Abstract
We have recently shown that the expression of nestin, the neural stem cell marker protein, is expressed in bulge-area stem cells of the hair follicle. We used transgenic mice with GFP expression driven by the nestin regulatory element [nestin-driven GFP (ND-GFP)]. The ND-GFP stem cells give rise to the outer-root sheath of the hair follicle as well as an ND-GFP interfollicular vascular network. In this study, we demonstrate that ND-GFP stem cells isolated from the hair-follicle bulge area that are negative for the keratinocyte marker keratin 15 can differentiate into neurons, glia, keratinocytes, smooth muscle cells, and melanocytes in vitro. These pluripotent ND-GFP stem cells are positive for the stem cell marker CD34, as well as keratin 15-negative, suggesting their relatively undifferentiated state. The apparent primitive state of the ND-GFP stem cells is compatible with their pluripotency. Furthermore, we show that cells derived from ND-GFP stem cells can differentiate into neurons after transplantation to the subcutis of nude mice. These results suggest that hair-follicle bulge-area ND-GFP stem cells may provide an accessible, autologous source of undifferentiated multipotent stem cells for therapeutic application.
Keywords: bulge area, GFP, differentiation, glial cell, smooth muscle cell, transgenic mice
The hair follicle is dynamic, cycling between growth (anagen), regression (catagen), and resting (telogen) phases throughout life. Stem cells located in the hair-follicle bulge area give rise to the follicle structures during each anagen phase. Taylor et al. (1) reported that hair-follicle bulge stem cells are potentially bipotent because they can give rise not only to cells of the hair follicle but also to epidermal cells. Other experiments (2) also have provided evidence that the bulge-area stem cells differentiate into hair-follicle matrix cells, sebaceous-gland basal cells, and epidermis. A similar result was obtained by Fuchs and coworkers (3), who engineered transgenic mice to express histone H2B-GFP controlled by a tetracycline-responsive regulatory element as well as a keratinocyte-specific promoter. Bulge cells retained the GFP label, consistent with their role as stem cells. During anagen, newly formed GFP-positive populations derived from the bulge stem cells, form the outer-root sheath hair matrix cells and inner-root sheath. Also, in response to wounding, some GFP-labeled stem cells exited the bulge, migrated, and proliferated to repopulate the infundibulum and epidermis (3). Morris et al. (4) used a keratinocyte promoter to drive GFP expression in the hair-follicle bulge cells. They showed that bulge cells in adult mice generate all epithelial cell types within the intact follicle and hair during normal hair-follicle cycling. Toma et al. (5) reported that multipotent adult stem cells isolated from mammalian skin dermis, termed skin-derived precursors (SKPs), can proliferate and differentiate in culture to produce neurons, glia, smooth muscle cells, and adipocytes. However, the exact location of the SKP was not identified.
Fernandes et al. (6) reported the presence of pluripotent neural crest stem cells in the dermal papillae of adult mammalian hair follicles. Sieber-Blum et al. (7) reported that neural crest cells resided in the outer-root sheath from the bulge to the matrix at the base of the follicle. Bulge explants from adult mouse vibrissal follicles yielded migratory neural crest cells, including neurons, smooth muscle cells, rare Schwann cells, and melanocytes. However, the origin of the cells was not clearly defined (7).
We reported that nestin, a marker for neural progenitor cells, is also expressed in cells of the hair-follicle bulge (8). Follicle bulge cells, labeled with nestin-driven GFP (ND-GFP), behave as stem cells, differentiating to form much of the hair follicle during each hair growth cycle. Also, we observed that vessels in the skin express ND-GFP and originate from hair-follicle cells during the anagen phase in the ND-GFP mice (9). The ND-GFP vessels emerging from follicles help vascularize the dermis. The follicular origin of the blood vessels was most evident when transplanting ND-GFP-labeled follicles to unlabeled nude mice in which fluorescent new blood vessels originate only from the ND-GFP-labeled follicles. The vessels originating from the transplanted ND-GFP follicles also responded to presumptive angiogenic signals from healing wounds. The ability to form new blood vessels further demonstrated the pluripotency of hair-follicle stem cells.
In this study, we isolated bulge hair-follicle stem cells by using the ND-GFP marker. We then established that these ND-GFP-expressing stem cells are primitive, because they express the stem cell marker CD34 but do not express the keratinocyte marker keratin 15. We show that these ND-GFP stem cells can differentiate into neurons, glia, keratinocytes, smooth muscle cells, and melanocytes in vitro. Furthermore, we show that the ND-GFP-expressing stem cells can extensively differentiate into neurons after transplantation to the subcutis of nude mice.
Materials and Methods
ND-GFP Transgenic Mice. Transgenic mice carrying GFP under the control of the nestin second-intron enhancer (8–13) were obtained from G. Enikolopov (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY).
ND-GFP Cells Isolated from the Vibrissa-Follicle Bulge Area. To isolate the vibrissa follicles, the upper lip containing the vibrissa pad of ND-GFP mice was cut and its inner surface was exposed (9). The vibrissa follicles were dissected under a binocular microscope. We plucked the vibrissa from the pad by pulling them gently by the neck with fine forceps. The isolated vibrissae were washed in DMEM-F12 (GIBCO/BRL), containing B-27 (GIBCO/BRL) and 1% penicillin/streptomycin (GIBCO/BRL). All surgical procedures were done under a sterile environment. The vibrissa follicular bulge area contained ND-GFP-expressing cells. The cells were isolated under fluorescence microscopy. The isolated cells were suspended in 1 ml of DMEM-F12 containing B-27 with 1% methylcellulose (Sigma–Aldrich), and 20 ng·ml–1 basic FGF (bFGF) (Chemicon) (5, 14). Cells were cultured in 24-well tissue-culture dishes (Corning) at 37°C in a 5% CO2/95% air tissue-culture incubator. After 4 weeks, the ND-GFP bulge-area cells formed colonies.
For differentiation, the colonies were centrifuged, and the growth factor-containing DMEM-F12 medium was removed. The cells were resuspended into fresh RPMI medium 1640 (Cellgro, Herndon, VA) containing 10% FBS. The colonies were cultured in SonicSeal four-well chamber slides (Nunc) (5, 14).
For cloning experiments (14), ND-GFP cell colonies that had been cultured for 2 months were trypsinized and serially diluted into DMEM-F12 containing B-27 in 96-well uncoated tissue-culture dishes (BD Biosciences). Each chamber was assessed microscopically for the presence or absence of a single cell. The medium was then supplemented with 1% methylcellulose and 20 ng·ml–1 bFGF. The medium was changed every 2 days. After 4 weeks of clonal expansion, the ND-GFP colonies were switched to RPMI medium 1640 containing 10% FBS in the SonicSeal four-well chamber slides and differentiated. ND-GFP cells were labeled with BrdUrd for 7 days. The cells were then immunostained for anti-III-β-tubulin and anti-BrdUrd mAbs (see below).
ND-GFP Cells Isolated from Telogen Dorsal Skin Pelage Hair Follicles. ND-GFP-expressing hair follicles were isolated from telogen dorsal skin pelage hair follicles under fluorescence microscopy. The follicles were washed in DMEM-F12 containing B-27 and 1% penicillin/streptomycin. The bulge-area cells were suspended in 1 ml of DMEM-F12 containing B-27 and 20 ng·ml–1 bFGF. Cells were cultured in 24-well tissue-culture dishes at 37°C in 5% CO2/95% air. The cells formed ND-GFP colonies by 4 weeks. For differentiation, ND-GFP colonies were transferred to SonicSeal four-well chamber slides, where the colonies were resuspended in fresh RPMI medium 1640 containing 10% FBS. ND-GFP colonies were also transferred to SonicSeal four-well chamber slides in the presence of DMEM-F12 containing B-27 and 20 ng·ml–1 bFGF. Defined keratinocyte serum-free medium (GIBCO/BRL) was used for differentiation to keratinocytes.
Immunohistochemistry. The following primary Abs were used: anti-III-β-tubulin mAb (1:500, Tuj1 clone; Covance Research Products, Berkeley, CA); anti-neurofilament 200 polyclonal Ab (1:80; Sigma–Aldrich); anti-GABA polyclonal Ab (1:200; Chemicon); anti-neuronal-specific enolase mAb (1:800; Lab Vision, Fremont, CA); anti-tyrosine hydroxylase polyclonal Ab (1:100; Chemicon); anti-glial fibrillary acidic protein (GFAP) mAb (1:100; Molecular Probes); anti-2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) mAb (1:50; Lab Vision); anti-keratin 5/8 (K5/8) mAb (1:250; Chemicon); anti-keratin 15 (K15) mAb (1:100; Lab Vision); anti-smooth muscle actin (SMA) mAb (1:200; Lab Vision); anti-BrdUrd mAb (1:10; BD PharMingen); anti-CD31 mAb (1:50; Chemicon); and anti-CD34 mAb (1:10; BD PharMingen). The following secondary Abs were used: Alexa Fluor 568-conjugated goat anti-mouse (1:200; Molecular Probes); Alexa Fluor 568-conjugated goat anti-rabbit (1:200; Molecular Probes); and Alexa Fluor 647-conjugated chicken anti-rat (1:200; Molecular Probes). For BrdUrd immunocytochemistry, cells were treated as described above. For quantification of the percentage of cells producing a given marker protein, at least three fields were photographed in any given experiment, and the number of positive cells was determined relative to the total number of cells.
Immunocytochemical staining of III β-tubulin and K15 in the ND-GFP cells was detected with the Mouse-on-Mouse (MOM) immunodetection kit (Vector Laboratories). CD31 and CD34 were detected with the Ig horseradish peroxidase detection kit (BD PharMingen). Purple (VIP substrate kit, Vector Laboratories) or brown (chromogen 3,3′-diaminobenzidine substrate; BD PharMingen) staining was used for antigen detection.
Transplantation of ND-GFP-Expressing Cell Colonies into the Subcutis of Nude (nu/nu) Mice. The mice (6–8 weeks of age) (Harlan, San Diego) were anesthetized with tribromoethanol. ND-GFP cells isolated and cultured from the vibrissa-follicle bulge area were transplanted s.c. in the nu/nu mice. The incision was closed with nylon sutures (6–0). After 7 days, the subcutis of the transplanted mice was observed directly by fluorescence microscopy in a skin flap. After 14 days, the skin samples of the transplanted mice were excised and observed directly as frozen sections by fluorescence microscopy. The frozen sections were used for the immunohistochemistry staining of III β-tubulin and CD31, as described above.
Fluorescence Microscopy. The ND-GFP skin samples were directly observed with the epidermis up and dermis down under an Olympus (Melville, NY) IMT-2 inverted microscope equipped with a mercury-lamp power supply. The microscope had a GFP filter set (Chroma Technology, Brattleboro, VT).
Results
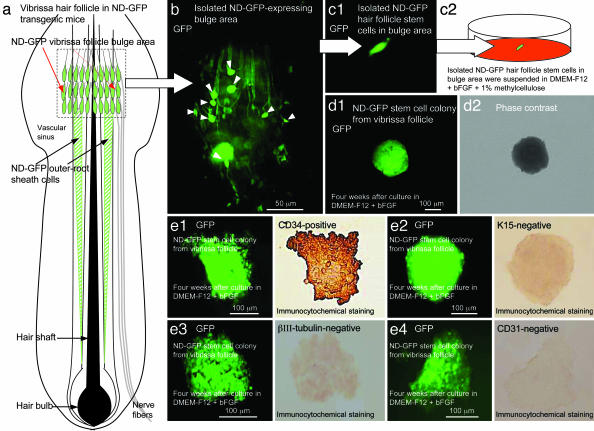
Isolated ND-GFP cells from the bulge area of vibrissa hair follicles were plated at low density in DMEM-F12 medium containing B-27 with 1% methylcellulose and bFGF. Colonies formed within 4 weeks. ND-GFP cells within the colonies derived from the vibrissa-follicle bulge were positive for the stem-cell marker CD34 but lacked many markers of differentiation (e.g., they were K15-negative and negative for the neuronal marker neural class III β-tubulin and negative for the endothelial marker CD31, indicating the relatively undifferentiated state of the cells) (Fig. 1).
Fig. 1.
Isolation, culture, and characterization of ND-GFP hair follicle stem cells. (a) Schematic representation of a vibrissa hair follicle in ND-GFP transgenic mice showing the position of the ND-GFP-expressing vibrissa follicular bulge area (red arrows) and ND-GFP-expressing outer-root sheath cells (black arrows). (b) Isolated ND-GFP-expressing vibrissa follicular bulge area contains ND-GFP-expressing hair-follicle stem cells (white arrow heads). (c1 and c2) The ND-GFP-expressing hair-follicle stem cells in the vibrissa follicular bulge area were isolated and suspended in DMEM-F12 containing B-27 and 1% methylcellulose supplemented with bFGF every 2 days. (d1 and d2) After 4 weeks, ND-GFP-expressing hair-follicle stem cells from the vibrissa follicular bulge area formed the ND-GFP-expressing cell colony. (e) ND-GFP-expressing cells within the colony from the vibrissa follicular bulge area were CD34-positive (e1), and the ND-GFP-expressing cells within the colony were K15- (e2), III β-tubulin- (e3), and CD31-negative (e4).
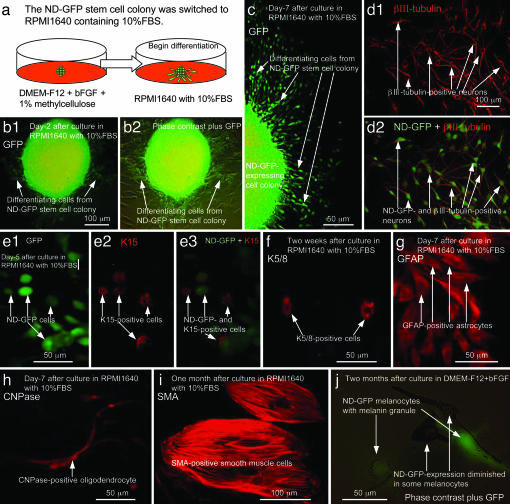
The ND-GFP cells were then transferred to RPMI medium 1640 containing 10% FBS. The ND-GFP cells began to migrate within 2 days. By 5 days, they began to differentiate to K15-positive cells. By 7 days, some ND-GFP cells differentiated to neural class III β-tubulin-positive neurons, which continued to express GFP. Most of the neural class III β-tubulin-positive neurons also had incorporated BrdUrd, indicating that they had continued to divide. Some ND-GFP cells differentiated to GFAP-positive astrocytes and 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase)-positive oligodendrocytes by 7 days. At 2 weeks after transfer to RPMI medium 1640, some ND-GFP cells differentiated to GABAergic neurons, which continued to express GFP. At 2 weeks after transfer to RPMI medium 1640, some ND-GFP cells also differentiated to K5/8-positive keratinocytes and tyrosine hydroxylase-positive neurons. ND-GFP-expression diminished in some tyrosine hydroxylase-positive neurons. At 1 month after culture in RPMI medium 1640, some ND-GFP cells became SMA-positive, indicating that smooth muscle cells were formed (Fig. 2 and Table 1).
Fig. 2.
Differentiation of ND-GFP hair follicle stem cells in vitro. (a) The ND-GFP-expressing cell colony was switched to RPMI medium 1640 containing 10% FBS from DMEM-F12 containing B-27 and 1% methylcellulose supplemented with bFGF every 2 days. (b1 and b2) At 2 days after switching into RPMI medium 1640 containing 10% FBS, differentiating cells migrated out of the ND-GFP-expressing cell colony. (c) At 7 days after switching to RPMI medium 1640, many differentiating cells migrated out of the ND-GFP-expressing cell colony. (d1 and d2) ND-GFP-expressing cells differentiated to III β-tubulin-positive neurons which maintain ND-GFP-expression. (e1–e3) At 5 days after switching to RPMI medium 1640, ND-GFP-expressing cells differentiated to K15-positive cells (red fluorescence, arrows) (e2). The K15-positive cells still expressed ND-GFP. (f) ND-GFP-expressing cells differentiated to K5/8-positive cells 2 weeks after switching to RPMI medium 1640. (g) At 7 days after switching to RPMI medium 1640, ND-GFP-expressing cells differentiated to GFAP-positive astrocytes. (h) At 7 days after switching to RPMI medium 1640, ND-GFP-expressing cells differentiated to 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase)-positive oligodendrocytes. (i) At 1 month after culture in RPMI medium 1640 containing 10% FBS, ND-GFP-expressing cells differentiated to SMA-positive smooth muscle cells. (j) At 2 months after culture in DMEM-F12 containing B-27 and 1% methylcellulose supplemented with bFGF every 2 days, ND-GFP-expressing cells differentiated to melanocytes containing melanin. Some melanocytes still expressed ND-GFP.
Table 1. Differentiated cells derived from ND-GFP hair-follicle bulge stem cells.
| Differentiated cell type | Vibrissa hair follicles, % | Dorsal pelage hair follicles, % |
|---|---|---|
| Neurons* | 48 ± 8 | 68 ± 7 |
| Glial cells* | 30 ± 8 | 16 ± 4 |
| Keratinocytes† | 18 ± 5 | 10 ± 4 |
| Smooth muscle cells‡ | 2 ± 2 | 2 ± 2 |
| Melanocytes§ | 2 ± 2 | 2 ± 2 |
At 7 days after transfer to RPMI medium 1640 containing 10% FBS.
At 2 weeks after transfer to RPMI medium 1640 containing 10% FBS.
At 1 month after transfer to RPMI medium 1640 containing 10% FBS.
At 2 months after culture in DMEM-F12 containing B-27 plus bFGF.
At 2 months after culture in the DMEM-F-12 medium, some ND-GFP cells differentiated to melanocytes. Some melanocytes continued to express ND-GFP (Fig. 2).
At 2 months after culture in the DMEM-F12 medium, single cells were isolated from the ND-GFP-expressing colonies, which were derived from the bulge area of vibrissa follicles. The single cells were cultured in the same medium and formed clones. The clones were transferred to RPMI medium 1640 and differentiated to neurons, astrocytes, oligodendrites, keratinocytes, and smooth muscle cells, as described above (data not shown).
ND-GFP cells were isolated from the bulge area of telogen pelage hair follicles from dorsal skin. The cells were cultured in DMEM-F12 containing B-27 with 1% methylcellulose and bFGF, as described above. By 4 weeks, the ND-GFP bulge-area cells had formed colonies. The ND-GFP colonies were transferred to RPMI medium 1640 containing 10% FBS. At 2 days after transfer, the ND-GFP cells started to migrate. At 5 days after transfer, some ND-GFP cells differentiated to K15-positive cells. Some ND-GFP-expressing cells differentiated to neural class III β-tubulin-positive neurons after 7 days. Most of the neural class III β-tubulin-positive neurons were also BrdUrd-positive, indicating that they were dividing. At 7 days after transfer to RPMI medium 1640, the ND-GFP cells differentiated to GFAP-positive astrocytes. At 2 weeks after transfer, some ND-GFP cells differentiated to GABAergic neurons, NF200-positive neurons, and neuronal-specific enolase-positive neurons. At 2 weeks after culture in defined keratinocyte medium, many round cells appeared that were positive for the keratinocyte marker K5/8. At 1 month after culture in RPMI medium 1640, some ND-GFP-expressing cells differentiated to SMA-positive cells indicating smooth muscle cells were formed. At 2 months after culture in the DMEM-F-12 medium, the ND-GFP-expressing cells differentiated to melanocytes containing melanin. Some melanocytes still express ND-GFP. Approximately 48% of the vibrissa bulge cells and 68% of the dorsal-follicle bulge cells produced neuronal markers (Table 1).
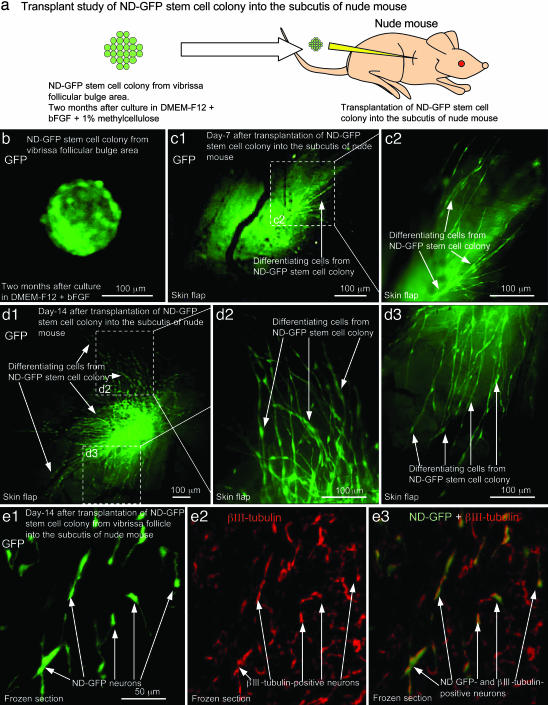
The ND-GFP cells originating from the vibrissa that were cultured in the DMEM-F12 medium for 2 months were transplanted s.c. into nude mice. At 7 days after transplantation, the ND-GFP cells began to migrate within the subcutis. At 14 days after transplantation, the ND-GFP cells differentiated to neurons, which were neural class III β-tubulin-positive and CD31-negative (Fig. 3).
Fig. 3.
Differentiation of ND-GFP hair follicle stem cells after transplantation. ND-GFP-expressing cell colony from the vibrissa follicular bulge area was cultured in DMEM-F12 containing B-27 and 1% methylcellulose supplemented with bFGF every 2 days for 2 months. (a) The ND-GFP-expressing cell colony was transplanted to the subcutis in nude mice. (b) ND-GFP-expressing cell colony before transplantation. (c1 and c2) At 7 days after transplantation into the subcutis in nude mice, ND-GFP-expressing cells migrated from the ND-GFP-expressing cell colony. External image in skin flap. (c2) High magnification of the area in c1 indicated by the white dashed box. (d1–d3) At 14 days after transplantation into the subcutis, many ND-GFP-expressing cells migrated from the ND-GFP-expressing cell colony. An external image was made through a skin flap. (d2 and d3) Higher magnification of the areas of d1 indicated by the white dashed boxes. (e) Immunofluorescence staining of III β-tubulin in a frozen section. The ND-GFP-expressing cell colony differentiated to ND-GFP- and III β-tubulin-positive neurons (arrows) (e1–e3) in the subcutis of nude mice.
Discussion
The following major conclusions are supported by our data: The hair-follicle ND-GFP bulge-area stem cells are relatively primitive because they are K15-negative and CD34-positive and remain so even after 4 weeks of culture in DMEM-F12 medium. The ND-GFP bulge-area cells can readily differentiate into many lineages as well as hair follicle cells after transfer to RPMI medium 1640. The ND-GFP cells differentiated to K15-positive cells after transfer to RPMI medium 1640 containing FBS. These cells are in the keratinocyte lineage. Other cells differentiated to the neural lineage, including neurons and glia. These ND-GFP stem cells also differentiated to smooth muscle cells. The ND-GFP cells differentiated into melanocytes in the DMEM-F12 medium, suggesting a different mechanism. Recently, the identification of melanocyte stem cells has been an area of intense interest (15, 16). The disappearance of melanocyte stem cells has been suggested as the cause of age-related hair graying (16).
Importantly, the in vivo niche for these pluripotent ND-GFP stem cells is in the hair-follicle bulge. Previously, we have shown that these ND-GFP stem cells can differentiate into the hair follicle as well as hair-follicle-associated blood vessels. ND-GFP expression was critical for the isolation of the primitive, pluripotent bulge-area stem cells. Whether the bulge-area stem cells spontaneously form neural and other cell types in their niche is open to question. The fact that the ND-GFP bulge-area stem cells could form neurons and other cell types after transplantation in vivo as well as in vitro raises possibilities of their therapeutic applications.
Author contributions: Y.A., L.L., and R.M.H. designed research; Y.A. and L.L. performed research; Y.A., L.L., K.K., S.P., and R.M.H. analyzed data; and Y.A., S.P., and R.M.H. wrote the paper.
Abbreviations: SMA, smooth muscle actin; bFGF, basic FGF; ND-GFP, nestin-driven GFP; GFAP, glial fibrillary acidic protein.
References
- 1.Taylor, G., Lehrer, M. S., Jensen, P. J., Sun, T.-T. & Lavker, R. M. (2000) Cell 102, 451–461. [DOI] [PubMed] [Google Scholar]
- 2.Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K. & Barrandon, Y. (2001) Cell 104, 233–245. [DOI] [PubMed] [Google Scholar]
- 3.Tumbar, T., Guasch, G., Greco, V., Blanpain, C., Lowry, W. E., Rendl, M. & Fuchs, E. (2004) Science 303, 359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris, R. J., Liu, Y., Marles, L., Yang, Z., Trempus, C., Li, S., Lin, J. S., Sawicki, J. A. & Cotsarelis, G. (2004) Nat. Biotechnol. 22, 411–417. [DOI] [PubMed] [Google Scholar]
- 5.Toma, J. G., Akhavan, M., Fernandes, K. J., Barnabe-Heider, F., Sadikot, A., Kaplan, D. R. & Miller, F. D. (2001) Nat. Cell Biol. 3, 778–784. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes, K. J. L., McKenzie, I. A., Mill, P., Smith, K. M., Akhavan, M., Barnabé-Heider, F., Biernaskie, J., Junek, A., Kobayashi, N. R., Yoma, J. G., et al. (2004) Nat. Cell Biol. 6, 1082–1093. [DOI] [PubMed] [Google Scholar]
- 7.Sieber-Blum, M., Grim, M., Hu, Y. F. & Szeder, V. (2004) Dev. Dyn. 231, 258–269. [DOI] [PubMed] [Google Scholar]
- 8.Li, L., Mignone, J., Yang, M., Matic, M., Penman, S., Enikolopov, G. & Hoffman, R. M. (2003) Proc. Natl. Acad. Sci. USA 100, 9958–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amoh, Y., Li, L., Yang, M., Moossa, A. R. Katsuoka, K., Penman, S. & Hoffman, R. M. (2004) Proc. Natl. Acad. Sci. USA 101, 13291–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lendahl, U., Zimmerman, L. B. & McKay, R. D. G. (1990) Cell 60, 585–595. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman, L., Parr, B., Lendahl, U., Cunningham, M., McKay, R., Gavin, B., Mann, J., Vassileva, G. & McMahon, A. (1994) Neuron 12, 11–24. [DOI] [PubMed] [Google Scholar]
- 12.Yaworsky, P. J. & Kappen, C. (1999) Dev. Biol. 205, 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mignone, J. L., Kukekov, V., Chiang, A. S., Steindler, D. & Enikolopov, G. (2004) J. Comp. Neurol. 469, 311–324. [DOI] [PubMed] [Google Scholar]
- 14.Sawamoto, K., Nakao, N., Kakishita, K., Ogawa, Y., Toyama, Y., Yamamoto, A., Yamaguchi, M., Mori, K., Goldman, S. A., Itakura, T. & Okano, H. (2001) J. Neurosci. 21, 3895–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimura, E. K., Jordan, S. A., Oshima, H., Yoshida, H., Osawa, M., Moriyama, M., Jackson, I. J., Barrandon, Y., Miyachi, Y. & Nishikawa, S. (2002) Nature 416, 854–860. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura, E. K., Granter, S. R. & Fisher, D. E. (2005) Science 301, 720–724. [DOI] [PubMed] [Google Scholar]