Abstract
Objective
To compare the PO2 distribution in different regions in the eyes of patients undergoing intraocular surgery.
Methods
Before initiation of intraocular cataract and/or glaucoma surgery, an optical oxygen sensor was introduced into the anterior chamber via a peripheral corneal paracentesis. The tip of the flexible fiberoptic probe was positioned by the surgeon for 3 measurements in all patients: (1) near the central corneal endothelium, (2) in the mid–anterior chamber, and (3) in the anterior chamber angle. In patients scheduled to undergo cataract extraction, PO2 was also measured (4) at the anterior lens surface and (5) in the posterior chamber just behind the iris. Oxygen measurements at the 5 locations were compared using a 2-tailed unpaired t test and multivariate regression.
Results
The PO2 value was significantly higher in African American patients at all 5 locations compared with Caucasian patients. Adjusting for age increased the significance of this association. Adjusting for race revealed that age was associated with increased PO2 beneath the central cornea.
Conclusions
Racial differences in oxygen levels in the human eye reflect an important difference in oxidative metabolism in the cornea and lens and may reflect differences in systemic physiologic function. Increased oxygen or oxygen metabolites may increase oxidative stress, cell damage, intraocular pressure, and the risk of developing glaucoma. Oxygen use by the cornea decreases with age.
Glaucoma is one of the leading causes of blindness and visual disability worldwide, affecting more than 60 million individuals.1 The prevalence of glaucoma is disproportionately higher in African populations.2 In the United States, the Baltimore Eye Survey3 found that the prevalence of glaucoma in individuals of African descent was 6 times more than the levels in the Caucasian population in some age groups. Primary open-angle glaucoma is the leading cause of blindness in the African American population, where it is 16 times more likely to result in blindness than in Caucasian Americans.4,5 Glaucoma is also diagnosed approximately 10 years earlier and shows more rapid progression in the African American compared with the Caucasian population.6 Ocular hypertension, the most important risk factor for the development of primary open-angle glaucoma, occurs 12 years earlier in this population, with a higher percentage of African Americans compared with Caucasians progressing to the development of glaucoma.6 Population-based studies outside the United States found similar results. The Barbados Eye Study showed that 1 in 11 Afro-Caribbeans older than 50 years and 1 in 6 older than 70 had open-angle glaucoma.7 On the West Indian island of St Lucia, the homogeneous Afro-Caribbean population has a prevalence of glaucoma significantly greater than that of the Caucasian populations in other studies.8 Investigations in Tanzania and South Africa confirmed the high prevalence of glaucoma in individuals of African descent.9,10
Several studies11–13 have suggested that oxidative damage contributes to the development of glaucoma. Oxidative damage may contribute to primary open-angle glaucoma by increasing intraocular pressure and by directly damaging retinal ganglion cells or their axons. Sources of oxidative damage include by-products of normal cellular metabolism, such as superoxide anion and hydrogen peroxide, which increase when cells are exposed to oxygen at higher than normal levels.
Studies14,15 have shown that removal of the vitreous body, the gel between the lens and the retina, during retinal surgery exposed the posterior surface of the lens to increased oxygen, and oxidative damage was associated with the rapid progression of nuclear cataracts. Chang16 found that vitrectomy also increased the risk of primary open-angle glaucoma, especially after cataract surgery. He hypothesized that oxygen or an oxygen metabolite, such as hydrogen peroxide, damaged cells in the outflow pathway, the main site of damage, leading to increased intraocular pressure. To test the predictions of the Chang hypothesis, we17 measured the PO2 in the eyes of patients undergoing surgical intervention for cataract and/or glaucoma. During this study, we noted significant differences in PO2 in the eyes of African American and Caucasian patients in a reference group of eyes that had not had previous cataract or vitrectomy operations. These differences in oxygen distribution reflect underlying differences in oxygen use that may be important contributors to the risk of ocular and, perhaps, systemic disease.
METHODS
STUDY DESIGN
The Human Resource Protection Office and the Institutional Review Board of the Washington University School of Medicine, in adherence with the tenets of the Declaration of Helsinki, approved this study, which is compliant with Health Insurance Portability and Accountability Act guidelines. Informed consent was obtained from the individuals after explanation of the nature and possible consequences of the study interventions. The study was designed to compare oxygen distribution in different regions of the eye in a reference group (no previous cataract or vitrectomy surgery) with oxygen distribution in patients with previous vitrectomy, cataract surgery, or both. Patients for the present analysis composed the reference group.
PATIENTS AND PO2 MEASUREMENTS
Patients undergoing cataract and/or open-angle glaucoma surgical procedures in the subspecialty practice of one of the authors (C.J.S.) were eligible for this study. Patients were excluded from the study if they had evidence of corneal endothelial dysfunction, ischemic ocular disease, anterior chamber angle closure, inflammatory disease, ocular neoplasia, or monocular status. Those who had undergone ocular surgical procedures, except for laser therapy and incisional glaucoma procedures, were also excluded from participation.
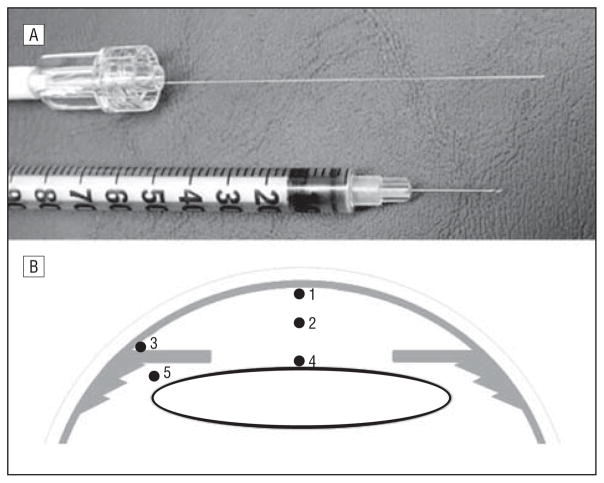
A complete general medical and ophthalmic history was obtained and a complete ophthalmic examination was performed. Central corneal thickness was measured by ultrasonography (Pachmate DGH 55; DGH Technology, Inc, Exton, Pennsylvania). Axial length measurements were recorded on patients undergoing cataract extraction (Zeiss IOLMaster; Carl Zeiss Meditec, Jena, Germany). Race was self-reported, using a standardized questionnaire. The pupils of patients undergoing cataract surgery were dilated, and pupil size was measured in all patients. As per usual surgical protocol, the patient was placed in the supine position and intravenous sedation was administered. Supplemental oxygen was provided by nasal cannula. The surgical field was separated from the cannula by an adhesive surgical drape to avoid any additional oxygen exposure to the eye. Arterial oxygen saturation monitoring was performed by continuous pulse oximetry. The surgical eye was prepped and draped and a lid speculum was placed. A sub-Tenon injection of 3 mL of lidocaine, 2%, and bupivacaine, 0.375% (50:50 ratio), was administered to provide local anesthesia. A 30-gauge needle was used for entry into the anterior chamber to fashion a peripheral corneal paracentesis, and the optical oxygen sensor (Oxylab pO2 optode; Oxford Optronix, Oxford, England) was carefully introduced into the anterior chamber without leakage of aqueous humor (Figure 1A). Instrument calibration was checked before and after each set of measurements. The tip of the flexible fiberoptic probe was positioned for 3 measurements in all patients by the surgeon (C.J.S.; Figure 1B): (1) near the central corneal endothelium, (2) in the mid–anterior chamber, and (3) in the anterior chamber angle. In patients scheduled to undergo cataract extraction, additional measurements were taken (4) at the anterior lens surface and (5) in the posterior chamber just behind the iris. This avoided the risk of damage to the lens in patients whose eyes were to remain phakic after the operative procedure. Patients were monitored postoperatively for any complications.
Figure 1.
Instrument and technique of PO2 measurement in the human eye in vivo. A, Optical oxygen sensor shown next to a standard 30-gauge tuberculin syringe needle. B, The locations in the eye where PO2 was measured: (1) beneath the central corneal endothelium, (2) mid–anterior chamber, (3) anterior chamber angle, (4) anterior lens surface, and (5) posterior chamber.
STATISTICAL ANALYSIS
Results are expressed as mean (SE). Outliers were defined as 1.5 times the interquartile range above or below the 75th or 25th percentile, respectively. Only 1 outlier was detected; removing it had no effect on the statistical significance of any of the results. Statistical analyses were performed using commercial software (SPSS, version 17.0; SPSS, Inc, Chicago, Illinois). Multivariate regression analyses were performed, with adjustment for all potential confounding variables measured. A 2-tailed unpaired t test was used to compare PO2 in the eyes of both groups. P < .05 was considered statistically significant.
RESULTS
Characteristics of the 72 patients who participated in the study are noted in Table 1. There were no significant differences in sex, and similar numbers of patients underwent surgical intervention for glaucoma, cataract, or the combined procedure. The PO2 in different regions of the eye was not related to surgical diagnosis, whether the 3 groups were compared (glaucoma only, cataract only, and cataract and glaucoma) or divided into all glaucoma vs no glaucoma or all cataract vs no cataract. The study included patients with no previous ocular surgery (48 eyes) and those with glaucoma (24 eyes) who had previously undergone a glaucoma surgical procedure (laser trabeculoplasty, iridotomy, or trabeculectomy). Excluding eyes that had undergone previous glaucoma operations had no significant effect on comparison of eyes of African Americans and Caucasians. The African American patients were significantly younger, but central corneal thickness, axial length, and arterial oxygen saturation during surgery were not significantly different between racial groups (P > .10 for all comparisons). The classes and combinations of antiglaucoma medications were similar in the 2 racial groups. Some participants had diabetes mellitus (6 Caucasian [13%] and 6 African American [32%]), but none had ischemic diabetic retinopathy. Diabetes was not significantly related to oxygen levels in the anterior segment of the eye. None of the patients regularly wore contact lenses.
Table 1.
Patient Characteristics
| Characteristic | Group
|
P Value | |||
|---|---|---|---|---|---|
| African American (n = 19) | Caucasian (n = 53) | All Patients (N = 72) | |||
| Age, mean (SD), y | 64.1 (12.5) | 72.0 (10.1) | 69.9 (11.2) | .02a | |
| Sex, No. | |||||
| Male | 10 | 26 | 36 |
|
.79b |
| Female | 9 | 27 | 36 | ||
| Surgical diagnosis, No. | |||||
| Cataract | 7 | 17 | 24 |
|
.02b |
| Glaucoma | 9 | 10 | 19 | ||
| Cataract and glaucoma | 3 | 26 | 29 | ||
By t test.
By χ2 test.
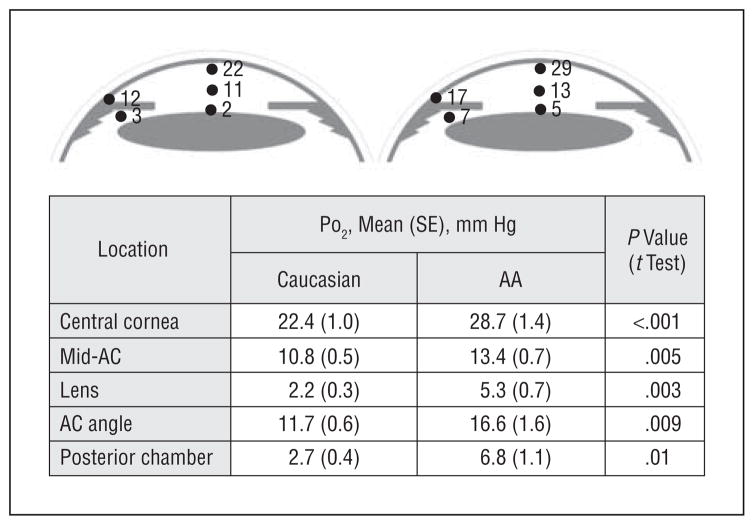
Steep oxygen gradients were identified in all eyes (Figure 2). Subsequent measurements in the same patient were within 1 mm Hg, illustrating the stability of the oxygen gradients when the probe was manipulated in the eye. Measurements in rabbits also demonstrated that intraocular oxygen gradients were stable and showed that they were not affected by convective mixing due to the position of the head (supine vs upright; unpublished data, July 2009). The approximate PO2 of air is 160 mm Hg (21 kPa). There was a 5-fold to 7-fold PO2 steady-state gradient across the cornea. A steep oxygen gradient was also present in the anterior chamber between the inner corneal surface and the anterior lens surface in both groups, with intermediate PO2 in the mid–anterior chamber. The mean PO2 measured in the anterior chamber angle was significantly lower than the measurement at the central cornea. The posterior chamber measurement of PO2 behind the iris was similar to the PO2 anterior to the lens.
Figure 2.
Diagram of PO2 at 5 location in the eye (see Figure 1). Measured values are shown next to those locations in the illustration. The table provides the mean (SE) and statistical significance of the differences in PO2 between Caucasian and African American (AA) participants, unadjusted for race or age. All differences were statistically significant. AC indicates anterior chamber.
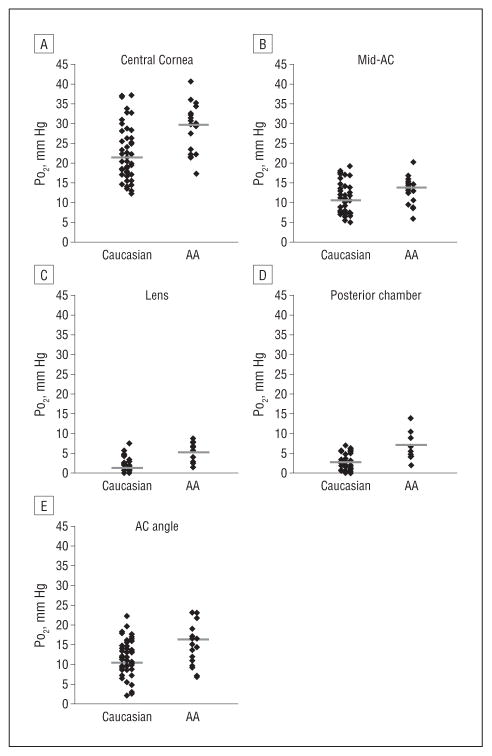
Statistical comparison revealed significantly increased PO2 in each of the 5 locations in the eyes of African American compared with Caucasian patients (Figure 2 and Figure 3). After adjusting for age, the differences in PO2 between the racial groups became more significant at all 5 locations (Table 2 and the eTable [http://www.archophthalmol.com]). Adjusting for race revealed a significant age-dependent increase in PO2 beneath the central cornea and in the central anterior chamber. No age-related change in oxygen level was detected near the lens, in the anterior chamber angle, or in the posterior chamber.
Figure 3.
Scatterplots of PO2 in Caucasian and African American (AA) patients at each of 5 intraocular locations. Solid black bars identify the mean value of the distribution. AC indicates anterior chamber.
Table 2.
Differences in PO2 at Each Location in the Eye After Adjusting for Race or Age
| Location | Adjusted for Racea
|
|||
|---|---|---|---|---|
| Effect of Age
|
Effect of Race
|
|||
| Regression Coefficient | P Value | Regression Coefficient | P Value | |
| Central cornea | 0.19 | .01 | 7.80 | <.001 |
| Mid-AC | 0.09 | .03 | 3.32 | .001 |
| Lens | 0.01 | .85 | 3.06 | <.001 |
| AC angle | 0.03 | .63 | 5.05 | .001 |
| Posterior chamber | 0.02 | .61 | 4.17 | <.001 |
Abbreviation: AC, anterior chamber.
Statistically significant P values are shown in bold.
COMMENT
RACIAL DIFFERENCES IN OCULAR OXIDATIVE METABOLISM
The significantly higher intraocular PO2 detected in African American patients is likely to reflect an important, underlying physiologic difference. The amount and distribution of oxygen in the eye are an indication of the metabolic activity of the ocular tissues. For example, oxygen diffuses from the air across the avascular cornea. The steady-state PO2 in the aqueous humor at the inner surface of the cornea depends on the diffusion constant of oxygen, diffusion distance, and the consumption of oxygen by the cornea. The diffusion constant of oxygen in corneal tissue would be expected to vary little among individuals with a normal corneal stroma and, in the study group, the PO2 beneath the central cornea was not related to corneal thickness. Therefore, the PO2 at this location is determined primarily by the consumption of oxygen by corneal cells. If oxygen consumption were lower, the PO2 at the inner surface of the cornea would be higher. Therefore, the greater mean PO2 at the inner surface of the cornea in African Americans reflects decreased oxygen consumption by the corneal cells. The corneas of the patients enrolled in this study were clear and without obvious corneal disease. Therefore, the African Americans in this study maintained normal corneal function while consuming less oxygen. Similarly, the higher PO2 measured anterior to the lens and at the other locations in the eyes of African Americans is likely to be the consequence of decreased oxygen consumption by the lens epithelium and other metabolically active tissues. Decreased oxygen consumption could be the result of increased efficiency of oxidative metabolism (less oxygen consumed per mole of adenosine triphosphate produced) or an altered balance between oxidative and glycolytic metabolism (more glucose and less oxygen consumed per mole of adenosine triphosphate produced). It is currently unclear which of these explanations accounts for the observed differences. Altered mitochondrial oxidative metabolism may be associated with diabetes,18 but we did not find any significant differences in oxygen levels between patients with and those without diabetes in this study.
Oxidative stress has been implicated as an important contributor to age-related cataract and open-angle glaucoma.15,19–21 Because all patients in this study had cataract, glaucoma, or both, the results would not reveal whether the elevated PO2 in the eyes of African Americans predisposed them to either disease. However, persons of African descent have a significantly greater risk of developing glaucoma compared with Caucasian individuals.4,5,7,8 Those of African descent also have increased risk for age-related cortical cataracts.22 The risk of these diseases could be affected by exposure to increased intraocular oxygen. Oxygen reacts with the high ascorbate in the ocular fluids,23 leading to the production of hydrogen peroxide.24,25 Peroxide in the aqueous can be degraded by catalase, but excess production could overwhelm this protective mechanism. Excess oxygen could also directly increase production of reactive oxygen species in the cells of the lens and the aqueous outflow system, the trabecular meshwork. The trabecular meshwork cells regulate intraocular pressure and are damaged and depleted in patients with open-angle glaucoma.11,26 If the differences in oxygen metabolism detected in the anterior segment of the eye reflect similar differences in the retina or optic nerve, increased oxidative stress in these tissues could also contribute to racial differences in glaucoma susceptibility and progression.
The racial differences in oxygen metabolism detected in this study may reflect genetic variation or arise from other factors. However, there is significant genetic contribution to the risk of developing glaucoma.27,28 Several studies29–33 have mapped chromosomal regions associated with dominantly inherited increased risk of open-angle glaucoma, including a recent study of individuals of African descent from Barbados.29 However, the causative genetic modifications at these loci have not been identified, and the functions of all the genes lying within the mapped intervals are not known. It would be particularly interesting if one or more genes residing at these loci were involved in oxidative metabolism or susceptibility to oxidative damage.
Results of studies that examined racial differences in oxidative metabolism are consistent with the possibility that the differences in oxygen use detected in the present study reflect differences in systemic physiology. Several studies identified lower maximal oxygen consumption (V̇O2max) in African American than in Caucasian individuals. This racial difference was present in prepubertal children34; adolescent girls35; sedentary, premenopausal women36; and similarly trained male distance runners.37 In one study,36 physiologic analysis revealed that mitochondrial oxidative capacity and oxygen delivery capability accounted for most of the measured differences in maximum oxygen consumption. It is unclear whether similar biochemical differences account for the racial variation in oxygen metabolism in the eye. However, the anterior segment of the eye provides an excellent location to study the biochemical factors influencing oxygen use since the avascular nature of the lens and cornea permits measurements that are not influenced by changes in local or systemic blood flow.
Although the racial differences in PO2 were highly significant at all 5 locations measured, the study was not designed to measure racial differences. The number of African Americans enrolled was, therefore, relatively small. In addition, self-reported race does not accurately reflect the extent of admixture in the African American population.38 In future studies, genetic classification would provide a more objective and accurate definition of racial background to evaluate disease association. Confirmatory studies are warranted. Further analysis of corneal oxygen consumption by noninvasive methods is planned.39 If such noninvasive tests correctly predict intraocular PO2 in patients, they could be useful for larger-scale clinical, epidemiologic, and genetic analyses.
Topical antiglaucoma medications, many of which inhibit the secretion of aqueous humor, were used by most of the patients in both racial groups before the glaucoma operation. Common glaucoma medications, such as carbonic anhydrase inhibitors, adrenergic agonists, and β-blockers, could alter oxygen consumption in the ciliary epithelium. Carbonic anhydrase inhibitors could also affect metabolism in the corneal endothelium. However, there were no differences between races in the classes of medications or the combinations used.
Central corneal thickness is a highly hereditable trait that has been linked40,41 to increased risk of glaucoma and progression of glaucomatous damage. Individuals of African descent have been reported42 to have thinner corneas. However, for the relatively small number of African Americans in the present study, central corneal thickness was not significantly associated with race or PO2 beneath the central cornea.
INFLUENCE OF AGE ON PO2 AND CORNEAL FUNCTION
As a group, the African Americans in this study were significantly younger than the Caucasian patients. Adjustment for age substantially increased the statistical significance of the racial differences in intraocular PO2, revealing an effect of age on ocular oxygen metabolism. After adjusting for race, increasing age was associated with a significant increase in PO2 beneath the central cornea and in the mid-anterior chamber. This observation may be consistent with reports43 of age-related decline in the number of corneal endothelial cells. It raises the possibility of a similar decline in the oxidative metabolism of the cornea, which may be linked to or separate from the decline in corneal endothelial cell density. We believe that this is the first report of an age-related change in corneal oxidative metabolism.
PHYSIOLOGIC DIFFERENCES AND RACIAL VARIATION IN DISEASE SUSCEPTIBILITY
Although we do not yet know the biochemical basis for the physiologic variations revealed by measuring intra-ocular PO2, the differences identified in this study may be significant in areas other than the eye. Racial differences in oxidative metabolism at the cellular level or in isolated mitochondria would appear to be worthy of further study. Understanding the biochemical and genetic basis of differences in oxygen metabolism could be relevant to racial variation in the prevalence of systemic diseases or drug metabolism and function.
Acknowledgments
Funding/Support: This research was supported by the American Health Assistance Foundation–National Glaucoma Research Grant (Dr Siegfried), National Eye Institute grant EY015863 (Dr Beebe), and an unrestricted grant from Research to Prevent Blindness to the Washington University Department of Ophthalmology and Visual Sciences.
Footnotes
Author Contributions: Drs Siegfried and Shui had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Siegfried and Shui contributed equally to this work.
Financial Disclosure: None reported.
Online-Only Material: The eTable is available at http://www.archophthalmol.com.
Role of the Sponsor: The funding organizations had no role in the design or conduct of this research, data analysis, or manuscript preparation.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosoko-Lasaki O, Gong G, Haynatzki G, Wilson MR. Race, ethnicity and prevalence of primary open-angle glaucoma. J Natl Med Assoc. 2006;98(10):1626–1629. [PMC free article] [PubMed] [Google Scholar]
- 3.Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma: the Baltimore Eye Survey. JAMA. 1991;266(3):369–374. [PubMed] [Google Scholar]
- 4.Javitt JC, McBean AM, Nicholson GA, Babish JD, Warren JL, Krakauer H. Undertreatment of glaucoma among black Americans. N Engl J Med. 1991;325(20):1418–1422. doi: 10.1056/NEJM199111143252005. [DOI] [PubMed] [Google Scholar]
- 5.Muñoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: the Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000;118(6):819–825. doi: 10.1001/archopht.118.6.819. [DOI] [PubMed] [Google Scholar]
- 6.Wilson R, Richardson TM, Hertzmark E, Grant WM. Race as a risk factor for progressive glaucomatous damage. Ann Ophthalmol. 1985;17(10):653–659. [PubMed] [Google Scholar]
- 7.Leske MC, Connell AM, Schachat AP, Hyman L. The Barbados Eye Study: prevalence of open angle glaucoma. Arch Ophthalmol. 1994;112(6):821–829. doi: 10.1001/archopht.1994.01090180121046. [DOI] [PubMed] [Google Scholar]
- 8.Mason RP, Kosoko O, Wilson MR, et al. National survey of the prevalence and risk factors of glaucoma in St. Lucia, West Indies, part I: prevalence findings. Ophthalmology. 1989;96(9):1363–1368. doi: 10.1016/s0161-6420(89)32708-4. [DOI] [PubMed] [Google Scholar]
- 9.Buhrmann RR, Quigley HA, Barron Y, West SK, Oliva MS, Mmbaga BB. Prevalence of glaucoma in a rural East African population. Invest Ophthalmol Vis Sci. 2000;41(1):40–48. [PubMed] [Google Scholar]
- 10.Rotchford AP, Kirwan JF, Muller MA, Johnson GJ, Roux P. Temba glaucoma study: a population-based cross-sectional survey in urban South Africa. Ophthalmology. 2003;110(2):376–382. doi: 10.1016/S0161-6420(02)01568-3. [DOI] [PubMed] [Google Scholar]
- 11.Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981;21(5):714–727. [PubMed] [Google Scholar]
- 12.Izzotti A, Bagnis A, Saccà SC. The role of oxidative stress in glaucoma. Mutat Res. 2006;612(2):105–114. doi: 10.1016/j.mrrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Saccà SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123(4):458–463. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- 14.Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139(2):302–310. doi: 10.1016/j.ajo.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 15.Truscott RJ. Age-related nuclear cataract—oxidation is the key. Exp Eye Res. 2005;80(5):709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Chang S. LXII Edward Jackson lecture: open angle glaucoma after vitrectomy. Am J Ophthalmol. 2006;141(6):1033–1043. doi: 10.1016/j.ajo.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Siegfried CJ, Shui YB, Holekamp NM, Bai F, Beebe DC. Oxygen distribution in the human eye: relevance to the etiology of open-angle glaucoma after vitrectomy. Invest Ophthalmol Vis Sci. 2010;51(11):5731–5738. doi: 10.1167/iovs.10-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212(2):167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Holekamp NM, Shui Y-B, Beebe D. Lower intraocular oxygen tension in diabetic patients: possible contribution to decreased incidence of nuclear sclerotic cataract. Am J Ophthalmol. 2006;141(6):1027–1032. doi: 10.1016/j.ajo.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004;137(1):62–69. doi: 10.1016/s0002-9394(03)00788-8. [DOI] [PubMed] [Google Scholar]
- 21.Saccà SC, Izzotti A. Oxidative stress and glaucoma: injury in the anterior segment of the eye. In: Nucci C, Cerulli L, Giacinto B, editors. Progress in Brain Research. Vol. 173. Amsterdam, the Netherlands: Elsevier; 2008. pp. 385–407. [DOI] [PubMed] [Google Scholar]
- 22.Congdon N, West SK, Buhrmann RR, Kouzis A, Muñoz B, Mkocha H. Prevalence of the different types of age-related cataract in an African population. Invest Ophthalmol Vis Sci. 2001;42(11):2478–2482. [PubMed] [Google Scholar]
- 23.Rose RC, Bode AM. Ocular ascorbate transport and metabolism. Comp Biochem Physiol A Comp Physiol. 1991;100(2):273–285. doi: 10.1016/0300-9629(91)90470-w. [DOI] [PubMed] [Google Scholar]
- 24.Spector A, Ma W, Wang RR. The aqueous humor is capable of generating and degrading H2O2. Invest Ophthalmol Vis Sci. 1998;39(7):1188–1197. [PubMed] [Google Scholar]
- 25.Shui Y-B, Holekamp NM, Kramer BC, et al. The gel state of the vitreous and ascorbate-dependent oxygen consumption: relationship to the etiology of nuclear cataracts. Arch Ophthalmol. 2009;127(4):475–482. doi: 10.1001/archophthalmol.2008.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grierson I, Howes RC. Age-related depletion of the cell population in the human trabecular meshwork. Eye (Lond) 1987;1(pt 2):204–210. doi: 10.1038/eye.1987.38. [DOI] [PubMed] [Google Scholar]
- 27.Wolfs RC, Klaver CC, Ramrattan RS, van Duijn CM, Hofman A, de Jong PT. Genetic risk of primary open-angle glaucoma: population-based familial aggregation study. Arch Ophthalmol. 1998;116(12):1640–1645. doi: 10.1001/archopht.116.12.1640. [DOI] [PubMed] [Google Scholar]
- 28.Hulsman CA, Houwing-Duistermaat JJ, Van Duijn CM, et al. Family score as an indicator of genetic risk of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(12):1726–1731. doi: 10.1001/archopht.120.12.1726. [DOI] [PubMed] [Google Scholar]
- 29.Jiao X, Yang Z, Yang X, et al. Common variants on chromosome 2 and risk of primary open-angle glaucoma in the Afro-Caribbean population of Barbados. Proc Natl Acad Sci U S A. 2009;106(40):17105–17110. doi: 10.1073/pnas.0907564106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allingham RR, Wiggs JL, Hauser ER, et al. Early adult-onset POAG linked to 15q11-13 using ordered subset analysis. Invest Ophthalmol Vis Sci. 2005;46(6):2002–2005. doi: 10.1167/iovs.04-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wirtz MK, Samples JR, Kramer PL, et al. Mapping a gene for adult-onset primary open-angle glaucoma to chromosome 3q. Am J Hum Genet. 1997;60(2):296–304. [PMC free article] [PubMed] [Google Scholar]
- 32.Wirtz MK, Samples JR, Rust K, et al. GLC1F, a new primary open-angle glaucoma locus, maps to 7q35-q36. Arch Ophthalmol. 1999;117(2):237–241. doi: 10.1001/archopht.117.2.237. [DOI] [PubMed] [Google Scholar]
- 33.Monemi S, Spaeth G, DaSilva A, et al. Identification of a novel adult-onset primary open-angle glaucoma (POAG) gene on 5q22.1. Hum Mol Genet. 2005;14(6):725–733. doi: 10.1093/hmg/ddi068. [DOI] [PubMed] [Google Scholar]
- 34.Trowbridge CA, Gower BA, Nagy TR, Hunter GR, Treuth MS, Goran MI. Maximal aerobic capacity in African-American and Caucasian prepubertal children. [Accessed July 2009];Am J Physiol. 1997 273(4 pt 1):E809–E814. doi: 10.1152/ajpendo.1997.273.4.E809. http://ajpendo.physiology.org/content/273/4/E809.long. [DOI] [PubMed] [Google Scholar]
- 35.Pivarnik JM, Bray MS, Hergenroeder AC, Hill RB, Wong WW. Ethnicity affects aerobic fitness in US adolescent girls. Med Sci Sports Exerc. 1995;27(12):1635–1638. [PubMed] [Google Scholar]
- 36.Roy JL, Hunter GR, Fernandez JR, et al. Cardiovascular factors explain genetic background differences in VO2max. Am J Hum Biol. 2006;18(4):454–460. doi: 10.1002/ajhb.20509. [DOI] [PubMed] [Google Scholar]
- 37.Weston AR, Mbambo Z, Myburgh KH. Running economy of African and Caucasian distance runners. Med Sci Sports Exerc. 2000;32(6):1130–1134. doi: 10.1097/00005768-200006000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Yaeger R, Avila-Bront A, Abdul K, et al. Comparing genetic ancestry and self-described race in African Americans born in the United States and in Africa. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1329–1338. doi: 10.1158/1055-9965.EPI-07-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonanno JA, Clark C, Pruitt J, Alvord L. Tear oxygen under hydrogel and silicone hydrogel contact lenses in humans. Optom Vis Sci. 2009;86(8):E936–E942. doi: 10.1097/OPX.0b013e3181b2f582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Surv Ophthalmol. 2008;53(6 suppl 1):S3–S10. doi: 10.1016/j.survophthal.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Dimasi DP, Burdon KP, Craig JE. The genetics of central corneal thickness. Br J Ophthalmol. 2010;94(8):971–976. doi: 10.1136/bjo.2009.162735. [DOI] [PubMed] [Google Scholar]
- 42.La Rosa FA, Gross RL, Orengo-Nania S. Central corneal thickness of Caucasians and African Americans in glaucomatous and nonglaucomatous populations. Arch Ophthalmol. 2001;119(1):23–27. [PubMed] [Google Scholar]
- 43.Niederer RL, Perumal D, Sherwin T, McGhee CN. Age-related differences in the normal human cornea: a laser scanning in vivo confocal microscopy study. Br J Ophthalmol. 2007;91(9):1165–1169. doi: 10.1136/bjo.2006.112656. [DOI] [PMC free article] [PubMed] [Google Scholar]