them unusable in the assay. Clusters were washed twice with 1X buffer D (from above) supplemented with protease and phosphatase inhibitors, by spinning at 1000g for 2 min. Chaperone proteins (which were previously dialyzed in 1X buffer D) of appropriate amounts (Figure 3B: 1, 3 and 6 µg, Figure 3C: 6 and 12 µg) were added and diluted with wash buffer to a final volume of 50 µl. Due to the extremely fragile nature of the chromosomal clusters, assays were optimized at different temperatures and the optimal assay temperature was in a range of 4–15 °C, with the higher temperature ranges giving better eviction efficiencies. Incubations were carried out in an eppendorf thermomixer (800–1000 rpm). After incubation for 1 h, assay mixtures were spun at 1000g for 3 min and supernatants were carefully harvested for western blotting. Pellets were also utilized for H3 immunoblotting (loading control). To compare relative eviction between samples, intensities were first normalized to H3 (pellet), and these values were normalized again to the peak (or highest) value. Absolute eviction efficiencies calculated by dividing the amount evicted in supernatant by the total amount (supernatant + pellet) ranged from 5–15%. This lowered efficiency is most likely because the isolated chromosome clusters are highly condensed and bundled, where accessibility and conformation may not recapitulate those observed in the cells. However, we observed robust specificity (for the target proteins) and selection (for the presence/absence of chaperone activity) in the assay, under multiple conditions and from chromosomes isolated from different cell lines.
For the kinase assays on chromosome clusters, 15 µg GST-PLK1 was incubated with DMSO or 0.5 mM BI2536 and incubated on ice for 20 min. Chromosome clusters were homogenized and washed twice with kinase buffer 1 (20 mM Tris, pH 7.5, 2 mM EGTA, 5 mM MgSO4, 50 mM NaCl and 0.05% IGEPAL-CA630). Clusters were resuspended in kinase buffer 2 (kinase buffer 1 + 1 mM DTT, 100 µM ATP and/or 1 µM γP32-ATP, where applicable). GST-PLK1 (active or inactive) was added, and the reaction was incubated in an eppendorf mixer (800 rpm) for 30 min at 25 °C. Clusters were washed three times in kinase buffer 1 and split into two tubes. To one tube, 1X buffer D was added and to the other, 6 µg SET was added, and incubated in the mixer for 1 h at 15 °C. Supernatants were analyzed as before. For kinase assay with GST-SGOL2, 5 µg of GST-SGOL2 was mixed with 2 µg of GST-PLK1 in the buffers described above, and incubated at 25 °C for 20 min.
CRISPR-Cas9 experiments
Guide RNAs targeting intron 2 and exon 3 of the SET genomic locus were designed based on the MIT CRISPSR server (http://crispr.mit.edu/). Cloning of the guide RNAs was done in the pLENTI CRISPR v2 plasmids (a gift from Feng Zhang, Addgene plasmid # 52961) as described previously (Sanjana et al., 2014). Lentiviruses were generated in 293 FT cells using the packaging plasmids pMD2.G and psPAX 2, and RPE1 cells were transduced with either the intron guide viruses or exon guide viruses. Cells were selected with 2.5 µg/ml puromycin for 1 week following which, they were single cell sorted into a 96-well plate and supplemented with RPE1 conditioned media. Due to poor survival, at least 10 × 96 well plates were sorted to ensure the availability of 50+ clones for screening. Clones were genotyped by amplifying a 680 bp fragment spanning both intron 2 and exon 3 and the PCR products were sequenced to determine desired clones.
H1 purification, assembly and chaperone assay
Nuclear extracts from 0.5 × 108 HeLa cells in Dignam buffer (20 mM Tris, pH 7.9, 25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.05% IGEPAL-CA630) were subjected to TCA precipitation as described previously (Reeves and Nissen, 1999). Briefly, nuclear extracts were treated with 2% TCA and incubated on ice for 30 min. After spinning at 30,000g for 30 min, the supernatant was harvested and treated with a final concentration of 25% TCA. The sample was then incubated on ice for at least 1 h or overnight at 4 °C. After centrifugation at 30,000g for 30 min, the pellet was washed twice with ice-cold acetone, and air dried to remove all traces of acetone. The pellet was dissolved in 3 ml of 6 M guanidinium hydrochloride, 20 mM Tris, pH 7.5, 1 mM EDTA and 5 mM 2-mercaptoethanol and spun briefly to remove insoluble material. The sample was dialyzed first against 2M NaCl, 20 mM Tris, pH 7.5, 1 mM EDTA and 5 mM 2-mercaptoethanol overnight and an additional 2 h with two buffer changes. The salt was reduced by dialysis to 0.1 M NaCl in the same buffer conditions, and the sample was loaded onto a Mono S column, and eluted with a linear gradient up to 1 M NaCl. Since the A280nm of H1 is very low, a peak may or may not be visible and alternate fractions are checked by SDS-PAGE to identify those that contain H1. The H1 containing fractions were pooled and dialyzed against 20 mM Tris, pH 7.5, 100 mM NaCl and 5% glycerol, and stored at −80 °C.
For assembly of H1 on nucleosomal templates, histone octamers were first assembled on a plasmid containing 12X 601 sequences by a progressive dilution of octamers+DNA in 2 M NaCl to 100 mM NaCl using a no-salt buffer. The assembled nucleosomes were treated with H1 in a molar ratio of 100:1 (H1: nucleosome core particles) at 37 °C for 1 h. To test for H1 assembly, the above assembled templates were digested with MNase for 1 min, the reaction was stopped using buffer containing 1% SDS, 0.25 mg/ml glycogen, 0.2 M NaCl and 20 mM EDTA. DNA was extracted by phenol-chloroform and subjected to ethanol precipitation, loaded on a 2% agarose gel and stained with ethidium bromide.
For EMSA-chaperone assay, H1 containing nucleosomal templates (prepared by assembling histone octamers and a 190 bp of 5s RNA template DNA) were incubated with H1 in the same ratio as above at 37 °C for 1 h. Chaperones were added to these assembled templates in a ratio of 5:1 (chaperone: H1) and incubated for an additional 1 h at 37 °C. Reactions were loaded on a 5% native polyacrylamide gel and stained with SYBR GOLD.
QUANTIFICATION AND STATISTICAL ANALYSIS
For all quantification analyses, sample size (N) and statistical tests performed are described in the corresponding figure legends. Quantification of interchromatid distances and description of colocalization analysis is provided above. Western blot and fluorescence intensity quantification was performed on ImageJ. All statistical analysis was performed using GraphPad prism.
DATA AND SOFTWARE AVAILABILITY
Raw data deposited at: http://dx.doi.org/10.17632/h22sb48h79.1
Supplementary Material
Figure S1 – Related to Figure 1
Figure S2 – Related to Figure 2
Figure S3 – Related to Figure 3
Figure S4 – Related to Figure 4
Figure S5 – Related to Figure 5
Figure S6 – Related to Figure 5
Table S1- Peptide count numbers of SET TAP-MS analysis from asynchronous iMEFs and RPE1 cells (Related to Figure 1)
Acknowledgments
We thank Dr. L.Vales for critical comments, past and current members of the Reinberg lab for insightful discussions, H.Zheng, C.Zhao and M.Qian for the initial MS analysis and A.Paradhkar for technical assistance. We also thank the NYU Microscopy Core (grant: NCRR S10 RR023704-01A1) for use of the confocal microscope and the NYU Flow Cytometry core (grant: NIH/NCI P30CA016087) for analysis. This work in the D.R. laboratory was supported by the Howard Hughes Medical Institute (HHMI) and work in the M.V. lab is supported by grants from the Netherlands organization for Scientific Research (NWO-VIDI grant no 864.09.003 and NWO-Gravitation program CGC.nl).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
S.K. conceptualized and carried out the experiments. A.H.S performed the quantitative mass spectrometry studies in M.V. lab. S.K. wrote the manuscript with assistance from D.R.
References
- Adachi Y, Pavlakis GN, Copeland TD. Identification and Characterization of Set, a Nuclear Phosphoprotein Encoded by the Translocation Break Point in Acute Undifferentiated Leukemia. J Biol Chem. 1994;269:2258–2262. [PubMed] [Google Scholar]
- Belotserkovskaya R, Oh S, Bondarenko VA, Orphanides G, Studitsky VM, Reinberg D. FACT facilitates transcription-dependent nucleosome alteration. Science. 2003;301:1090–1093. doi: 10.1126/science.1085703. [DOI] [PubMed] [Google Scholar]
- Burgess RJ, Zhang ZG. Histone chaperones in nucleosome assembly and human disease. Nature structural & molecular biology. 2013;20:14–22. doi: 10.1038/nsmb.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos EI, Fillingham J, Li G, Zheng H, Voigt P, Kuo WH, Seepany H, Gao Z, Day LA, Greenblatt JF, et al. The program for processing newly synthesized histones H3.1 and H4. Nature structural & molecular biology. 2010;17:1343–1351. doi: 10.1038/nsmb.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos EI, Smits AH, Kang YH, Landry S, Escobar TM, Nayak S, Ueberheide BM, Durocher D, Vermeulen M, Hurwitz J, et al. Analysis of the Histone H3.1 Interactome: A Suitable Chaperone for the Right Event. Mol Cell. 2015;60:697–709. doi: 10.1016/j.molcel.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon JP, Touati SA, Berneau S, Cladiere D, Hebras C, Groeme R, McDougall A, Wassmann K. The PP2A inhibitor I2PP2A is essential for sister chromatid segregation in oocyte meiosis II. Current biology : CB. 2013;23:485–490. doi: 10.1016/j.cub.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Chu CS, Hsu PH, Lo PW, Scheer E, Tora L, Tsai HJ, Tsai MD, Juan LJ. Protein Kinase A-mediated Serine 35 Phosphorylation Dissociates Histone H1.4 from Mitotic Chromosome. J Biol Chem. 2011;286:35843–35851. doi: 10.1074/jbc.M111.228064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AS, Tang TT, Ooi DL, Orr-Weaver TL. POLO kinase regulates the Drosophila centromere cohesion protein MEI-S332. Dev Cell. 2005;8:53–64. doi: 10.1016/j.devcel.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Coelho PA, Queiroz-Machado J, Sunkel CE. Condensin-dependent localisation of topoisomerase II to an axial chromosomal structure is required for sister chromatid resolution during mitosis. J Cell Sci. 2003;116:4763–4776. doi: 10.1242/jcs.00799. [DOI] [PubMed] [Google Scholar]
- Dailey L, Caddle MS, Heintz N, Heintz NH. Purification of Rip60 and Rip100, Mammalian Proteins with Origin-Specific DNA-Binding and Atp-Dependent DNA Helicase Activities. Molecular and cellular biology. 1990;10:6225–6235. doi: 10.1128/mcb.10.12.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das C, Tyler JK, Churchill MEA. The histone shuffle: histone chaperones in an energetic dance. Trends Biochem Sci. 2010;35:476–489. doi: 10.1016/j.tibs.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1077. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- Fan Z, Beresford PJ, Oh DY, Zhang D, Lieberman J. Tumor suppressor NM23-H1 is a granzyme A-activated DNase during CTL-mediated apoptosis, and the nucleosome assembly protein SET is its inhibitor (vol 112, pg 659, 2000) Cell. 2003:115. doi: 10.1016/s0092-8674(03)00150-8. [DOI] [PubMed] [Google Scholar]
- Fu G, Hua S, Ward T, Ding X, Yang Y, Guo Z, Yao X. D-box is required for the degradation of human Shugoshin and chromosome alignment. Biochem Biophys Res Commun. 2007;357:672–678. doi: 10.1016/j.bbrc.2007.03.204. [DOI] [PubMed] [Google Scholar]
- Gadad SS, Senapati P, Syed SH, Rajan RE, Shandilya J, Swaminathan V, Chatterjee S, Colombo E, Dimitrov S, Pelicci PG, et al. The Multifunctional Protein Nucleophosmin (NPM1) Is a Human Linker Histone H1 Chaperone. Biochemistry. 2011;50:2780–2789. doi: 10.1021/bi101835j. [DOI] [PubMed] [Google Scholar]
- Gao ZH, Zhang J, Bonasio R, Strino F, Sawai A, Parisi F, Kluger Y, Reinberg D. PCGF Homologs, CBX Proteins, and RYBP Define Functionally Distinct PRC1 Family Complexes. Mol Cell. 2012;45:344–356. doi: 10.1016/j.molcel.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser SM, Laemmli UK. Improved Methods for the Isolation of Individual and Clustered Mitotic Chromosomes. Exp Cell Res. 1987;173:85–98. doi: 10.1016/0014-4827(87)90334-x. [DOI] [PubMed] [Google Scholar]
- Gurard-Levin ZA, Quivy JP, Almouzni G. Histone Chaperones: Assisting Histone Traffic and Nucleosome Dynamics. Annu Rev Biochem. 2014;83:487. doi: 10.1146/annurev-biochem-060713-035536. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Caballero C, Cebollero LR, Pendas AM. Shugoshins: from protectors of cohesion to versatile adaptors at the centromere. Trends Genet. 2012;28:351–360. doi: 10.1016/j.tig.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Harshman SW, Young NL, Parthun MR, Freitas MA. H1 histones: current perspectives and challenges. Nucleic Acids Res. 2013;41:9593–9609. doi: 10.1093/nar/gkt700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergeth SP, Dundr M, Tropberger P, Zee BM, Garcia BA, Daujat S, Schneider R. Isoform-specific phosphorylation of human linker histone H1.4 in mitosis by the kinase Aurora B. J Cell Sci. 2011;124:1623–1628. doi: 10.1242/jcs.084947. [DOI] [PubMed] [Google Scholar]
- Hergeth SP, Schneider R. The H1 linker histones: multifunctional proteins beyond the nucleosomal core particle. Embo Rep. 2015;16:1439–1453. doi: 10.15252/embr.201540749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann P. Phosphorylation of H1-Histones. Mol Cell Biochem. 1983;57:81–92. doi: 10.1007/BF00223526. [DOI] [PubMed] [Google Scholar]
- Jeppesen P. Immunofluorescence in cytogenetic analysis: method and applications. Genet Mol Biol. 2000;23:1107–1114. [Google Scholar]
- Kalousi A, Hoffbeck AS, Selemenakis PN, Pinder J, Savage KI, Khanna KK, Brino L, Dellaire G, Gorgoulis VG, Soutoglou E. The Nuclear Oncogene SET Controls DNA Repair by KAP1 and HP1 Retention to Chromatin. Cell reports. 2015;11:149–163. doi: 10.1016/j.celrep.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Karamysheva Z, Diaz-Martinez LA, Crow SE, Li B, Yu H. Multiple anaphase-promoting complex/cyclosome degrons mediate the degradation of human Sgo1. J Biol Chem. 2009;284:1772–1780. doi: 10.1074/jbc.M807083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Okuwaki M, Nagata K. Role of Template Activating Factor-I as a chaperone in linker histone dynamics. J Cell Sci. 2011;124:3254–3265. doi: 10.1242/jcs.083139. [DOI] [PubMed] [Google Scholar]
- Kawashima SA, Yamagishi Y, Honda T, Ishiguro K, Watanabe Y. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science. 2010;327:172–177. doi: 10.1126/science.1180189. [DOI] [PubMed] [Google Scholar]
- Kepert JF, Mazurkiewicz J, Heuvelman GL, Toth KF, Rippe K. NAP1 modulates binding of linker histone H1 to chromatin and induces an extended chromatin fiber conformation. J Biol Chem. 2005;280:34063–34072. doi: 10.1074/jbc.M507322200. [DOI] [PubMed] [Google Scholar]
- Kim K, Lee B, Kim J, Choi J, Kim JM, Xiong Y, Roeder RG, An W. Linker Histone H1.2 cooperates with Cul4A and PAF1 to drive H4K31 ubiquitylation-mediated transactivation. Cell reports. 2013;5:1690–1703. doi: 10.1016/j.celrep.2013.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima TS, Sakuno T, Ishiguro K, Iemura S, Natsume T, Kawashima SA, Watanabe Y. Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature. 2006;441:46–52. doi: 10.1038/nature04663. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Collazo E, Ortiz-Tello PA, Trievel RC. Purification and assay protocols for obtaining highly active Jumonji C demethylases. Anal Biochem. 2012;420:48–53. doi: 10.1016/j.ab.2011.08.034. [DOI] [PubMed] [Google Scholar]
- Kueng S, Hegemann B, Peters BH, Lipp JJ, Schleiffer A, Mechtler K, Peters JM. Wapl controls the dynamic association of cohesin with chromatin. Cell. 2006;127:955–967. doi: 10.1016/j.cell.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Lenart P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, Rettig WJ, Kraut N, Peters JM. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Current Biology. 2007;17:304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- Li M, Damuni Z. I1PP2A and I2PP2A. Two potent protein phosphatase 2A-specific inhibitor proteins. Methods in molecular biology. 1998;93:59–66. doi: 10.1385/0-89603-468-2:59. [DOI] [PubMed] [Google Scholar]
- Li M, Guo H, Damuni Z. Purification and characterization of two potent heat-stable protein inhibitors of protein phosphatase 2A from bovine kidney. Biochemistry. 1995;34:1988–1996. doi: 10.1021/bi00006a020. [DOI] [PubMed] [Google Scholar]
- Li M, Makkinje A, Damuni Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J Biol Chem. 1996;271:11059–11062. doi: 10.1074/jbc.271.19.11059. [DOI] [PubMed] [Google Scholar]
- Macurek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, Clouin C, Taylor SS, Yaffe MB, Medema RH. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008;455:119–U188. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- Marston AL. Shugoshins: tension-sensitive pericentromeric adaptors safeguarding chromosome segregation. Molecular and cellular biology. 2015;35:634–648. doi: 10.1128/MCB.01176-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Nagata K, Miyaji-Yamaguchi M, Kikuchi A, Tsujimoto M. Sperm chromatin decondensation by template activating factor I through direct interaction with basic proteins. Molecular and cellular biology. 1999;19:6940–6952. doi: 10.1128/mcb.19.10.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. European journal of biochemistry. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- Mortlock AA, Foote KM, Heron NM, Jung FH, Pasquet G, Lohmann JJM, Warin N, Renaud F, De Savi C, Roberts NJ, et al. Discovery, synthesis, and in vivo activity of a new class of pyrazoloquinazolines as selective inhibitors of aurora B kinase. J Med Chem. 2007;50:2213–2224. doi: 10.1021/jm061335f. [DOI] [PubMed] [Google Scholar]
- Moshkin YM, Doyen CM, Kan TW, Chalkley GE, Sap K, Bezstarosti K, Demmers JA, Ozgur Z, van Ijcken WFJ, Verrijzer CP. Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A. Plos Genet. 2013:9. doi: 10.1371/journal.pgen.1003719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto S, Senda M, Akai Y, Sato L, Suzuki T, Nagai R, Senda T, Horikoshi M. Relationship between the structure of SET/TAF-Ibeta/INHAT and its histone chaperone activity. Proc Natl Acad Sci U S A. 2007;104:4285–4290. doi: 10.1073/pnas.0603762104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, Gnad F, Cox J, Jensen TS, Nigg EA, et al. Quantitative Phosphoproteomics Reveals Widespread Full Phosphorylation Site Occupancy During Mitosis. Sci Signal. 2010:3. doi: 10.1126/scisignal.2000475. [DOI] [PubMed] [Google Scholar]
- Ozbek U, Kandilci A, van Baal S, Bonten J, Boyd K, Franken P, Fodde R, Grosveld GC. SET-CAN, the product of the t(9;9) in acute undifferentiated leukemia, causes expansion of early hematopoietic progenitors and hyperproliferation of stomach mucosa in transgenic mice. Am J Pathol. 2007;171:654–666. doi: 10.2353/ajpath.2007.060934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi ST, Wang ZB, Ouyang YC, Zhang QH, Hu MW, Huang X, Ge ZJ, Guo L, Wang YP, Hou Y, et al. Overexpression of SET beta, a protein localizing to centromeres, causes precocious separation of chromatids during the first meiosis of mouse oocytes. J Cell Sci. 2013;126:1595–1603. doi: 10.1242/jcs.116541. [DOI] [PubMed] [Google Scholar]
- Quentmeier H, Schneider B, Rohrs S, Romani J, Zaborski M, MacLeod RAF, Drexler HG. SET-NUP214 fusion in acute myeloid leukemia- and T-cell acute lymphoblastic leukemia-derived cell lines. J Hematol Oncol. 2009:2. doi: 10.1186/1756-8722-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R, Nissen MS. Purification and assays for high mobility group HMG-I(Y) protein function. Methods in enzymology. 1999;304:155–188. doi: 10.1016/s0076-6879(99)04011-2. [DOI] [PubMed] [Google Scholar]
- Richardson RT, Batova IN, Widgren EE, Zheng LX, Whitfield M, Marzluff WF, O’Rand MG. Characterization of the histone H1-binding protein, NASP, as a cell cycle-regulated somatic protein. J Biol Chem. 2000;275:30378–30386. doi: 10.1074/jbc.M003781200. [DOI] [PubMed] [Google Scholar]
- Rosati R, La Starza R, Barba G, Gorello P, Pierini V, Matteucci C, Roti G, Crescenzi B, Romoli S, Aloisi T, et al. Cryptic chromosome 9q34 deletion generates TAF-I alpha/CAN and TAF-I beta/CAN fusion transcripts in acute myeloid leukemia. Haematol-Hematol J. 2007;92:232–235. doi: 10.3324/haematol.10538. [DOI] [PubMed] [Google Scholar]
- Saito S, Miyaji-Yamaguchi M, Nagata K. Aberrant intracellular localization of set-can fusion protein, associated with a leukemia, disorganizes nuclear export. Int J Cancer. 2004;111:501–507. doi: 10.1002/ijc.20296. [DOI] [PubMed] [Google Scholar]
- Salic A, Waters JC, Mitchison TJ. Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell. 2004;118:567–578. doi: 10.1016/j.cell.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria A, Bin W, Elowe S, Malik R, Zhang F, Bauer M, Schmidt A, Sillje HHW, Koorner R, Nigg EA. The Plk1-dependent Phosphoproteome of the Early Mitotic Spindle. Mol Cell Proteomics. 2011:10. doi: 10.1074/mcp.M110.004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintomi K, Iwabuchi M, Saeki H, Ura K, Kishimoto T, Ohsumi K. Nucleosome assembly protein-1 is a linker histone chaperone in Xenopus eggs. P Natl Acad Sci USA. 2005;102:8210–8215. doi: 10.1073/pnas.0500822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegmaier M, Hoffmann M, Baum A, Lenart P, Petronczki M, Krssak M, Gurtler U, Garin-Chesa P, Lieb S, Quant J, et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Current Biology. 2007;17:316–322. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Strunnikov AV. One-hit wonders of genomic instability. Cell Div. 2010:5. doi: 10.1186/1747-1028-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumara I, Vorlaufer E, Stukenberg PT, Kelm O, Redemann N, Nigg EA, Peters JM. The dissociation of cohesin from chromosomes in prophase is regulated by polo-like kinase. Mol Cell. 2002;9:515–525. doi: 10.1016/s1097-2765(02)00473-2. [DOI] [PubMed] [Google Scholar]
- Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell. 2004;116:51–61. doi: 10.1016/s0092-8674(03)01064-x. [DOI] [PubMed] [Google Scholar]
- Tanno Y, Kitajima TS, Honda T, Ando Y, Ishiguro K, Watanabe Y. Phosphorylation of mammalian Sgo2 by Aurora B recruits PP2A and MCAK to centromeres. Genes Dev. 2010;24:2169–2179. doi: 10.1101/gad.1945310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen JWF, Petrini JHJ. Methods for studying the cellular response to DNA damage: Influence of the Mre11 complex on chromosome metabolism. Method Enzymol. 2006;409:251. doi: 10.1016/S0076-6879(05)09015-4. [DOI] [PubMed] [Google Scholar]
- Thorslund T, Ripplinger A, Hoffmann S, Wild T, Uckelmann M, Villumsen B, Narita T, Sixma TK, Choudhary C, Bekker-Jensen S, et al. Histone H1 couples initiation and amplification of ubiquitin signalling after DNA damage. Nature. 2015;527:389. doi: 10.1038/nature15401. [DOI] [PubMed] [Google Scholar]
- Vasudevan NT, Mohan ML, Gupta MK, Hussain AK, Prasad SVN. Inhibition of Protein Phosphatase 2A Activity by PI3K gamma Regulates beta-Adrenergic Receptor Function. Mol Cell. 2011;41:636–648. doi: 10.1016/j.molcel.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Cetin B, Anger M, Cho US, Helmhart W, Nasmyth K, Xu W. Structure and function of the PP2A-shugoshin interaction. Mol Cell. 2009;35:426–441. doi: 10.1016/j.molcel.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi Y, Honda T, Tanno Y, Watanabe Y. Two histone marks establish the inner centromere and chromosome bi-orientation. Science. 2010;330:239–243. doi: 10.1126/science.1194498. [DOI] [PubMed] [Google Scholar]
- Zachariae W, Nasmyth K. Whose end is destruction: cell division and the anaphase-promoting complex. Gene Dev. 1999;13:2039–2058. doi: 10.1101/gad.13.16.2039. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Giebler HA, Isaacson MK, Nyborg JK. Eviction of linker histone H1 by NAP-family histone chaperones enhances activated transcription. Epigenet Chromatin. 2015:8. doi: 10.1186/s13072-015-0022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 – Related to Figure 1
Figure S2 – Related to Figure 2
Figure S3 – Related to Figure 3
Figure S4 – Related to Figure 4
Figure S5 – Related to Figure 5
Figure S6 – Related to Figure 5
Table S1- Peptide count numbers of SET TAP-MS analysis from asynchronous iMEFs and RPE1 cells (Related to Figure 1)